Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104842
Revised: March 20, 2025
Accepted: April 1, 2025
Published online: May 15, 2025
Processing time: 132 Days and 4.2 Hours
This editorial focuses on the recent article by Yang et al in the World Journal of Gastrointestinal Oncology, which highlights the role of interlukin-17A in promoting hepatocellular carcinoma (HCC) progression by up-regulated programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PD-L1) expression. Previous, the high PD-1/PD-L1 level was due to hepatitis virus infection leading to systemic innate immune tolerance and cluster of differentiation 8 + T cells exhaustion, ultimately leading to HCC. Recently, interesting studies have found that the malignant progression of metabolic dysfunction-associated steatotic/fatty liver disease (MASLD/MAFLD), that is former nonalcoholic fatty liver disease, was achieved by up-regulated PD-L1 level that was activated the cGAS-STING pathway under lipid accumulation with mito
Core Tip: Hepatocellular carcinoma (HCC) is associated with hepatitis virus (hepatitis B virus or hepatitis C virus) infection and increasing metabolic dysfunction-associated steatotic/fatty liver disease (MASLD/MAFLD). Virus vaccines and antiviral therapy are considered key prevention and anti-viral treatments for HCC. Recently, interesting observation was that MASLD malignant progression was associated with higher programmed cell death protein-1/its ligand 1 (PD-1/PD-L1) expression. Therefore, inhibiting PD-1/PD-L1 except of HCC therapy could be a promising target to prevent or delay the malignant transformation of hepatocytes.
- Citation: Xu M, Ruan TT, Tang H, Fang RF, Sai WL, Xie Q, Yao DF, Yao M. Up-regulated programmed cell death protein-1/its ligand 1 expression promotes metabolic dysfunction-associated steatotic liver disease malignant progression. World J Gastrointest Oncol 2025; 17(5): 104842
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104842.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104842
Human hepatocellular carcinoma (HCC) is still one of the most common malignancies worldwide and a major cause of cancer-related death[1,2], including higher prevalence or mortality rate in the inshore area of Yangtze River[3,4]. Early monitoring of HCC occurrence and effective treatment of advanced liver cancer are extremely important. Although surgical resection, local ablation, and liver transplantation can cure some early-stage HCC patients, the lack of effective screening methods means most HCC patients are diagnosed at an advanced stage and miss the opportunity for curative treatment. Prognosis of cases with advanced HCC is very poor, with less than 10% for a 5-year survival rate[5]. In recent 20 years, with the rapid development of molecular biology and tumor immunology, new treatment represented by molecular targeted drugs and immunotherapy have emerged one after another, bringing hope for the systemic treatment of advanced HCC patients. Sorafenib monotherapy has long been the first-line treatment for advanced HCC. However, there are still many deficiencies[6].
Recently, the prognostic significance of programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PD-L1) in HCC has been systematically reviewed[7,8]. The PD-1/PD-L1 expression was closely related to tumor-associated macrophages in HCC pathogenesis is poorly understood[9,10]. Most studies rely on platforms that deplete hepatic macrophages before evaluation. To combat T cell activation, the PD-1/PD-L1 axis is exposed on the surfaces of T cells and hepatocytes, and specific monoclonal antibodies (mAb) such as nivolumab, pembizumab have been used to target HCC[11,12]. Alterations of the PD-1/PD-L1 axis in the immune microenvironment or clinical trials of mono- or combined- therapy have demonstrated the efficacy for HCC, with dual-inhibitors in a syngeneic model[13,14]. However, how to prevent immune escape or the emergence of multi-drug resistance (MDR) or immune escape[9,12], a better understanding of early intervention in PD-L1 expression to prevent or delay the malignant transformation of hepatocytes, using PD-1/PD-L1 inhibitors might be a promising approach for the treatment of HCC.
PDCD1-encoded PD-1 (or cluster of differentiation 279) is continuously expressed on the surface of immune cells[15]. PD-1 as an immune checkpoint molecule can bind to PD-L1 in cancer cells, leading to immune evasion occurrence by downregulating immune system function promoting immune tolerance. Both of anti-PD-1 and anti-PD-L1 are critical components of cancer immunotherapy. PD-L1 contributes to cancer immune evasion by to PD-1, leading to treatment failure[16]. Anti-PD-1/PD-L1 mAb restore the anti-tumor immune of T lymphocytes by blocking the PD-1/PD-L1 pathway, and have shown good efficacy in various malignant tumors. Currently, several clinical trials of immune checkpoint inhibitors (ICI) monotherapy are being conducted for HCC[17].
For most HCC patients, the PD-1/PD-L1 pathway isn’t the only rate-limiting factor for anti-tumor, and it isn’t enough to stimulate effective anti-HCC immune response by blocking the PD-1/PD-L1 axis alone; therefore, combination therapy be a better choice[18]. Currently, dual-ICI of combined anti-PD-1/PD-L1 with anti-cytotoxic T lymphocyte-associated antigen-4 (anti-CTLA-4) mAb are being evaluated for a broad range of cancer histologies[19]. In addition, some ongoing clinical trials aim to study combined ICI in combination with traditional treatment modalities, such as chemotherapy, surgery, and radiotherapy[20].
IMbrave150 is a groundbreaking global multicenter, open-label phase III clinical trial aimed at the efficacy and safety of PD-L1 mAb atezolizumab combined with anti-vascular endothelial growth factor mAb beizumab as first-line treatment for advanced HCC[21]. The study included 501 advanced HCC cases without received systemic treatment. According to the protocol, they were randomly assigned in a 2:1 ratio to atezolizumab + bevacizumab or sorafenib group. Compared with Sorafenib group, the combined Atezolizumab plus Bevacizumab significantly prolonged the median overall survival (OS) [19.0 vs 13.4 months, hazard ratio (HR) = 0.66] and median progression-free survival (PFS) (6.9 vs 4.3 months, HR = 0.59) of HCC, increased the objective response rate (ORR) (27% vs 12%, P < 0.001) and complete response (6% vs < 1%). In PD-L1 positive (tumor proportion score ≥ 1%) patients, the ORR in the combined group was as 41%[22]. ORIENT-32 conducted by Chinese scholars, is a phase II-III clinical trial (NCT0379440) designed to evaluate the efficacy and safety of PD-1 inhibitor sintilimab plus bevacizumab biosimilar (BI305) as first-line treatment for advanced HCC. Compared to sorafenib, the combined sintilimab and BI305 significantly prolonged OS (not reached vs 10.4 months, HR = 0.57) and PFS (4.6 vs 2.8 months, HR = 0.56) in the interim analysis[23,24]. The combining ICIs and anti-angiogenic therapy could be a generally applicable treatment model for advanced HCC.
CARES-310 is an open-label, multi-center, randomized, phase III trial conducted in China to evaluate the efficacy and safety of PD-1 inhibitor camrelizumab + apatinib as first-line treatment for unresectable HCC. A total of 543 cases with unresectable or metastatic HCC were randomized 1:1 to receive camrelizumab + apatinib or sorafenib. The combination therapy demonstrated advantages in both PFS and OS, with a median PFS of 0.6 months and a median OS of 22.1 months. In terms of safety, the incidence of grade 3 or higher treatment-related adverse events was 81% in the camrelizumab plus apatinib group[23]. Based on the study, the National Medical Products Administration of China approved camrelizumab + apatinib for the first-line treatment for unresectable or metastatic HCC. CheckMate 040 is a multicohort, open-label, phase I/II trial enrolling cases with advanced HCC aiming to evaluate the anti-tumor activity and safety of the PD-1 inhibitor nivolumab (O drug), with the ORR for receiving nivolumab is 15% to 20%, the disease control rate is 58% to 64%, the median duration of is 17 months, and the median OS is 15 to 16 months[24].
Bispecific antibodies are a novel that targets two different tumor-related molecules simultaneously, such as PD-1/LAG-3 and PD-1/CTLA-4, to enhance the tumor immune response[25]. T cell immuno-receptor with immunoglobulin (Ig) or immunoreceptor tyrosine-based inhibitory motif (ITIM) domains (TIGIT) inhibitors are another class of ICIs that target T lymphocyte Ig and ITIM domains. These inhibitors enhance anti-tumor immunity by activating natural killer (NK) cells and T lymphocytes. TIGIT acts synergistically with PD-L1, and its inhibitors are expected to improve the survival rate of HCC cases[26].
To evaluate the immunohistochemical expression of PD-L1 between the tumor cells group and the tumor-infiltrating immune cells group in HCC patients, the high PD-1/PD-L1 levels were associated with poor prognosis in HCC, with lower promoter methylation in HCC tissues[27,28]. Total 149 HCC cases were included, with 66 cases showing high hepatitis B virus (HBV)-DNA level (> 500 IU/mL) and 83 cases ≤ 500 IU/mL. High HBV-DNA cohort had a greater rate of hepatitis Be antigen positivity, HCC size ≥ 10 cm, and vascular invasion. Before propensity score matching, the median PFS and OS were 6.1 months and 17.5 months in the high HBV-DNA cohort and 6.7 months and 19.3 months in the low HBV-DNA cohort and indicated that HBV-DNA level be one of the most important factors for HBV-related HCC therapy[29]. A synergistic effect was found, in which the angiogenesis inhibitor could convert cold tumors into hot tumors and enhance the efficacy of anti-PD-1/PD-L1, leading to a highly effective combination therapy[30].
Several immunotherapy approaches including cytokines, ICI, adoptive cellular transfer and vaccines have been analyzed in HCC treatment[31,32]. In the second-line setting, monotherapy with PD-1/PD-L1 agents resulted in an overall response rate of 15%-20%, with 60% of HCC controlled[33]. Anti-angiogenic drug was found to have promising activity in various solid tumors, including advanced HCC. As an effective locoregional therapy, transarterial chemoembolization could induce vascular endothelial growth factor and PD-1/PD-L1 up-regulation, accompanied by a reduction in tumor burden. Anti-PD-L1 immunotherapy for HCC demonstrated a mixed response. PD-1 level had a significant positive correlation with CTLA-4, PD-L1 and PD-L2. Higher PD-1/PD-L1 expression promoted cancer cell proliferation or migration while inhibiting the apoptosis of HCC cells. Identification of the in situ correlation of PD-L1 and PD-L2 suggest their cumulative immunosuppressive role in HCC[34,35].
Chronic hepatitis C virus (HCV) infection appears to be associated with about 30% of HCC. More than one-sixth of cases are at an advanced stage of HCC by the time of diagnosis. For these cases, systemic therapy remains the treatment approach[36,37]. HCV induces multi-step alterations in liver, involving metabolic disorders, steatosis, liver cirrhosis and HCC. Recently, the novel studies about the regulating the immune response in HCC via DNA methylation, and the integration of methylation with tumor microenvironment (TME) and immunotherapy have been reported by applying a unique scoring system [DNA methylation score, (DM score)] evaluating methylation modifications in the three differentiation patterns of differentially expressed genes in TME infiltration and to predict different subtypes, cancerous TME infiltration, and prognosis of HCC cases.
If HCC cases with a lower DM score, characterized by the patients with elevated TMB (tumor mutation burden), HBV/HCV infection, and immune function activation, indicated an TME type, with a 5-year survival rate of 7.8%. Additionally, the lower DM score seemed to increase the efficacy of anti-CTLA-4/PD-1/PD-L1 immunotherapy, in order to better understand the correlation between DNA methylation and TME in HCC, could possibly act as a surrogate biomarker for prognosis or immune therapeutic efficacy of HCC patients[38].
Compared with the TME of HCV-induced HCC patients, the TME of HBV-induced HCC cases has a degree of vascularization and presents different immunity, resulting in similar tumor immunosuppression[35]. The combined risk ratio for OS [HR = 0.71; 95% confidence interval (CI): 0.60-085] and PFS (HR = 0.64; 95%CI: 0.53-0.77) that the treatment effect of PD-1/PD-L1 inhibitors in combination group was significantly better than that of targeted monotherapy in the treatment of unresectable HCC. HCV-infected patients were divided into four cohorts: that is, chronic HCV, HCV-related cirrhosis, HCV-related HCC and healthy control group. Among 4 cohorts, the IC analysis confirmed that GPN-loop GTPase 1 level was associated with PD-1/PD-L1 and CTLA-4, suggesting that the PD-1/PD-L1 could be a potential biomarker for predicting the immune-subtype and immunotherapy response of HCC patients.
The positive rate of PD-L1 expression was detected in 85% of HCC tissues, which was first used for HCC cases pembrolizumab at doses of 200 mg anti-PD-L1 mAb every 21 days through intravenous infusion, showing the inhibitory efficacy on HCC growth. Anti-PD-1 mAb treatment could inhibit HCC progression in mouse model or in HCC cases[34,39] and synergize enhanced immune response via phosphatidylinositol 3-kinase/protein kinase B and Janus kinase/signal transducer and activator of transcription signaling pathways[40].
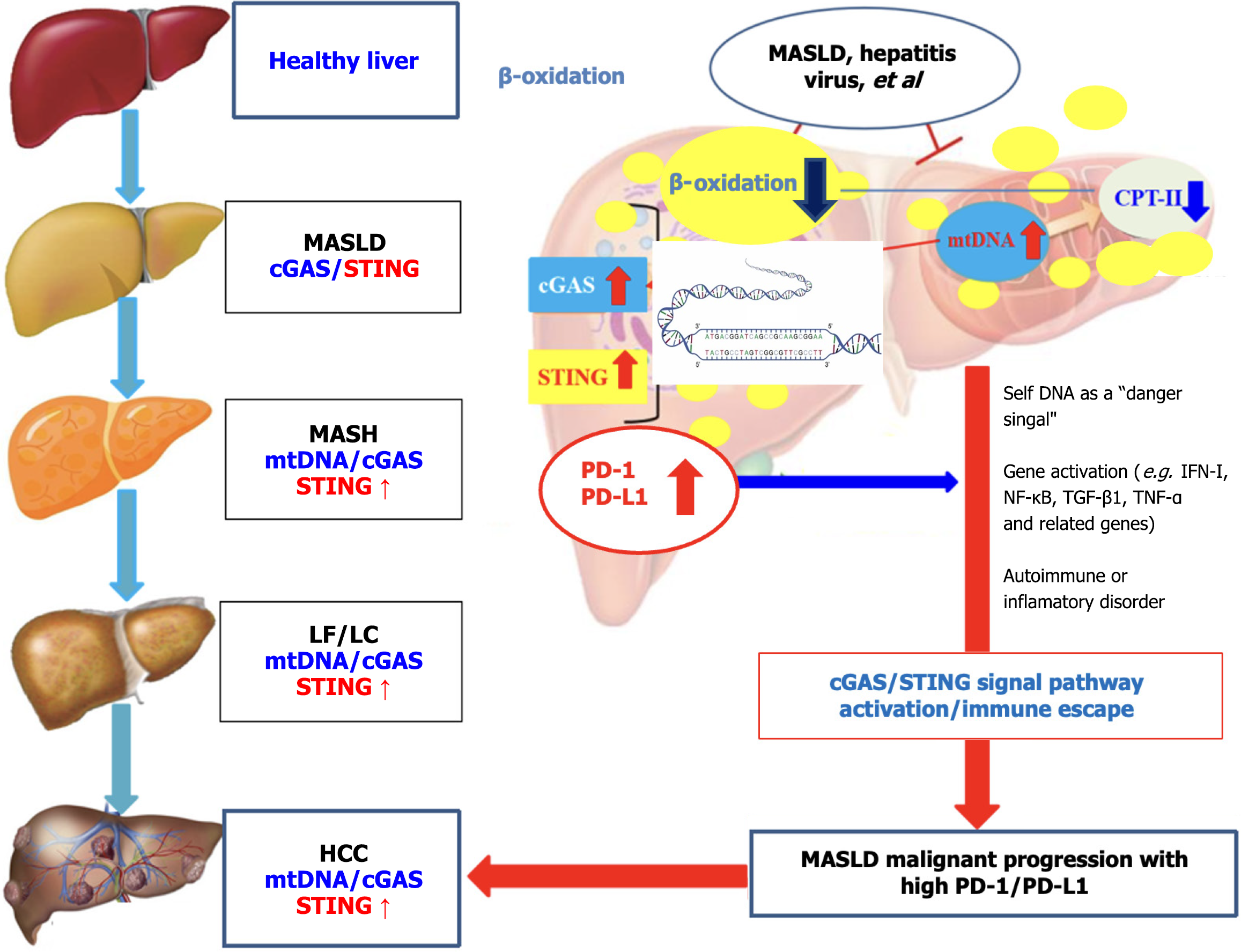
Chronic HBV or HCV infection, aflatoxin B1 exposure, and metabolic dysfunction-associated steatotic liver disease (MASLD), that is previously named nonalcoholic fatty liver disease have been established as the major etiologic for HCC occurrence[41]. MASLD is a multi-factorial disease which fat metabolic, genetic, and environmental risk factors play a major role in chronic liver disease[42,43]. A PD-1/PD-L1 axis has been reported to modulate hepatocyte inflammation and progression to HCC in MASLD and the important predictive or prognostic markers detected in immunotherapy clinical trials in HCC patients[44,45]. Within the liver, a variety of parenchymal, non-parenchymal, and immune cells maintain immune homeostasis, the activation of innate and adaptive immunity through different regulatory pathways. PD-1/PD-L1 signaling contributes to the balance between maintaining immune response and tissue immuneostasis (see Figure 1), promoting self-tolerance via activated T cells[46].
Recently, PD-1 has attracted widespread attention for its role in inducing an exhausted T-cells, which also promotes HCC evasion of immune response. Actually, clinical and basic research has proven that excessive fat accumulation in the liver of MASLD patients could disrupt the immune system, increase cytotic lymphocytes, and reduce their cytolytic activity[47].
In a high-fat environment, T cells aggravated liver functional damage and promoted the progression of MASLD, and its immune escape was conducive to the ant progression of hepatocytes into HCC became possible. Therefore, targeted intervention of PD-1/PD-L1 may help to activate T cells and reinvigorate immune surveillance against HCC or prevent hepatocyte malignant transformation become possible[48,49]. The novel discovery of PD-1/PD-L1 as biomarkers except of alpha fetoprotein play an extremely important role in MASLD, especially in predicting drug efficacy of anti-HCC immunotherapy or monitoring MASLD malignant progression[50]. PD-L1/PD-L1 and its correlation with HCC patient response in future should be also a research hotspot for metabolic or viral chronic liver disease malignant progression.
In the high-lipid and hypoxic TME, liver cancer cells mainly produce lactic acid via glycolysis, which contributes to immune escape[51]. This special TME, composed of various types of cells including cancer cells, immune cells, fibroblasts and Tregs[52].
A low glucose, high lactate microenvironment is not only harmless to normal survival of Tregs but also promotes the proliferation and differentiation of cancers. Because Tregs absorb lactate via monocarboxylate transporter 1 and increase PD-1 expression, thus activating the PD-1 + Treg subset. A large amount of lipid accumulation in liver tissue damages mitochondrial DNA, causes chronic inflammation of hepatocytes with the production of reactive oxygen species, nuclear factor kappa-B (NF-κB), interferon-γ, tumor necrosis factor-α, PD-1/PD-L1, and results in innate immunity activation. Continuous inflammatory activates the adaptative immune response, induces increasing Tregs and cluster of differentiation 8 (CD8) + T cells, and promotes macrophages polarization into the M1 phenotype. MASLD was achieved by up-regulated PD-L1 by activating cGAS-STING signal pathway[53,54]. Persistent inflammation occurs during the progression of MASLD, especially during the transition from metabolic dysfunction-associated steatohepatitis to HCC, leading to the differentiation of CD8 + T cells into exhausted T lymphocytes (immune escape)[55,56]. These data suggest that the subset suppresses the function of effector cells or cGAS-STING signaling pathway, thus rendering anti-PD-1 treatment ineffective.
Activated cGAS-STING is an important mechanism for the immune system to sense cytoplasmic DNA and initiate immune responses. large number of pre-clinical studies have found that activation of the cGAS-STING pathway induces type I interferon-dependent antitumor immune effects. However, clinical using STING agonists in a variety of solid tumors have found that the clinical efficacy of STING agonists alone and in combination with ICI is very limited and there is an urgent need to deeply and comprehensively elucidate the in vivo mechanism of STING action to change clinical dosing strategies[44,57]. STING agonists up-regulation of endogenous PD-L1 expression by activating IRF3-type I interferon (IRF3-IFN) signaling axis in monocytes in the TME; monocytes high intracellular PD-L1 expression can’t be inhibited by surface PD-1 antibodies and significantly promote MASLD progression, weakening anti-tumor immune effects mediated STING agonists. Mechanistic studies have found that while downstream IRF3-IFN signaling axis of STING activated and NF-κB pathway significantly inhibited. Thereby down-regulating the expression of endogenous PD-L1 mediated by STING, and this downstream signal conversion effect does occur in dendritic cells, nor does it affect the functional activity of T cells and NK cells[58]. Effectively resist the immunosuppression mediated by monocytes with PD-L1 expression, and more produce significantly enhanced local and systemic anti-tumor immune responses[59,60], indicated that the key mechanism of resistance to STING agonist therapy provides theoretical basis for its development and application and clinical program formulation.
The diagnosis, screening, long-term management and prevention of a large number of MASLD are the front line to achieve early detection and treatment of the disease, to block MASLD progression and to improve the quantity and quality of diagnosis and management are directly to the health outcomes and burden of the majority of metabolic dysfunction-associated fatty liver disease patients. Alterations in HCC immune microenvironment and clinical trials of various mono- or combined-therapy have demonstrated the efficacy of anti-PD-1/PD-L1 therapies in advanced HCC. However, how to prevent immune escape for hepatocarcinogenesis and improve MDR could be better understood as PD-1/PD-L1 blockade as a promising therapy. The elevated levels of PD-1/PD-L1 during MASLD progression are associated with malignant transformation of hepatocytes or chronic liver diseases. Therefore, down-regulated or inhibited PD-1/PD-L1 expression is necessary to delay or prevent metabolic or viral chronic liver disease malignant progression.
The authors would like to thank the staff of the Research Center of Medical Research, Affiliated Hospital of Nantong University, China.
| 1. | Rich NE. Changing Epidemiology of Hepatocellular Carcinoma Within the United States and Worldwide. Surg Oncol Clin N Am. 2024;33:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:1295-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 3. | Chen JG, Zhang YH, Lu JH, Kensler TW. Liver Cancer Etiology: Old Issues and New Perspectives. Curr Oncol Rep. 2024;26:1452-1468. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14:986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Timperi E, Barnaba V. Viral Hepatitides, Inflammation and Tumour Microenvironment. Adv Exp Med Biol. 2020;1263:25-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Radmanić L, Zidovec-Lepej S. The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD. Life (Basel). 2022;12:2072. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Kinsey E, Lee HM. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers (Basel). 2024;16:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 9. | Ailia MJ, Heo J, Yoo SY. Navigating through the PD-1/PDL-1 Landscape: A Systematic Review and Meta-Analysis of Clinical Outcomes in Hepatocellular Carcinoma and Their Influence on Immunotherapy and Tumor Microenvironment. Int J Mol Sci. 2023;24:6495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Millian DE, Saldarriaga OA, Wanninger T, Burks JK, Rafati YN, Gosnell J, Stevenson HL. Cutting-Edge Platforms for Analysis of Immune Cells in the Hepatic Microenvironment-Focus on Tumor-Associated Macrophages in Hepatocellular Carcinoma. Cancers (Basel). 2022;14:1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Reiter FP, Rau M, Kunzmann V, Kickuth R, Klein I, Neumann O, Stenzinger A, Schirmacher P, Geier A. Profound tumor response to combined CTLA-4 and PD-1 inhibition in systemic fourth line therapy observed in a patient with hepatocellular carcinoma harboring SETD2 and LRP1B mutations. Z Gastroenterol. 2023;61:71-75. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Dyhl-Polk A, Mikkelsen MK, Ladekarl M, Nielsen DL. Clinical Trials of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. J Clin Med. 2021;10:2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Yang ZX, Zhang LT, Liu XJ, Peng XB, Mao XR. Interleukin-17A facilitates tumor progression via upregulating programmed death ligand-1 expression in hepatocellular carcinoma. World J Gastrointest Oncol. 2025;17:97831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (4)] |
| 14. | Lombardi R, Piciotti R, Dongiovanni P, Meroni M, Fargion S, Fracanzani AL. PD-1/PD-L1 Immuno-Mediated Therapy in NAFLD: Advantages and Obstacles in the Treatment of Advanced Disease. Int J Mol Sci. 2022;23:2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Chen M, Bie L, Ying J. Cancer cell-intrinsic PD-1: Its role in malignant progression and immunotherapy. Biomed Pharmacother. 2023;167:115514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 16. | Helmy DO, Khattab F, Hegazy AE, Sabry RM. Immunohistochemical expression of immune check point protein PDL-1 in hepatocellular carcinoma denotes its prognostic significance and association with survival. J Immunoassay Immunochem. 2023;44:213-228. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, Impera V, Dubois M, Persano M, Migliari M, Pretta A, Liscia N, Astara G, Scartozzi M. Immune Checkpoint Inhibitors in the Treatment of HCC. Front Oncol. 2020;10:601240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Kamal MA, Badary HA, Omran D, Shousha HI, Abdelaziz AO, El Tayebi HM, Mandour YM. Virtual Screening and Biological Evaluation of Potential PD-1/PD-L1 Immune Checkpoint Inhibitors as Anti-Hepatocellular Carcinoma Agents. ACS Omega. 2023;8:33242-33254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 19. | Wang X, Yang T, Shi S, Xu C, Wang F, Dai D, Guan G, Zhang Y, Wang S, Wang J, Zhang B, Liu P, Bai X, Jin Y, Li X, Zhu C, Chen D, Xu Q, Guo Y. Heterogeneity-induced NGF-NGFR communication inefficiency promotes mitotic spindle disorganization in exhausted T cells through PREX1 suppression to impair the anti-tumor immunotherapy with PD-1 mAb in hepatocellular carcinoma. Cancer Med. 2024;13:e6736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Zhang JX, Hua HJ, Cheng Y, Liu S, Shi HB, Zu QQ. Role of Transarterial Chemoembolization in the Era of Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor Combination Therapy for Unresectable Hepatocellular Carcinoma: A Retrospective Propensity Score Matched Analysis. Acad Radiol. 2024;31:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Singh A, Beechinor RJ, Huynh JC, Li D, Dayyani F, Valerin JB, Hendifar A, Gong J, Cho M. Immunotherapy Updates in Advanced Hepatocellular Carcinoma. Cancers (Basel). 2021;13:2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Cerniglia M, Klepadlo M, Sheneman D, Kim SS. Response to PD-1 inhibitor after progression on PD-L1 inhibitor in advanced HCC. BMJ Case Rep. 2022;15:e250009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Li J, Liao C, Liu Z, Xiong H, Cai J, Liu T. PD-1/PD-L1 Inhibitors Plus Antiangiogenic Drugs Versus Sorafenib as the First Line Treatment for Advanced Hepatocellular Carcinoma: A Phase 3 RCTs Based Meta-Analysis. Technol Cancer Res Treat. 2024;23:15330338241305700. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Zhou T, Wang X, Cao Y, Yang L, Wang Z, Ma A, Li H. Cost-effectiveness analysis of sintilimab plus bevacizumab biosimilar compared with lenvatinib as the first-line treatment of unresectable or metastatic hepatocellular carcinoma. BMC Health Serv Res. 2022;22:1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 26. | Liu WN, Harden SL, Tan SLW, Tan RJR, Fong SY, Tan SY, Liu M, Karnik I, Shuen TWH, Toh HC, Fan Y, Lim SG, Chan JKY, Chen Q. Single-cell RNA sequencing reveals anti-tumor potency of CD56(+) NK cells and CD8(+) T cells in humanized mice via PD-1 and TIGIT co-targeting. Mol Ther. 2024;32:3895-3914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Yan F, Zhu B, Shi K, Zhang Y, Zeng X, Zhang Q, Yang Z, Wang X. Prognostic and therapeutic potential of imbalance between PD-1+CD8 and ICOS+Treg cells in advanced HBV-HCC. Cancer Sci. 2024;115:2553-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 28. | Wang R, Tan G, Lei D, Li Y, Gong J, Tang Y, Pang H, Luo H, Qin B. Risk of HBV reactivation in HCC patients undergoing combination therapy of PD-1 inhibitors and angiogenesis inhibitors in the antiviral era. J Cancer Res Clin Oncol. 2024;150:158. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Chen QJ, Lin KY, Lin ZW, Zhang B, Liu MQ, Zhang JX, Huang QZ, Lin KC, Zhang JY, Wei FQ, You PH, You S, Jiang YB, Zhang H, Cheng ZQ, Wang CR, Zeng YY. Association of hepatitis B virus DNA levels with efficacy and safety outcomes in patients with hepatitis B virus-associated advanced hepatocellular carcinoma receiving tyrosine kinase inhibitor plus anti-PD-1 antibody: a multicenter propensity-matched study. Int Immunopharmacol. 2023;125:111098. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Liu L, Liu J, Li P, Luo J, Qin R, Peng Q, Li B, Wei X, Wang T, Shi H, Wang MD, Li C, Fang W, Chen W, Xu X, Yang T, Yin W, Zeng X. Single-cell analysis reveals HBV-specific PD-1(+)CD8(+) TRM cells in tumor borders are associated with HBV-related hepatic damage and fibrosis in HCC patients. J Exp Clin Cancer Res. 2023;42:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 31. | Luo JX, Zhang Y, Hu XY, Xiang N. Interferon therapy improves survival in patients with hepatitis B virus-related hepatocellular carcinoma after curative surgery: a meta-analysis. Hepatol Int. 2024;18:63-72. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Chen S, Huang C, Liao G, Sun H, Xie Y, Liao C, Wang J, He M, Hu H, Dai Z, Ren X, Zeng X, Lin Z, Zhang GP, Xie W, Shen S, Li S, Peng S, Kuang DM, Zhao Q, Duda DG, Kuang M. Distinct single-cell immune ecosystems distinguish true and de novo HBV-related hepatocellular carcinoma recurrences. Gut. 2023;72:1196-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Wu WC, Lin TY, Chen MH, Hung YP, Liu CA, Lee RC, Huang YH, Chao Y, Chen SC. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40:789-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Huang D, Ke L, Cui H, Li S. Efficacy and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic therapy for the unresectable hepatocellular carcinoma and the benefit for hepatitis B virus etiology subgroup: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2023;23:474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 35. | Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H, Tong Z, Liu L, Zheng Y, Zhao P, Jiang W, Fang W. Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review. Front Immunol. 2020;11:1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol. 2019;9:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 37. | Mocan T, Sparchez Z, Craciun R, Bora CN, Leucuta DC. Programmed cell death protein-1 (PD-1)/programmed death-ligand-1 (PD-L1) axis in hepatocellular carcinoma: prognostic and therapeutic perspectives. Clin Transl Oncol. 2019;21:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Zhao J, Liu Z, Yang K, Shen S, Peng J. DNA methylation regulator-based molecular subtyping and tumor microenvironment characterization in hepatocellular carcinoma. Front Immunol. 2024;15:1333923. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Park SJ, Hahn YS. Hepatocytes infected with hepatitis C virus change immunological features in the liver microenvironment. Clin Mol Hepatol. 2023;29:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Ramadan HK, Badr G, Ramadan NK, Sayed A. Enhanced immune responses, PI3K/AKT and JAK/STAT signaling pathways following hepatitis C virus eradication by direct-acting antiviral therapy among Egyptian patients: a case control study. Pathog Dis. 2021;79:ftab008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Zeng L, Zhu L, Fu S, Li Y, Hu K. Mitochondrial Dysfunction-Molecular Mechanisms and Potential Treatment approaches of Hepatocellular Carcinoma. Mol Cell Biochem. 2025;480:2131-2142. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Li A, Wang R, Zhao Y, Zhao P, Yang J. Crosstalk between Epigenetics and Metabolic Reprogramming in Metabolic Dysfunction-Associated Steatotic Liver Disease-Induced Hepatocellular Carcinoma: A New Sight. Metabolites. 2024;14:325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Kalligeros M, Henry L, Younossi ZM. Metabolic dysfunction-associated steatotic liver disease and its link to cancer. Metabolism. 2024;160:156004. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Pipitone RM, Lupo G, Zito R, Javed A, Petta S, Pennisi G, Grimaudo S. The PD-1/PD-L1 Axis in the Biology of MASLD. Int J Mol Sci. 2024;25:3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 45. | Pipitone RM, Malvestiti F, Pennisi G, Jamialahmadi O, Dongiovanni P, Bertolazzi G, Pihlajamäki J, Yki-Järvinen H, Vespasiani-Gentilucci U, Tavaglione F, Maurotti S, Bianco C, Di Maria G, Enea M, Fracanzani AL, Kärjä V, Lupo G, Männistö V, Meroni M, Piciotti R, Qadri S, Zito R, Craxì A, Di Marco V, Cammà C, Tripodo C, Valenti L, Romeo S, Petta S, Grimaudo S. Programmed cell death 1 genetic variant and liver damage in nonalcoholic fatty liver disease. Liver Int. 2023;43:1761-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Wang LL, Lu YM, Wang YH, Wang YF, Fang RF, Sai WL, Yao DF, Yao M. Carnitine palmitoyltransferase-II inactivity promotes malignant progression of metabolic dysfunction-associated fatty liver disease via liver cancer stem cell activation. World J Gastroenterol. 2024;30:5055-5069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Gok Yavuz B, Hasanov E, Lee SS, Mohamed YI, Curran MA, Koay EJ, Cristini V, Kaseb AO. Current Landscape and Future Directions of Biomarkers for Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Zou Y, Ruan S, Jin L, Chen Z, Han H, Zhang Y, Jian Z, Lin Y, Shi N, Jin H. CDK1, CCNB1, and CCNB2 are Prognostic Biomarkers and Correlated with Immune Infiltration in Hepatocellular Carcinoma. Med Sci Monit. 2020;26:e925289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 49. | Wu ST, Zhu L, Feng XL, Wang HY, Li F. Strategies for discovering novel hepatocellular carcinoma biomarkers. World J Hepatol. 2025;17:101201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 50. | Liang J, Kim N, Yang JD. Hepatocellular carcinoma risk prediction and early detection in patients with metabolic dysfunction associated steatotic liver disease. Transl Gastroenterol Hepatol. 2024;9:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Cadenas-De Miguel S, Lucianer G, Elia I. The metabolic cross-talk between cancer and T cells. Trends Biochem Sci. 2023;48:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 52. | Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201-218.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 499] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 53. | Tan S, Liu M, Feng F, Li R, Tian R, Nie Z. Exploring the pathogenesis and immunological profiles of psoriasis complicated with MASLD. PLoS One. 2024;19:e0305217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Chen M, Li Y, Zhu JY, Mu WJ, Luo HY, Yan LJ, Li S, Li RY, Yin MT, Li X, Chen HM, Guo L. Exercise-induced adipokine Nrg4 alleviates MASLD by disrupting hepatic cGAS-STING signaling. Cell Rep. 2025;44:115251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 55. | Xu D, Tian Y, Xia Q, Ke B. The cGAS-STING Pathway: Novel Perspectives in Liver Diseases. Front Immunol. 2021;12:682736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Tao S, Liang S, Zeng T, Yin D. Epigenetic modification-related mechanisms of hepatocellular carcinoma resistance to immune checkpoint inhibition. Front Immunol. 2022;13:1043667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 57. | Song H, Chen L, Pan X, Shen Y, Ye M, Wang G, Cui C, Zhou Q, Tseng Y, Gong Z, Zhong B, Cui H, Mo S, Zheng J, Jin B, Zheng W, Luo F, Liu J. Targeting tumor monocyte-intrinsic PD-L1 by rewiring STING signaling and enhancing STING agonist therapy. Cancer Cell. 2025;43:503-518.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Gu L, Zhu Y, Lee M, Nguyen A, Ryujin NT, Huang JY, Pandit SK, Chamseddine S, Xiao L, Mohamed YI, Kaseb AO, Karin M, Shalapour S. Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells. Proc Natl Acad Sci U S A. 2023;120:e2300706120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Singh A, Anjum B, Naz Q, Raza S, Sinha RA, Ahmad MK, Mehdi AA, Verma N. Night shift-induced circadian disruption: links to initiation of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and risk of hepatic cancer. Hepatoma Res. 2024;2024:2394-5079.2024.88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Ren S, Zhang Y, Wang X, Su J, Wang X, Yuan Z, He X, Guo S, Chen Y, Deng S, Wu X, Li M, Du F, Zhao Y, Shen J, Hu W, Li X, Xiao Z. Emerging insights into the gut microbiota as a key regulator of immunity and response to immunotherapy in hepatocellular carcinoma. Front Immunol. 2025;16:1526967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |