Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.107526
Revised: May 8, 2025
Accepted: July 8, 2025
Published online: August 15, 2025
Processing time: 127 Days and 21.1 Hours
Colorectal cancer (CRC) frequently metastasizes to the liver, significantly compromising patient survival. While surgical resection improves outcomes for resectable cases, many patients have limited therapeutic options.
To evaluate the role of laparoscopic ultrasound in resection and ablation of colorectal liver metastases (CRLM).
Between June 2018 and June 2020, 300 patients with CRC and liver metastases were admitted to our hospital. They were divided into two groups (150 cases each) based on treatment method: The control group (ethoxybenzyl diethylenetriamine penta-acetic acid enhanced magnetic resonance imaging) and the observation group [contrast-enhanced ultrasound with Sonazoid (S-CEUS)].
The study group demonstrated better efficacy (P < 0.05), fewer adverse events (P < 0.05), and better survival outcomes compared to the control group (1-year: 80% vs 62%; 3-year: 54% vs 33%; 5-year: 32% vs 18%; median survival: 48 months vs 30 months; hazard ratio = 0.63, 95%CI: 0.48-0.83, P < 0.001). Although Karnofsky Performance Status scores improved in both groups, the scores were significantly higher in the observation group (P < 0.05). Multivariate analysis confirmed intraoperative S-CEUS and tumor differentiation as independent prognostic factors (P < 0.05).
Laparoscopic ultrasound-guided resection/ablation improved outcomes in CRLM, reducing complications and enhancing survival. Intraoperative S-CEUS was an independent prognostic factor, supporting its clinical value.
Core Tip: This study demonstrates that intraoperative laparoscopic ultrasound [contrast-enhanced ultrasound with Sonazoid (S-CEUS)]-guided surgical resection and microwave ablation significantly enhances clinical outcomes in colorectal cancer liver metastasis patients compared to preoperative ethoxybenzyl diethylenetriamine penta-acetic acid enhanced magnetic resonance imaging planning. The S-CEUS group exhibited superior oncologic efficacy (higher 5-year survival: 32% vs 18%), fewer adverse events, and improved quality of life (Karnofsky Performance Status scores). Multivariate analysis confirmed intraoperative S-CEUS utilization and primary tumor differentiation as independent prognostic factors. These findings highlight S-CEUS-guided strategies as a critical advance in optimizing oncologic precision and long-term survival in metastatic liver surgery, warranting broader clinical adoption.
- Citation: Wu HR, Bu H, Liu YY, Zhou HP, Ye JS, Chen H. Intraoperative laparoscopic ultrasound-guided resection and microwave ablation for colorectal liver metastases. World J Gastrointest Oncol 2025; 17(8): 107526
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/107526.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.107526
Colorectal cancer (CRC) is one of the most common malignant tumors, characterized by high incidence and mortality rates[1]. According to the 2022 epidemiological analysis report of malignant tumors in China[2], CRC is the third most commonly diagnosed malignancy and fifth in cancer-related mortality nationwide. Among CRC patients, the liver is the most frequent site of distant metastasis, with approximately 50% of patients developing liver metastases during disease progression. Colorectal liver metastases (CRLM) represent one of the critical factors contributing to patient mortality, and the prognosis remains challenging. In CRLM patients, the median survival time typically ranges from 6 to 12 months[3-6]. While surgical resection remains the primary treatment modality for CRLM, particularly for resectable liver metastases where it can significantly prolong patient survival. However, surgical options are limited for initially unresectable or multifocal metastatic malignancies[7-10].
With advances in minimally invasive techniques, microwave ablation has emerged as a local minimally invasive treatment modality offering distinct advantages including minimal trauma, rapid recovery, and excellent repeatability[11,12]. Laparoscopic techniques in CRC surgery have gained widespread clinical acceptance due to their potential benefits, including reduced trauma, accelerated postoperative recovery, and lower complications rates. The incorporation of intraoperative ultrasound (IOUS) guidance in laparoscopic procedures allows for more precise localization of liver metastases, thereby improving both surgical accuracy and patient safety. Specifically, IOUS provides real-time visualization of hepatic lesions, allowing surgeons to better monitor and localize metastases during laparoscopy, thereby optimizing surgical planning and ablation ranges. Furthermore, laparoscopic microwave ablation enables direct visualization, allowing for effective isolation and ablation of superficial hepatic metastases or lesions near critical structures while minimizing surrounding tissue damage. Compared to traditional staged treatment approaches, the one-stop treatment model combining laparoscopic CRC radical surgery with synchronous microwave ablation or resection of liver metastases reduces risks associated with repeated anesthesia and multiple surgeries while decreasing overall hospitalization periods[13-16].
This study compares the efficacy of preoperative exclusive use of ethoxybenzyl diethylenetriamine penta-acetic acid enhanced magnetic resonance imaging (EOB-MRI) technology and intraoperative combined application of contrast-enhanced ultrasound with Sonazoid (S-CEUS). The findings are reported as follows.
A total of 300 patients with colorectal malignancies and liver metastases admitted to the hospital from June 2018 to June 2020 were enrolled as study subjects. They were divided into two groups based on treatment methods: (1) A control group (preoperative exclusive use of EOB-MRI); and (2) An observation group (intraoperative combined application of S-CEUS in addition to the control group protocol), with 150 cases in each group. There were no statistically significant differences of baseline characteristics between the two groups (Table 1) (P > 0.05). This study was conducted after approval by the hospital’s ethics committee.
| Group | n | Gender (n) | Age (years) | Body mass index (kg/m2) | Number of liver metastases | Primary tumor location | |||
| Male | Female | Single | Multiple | Rectal cancer | Colon cancer | ||||
| Control | 150 | 74 | 76 | 59.60 ± 2.15 | 19.52 ± 0.77 | 81 | 69 | 79 | 71 |
| Observation | 150 | 77 | 73 | 59.98 ± 2.74 | 19.49 ± 0.83 | 83 | 67 | 76 | 74 |
| t/χ² | 0.120 | 1.336 | 0.325 | 0.054 | 0.120 | ||||
| P value | 0.729 | 0.183 | 0.746 | 0.817 | 0.729 | ||||
Inclusion criteria: (1) Age 18-80 years; (2) Adequate future liver remnant (FLR), defined as: FLR > 30% in patients without underlying liver disease, FLR > 50% in patients with underlying liver disease; (3) Presence of resectable extrahepatic metastases; (4) Preoperative EOB-MRI confirmation of CRLM; (5) Requiring radical hepatectomy, with ≥ 2 but < 10 Lesions; and (6) Informed consent obtained from patients and their families.
Exclusion criteria: (1) Inability to undergo required imaging examinations, including contrast-enhanced computed tomography, EOB-MRI, contrast-enhanced ultrasound (CEUS), IOUS, or contrast-enhanced IOUS; (2) Preoperative diagnosis of ≥ 10 CRLM lesions; (3) Previous treatment of liver lesions (e.g., local ablation therapy or transarterial chemoembolization); (4) Severe cardiac or pulmonary dysfunction; and (5) Lactating or pregnant women.
Control group: Patients underwent preoperative evaluation with EOB-MRI alone. Preoperative routine MRI was performed to determine tumor staging, location, and number of liver lesions caused by metastases. Subsequently, laparoscopic CRC radical surgery combined with ultrasound-guided liver metastasis resection or microwave ablation was conducted.
Observation group: Patients underwent intraoperative combined application of S-CEUS in addition to the control group protocol. Preoperative routine magnetic resonance imaging (MRI) was performed to evaluate tumor staging, lesion, and number of liver metastases. During surgery, CEUS was employed to compare with preoperative MRI findings, detect any radiographically occult CRLM lesions, and guide subsequent laparoscopic CRC radical surgery with ultrasound-guided liver metastasis resection or microwave ablation.
Laparoscopic radical resection of CRC was performed as follows: After establishing a pneumoperitoneum using high-definition laparoscopic systems with ultrasonic scalpels, energy platforms and other instruments, the abdominal cavity was explored via laparoscopy to identify the tumor location, dimensions and anatomical relationship to surrounding tissues. The procedure was systematically performed in three key steps. Lymph node dissection was performed along the root of the mesenteric vessels. The mesenteric vessels were transected using instruments such as ultrasonic scalpels, followed by complete resection of the tumor-bearing intestinal segment. Finally, intestinal anastomosis was performed.
Ultrasonically guided resection of hepatic metastases: The procedure was performed using ultrasonic diagnostic apparatus, laparoscopic surgical instruments, etc. Using ultrasonic guidance, the exact location of the hepatic metastases was determined. Following laparoscopic access, the liver tissue was resected along the predetermined tangent line to ensure complete removal of metastases while maximizing preservation of functional hepatic tissue.
Microwave ablation: The power of microwave ablation was set to 30-50 W with a duration of 5-10 minutes. The specific parameters were adjusted according to lesion size and location. Under ultrasonic guidance, the microwave ablation needle was accurately inserted into the hepatic metastases. The microwave ablation device was activated, and the tumor tissue underwent coagulative necrosis due to generation of localized hyperthermia by the microwaves.
EOB-MRI protocol, MRI system: ≥ 1.5 T. (1) Contrast agent: Gd-EOB-DTPA (Gadoxetic Acid Disodium, Beijing Takeda Pharmaceutical Co., Ltd.) was injected via the antecubital vein as a bolus at a dose of 0.1 mL/kg, administered at a flow rate of 2 mL/second, followed by 20 mL saline flush; (2) Scanning sequence: Three-dimensional fat-suppressed gradient echo sequence; and (3) Data management: Raw imaging data were recorded and archived for subsequent analysis.
S-CEUS protocol, target lesion selection: The clearest target lesion was identified and fixed. (1) Contrast agent: Sonazoid (GE Healthcare, United States) injected via the antecubital vein as a bolus at a dose of 0.01 mL/kg, administered at 1 mL/second, followed by 5–10 mL saline flush; (2) Imaging phases: Vascular phase (arterial, portal, and delayed phases) and post-vascular phase imaging; (3) Lesion evaluation: During the post-vascular phase, whole-liver imaging was performed to detect preoperatively confirmed lesions and newly identified lesions. Data on hepatic lesions were observed and recorded; and (4) Data management: Raw CEUS imaging data were recorded and archived for subsequent analysis.
In EOB-MRI, the lesion signal intensity was observed during the vascular phase (arterial phase or portal venous phase) and evaluated for signal changes during the delayed phase or hepatobiliary phase. Hepatic metastases were radiologically confirmed when the lesions exhibited hyperintensity during the vascular phase concurrent with hypointensity in the delayed phase, or hyperintensity in the vascular phase and hypointensity during the hepatobiliary phase.
In S-CEUS, lesion enhancement was observed during the arterial phase followed by washout in either the delayed phase or Kupffer cell phase. Hepatic metastases were radiologically confirmed when the lesion showed arterial-phase hyperenhancement followed by washout in either the delayed phase (2-3 minutes) or the Kupffer cell phase (after 10 minutes).
Measurement of tumor size: Tumor dimensions were measured using electronic calipers to record the longest axial diameters and shortest perpendicular diameter in millimeters. For tumors with an irregular shape, a multi-diameter measurement approach was employed to comprehensively evaluate tumor size.
S-CEUS diagnosis (meeting either criterion): (1) Lesion showed hyperenhancement in the arterial phase and washout in the delayed phase (2–3 minutes); and (2) Lesion showed hyperenhancement in the arterial phase and washout in the Kupffer phase (10 minutes post-injection).
EOB-MRI diagnosis (meeting either criterion): (1) Lesion hyperintensity in the vascular phase (arterial or portal phase) and hypointensity in the delayed phase; and (2) Lesion hyperintensity in the vascular phase (arterial or portal phase) and hypointensity in the hepatobiliary phase.
Patients underwent systematic postoperative surveillance via telephone interviews, outpatient reviews, and re-hospitalization records starting from the first postoperative month and every 2 months thereafter until tumor recurrence, patient death, or study termination.
Efficacy comparison: The efficacy was evaluated for each group according to the Response Evaluation Criteria in Solid Tumors[7].
Complete response: Disappearance of all target lesions with no evidence of new lesions during a follow-up period of at least 4 weeks.
Partial response: ≥ 30% reduction in the sum of the maximum diameters of target lesions compared to baseline, sustained for at least 4 weeks.
Stable disease: Reduction in the sum of lesion diameters between 20% and 30% (excluding 30%) or an increase ≤ 20% compared to baseline.
Progressive disease: > 20% increase in the sum of lesion diameters or the appearance of new lesions.
Overall response rate was calculated as: Overall response rate = (number of complete response cases + number of partial response cases)/total number of cases × 100%. Comparison of adverse reactions between groups[8].
The incidence of adverse reactions was calculated as: Incidence (%) = (number of patients with hepatic dysfunction + number of patients with anemia + number of patients with gastrointestinal reactions + number of patients with neurotoxicity)/total number of patients × 100%.
Comparison of Karnofsky Performance Status scores between groups: The Karnofsky Performance Status (KPS) scoring system was employed to assess the quality of life in both groups before treatment and 1-month post-treatment. The evaluation comprehensively measured the following key domains: (1) Ability to perform activities of daily living; (2) Physical activity level; (3) Work capacity; (4) Ability to tolerate treatment; and (5) The total score ranges from 0 to 100 points, with higher scores indicating better functional status.
Analysis of prognostic risk factors in patients with CRC complicated by liver metastases: (1) Univariate regression analysis; and (2) Multivariate regression analysis.
Data analysis was performed using Statistical Package for the Social Sciences 25.0 software. Quantitative data were expressed as mean ± SD and analyzed using t-tests. Qualitative data were presented as frequency and percentage (%) and analyzed using the χ² test. Univariate and multivariate logistic regression analyses were conducted to identify prognostic factors influencing outcomes in patients with CRC concurrent with liver metastases. P value < 0.05 was considered statistically significant.
The observation group demonstrated significantly higher therapeutic efficacy compared to the control group (P < 0.05), as shown in Table 2.
| Group | n | Complete response | Partial response | Stable disease | Progressive disease | Overall response rate |
| Control | 150 | 15 (10.00) | 61 (40.67) | 35 (23.33) | 39 (26.00) | 76 (50.67) |
| Observation | 150 | 17 (11.33) | 89 (59.33) | 31 (20.67) | 13 (8.67) | 106 (70.67) |
| χ² | 12.572 | |||||
| P value | 0.000 |
The incidence of adverse reactions in the observation group was significantly lower than that in the control group (P < 0.05), as detailed in Table 3.
| Group | n | Hepatic dysfunction | Anemia | Gastrointestinal reactions | Neurotoxicity | Incidence (%) |
| Control | 150 | 27 (18.00) | 10 (6.67) | 13 (8.67) | 11 (7.33) | 61 (40.67) |
| Observation | 150 | 13 (8.67) | 5 (3.33) | 3 (2.00) | 3 (2.00) | 24 (16.00) |
| χ² | 22.473 | |||||
| P value | 0.000 |
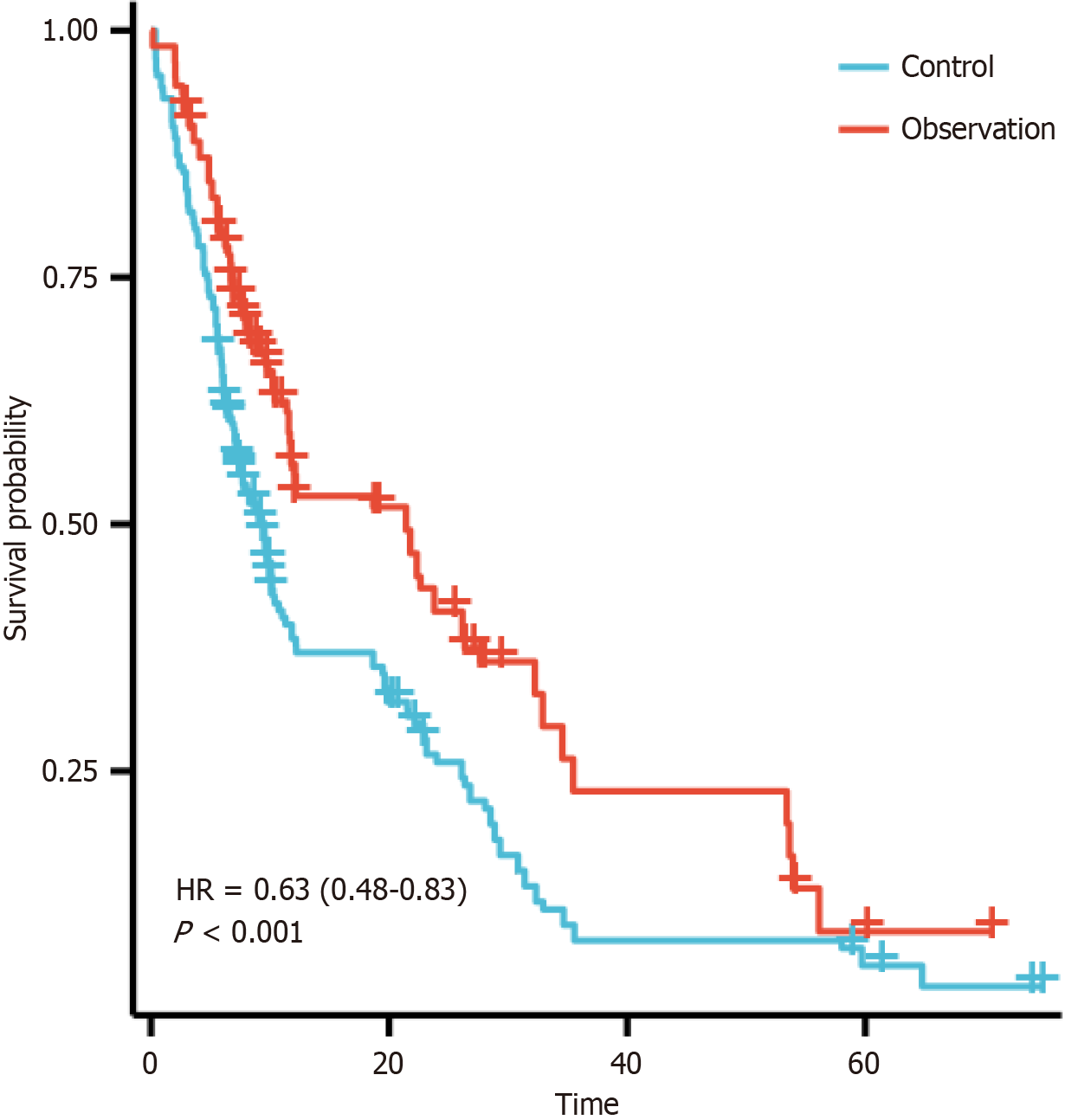
This figure represents the comparative survival outcomes between the control group (using only EOB-MRI before surgery) and the observation group (using EOB-MRI before surgery and combining with S-CEUS during surgery). The control group (blue curve) demonstrated a 1-year survival rate of approximately 36%, a 3-year survival rate of approximately 14%, and a 5-year survival rate of approximately 4%, with a median survival period of approximately 16 months. The observation group (red curve) had a 1-year survival rate of approximately 50%, a 3-year survival rate of approximately 25%, and a 5-year survival rate of approximately 8%. The median survival period was approximately 22 months. While comparing the two groups, the overall survival rate of the observation group was higher than that of the control group, and the difference was statistically significant (hazard ratio = 0.63, 95%CI: 0.48-0.83, P < 0.001). A total of 300 patients were included in this study, with 150 patients in the control group and 150 patients in the observation group. During the follow-up process, 79 patients were lost to follow-up, and the number of dropouts was 45 (Figure 1).
After treatment, KPS scores significantly increased in both groups, with a more pronounced improvement in the observation group (P < 0.05), as presented in Table 4.
Univariate regression analysis: Univariate analysis revealed that intraoperative application of S-CEUS, postoperative N stage, number of liver metastases, and degree of primary tumor differentiation grade all showed significant prognostic value (P < 0.05), as shown in Table 5.
| Factor | n | 3-year overall survival (%) | 95%CI | χ² | P value |
| Gender | 0.077 | 0.898 | |||
| Male | 151 | 34.45 | 27.31-38.92 | ||
| Female | 149 | 42.27 | 27.12-41.13 | ||
| Age | 0.327 | 0.742 | 0.393 | ||
| ≤ 60 years | 162 | 39.87 | 29.51-41.77 | ||
| > 60 years | 138 | 31.65 | 24.31-37.65 | ||
| American Society of Anesthesiologists grade | 0.043 | 0.992 | |||
| 1 | 56 | 47.92 | 22.17-44.91 | ||
| 2 | 213 | 34.63 | 28.21-38.94 | ||
| 3 | 31 | 25.18 | 18.81-41.56 | ||
| Intraoperative contrast-enhanced ultrasound with Sonazoid | 0.798 | 0.000 | |||
| Yes | 150 | 54.87 | 19.46-41.17 | ||
| No | 150 | 33.27 | 28.32-37.91 | ||
| Postoperative T stage | 0.463 | 0.521 | |||
| T2-T3 | 229 | 43.31 | 29.69-40.28 | ||
| T4 | 71 | 21.24 | 21.36-38.77 | ||
| Postoperative N stage | 7.954 | 0.020 | |||
| N0 | 114 | 54.31 | 33.22-47.51 | ||
| N1 | 118 | 26.42 | 25.47-39.69 | ||
| N2 | 68 | 26.38 | 14.78-32.91 | ||
| Number of liver mets | 5.269 | 0.033 | |||
| ≤ 3 | 182 | 49.12 | 31.92-44.93 | ||
| >3 | 118 | 24.63 | 22.31-34.17 | ||
| Tumor differentiation | 6.199 | 0.024 | |||
| Low | 142 | 52.37 | 33.12-45.64 | ||
| Moderate/high | 158 | 22.65 | 21.42-33.57 |
Multivariate logistic regression analysis: Multivariate logistic regression analysis confirmed intraoperative application of S-CEUS and degree of primary tumor differentiation as independent prognostic factors (P < 0.05), as summarized in Table 6.
| Factor | Coding | Hazard ratio | 95%CI | P value |
| Intraoperative contrast-enhanced ultrasound with Sonazoid application | No = 0; yes = 1 | 2.134 | 1.162-3.929 | 0.017 |
| Postoperative N stage | N0 = 0; N1 = 1; N2 = 2 | 1.459 | 0.995-2.169 | 0.176 |
| Number of liver metastases | ≤ 3 = 0; > 3 = 1 | 1.480 | 0.913-2.524 | 0.251 |
| Differentiation of primary tumor | Low = 0; moderate/high = 1 | 0.521 | 0.312-0.914 | 0.017 |
CRC is a common malignant tumor of the digestive system. Although its exact etiology remains unclear, it is closely associated with multiple factors including unhealthy dietary habits, genetic predisposition, chronic inflammatory stimulation (such as ulcerative colitis and Crohn's disease), and colorectal polyps[17-19]. Epidemiological data indicate that CRC is the third most commonly diagnosed malignancy in terms of incidence and the second leading cause of cancer-related deaths worldwide, posing a significant threat to human health due to its high morbidity and mortality rates[20]. In current clinical practice, conventional treatment modalities for CRC encompass surgical intervention, chemotherapy, radiotherapy, immunotherapy, targeted therapy, and endoscopic treatment. Surgical resection remains the cornerstone of treatment, primarily involving radical resection procedures such as total mesorectal excision and radical colectomy to remove malignant tissues[21-23]. Early screening through regular colonoscopy and fecal occult blood testing facilitates early detection of precancerous lesions and early-stage cancers, thereby substantially improving therapeutic efficacy and patient long-term survival rates. However, despite their therapeutic benefits, radiotherapy and chemotherapy are associated with notable adverse effects in CRC patients, including gastrointestinal discomfort, oral mucositis, diarrhea, abdominal pain, and hepatic impairment[24]. For patients with CRC liver metastasis undergoing localized therapy, the dual imaging approach combining preoperative EOB-MRI (ethoxybenzyl MRI) with intraoperative S-CEUS (CEUS) offers distinct clinical benefits. EOB-MRI exhibits high accuracy in detecting hepatic metastases from CRC, providing clear visualization of hepatic anatomical structures and tumor morphology to facilitate precise clinical evaluation and treatment planning. Meanwhile, S-CEUS enhances diagnostic accuracy through real-time imaging of tumor vascularity and morphological changes. The combined application of these two modalities provides physicians with more comprehensive imaging information, enabling better assessment of tumor staging and resectability. This integrated approach not only improves therapeutic efficacy but also extends patient survival[25].
Together, the results demonstrated that the observation group exhibited higher therapeutic efficacy and a lower incidence of adverse reactions compared to the control group, (P < 0.05), indicating that the treatment regimen of ultrasonic guidance during laparoscopic surgery combined with surgical resection and microwave ablation improves therapeutic efficacy, reduces the risk of adverse reactions, and enhances treatment safety and tolerability. The analysis suggests that the application of S-CEUS can significantly improve surgical precision. This approach achieves dual therapeutic benefits by simultaneously enhancing tumor removal efficiency and reducing damage to healthy hepatic tissues, thereby optimizing overall oncologic outcomes. Moreover, the precise treatment approach significantly reduced systemic adverse effects on the body, resulting in a lower incidence of treatment-related complications. In addition, S-CEUS provides superior visualization of the anatomical relationship between the tumor and the portal venous system during the portal venous phase imaging, thereby reducing the possibility of missed lesions, which is an important anatomical basis for optimizing therapeutic efficacy.
The overall survival rate of the observation group was higher than that of the control group. At 1-month post-treatment, both groups showed significant improvement in KPS scores, with markedly higher significance in the observation group (P < 0.05), indicating that the treatment regimen of the observation group prolonged survival and significantly improved their quality of life. These outcomes indicate that the higher survival rate and improved quality of life are related to the more thorough tumor tissue removal and reduced tumor burden associated with this treatment regimen, thus prolonging patient survival time. Furthermore, due to the lower incidence of adverse reactions during treatment, the physical status and body functions of patients can be better restored and maintained, further promoting the quality of life.
However, Li’s study[26] found that there was no significant difference in survival rate between thermal ablation combined with systemic treatment and simple thermal ablation treatment. This variation may be attributed due to distinct patient populations. All the patients included in this study were patients with solely liver metastases from CRC, while Li's study[26] included patients with various types of liver metastases. Multivariate Logistic regression analysis showed that whether S-CEUS was applied during the operation and the degree of differentiation of the primary tumor were independent influencing factors for the prognosis of patients with colorectal malignant tumors complicated by liver metastases (P < 0.05), indicating that ultrasonic guidance during the operation and the degree of differentiation of the primary tumor play crucial roles in the prognosis of patients with liver metastases from CRC. These two factors can be used as an important indicators for evaluating the prognosis of patients. These outcomes demonstrated that the application of S-CEUS during the operation can provide more accurate intraoperative imaging information, guiding doctors to more clearly visualize tumor boundaries and vascular structures. While concurrent application of the scope of surgical resection with the positioning of microwave ablation, treatment precision and efficacy can be improved, thereby improving patient prognosis. Patients with poorly differentiated primary tumors exhibit stronger tumor invasiveness and proliferation ability, indicating a worse prognosis. Therefore, the high-risk subgroup warrants a more aggressive treatment approach and strategies to improve the prognosis of these patients.
The treatment regimen of this study has significant effects in improving the survival rate of patients with liver metastases from CRC, reducing the incidence of adverse reactions, and improving the quality of life. However, there are still the following limitations: (1) The requirements for advanced equipment and technology currently restricts widespread adoption and application at the grassroots level; (2) The follow-up time is short, and long-term evaluation is required for evaluating survival outcomes; and (3) Lack of molecular profiling limits biological correlation analysis. In addition, there is a loss of the sample size in this study, which may affect the results of the survival analysis. Therefore, future studies should perform sensitivity analysis to validate the findings of the long-term efficacy and prognostic value of the treatment regimen.
The combined application of preoperative EOB-MRI technology combined with the intraoperative use of S-CEUS can not only effectively improve patients' survival rate and quality of life but also reduce the incidence of adverse reactions. Additionally, the intraoperative application of S-CEUS and the degree of differentiation of the primary tumor are independent factors influencing patient prognosis, providing crucial references for clinical treatment and prognostic assessment. Future studies should expand the sample size, extend the follow-up period, explore advanced techniques and personalized treatment regimens in conjunction with tumor biological characteristics, and strengthen training at the grassroots level to optimize treatment protocols.
| 1. | Han L, Wu XL, Guo F, Xi YN, Chang XY, Zhang CZ, Zhang JF, Ma PC. [Clinical Application of Microwave Ablation in Potentially Resectable Colorectal Cancer With Simultaneously Multiple Liver Metastases]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2024;46:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Unterrainer M, Deroose CM, Herrmann K, Moehler M, Blomqvist L, Cannella R, Caramella C, Caruso D, Chouhan MD, Denecke T, De la Pinta C, De Geus-Oei LF, Dulskas A, Eisenblätter M, Foley KG, Gourtsoyianni S, Lecouvet FE, Lopci E, Maas M, Obmann MM, Oprea-Lager DE, Verhoeff JJC, Santiago I, Terraz S, D'Anastasi M, Regge D, Laghi A, Beets-Tan RGH, Heinemann V, Lordick F, Smyth EC, Ricke J, Kunz WG; European Organisation for Research and Treatment of Cancer (EORTC) Imaging Group; European Organisation for Research and Treatment of Cancer (EORTC) Gastrointestinal Tract Cancer Group; European Society of Oncologic Imaging (ESOI) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR). Imaging standardisation in metastatic colorectal cancer: A joint EORTC-ESOI-ESGAR expert consensus recommendation. Eur J Cancer. 2022;176:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Cui R, Zhou J, Yang W, Chen Y, Chen L, Tan L, Zhang F, Liu G, Yu J. Ultrasound-Triggered Nanogel Boosts Chemotherapy and Immunomodulation in Colorectal Cancer. ACS Appl Mater Interfaces. 2025;17:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Li J, Pang C, Liu G, Xie X, Zhang DZ, Li K, Li Z, He G, Xu E, Zhong H, Yang H, Lu M, Lou K, Xie X, Lan S, Li Q, Dai G, Yu J, Liang P. Thermal ablation with and without adjuvant systemic therapy: a nationwide multicenter observational cohort study of solitary colorectal liver metastases. Int J Surg. 2024;110:4240-4248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Lian J, Li J, Liu C, Luan B, Miao Y. Research progress of robot and laparoscope in postoperative complications of rectal cancer. J Robot Surg. 2024;18:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Xie Y, Wang P, Chen Y, Qin S, Li F, Wu Y, Huang M, Hou Z, Cai Y, He X, Lin H, Hu B, Qin Q, Ma T, Tan S, Liao Y, Ke J, Zhang D, Lai S, Jiang Z, Wang H, Xiang J, Cai Z, Wang H, He X, Yang Z, Ren D, Wu X, Hong Y, Huang M, Luo Y, Liu G, Lin J. Evaluation of transrectal ultrasound-guided tru-cut biopsy as a complementary method for predicting pathological complete response in rectal cancer after neoadjuvant treatment: a phase II prospective and diagnostic trial. Int J Surg. 2024;110:3230-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Aishah Baharuddin S, Nadiah Abd Karim Shah N, Saiful Yazan L, Abd Rashed A, Kadota K, Al-Awaadh AM, Aniza Yusof Y. Optimization of Pluchea indica (L.) leaf extract using ultrasound-assisted extraction and its cytotoxicity on the HT-29 colorectal cancer cell line. Ultrason Sonochem. 2023;101:106702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Jiang Q, Chen M, Yuan L, Yao L. Laparoscopic posterior pelvic exenteration using the abdominal perineal resection (APR) technique for management of tumor invading both vagina and rectum. Int J Gynecol Cancer. 2023;33:1656-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Huang S, Ding D, Lan T, He G, Ren J, Liang R, Zhong H, Chen G, Lu X, Shuai X, Wei B. Multifunctional nanodrug performs sonodynamic therapy and inhibits TGF-β to boost immune response against colorectal cancer and liver metastasis. Acta Biomater. 2023;164:538-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 10. | Park SY, Ha GW, Lee SY, Kim CH, Son GM. Impact of non-muscle cutting periumbilical transverse incision on the risk of incisional hernia as compared to midline incision during laparoscopic colon cancer surgery: a study protocol for a multi-centre randomised controlled trial. Trials. 2023;24:152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Jones C, Badger SA, Ellis G. The role of microwave ablation in the management of hepatic colorectal metastases. Surgeon. 2011;9:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Lin J, Liu H, Liang S, Luo L, Guan S, Wu S, Liu Y, Xu S, Yan R, Xu E. Microwave ablation for colorectal liver metastases with ultrasound fusion imaging assistance: a stratified analysis study based on tumor size and location. Abdom Radiol (NY). 2025;50:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Tinguely P, Ruiter SJS, Engstrand J, de Haas RJ, Nilsson H, Candinas D, de Jong KP, Freedman J. A prospective multicentre trial on survival after Microwave Ablation VErsus Resection for Resectable Colorectal liver metastases (MAVERRIC). Eur J Cancer. 2023;187:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Li H, Gu GL, Li SY, Yan Y, Hu SD, Fu Z, Du XH. Multidisciplinary discussion and management of synchronous colorectal liver metastases: A single center study in China. World J Gastrointest Oncol. 2023;15:1616-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Manthe RL, Foy SP, Krishnamurthy N, Sharma B, Labhasetwar V. Tumor ablation and nanotechnology. Mol Pharm. 2010;7:1880-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 17. | Dupré A, Rivoire M, Metzger S, Cropet C, Vincenot J, Peyrat P, Chen Y, Pérol D, Melodelima D. Intra-operative High-Intensity Focused Ultrasound in Patients With Colorectal Liver Metastases: A Prospective Ablate-and-Resect Study. Ultrasound Med Biol. 2023;49:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zhang D, Chen Z, Zhang L, Qian X, Huang X, Zheng Z, Pan W. Application of Laparoscopic Hepatectomy Combined with Intraoperative Microwave Ablation in Colorectal Cancer Liver Metastasis. J Vis Exp. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Xie Y, Lin J, Zhang N, Wang X, Wang P, Peng S, Li J, Wu Y, Huang Y, Zhuang Z, Shen D, Zhu M, Liu X, Liu G, Meng X, Huang M, Yu H, Luo Y. Prevalent Pseudoprogression and Pseudoresidue in Patients With Rectal Cancer Treated With Neoadjuvant Immune Checkpoint Inhibitors. J Natl Compr Canc Netw. 2023;21:133-142.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 20. | Gao J, Wang H, Wang Z. Study on Ultrasonographic Diagnosis and Postoperative Comprehensive Nursing of Rectal Cancer in Preoperative Staging. Contrast Media Mol Imaging. 2022;2022:7671169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Nishimura A, Kawahara M, Kawachi Y, Hasegawa J, Makino S, Kitami C, Nakano T, Otani T, Nemoto M, Hattori S, Nikkuni K. Totally laparoscopic resection of right-sided colon cancer using transvaginal specimen extraction with a 10-mm-long abdominal incision. Tech Coloproctol. 2022;26:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Kalari P, Nirhale DS, Vajja R, Galam P. Comparison of Conventional Bipolar Electrocautery and Ultrasonic Harmonic Scalpel in Colorectal Cancer Surgeries. Cureus. 2022;14:e23255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Chen JY, Dai HY, Li CY, Jin Y, Zhu LL, Zhang TF, Zhang YX, Mai WH. Improved sensitivity and positive predictive value of contrast-enhanced intraoperative ultrasound in colorectal cancer liver metastasis: a systematic review and meta-analysis. J Gastrointest Oncol. 2022;13:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Sharifi N, Soleimanjahi H, Mokhtari-Dizaji M, Banijamali RS, Elhamipour M, Karimi H. Low-Intensity Ultrasound as a Novel Strategy to Improve the Cytotoxic Effect of Oncolytic Reovirus on Colorectal Cancer Model Cells. Intervirology. 2022;65:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Li Q, Yang X, Teng Q, Guo Q, Qin L, Lv Z, Zhou D, Ren M. Reasonable Collocation of Two Different Functional 3D Laparoscopes May Improve the Efficiency of Transanal Total Mesorectal Excision Surgery Using a Synchronous Two-Team Approach? J Laparoendosc Adv Surg Tech A. 2023;33:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Li XZ. Comparison of Efficacy of Thermal Ablation and Hepatic Resection in the Colorectal Liver Metastasis. Master's thesis. Shandong University, 2023. |