Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.105818
Revised: May 29, 2025
Accepted: July 1, 2025
Published online: August 15, 2025
Processing time: 110 Days and 22.8 Hours
Contrast-enhanced ultrasonography (CEUS) is utilized to assess the therapeutic efficacy of interventional therapy in liver cancer patients, offering insights into tumor blood flow changes, angiogenesis, and tumor markers.
To evaluate the use of CEUS in examining the effectiveness of interventional therapy for liver cancer, we aim to investigate its diagnostic utility for tumor perfusion patterns, microvessel density, perfusion recovery, blood flow enhan
The study involved 124 patients who underwent interventional therapy for liver cancer at Guangzhou First People’s Hospital from January 2022 to February 2024. All patients were examined using CEUS before treatment and at 1 month, 3 months, and 6 months, and the concentrations of tumor markers were collected and statistically analyzed using Statistical Package for the Social Sciences 25.0. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic efficacy of CEUS and analyze its sensitivity, specificity, and correlation with clinical indicators.
Before treatment, tumor blood flow was primarily enhanced. After treatment, enhanced perfusion declined, while uniform and non-uniform perfusion increased, indicating reduced tumor activity. Enhanced perfusion decreased from 68.25% before treatment to 53.75% at 6 months post-treatment (F = 6.123, P = 0.016), indicating reduced tumor activity. The microvessel density of the tumors decreased significantly after treatment (P < 0.05), and the proportion of low microvessel density increased. After treatment, perfusion recovery in the tumor area improved, the proportion of complete and partial responses gradually increased, and the proportion of stable lesions decreased (P < 0.05). The levels of alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9 decreased by 68.7%, 30.4%, and 41.6%, respectively, at 6 months post-treatment (P < 0.05). CEUS showed a sensitivity of 85.72%, specificity of 92.31%, and area under the curve of 0.911 (95%CI: 0.883–0.939) for evaluating treatment response. ROC curve analysis showed that CEUS had high sensitivity and specificity and could effectively evaluate the efficacy of interventional therapy for liver cancer.
CEUS has high diagnostic value in evaluating therapeutic effects in patients with liver cancer following interventional therapy. It can reflect changes in tumor blood flow, angiogenesis, and tumor marker levels, providing an effective basis for real-time monitoring of treatment outcomes.
Core Tip: This observational study highlights the value of contrast-enhanced ultrasonography (CEUS) in evaluating the therapeutic response of liver cancer patients to interventional therapy. CEUS enables real-time visualization of tumor perfusion patterns, microvessel density changes, and alterations in tumor markers. Among 124 patients, CEUS demonstrated high sensitivity (85.72%) and specificity (92.31%) in assessing treatment efficacy. Notably, tumor blood flow and tumor marker levels (alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19-9) significantly decreased post-treatment. These findings support CEUS as a reliable, noninvasive tool for monitoring treatment outcomes and guiding clinical decision-making in liver cancer management.
- Citation: Chen LP, Dong Y, He JG, Yang QQ, Hu ZW. Contrast-enhanced ultrasound in evaluating the curative effect of interventional therapy in patients with liver cancer. World J Gastrointest Oncol 2025; 17(8): 105818
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/105818.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.105818
Liver cancer, particularly hepatocellular carcinoma (HCC), poses a significant global health challenge due to its high morbidity and mortality rates. While computed tomography (CT) and magnetic resonance imaging (MRI) have been conventional methods for assessing responses, their drawbacks, including ionizing radiation, high costs, and limited real-time perfusion evaluation, restrict their frequent use in post-interventional monitoring. Conventional ultrasound, though noninvasive, lacks sensitivity in detecting crucial microvascular changes essential for early treatment response evaluation in liver cancer. Diagnosis and treatment typically rely on imaging tests, such as CT, MRI, and conventional ultrasound[1]. However, these methods have limitations in evaluating therapeutic effects and lesion dynamics, emphasizing the need for an accurate, non-invasive, and repeatable evaluation method for monitoring liver cancer treatment outcomes. Interventional therapy is an important means of treating liver cancer. Common therapeutic methods include percutaneous liver tumor ablation (such as radiofrequency ablation and microwave ablation) and transcatheter hepatic arterial chemoembolization[2]. These treatments can achieve the goal of shrinking the tumor or controlling its growth by directly interfering with the tumor's blood supply and tissue structure[3]. Because liver cancer has a rich vascular network, the blood flow changes in the tumor after treatment are directly related to the evaluation of the efficacy, and it is often difficult for traditional imaging methods to dynamically and accurately reflect this small blood flow change[4]. Accurate monitoring of blood flow and perfusion in liver cancer after interventional therapy has become the key to evaluating therapeutic effects. Contrast-enhanced ultrasonography (CEUS) is an emerging imaging technology that enhances the response of ultrasonic images using a microbubble contrast agent so that liver blood flow can be displayed more clearly[5]. CEUS has shown unique advantages in the treatment of patients with liver cancer, particularly in the evaluation of the therapeutic effect of liver cancer[6]. Unlike traditional ultrasound, CEUS offers real-time insights into tumor blood flow, accurately identifying vascular status, perfusion changes, and potential recurrence or metastasis[7]. Post-interventional treatment, CEUS can effectively monitor blood perfusion in the treatment area, aiding doctors in evaluating efficacy more intuitively and accurately[8]. While CEUS was initially used to evaluate treatment outcomes in some tumors, its full potential in assessing liver cancer treatment efficacy post-interventional therapy has not been fully explored. CEUS provides real-time, non-ionizing, and cost-effective advantages over CT, MRI, and conventional ultrasound for evaluating therapeutic effects in liver cancer, enabling dynamic monitoring of tumor blood flow. Therefore, this study aims to investigate the role of CEUS in evaluating the efficacy of patients with liver cancer after interventional therapy. Specifically, it will assess the accuracy, timeliness, and clinical value of CEUS in the evaluation of the therapeutic effect of liver cancer, with the goal of providing a scientific basis for optimizing treatment strategies for these patients. Simultaneously, the potential of CEUS in the early detection of recurrence or metastasis will be explored to provide a more reliable basis for clinical treatment decisions.
This study evaluated the effectiveness of CEUS following interventional therapy in patients with liver cancer. A total of 124 patients who underwent interventional therapy for liver cancer at our hospital between January 2022 and February 2024 were included. Patients meeting specific inclusion criteria were screened, while those not meeting these criteria were excluded. Inclusion criteria comprised: (1) A confirmed diagnosis of HCC via imaging (CT/MRI) or histopathology; (2) A planned intervention such as radiofrequency ablation, microwave ablation, or transarterial chemotherapy embolization [transcatheter hepatic arterial chemoembolization (TACE)]; (3) Age 18 years or above, with complete clinical data; (4) No other relevant treatments (e.g., surgery or radiotherapy) within at least one month before treatment; and (5) Patients who signed informed consent forms and were willing to participate in this study. Exclusion criteria included: (1) Severe liver failure (Child-Pugh score ≥ C or advanced liver cirrhosis); (2) Coexisting malignant tumors (e.g., gastric cancer, colon cancer); (3) Significant cardiopulmonary or systemic diseases unsuitable for interventional therapy; (4) Pregnant or lactating women; and (5) Patients who are allergic to contrast agents or have serious adverse reactions during CEUS examinations. This study received approval from the Ethics Committee of Guangzhou First People’s Hospital and adhered to the Declaration of Helsinki.
The patients were instructed to fast for at least 6 hours to minimize the impact of gastrointestinal gases on liver ultrasonic images. They were positioned supine with the abdomen exposed, and an ultrasound gel was applied to enhance transmission. Sonazoid (0.5 mL) was administered intravenously as a microbubble contrast agent, and both liver areas and low-concentration gas microbubbles were scanned. Imaging commenced immediately post-injection using a low-frequency ultrasound probe (1.5–2.5 MHz) for dynamic visualization of the liver and tumor areas. CEUS initiates with a standard liver ultrasound scan to ascertain tumor location, size, and borders. Immediately after injection of the sonazoid microbubble contrast agent, the liver was dynamically monitored with an ultrasound probe to record the perfusion of the contrast agent into the liver and tumor site. Blood flow changes in the tumor area were recorded by continuous scanning, with special attention paid to perfusion within the tumor and changes in marginal blood flow. Each scan lasted 3 minutes and was typically followed by contrast imaging at 1 week, 1 month, 3 months, and 6 months post-treatment. A scan at 1-week post-treatment aimed to identify residual tumor blood flow or treatment-induced vascular damage promptly, crucial for timely adjuvant therapy.
Baseline patient data, including sex, age, weight, height, medical history (e.g., hypertension and diabetes), family history, and smoking and drinking history were gathered. Prior to enrollment, data were obtained at the initial follow-up through questionnaires or interviews and recorded in an electronic medical record system. The presence of other comorbidities (such as hypertension, diabetes, and heart disease) and the management of these conditions significantly influence the treatment outcomes for liver cancer and the overall health of the patient. This assessment was performed by the study physician based on the patient's past and present medical history and was documented in conjunction with relevant findings. Baseline data were collected to assess potential confounders (e.g., comorbidities and lifestyle factors) and ensure homogeneity of the study population, thus minimizing bias in the evaluation of treatment effects.
Tumor blood perfusion pattern: (1) Normal perfusion (enhanced): The tumor exhibits adequate blood supply with significantly enhanced perfusion, typically seen in larger or more malignant tumors; (2) Hypoperfusion (uniform/non-uniform): Hypoperfusion is further categorized into uniform hypoperfusion (homogeneous reduced blood flow) and nonuniform hypoperfusion (heterogeneous blood flow with necrotic areas). Reduced blood flow is evident in the tumor area, showing either uniform or nonuniform enhancement, possibly indicating necrotic or hypovascular areas within the tumor; (3) Enhanced (adequate blood supply): An active tumor with good perfusion, usually indicating a malignancy; and (4) Uniform/non-uniform (poor blood supply): This suggests that the tumor has a poor blood supply and may have received some treatment or had a low malignancy.
Tumor microvascular density: Microvascular density indicates angiogenesis in the tumor region and is evaluated by blood perfusion intensity. A high microvessel density typically signifies increased tumor activity or malignancy. (1) High microvascular density: Intense tumor perfusion results in rapid enhancement, indicating robust angiogenesis and potential malignancy; and (2) Low microvascular density: Weak tumor perfusion suggests a degenerative or stable tumor state.
Perfusion recovery of the tumor area: Perfusion recovery of the tumor area after treatment is an important basis for evaluating efficacy. When treatment is effective, tumor perfusion should be significantly reduced, or perfusion recovery should be incomplete. (1) Complete response: The tumor area showed no enhancement, indicating that the blood flow in the tumor area completely disappeared after treatment, with no significant manifestation of perfusion, and the tumor was cured or completely atrophic; (2) Partial response: The perfusion in the tumor area was reduced by > 50%, the tumor volume was significantly reduced, and the reduction in perfusion reflected a good therapeutic effect; (3) Stable lesion: No significant changes in tumor perfusion and tumor volume were observed, suggesting a stable condition; and (4) Progressive lesions: The perfusion of the tumor area was enhanced, and the tumor volume increased, or new lesions appeared, indicating tumor recurrence or progression.
Enhancement of tumor blood flow: During CEUS, blood flow enhancement was observed in the tumor area. After treatment, the flow enhancement decreased or disappeared, indicating the effectiveness of the treatment. (1) Complementarily absent enhancement response: After treatment, there was no enhancement of blood flow in the tumor area, indicating that the treatment was completely effective; and (2) Reduction of blood flow enhancement: After treatment, blood flow enhancement was reduced by more than 50%, indicating that the treatment was effective; however, it had not yet completely disappeared, and further treatment was required. There was no significant change in blood flow enhancement, which persisted after treatment, indicating ineffective or poor treatment.
Changes in tumor markers: The concentrations of tumor markers such as alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) are nonspecific markers for gastrointestinal and hepatic malignancies, whereas carbohydrate antigen 19-9 (CA 19-9) is associated with cholangiocarcinoma and advanced liver cancer. Elevation of these markers indicates tumor activity. (1) Decline in AFP: A decline in the AFP level by more than 50% indicated that the curative effect was good; and (2) Continuous increase in AFP: The level of AFP remains elevated or unchanged, suggesting poor treatment or the possibility of tumor recurrence. All indicators were tested before treatment and 1 month, 3 months, and 6 months after treatment.
Statistical analyses were conducted using IBM Statistical Package for the Social Sciences Statistics for Windows, version 25.0. Measurement data were presented as mean ± SD, and inter-group comparisons were assessed using an independent sample t-test. Enumeration data were reported as frequency and percentage, and intra-group comparisons were conducted using analysis of variance and χ2 test. P < 0.05 indicated that the difference was statistically significant. All data were subjected to normality test, and data conforming to normal distribution were subjected to parametric test. A receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic efficiency of CEUS technology by comparing CEUS diagnosis results with the clinical standard diagnosis post-treatment (including CT and MRI imaging examinations and changes in the tumor marker AFP). The diagnostic accuracy was assessed based on the area under the curve (AUC) value, with a value closer to 1 indicating higher diagnostic accuracy. Pearson’s correlation analysis was used to analyze the correlation between CEUS results and clinical indicators such as liver function and tumor markers to validate the efficacy of CEUS in assessing treatment outcomes.
There were 98 male patients (79.03%) and 26 female patients (20.97%). The average age of the patients was 56.42 ± 8.11 years. The mean body weight was 68.37 ± 9.15 kg, and the average height was 168.62 ± 6.52 cm (Table 1).
| Factor | Statistical values |
| Gender (male/female) | 98/26 |
| Age (years) | 56.42 ± 8.11 |
| Body weight (kg) | 68.37 ± 9.15 |
| Height (cm) | 168.62 ± 6.52 |
| History of hypertension | 40/84 |
| History of diabetes | 18/106 |
| Smoking history | 52/72 |
| Drinking history | 45/79 |
| Family history (liver cancer) | 6/118 |
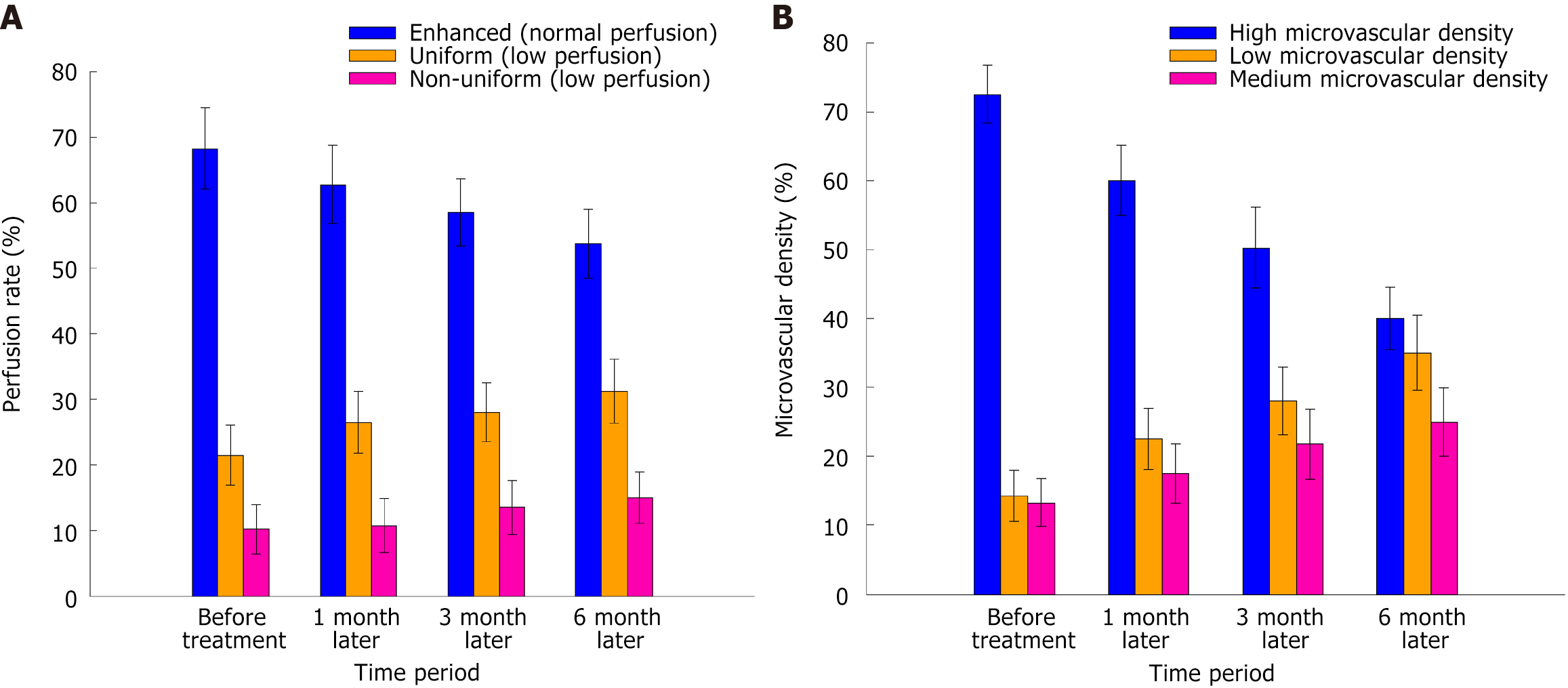
Before the treatment, the tumor predominantly exhibited an enhanced (normal) blood perfusion pattern. Over time following treatment, the proportion of enhanced perfusion significantly decreased (F = 4.378, P = 0.030), while the proportions of uniform and non-uniform low perfusion significantly increased (F = 5.187, P = 0.022 and F = 6.123, P = 0.016, respectively). These findings suggest a gradual alteration in intratumoral blood flow, with reduced perfusion indicating decreased tumor metabolic activity and potential progression toward hypoxia or necrosis in certain regions (Table 2, Figures 1 and 2).
| Perfusion type | Before treatment | 1 month after treatment | 3 months after treatment | 6 months after treatment | F value | P value |
| Enhanced (normal perfusion) | 68.25 ± 6.23 | 62.75 ± 5.99 | 58.50 ± 5.13 | 53.75 ± 5.28 | 4.378 | 0.03 |
| Uniform (low perfusion) | 21.50 ± 4.56 | 26.50 ± 4.72 | 28.00 ± 4.50 | 31.25 ± 4.89 | 5.187 | 0.022 |
| Non-uniform (low perfusion) | 10.25 ± 3.79 | 10.75 ± 4.12 | 13.50 ± 4.12 | 15.00 ± 3.89 | 6.123 | 0.016 |
| Total perfusion rate | 68.25 ± 6.23 | 62.75 ± 5.99 | 58.50 ± 5.13 | 53.75 ± 5.28 | 4.378 | 0.03 |
Before treatment, tumors exhibit high microvascular density, and most tumors are in a relatively active state with strong angiogenic characteristics. After treatment, the proportion of high microvessel density in the tumor decreased significantly (P < 0.05), and the proportion of low microvessel density increased (Table 3, Figure 1B).
| Time period | High microvascular density (%) | Low microvascular density (%) | Medium microvascular density (%) | Total (%) | F value | P value |
| Before treatment | 72.50 ± 4.23 | 14.25 ± 3.65 | 13.25 ± 3.45 | 72.50 ± 4.23 | ||
| 1 month after treatment | 60.00 ± 5.12 | 22.50 ± 4.43 | 17.50 ± 4.25 | 60.00 ± 5.12 | 5.784 | 0.019 |
| 3 months after treatment | 50.25 ± 5.89 | 28.00 ± 4.95 | 21.75 ± 5.12 | 50.25 ± 5.89 | 7.450 | 0.008 |
| 6 months after treatment | 40.00 ± 4.50 | 35.00 ± 5.45 | 25.00 ± 4.92 | 40.00 ± 4.50 | 9.236 | 0.003 |
All patients presented with stable lesions, strong tumor perfusion, significantly enhanced tumor area, and large tumor size before treatment. None of the patients exhibited a complete or partial response to the treatment. Following treatment, the proportion of complete response, partial response, and progressive lesions increased, while the proportion of stable lesions decreased significantly (P < 0.05) (Table 4, Figure 3).
| Time period | Complete response (%) | Partial response (%) | Stable lesions (%) | Progressive lesions (%) | χ2 | P value |
| Before treatment | 0 | 0 | 100 | 0 | ||
| 1 month after treatment | 8.33 | 43.33 | 40 | 8.33 | 12.227 | 0.016 |
| 3 months after treatment | 12.5 | 46.67 | 30 | 10.83 | 11.559 | 0.02 |
| 6 months after treatment | 18.33 | 41.67 | 28.33 | 11.67 | 8.908 | 0.031 |
Before treatment, all patients exhibited a substantial increase in blood flow within their tumor regions. This hyperenhancement was consistent across all cases (100%), with no variability across patients. Tumor activity was elevated, and perfusion was strong before treatment. Following treatment, the completely disappeared enhanced response increased. Furthermore, the percentage decrease in blood flow enhancement initially increased and subsequently declined, while the unchanged percentage of blood flow enhancement decreased. This disparity was statistically significant (P < 0.05) (Table 5).
| Time period | Complete disappearance of enhanced response (%) | Decrease in blood flow enhancement (%) | No change in blood flow enhancement (%) | χ2 | P value |
| Before treatment | 0 | 0 | 100 | ||
| 1 month after treatment | 12.5 | 50 | 37.5 | 18.227 | 0.01 |
| 3 months after treatment | 20.83 | 45.83 | 33.33 | 14.891 | 0.021 |
| 6 months after treatment | 28.33 | 42.5 | 29.17 | 12.224 | 0.028 |
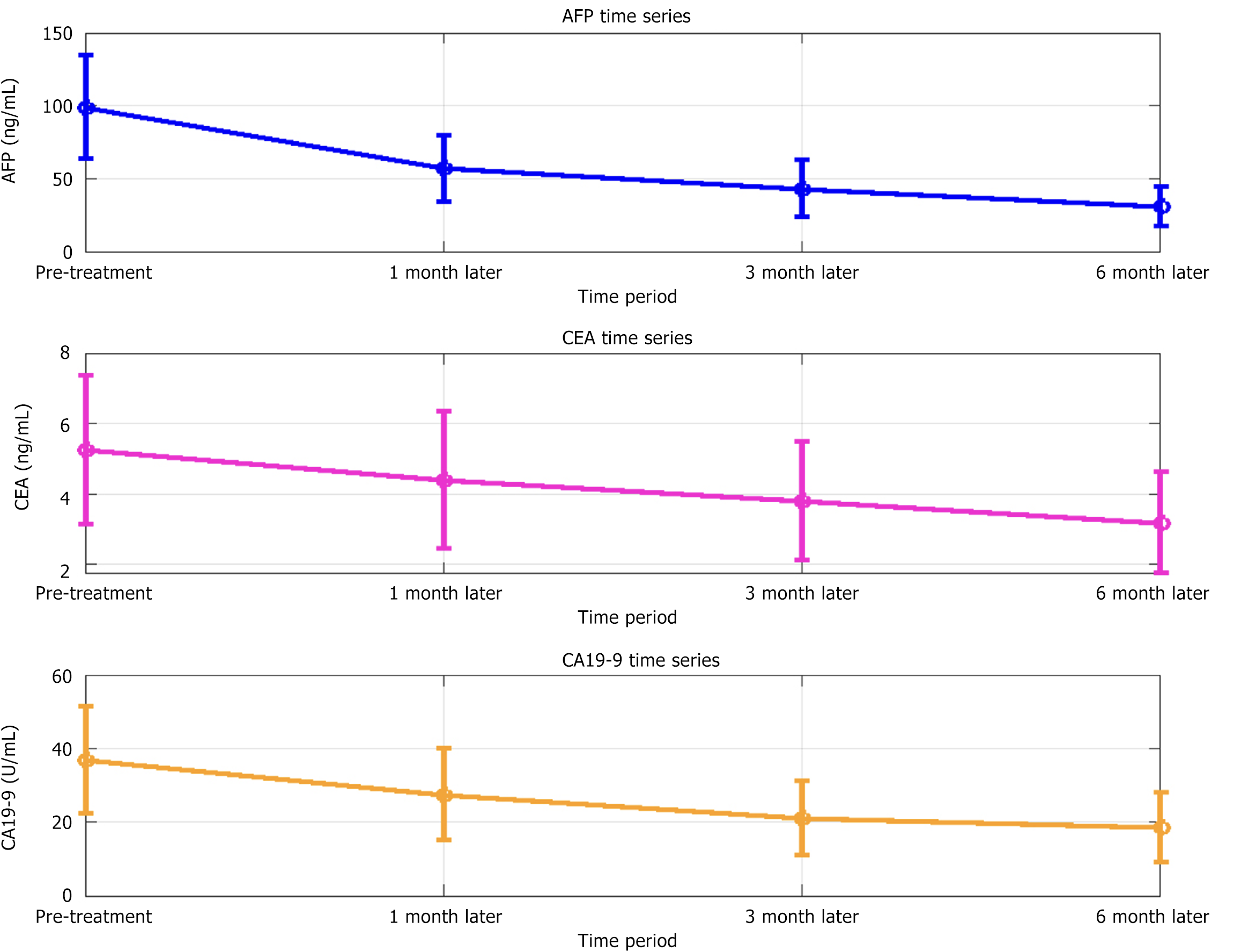
The levels of AFP, CEA, and CA 19-9 significantly decreased at different time points after treatment (P < 0.05) (Table 6, Figure 4).
| Time period | Alpha-fetoprotein (ng/mL) | Carcinoembryonic antigen (ng/mL) | Carbohydrate antigen 19-9 (U/mL) | F value | P value |
| Before treatment | 98.57 ± 34.89 | 5.24 ± 2.11 | 36.78 ± 14.57 | ||
| 1 month after treatment | 56.87 ± 22.31 | 4.38 ± 1.94 | 27.36 ± 12.50 | 25.271 | < 0.001 |
| 3 months after treatment | 43.28 ± 19.12 | 3.78 ± 1.68 | 21.04 ± 10.15 | 23.762 | < 0.001 |
| 6 months after treatment | 30.91 ± 13.45 | 3.16 ± 1.44 | 18.49 ± 9.42 | 30.184 | < 0.001 |
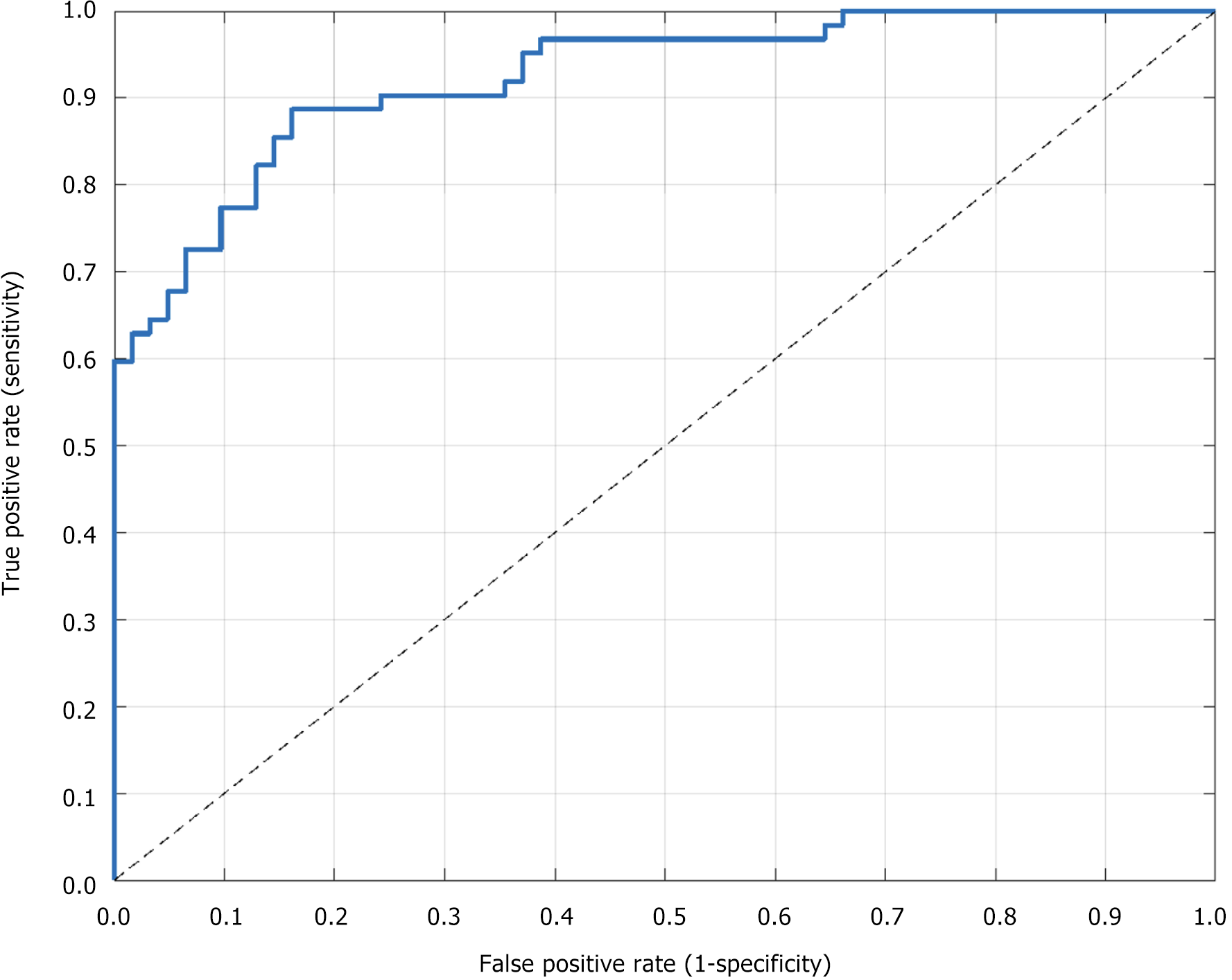
The sensitivity of CEUS in diagnosing therapeutically effective cases was 85.72%, indicating that 85.72% of the therapeutically effective cases could be correctly identified. The specificity was 92.31%, and CEUS accurately identified cases with no treatment effect or unchanged tumors, demonstrating high specificity. The AUC value was 0.911, the 95%CI: 0.883-0.939, which was close to 1, and Youden’s index was 0.780, indicating that CEUS had a high comprehensive diagnostic efficiency and could effectively distinguish between cases with effective and ineffective treatment (Figure 5).
Before treatment, the tumor area in most patients exhibited enhanced perfusion, indicating adequate tumor blood supply and high tumor activity. As treatment progressed, the enhanced perfusion ratio gradually decreased while the uniform and nonuniform perfusion ratios increased. This indicates that the activity of the tumor was gradually reduced after treatment, with potential necrosis or changes in the hypovascular area in some regions[9]. This phenomenon aligns with the changes in tumor perfusion after treatment of liver cancer, particularly the significant decrease in tumor blood flow post-radiofrequency ablation, microwave ablation, or TACE treatment[10]. Studies have shown that changes in tumor perfusion patterns are closely linked to treatment efficacy, with a decrease in blood flow typically indicating tumor cell death or volume reduction[11]. Therefore, CEUS can effectively assess treatment outcomes and offer crucial insights for dynamic tumor monitoring[12]. Our study found that the microvessel density of the tumor decreased significantly after treatment, and the proportion of low microvessel density increased, indicating that the treatment effectively inhibited tumor angiogenesis. The microvessel density of tumors is generally related to their growth rate and invasiveness[13]. High microvessel density usually indicates that the tumor is in an active state, whereas low microvessel density suggests that the tumor is in a degenerative or stable state. It is also generally believed in the existing literature that a reduction in tumor microvessel density is an effective marker for tumor treatment[14]. Therefore, CEUS can be used as an important tool to evaluate the therapeutic effects of liver cancer by monitoring changes in the tumor microvascular density[15]. Perfusion recovery post-treatment was used to evaluate tumor response. The results showed that with treatment, the proportion of complete and partial responses increased significantly, and the proportion of stable lesions decreased. This indicates that treatment can effectively reduce blood supply to the tumor, especially during tumor regression[16]. Similar studies have also indicated that a reduction in tumor perfusion is usually associated with good therapeutic effects. In particular, after TACE and ablation treatment, changes in blood perfusion have a high sensitivity for predicting therapeutic effects[17]. Before treatment, none of the patients exhibited significant changes in blood flow enhancement pre-treatment, consistent with high tumor activity and intense perfusion. However, as the treatment progressed, the tumor's blood flow enhancement reaction weakened significantly, with a gradual increase in completely disappeared enhancement reactions, indicating substantial inhibition of the tumor blood flow supply[18]. Literature also indicates that the reduction or disappearance of the tumor’s blood flow-enhancing reaction post-treatment typically signifies effective tumor control or cure[19]. Therefore, CEUS evaluation of flow-enhancing responses strongly supports efficacy asse
In summary, CEUS has significant clinical value in assessing the effectiveness of interventional therapy for liver cancer. It offers real-time evaluation of therapeutic effects by monitoring tumor perfusion patterns, microvessel density, perfusion recovery, blood flow enhancement response, and changes in tumor markers. Despite limitations, this study's results offer robust imaging support for monitoring the treatment of liver cancer patients and a scientific rationale for applying CEUS technology in clinical practice.
The authors sincerely thank all the patients and their families for their participation and cooperation in this study. We are also grateful to the clinical and technical staff of the Department of Medical Ultrasound at Guangzhou First People’s Hospital and The Second People’s Hospital of Baiyun for their invaluable support during the data collection and imaging procedures.
| 1. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 778] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 2. | Daher D, Seif El Dahan K, Cano A, Gonzales M, Ransom C, Jaurez E, Carranza O, Quirk L, Morgan T, Gopal P, Patel MS, Lieber S, Louissaint J, Cotter TG, VanWagner LB, Yang JD, Parikh ND, Yopp A, Rich NE, Singal AG. Hepatocellular Carcinoma Surveillance Patterns and Outcomes in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2024;22:295-304.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | van der Pol CB, McInnes MDF, Salameh JP, Levis B, Chernyak V, Sirlin CB, Bashir MR, Allen BC, Burke LMB, Choi JY, Choi SH, Forner A, Fraum TJ, Giamperoli A, Jiang H, Joo I, Kang Z, Kierans AS, Kang HJ, Khatri G, Kim JH, Kim MJ, Kim SY, Kim YY, Kwon H, Lee JM, Lewis SC, McGinty KA, Mulazzani L, Park MS, Piscaglia F, Podgórska J, Reiner CS, Ronot M, Rosiak G, Song B, Song JS, Tang A, Terzi E, Wang J, Wang W, Wilson SR, Yokoo T. CT/MRI and CEUS LI-RADS Major Features Association with Hepatocellular Carcinoma: Individual Patient Data Meta-Analysis. Radiology. 2022;302:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Kartalis N, Grigoriadis A. LI-RADS in Patients with Solitary Resected Hepatocellular Carcinoma: Glancing beyond Diagnosis. Radiology. 2024;310:e240161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Franzè MS, Bottari A, Caloggero S, Pitrone A, Barbera A, Lembo T, Caccamo G, Cacciola I, Maimone S, Alibrandi A, Pitrone C, Squadrito G, Raimondo G, Saitta C. Rate of hepatocellular carcinoma diagnosis in cirrhotic patients with ultrasound-detected liver nodules. Intern Emerg Med. 2021;16:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Huang Z, Xin JY, Wu LL, Luo HC, Li K. Dynamic contrast-enhanced ultrasonography with sonazoid predicts microvascular invasion in early-stage hepatocellular carcinoma. Br J Radiol. 2023;96:20230164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Moctezuma-Velazquez C, Abraldes JG. Editorial: The use of Baveno VI and VII criteria in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2023;58:478-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Zavadil J, Rohan T, Juráček J, Kiss I, Ostřížková L, Válek V, Slabý O, Andrašina T. Biomarkers as prognostic and predictive factors in patients with hepatocellular carcinoma undergoing radiological oncological interventions. Klin Onkol. 2023;36:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Kamal O, Horvat N, Arora S, Chaudhry H, Elmohr M, Khanna L, Nepal PS, Wungjramirun M, Nandwana SB, Shenoy-Bhangle AS, Lee J, Kielar A, Marks R, Elsayes K, Fung A. Understanding the role of radiologists in complex treatment decisions for patients with hepatocellular carcinoma. Abdom Radiol (NY). 2023;48:3677-3687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Russo FP, Zanetto A, Pinto E, Battistella S, Penzo B, Burra P, Farinati F. Hepatocellular Carcinoma in Chronic Viral Hepatitis: Where Do We Stand? Int J Mol Sci. 2022;23:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Nguyen SA, Merrill CD, Burrowes DP, Medellin GA, Wilson SR. Hepatocellular Carcinoma in Evolution: Correlation with CEUS LI-RADS. Radiographics. 2022;42:1028-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Li X, Han X, Li L, Su C, Sun J, Zhan C, Feng D, Cheng W. Dynamic Contrast-Enhanced Ultrasonography with Sonazoid for Diagnosis of Microvascular Invasion in Hepatocellular Carcinoma. Ultrasound Med Biol. 2022;48:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Kolarich A, Frangakis C, Yarchoan M, Hong K, Georgiades C. Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma with Intra-atrial Tumor Extension: Imaging Response and Oncologic Outcomes. J Vasc Interv Radiol. 2021;32:1203-1208.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Huang JY, Huang ZL, Yang Z, Zheng XP. Contrast-enhanced ultrasound predicts microvascular invasion in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2022;21:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Sakai M, Kawaguchi T, Koya S, Hirota K, Matsuse H, Torimura T. Subcutaneous Fat Thickness of the Lower Limb is Associated with Trunk Muscle Mass in Patients with Hepatocellular Carcinoma: A Simple Assessment for Sarcopenia Using Conventional Ultrasonography. Kurume Med J. 2022;67:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Hwang JA, Kang TW, Min JH, Kim YK, Kim SH, Sinn DH, Kim K. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Allaire M, Campion B, Demory A, Larrey E, Wagner M, Rudler M, Roux C, Blaise L, Carrie NG, Thabut D. Baveno VI and VII criteria are not suitable for screening for large varices or clinically significant portal hypertension in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2023;58:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Santambrogio R, Barabino M, D'Alessandro V, Galfrascoli E, Zappa MA, Piccolo G, Zuin M, Opocher E. Laparoscopic thermoablation for hepatocellular carcinoma in patients with liver cirrhosis: an effective procedure for tricky tumors. Med Oncol. 2020;37:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yoo SH, Lim TS, Lee HW, Kim JK, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Lee JI, Lee KS, Kim SU. Risk assessment of hepatocellular carcinoma and liver-related events using ultrasonography and transient elastography in patients with chronic hepatitis B. J Viral Hepat. 2021;28:1362-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Nakamura I, Hatano E, Tada M, Kawabata Y, Tamagawa S, Kurimoto A, Iwama H, Toriguchi K, Sueoka H, Iida K, Yoshida M, Nishimura T, Iijima H. Enhanced patterns on intraoperative contrast-enhanced ultrasonography predict outcomes after curative liver resection in patients with hepatocellular carcinoma. Surg Today. 2021;51:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Choi TW, Joo I, Kim HC. Association of dysmorphic intratumoral vessel with high lung shunt fraction in patients with hepatocellular carcinoma. Sci Rep. 2022;12:14248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Mendiratta-Lala M, Fetzer D, Kamaya A, Parikh ND, Singal AG. The Future Role of Abdominal US in Hepatocellular Carcinoma Surveillance. Radiology. 2024;311:e232624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |