Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.103275
Revised: January 14, 2025
Accepted: February 7, 2025
Published online: May 15, 2025
Processing time: 182 Days and 22 Hours
With gastric cancer ranking among the most prevalent and deadly malignancies worldwide, early detection and individualized prognosis remain essential for improving patient outcomes. This letter discusses recent advancements in arti
Core Tip: Artificial intelligence (AI) is transforming gastric cancer management by enhancing early detection and individualized treatment planning through advanced predictive models. This letter highlights recent innovations in AI, such as a computed tomography-based radiomic model that predicts responses to neoadjuvant immunochemotherapy in advanced gastric cancer patients. By integrating AI-driven analysis with clinical factors, these tools offer substantial predictive accuracy, promising improved patient outcomes. However, to fully realize AI’s potential, ongoing collaboration is essential to address ethical, technical, and validation challenges, ensuring AI’s responsible integration into clinical oncology for effective, transparent, and patient-centered care.
- Citation: Ardila CM, González-Arroyave D, Ramírez-Arbeláez J. Artificial intelligence as a predictive tool for gastric cancer: Bridging innovation, clinical translation, and ethical considerations. World J Gastrointest Oncol 2025; 17(5): 103275
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/103275.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.103275
Gastric cancer, a leading cause of cancer-related deaths, often presents at advanced stages due to limited early detection methods, resulting in poor prognosis and limited treatment options[1-3]. Current diagnostic methods, such as endoscopy and histopathological analysis, are often invasive, resource-intensive, and limited in their ability to provide comprehensive, personalized insights into disease progression. Recent developments in artificial intelligence (AI) have spurred research into predictive models that can provide earlier and more accurate risk assessments, potentially shifting the diagnostic and therapeutic paradigm for this disease. With capabilities that extend beyond traditional statistical methods, AI-driven models can analyze complex, multi-dimensional datasets, identifying predictive markers and patterns that support early diagnosis, prognostication, and personalized treatment planning[4-6] (Figure 1).
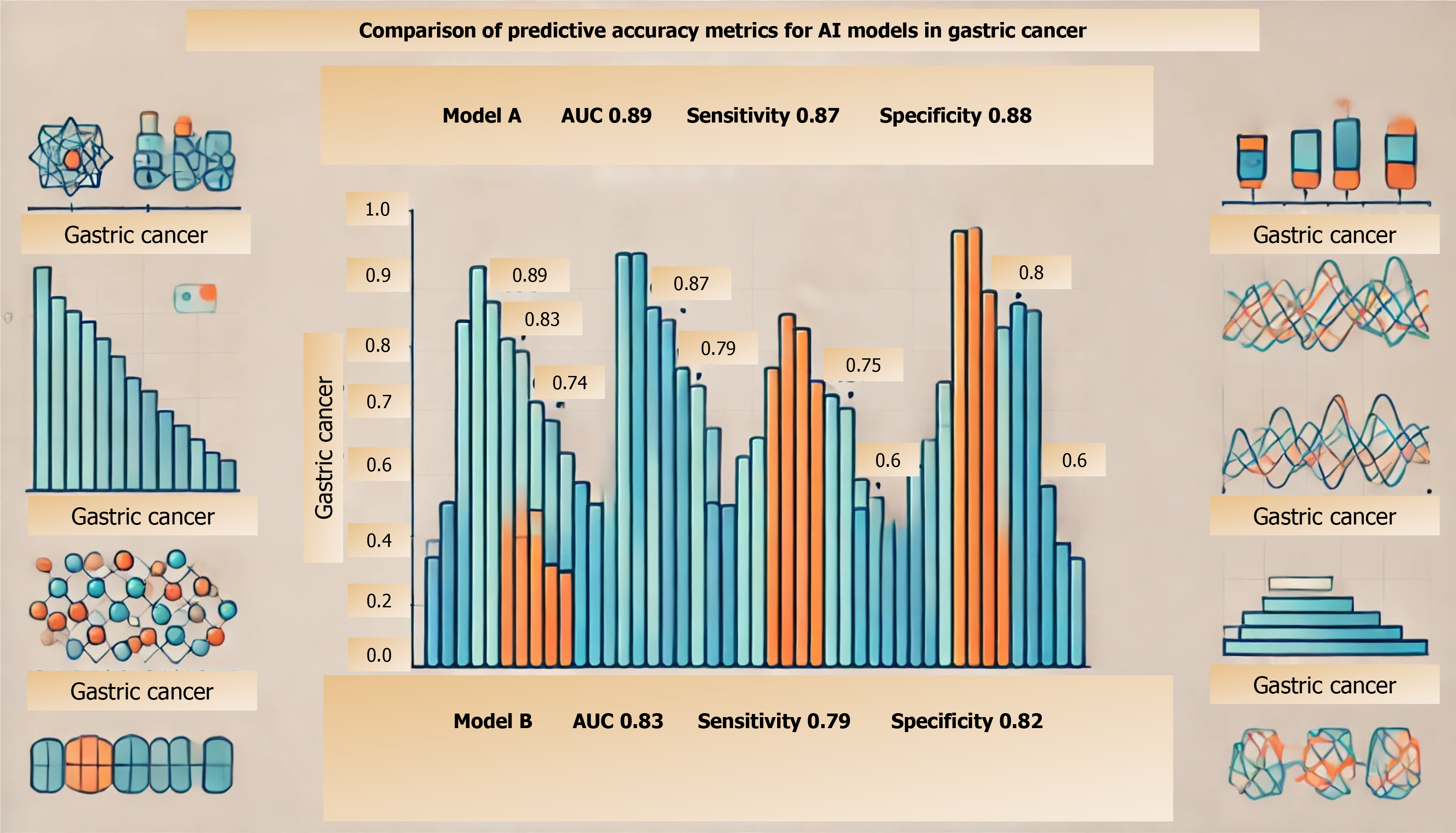
This bar chart compares the performance of different AI models in predicting gastric cancer. The models are evaluated based on three metrics: Area under the curve (AUC), sensitivity, and specificity. AUC measures the overall model performance, while sensitivity and specificity assess the model's ability to correctly identify true positive and true negative cases, respectively.
Despite its promise, integrating AI into clinical oncology faces multiple challenges, from technical and ethical hurdles to the need for effective model validation across diverse populations. AI models must be robust enough to handle the heterogeneity of gastric cancer presentations and treatment responses. Validation processes for these models commonly include performance metrics like sensitivity, specificity, and AUC. Additionally, external validation is performed using independent datasets from diverse cohorts to ensure the model’s reliability across varied clinical settings[5-7].
As evidenced by the above radiomic model[7], achieving high predictive accuracy relies on quality datasets and advanced validation methods, which are critical for bridging research and clinical use. The radiomic feature extraction process for computed tomography-based models typically involves segmenting the region of interest, extracting quantitative features (e.g., texture, shape, and intensity), and using feature selection algorithms to identify those most correlated with clinical outcomes. These steps ensure that extracted features meaningfully contribute to the model's predictive power (Figure 2).
This letter examines the state of AI in gastric cancer prediction and provides perspectives on both its clinical integration and ethical use. Addressing these topics is crucial for harnessing AI’s full potential while ensuring patient-centered care in the management of gastric cancer.
AI has shown notable success in oncology, particularly through ML and deep learning (DL) techniques that can predict patient outcomes by learning from large datasets[8-10]. For gastric cancer, these models have achieved promising accuracy in identifying high-risk individuals, predicting tumor aggressiveness, recurrence, and survival rates. For instance, convolutional neural networks and other DL models have demonstrated potential in analyzing endoscopic images, genomic data, and histopathology slides, aiding in both diagnosis and prognosis with levels of accuracy that rival traditional methods[11-13].
Moreover, predictive models trained on patient demographics, laboratory data, and genetic profiles have opened new possibilities for risk stratification and personalized treatment planning. Integrating data from multiple modalities (e.g., imaging, genetics, clinical) has enabled AI to provide a holistic assessment of a patient’s cancer status and potential progression, which could fundamentally change the timing and personalization of treatments[14-16].
However, translating these AI models from research into clinical practice is not without obstacles. One primary issue is data heterogeneity—variability in data quality, collection methods, and patient demographics across institutions can undermine model performance and generalizability. Additionally, the “black-box” nature of many AI algorithms, especially DL models, poses interpretability issues for clinicians, limiting trust in AI recommendations without a clear understanding of the decision-making process[17-19].
Another critical challenge is the need for high-quality, annotated datasets that are inclusive of diverse patient populations, considering that many AI models suffer from biases due to the limited representativeness of the training datasets. Models must be validated across multiple clinical centers to ensure that they perform accurately and consistently in real-world settings. Addressing these technical challenges will be necessary for achieving reliable, reproducible results in gastric cancer prediction[18-20].
To make a meaningful impact on patient outcomes, AI systems must be effectively integrated into clinical workflows, not as replacements for clinicians but as collaborative tools that enhance diagnostic and therapeutic precision. Integration includes mechanisms for presenting predictions to healthcare providers in user-friendly formats, such as visual dashboards or risk scores, to facilitate decision-making. Clinicians can incorporate these insights into their treatment planning processes, ensuring alignment with patient-specific needs and clinical judgment.
AI-driven models can act as an “early warning system”, flagging high-risk patients for further investigation and supporting clinicians in making data-informed decisions. This integration requires tailored, user-friendly interfaces that allow clinicians to review AI-generated predictions and insights efficiently[6,9,15].
Collaboration between AI developers and clinical oncologists is also vital for refining models based on practical insights and ensuring that AI systems complement rather than complicate clinical workflows. Clear protocols for clinician-AI interactions, ongoing training, and feedback loops will be essential to align the capabilities of AI with the unique needs of gastric cancer management[8,12,19].
As AI models are introduced into healthcare, ethical considerations must take precedence, particularly regarding patient privacy, transparency, and bias prevention. AI algorithms must operate with transparency, allowing clinicians to understand the basis for predictions and identify potential errors or biases. Algorithms trained on biased data can lead to disparities in care, affecting demographic groups differently. This issue necessitates careful data curation and ongoing validation to safeguard against unintended biases[2,11,20].
Moreover, the privacy of patient data must be rigorously protected, as AI models often require access to sensitive information for training and validation. Clear guidelines are needed to ensure that patient data is anonymized and securely stored, especially when working with multi-center datasets. Cross-disciplinary efforts involving AI researchers, ethicists, and oncologists are crucial for creating robust frameworks that prioritize patient welfare and equitable healthcare access.
AI presents a transformative opportunity in the fight against gastric cancer, with its potential to enable earlier detection, improve risk stratification, and personalize treatment strategies. However, realizing this potential will require addressing the technical, clinical, and ethical challenges associated with its use. Building on the strengths of recent technological advances, the medical community must foster interdisciplinary collaborations that promote transparency, inclusivity, and trust in AI tools. By prioritizing ethical standards and strengthening clinician-AI integration, we can pave the way for AI to serve as a reliable, patient-centered adjunct in gastric oncology. This approach aligns with the overarching goal of enhancing clinical decision-making while ensuring that AI applications in healthcare are both equitable and responsibly managed. The adoption of AI in gastric cancer care is not only a technological shift but a step towards a more proactive and personalized approach in oncology, promising significant advancements in patient outcomes.
| 1. | Chen H, Zheng Z, Yang C, Tan T, Jiang Y, Xue W. Machine learning based intratumor heterogeneity signature for predicting prognosis and immunotherapy benefit in stomach adenocarcinoma. Sci Rep. 2024;14:23328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Li N, Yang J, Li X, Shi Y, Wang K. Accuracy of artificial intelligence-assisted endoscopy in the diagnosis of gastric intestinal metaplasia: A systematic review and meta-analysis. PLoS One. 2024;19:e0303421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | El-Sherbini AH, Coroneos S, Zidan A, Othman M. Machine Learning as a Diagnostic and Prognostic Tool for Predicting Thrombosis in Cancer Patients: A Systematic Review. Semin Thromb Hemost. 2024;50:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Bao Z, Du J, Zheng Y, Guo Q, Ji R. Deep learning or radiomics based on CT for predicting the response of gastric cancer to neoadjuvant chemotherapy: a meta-analysis and systematic review. Front Oncol. 2024;14:1363812. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Adili D, Mohetaer A, Zhang W. Diagnostic accuracy of radiomics-based machine learning for neoadjuvant chemotherapy response and survival prediction in gastric cancer patients: A systematic review and meta-analysis. Eur J Radiol. 2024;173:111249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Fan H, Li L, Hou Y, Qian F, Zhuang M, Miao B, Fei S. The value of machine learning approaches in the diagnosis of early gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2024;22:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Wang Q, Guo TH, Gao W, Yu YM, Wang RF, Yu HL, Chen JJ, Sun LL, Zhang BY, Wang HJ. Computed tomography-based radiomic model for the prediction of neoadjuvant immunochemotherapy response in patients with advanced gastric cancer. World J Gastrointest Oncol. 2024;16:4115-4128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Klang E, Sourosh A, Nadkarni GN, Sharif K, Lahat A. Deep Learning and Gastric Cancer: Systematic Review of AI-Assisted Endoscopy. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | HajiEsmailPoor Z, Tabnak P, Baradaran B, Pashazadeh F, Aghebati-Maleki L. Diagnostic performance of CT scan-based radiomics for prediction of lymph node metastasis in gastric cancer: a systematic review and meta-analysis. Front Oncol. 2023;13:1185663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 10. | Wang X, Hu X, Xu Y, Yong J, Li X, Zhang K, Gan T, Yang J, Rao N. A systematic review on diagnosis and treatment of gastrointestinal diseases by magnetically controlled capsule endoscopy and artificial intelligence. Therap Adv Gastroenterol. 2023;16:17562848231206991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Oehring R, Ramasetti N, Ng S, Roller R, Thomas P, Winter A, Maurer M, Moosburner S, Raschzok N, Kamali C, Pratschke J, Benzing C, Krenzien F. Use and accuracy of decision support systems using artificial intelligence for tumor diseases: a systematic review and meta-analysis. Front Oncol. 2023;13:1224347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Zhao Y, Hu B, Wang Y, Yin X, Jiang Y, Zhu X. Identification of gastric cancer with convolutional neural networks: a systematic review. Multimed Tools Appl. 2022;81:11717-11736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Sy-Janairo MLL, Janairo JIB. Non-endoscopic Applications of Machine Learning in Gastric Cancer: A Systematic Review. J Gastrointest Cancer. 2024;55:47-64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Shibasaki S, Suda K, Hisamori S, Obama K, Terashima M, Uyama I. Robotic gastrectomy for gastric cancer: systematic review and future directions. Gastric Cancer. 2023;26:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Staal FCR, Aalbersberg EA, van der Velden D, Wilthagen EA, Tesselaar MET, Beets-Tan RGH, Maas M. GEP-NET radiomics: a systematic review and radiomics quality score assessment. Eur Radiol. 2022;32:7278-7294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Luo D, Kuang F, Du J, Zhou M, Liu X, Luo X, Tang Y, Li B, Su S. Artificial Intelligence-Assisted Endoscopic Diagnosis of Early Upper Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:855175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Alam MR, Abdul-Ghafar J, Yim K, Thakur N, Lee SH, Jang HJ, Jung CK, Chong Y. Recent Applications of Artificial Intelligence from Histopathologic Image-Based Prediction of Microsatellite Instability in Solid Cancers: A Systematic Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Chen PC, Lu YR, Kang YN, Chang CC. The Accuracy of Artificial Intelligence in the Endoscopic Diagnosis of Early Gastric Cancer: Pooled Analysis Study. J Med Internet Res. 2022;24:e27694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Dilaghi E, Lahner E, Annibale B, Esposito G. Systematic review and meta-analysis: Artificial intelligence for the diagnosis of gastric precancerous lesions and Helicobacter pylori infection. Dig Liver Dis. 2022;54:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Park JH, Kim EY, Luchini C, Eccher A, Tizaoui K, Shin JI, Lim BJ. Artificial Intelligence for Predicting Microsatellite Instability Based on Tumor Histomorphology: A Systematic Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |