Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2159
Peer-review started: November 15, 2023
First decision: January 9, 2024
Revised: February 6, 2024
Accepted: March 12, 2024
Article in press: March 12, 2024
Published online: May 15, 2024
Processing time: 175 Days and 23.9 Hours
The research findings suggest that the prognosis of children with Wilms tumor (WT) is affected by various factors. Some scholars have indicated that loss of heterozygosity (LOH) on chromosome 16q is associated with a poor prognosis in patients with WT.
To further elucidate this relationship, we conducted a meta-analysis.
This meta-analysis was registered in INPLASY (INPLASY2023100060). We systematically searched databases including Embase, PubMed, Web of Science, Cochrane, and Google Scholar up to May 31, 2020, for randomized trials reporting any intrapartum fetal surveillance approach. The meta-analysis was performed within a frequentist framework, and the quality and network inconsistency of trials were assessed. Odds ratios and 95%CIs were calculated to report the relationship between event-free survival and 16q LOH in patients with WT.
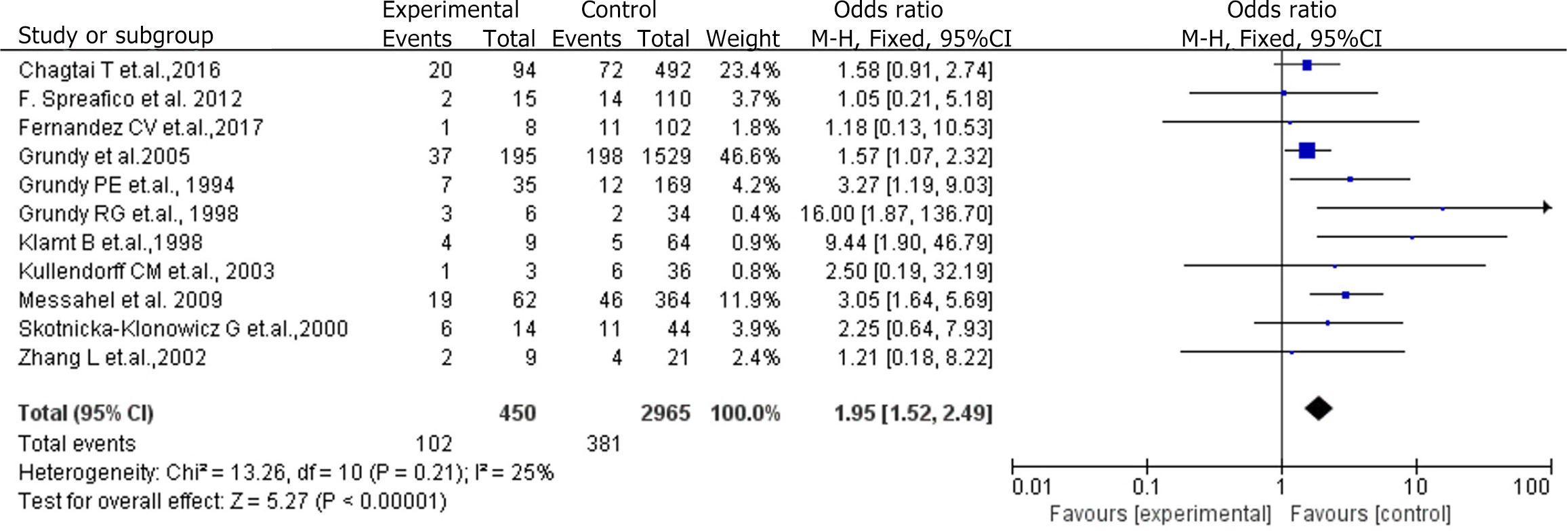
Eleven cohort studies were included in this meta-analysis to estimate the relationship between event-free survival and 16q LOH in patients with WT (I2 = 25%, P < 0.001). As expected, 16q LOH can serve as an effective predictor of event-free survival in patients with WT (risk ratio = 1.95, 95%CI: 1.52–2.49, P < 0.001).
In pediatric patients with WT, there exists a partial correlation between 16q LOH and an unfavorable treatment prognosis. Clinical detection of 16q chromosome LOH warrants increased attention to the patient’s prognosis.
Core Tip: This study explores the prognostic significance of 16q loss of heterozygosity (LOH) in the treatment of patients with Wilms tumor (WT) in recent years. Utilizing a meta-analysis, the overall predictive value of 16q LOH in patients with WT is assessed. The findings indicate that 16q LOH is associated with a poor prognosis in patients with WT. Following clinical treatment, it is crucial to pay increased attention to patients’ prognostic value while adjusting their survival expectations.
- Citation: Song YH, Li WL, Yang Z, Gao Y, Feng ZP. Loss of heterozygosity for chromosomes 16q in Wilms tumors predicts outcomes: A meta-analysis. World J Gastrointest Oncol 2024; 16(5): 2159-2167
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2159.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2159
Wilms tumor (WT) the most prevalent renal embryonic malignancy in children, presents as an abdominal mass with the potential for local and distant dissemination, with approximately 10% of cases manifesting bilaterally or multicentrically. The average onset age is 42–47 months unilaterally and 30–33 months bilaterally. Treatment primarily involves surgery, with advanced cases relying on renal dialysis or transplantation. Genetic and molecular analyses of WT patient genomes reveal consistent conclusions in recent studies. Sporadic familial cases differ in pathogenesis, and in patients with other syndromes, the possibility of sex chromosome mutation should be excluded. In over 90% of sporadic cases, WT results from a high-frequency mutation of multiple chromosomes and genes. Chromosomal variations in European and American patients with WT include loss of heterozygosity (LOH) at 16q, involving chromosomal structural changes and a decrease in allele number. This meta-analysis assesses the prognostic value of 16q LOH deletion in patients with WT.
Maw et al[1] initially reported the frequency of 16q LOH variation in 1992, confirming some cases with concurrent 1p LOH. Subsequent WT studies, National WT Study (NWTS)-3 and NWTS-4, revealed an adverse prognosis associated with 1p or 16q LOH. Similar confirmations of the association between 16q LOH and poor prognosis were reported in studies from the United Kingdom and Germany[2]. Another Italian study further confirmed the association of 1p LOH with poor prognosis[3-5]. Based on this foundation, the NWTS-5 research project by Grundy et al[6] evaluated over 1700 patients with WT, indicating an increased risk of recurrence or death with 1p or 16q LOH, especially when occurring simultaneously, affecting 5% of enrolled patients. Parallel research by Messahel et al[7] in the United Kingdom, involving 426 patients, affirmed the mutation frequency consistency with the NWTS-5 study results.
Two independent reviewers conducted a comprehensive literature search across three databases, adhering strictly to the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2009 Checklist Protocol. PubMed, Cochrane, Embase, Web of Science, and Google Scholar were used. Reports published before May 31, 2020, were retrieved utilizing these keywords: (16q [Mesh]) AND [(Wilms Tumor) OR [Mesh] (Tumor, Wilms) OR (Wilms Tumor) OR (Nephroblastoma) OR (Nephroblastomas) OR (Wilms’ Tumor) OR (Tumor, Wilms’) OR (Wilm Tumor) OR (Wilm’s Tumor) OR (Bilateral Wilms Tumor) OR (Tumor, Bilateral Wilms) OR (Wilms Tumor, Bilateral)]. Preselected findings were confined to English publications. Additionally, we performed manual searches to identify potentially eligible articles that may have been overlooked by computer-based searches[8]. For a detailed methodology, reference can be made to previous works[9-11].
Two investigators scrutinized the abstracts and titles of all literature identified using the search strategy to compile relevant articles. The full texts were subsequently examined by two different reviewers. Any disagreements were resolved through discussion or, if necessary, by the judgment of a third expert until a consensus was attained.
The data retrieved from the search were evaluated by two reviewers according to the title, abstract, and full text. The inclusion criteria encompassed cohort design, patients with WT, comparison of 16q LOH to a control group, survival as an outcome, and reporting of odds ratio, relative risk, hazard risk, or any data sufficient to estimate these parameters. Reviews, conference abstracts, case reports, papers, letters, and data overlapping with other studies were excluded.
The meta-analysis was performed using Cochrane Review Manager software (Rev Man 5.3). Continuous outcomes were calculated using standard mean differences, and dichotomous outcomes by risk ratios (RRs), both presented with 95%CIs. We assessed heterogeneity through forest plots and I2 calculation (> 50% considered extensive heterogeneity). A fixed-effects model was employed to collate results in case of minimal heterogeneity; otherwise, a random-effects model was applied. A qualitative examination of potential publication bias was conducted using funnel plots in Rev Man software when the CI distribution significantly deviated.
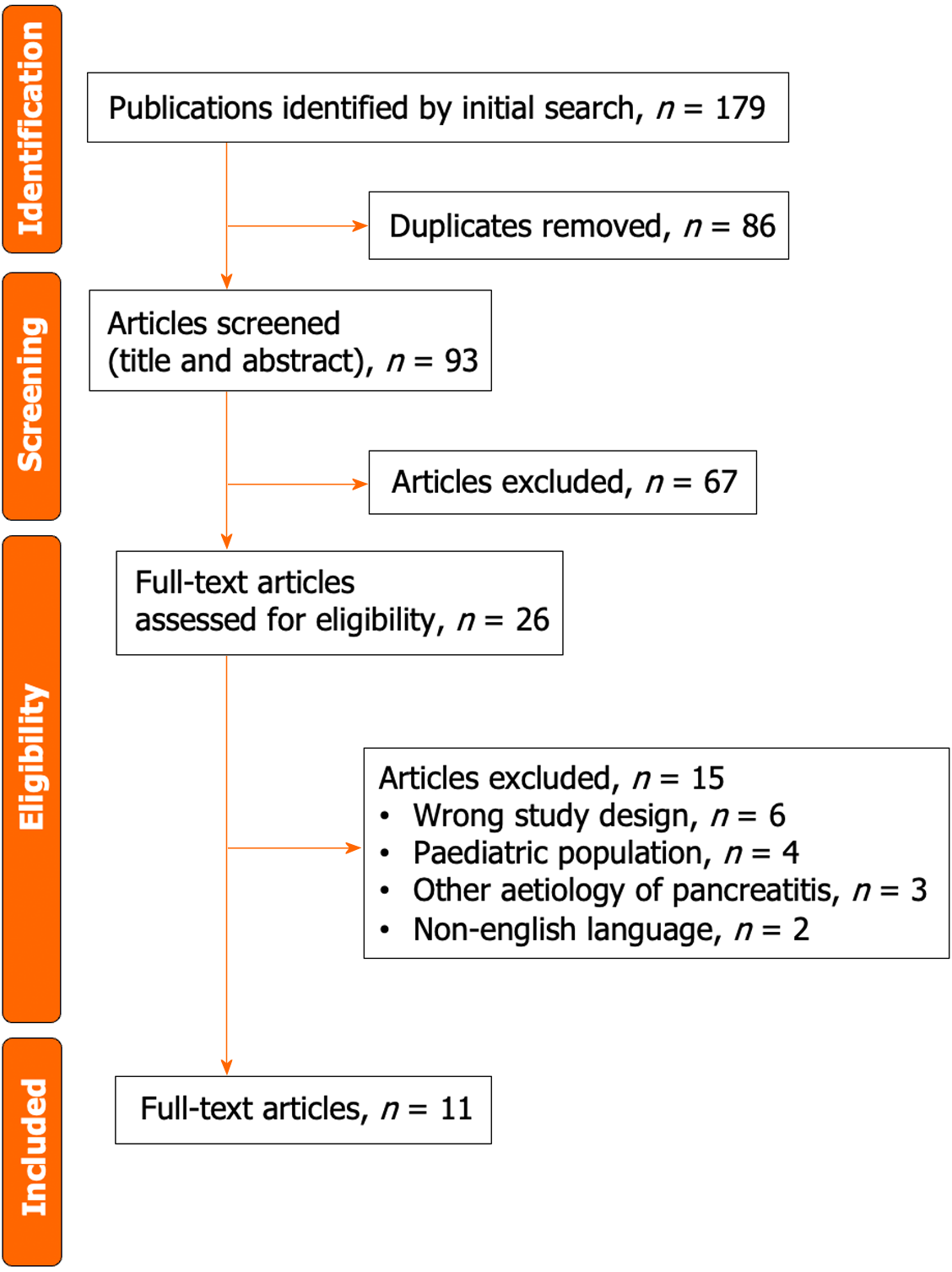
The literature retrieval process and results, based on research characteristics and quality evaluation, are depicted in Figure 1. Initially, 179 potentially relevant studies were identified. Eventually, 11 studies that met the inclusion criteria underwent meta-analysis. Table 1 presents the characteristics of the included studies, encompassing the number of patients, ages, follow-up time, and methods employed to detect 16q LOH. Of the 11 studies, 6 were of high quality, whereas others were of moderate quality (Table 2).
| Ref. | Country | Patients | Age | Follow-up | Method to Detect LOH 16q |
| Spreafico et al[27], 2013 | Italy | Nonanaplastic unilateral WT of stages I to IV | Median 40 months, range 1 to 172 months | Mean = 73 months, range 35 to 97 months | Microsatellite markers |
| Grundy et al[6], 2005 | Australia, New Zealand, Switzerland, and the Netherlands | 1724 patients younger than 16 years at diagnosis with specific WT | < 16 yr | 4 yr | PCR |
| Chagtai et al[35], 2016 | 26 countries: 24 in Europe, 1 in Australia, 1 in South America | 586 patients with WT of stages I to IV | Range 6 months to 18 years | M = 68 months | Multiplex Ligation-ependent Probe Amplification |
| Messahel et al[7], 2009 | United Kingdom | 426 patients with favorable-histology WT | Not known | 4 yr | Microsatellite markers |
| Grundy et al[2], 1994 | United States | 204 patients with histology WT | < 17 yr | M = 1.3 yr (LOH group) or 1.4 yr (control group) | PCR |
| Grundy et al[3], 1998 | United Kingdom | 40 patients with sporadic WT | Not known | ≥ 7 yr | PCR |
| Klamt et al[4], 1998 | Germany | 73 patients with WT | Mean = 3.48 yr | Not known | PCR |
| Skotnicka-Klonowicz et al[36], 2000 | Poland | 58 patients with WT | M = 39 months; range = 2 d to 13 yr | M = 42 months; range = 14–139 months | PCR |
| Zhang et al[37], 2002 | China | 30 patients with WT | M = 38 months; range = 2 months to 13 yr | Not known | PCR |
| Kullendorff et al[38], 2003 | Sweden | 39 patients with WT | M = 4.2 yr, range 5 months to 15 yr | Range 7 to 160 months | Not known |
| Fernandez et al[22], 2017 | United States, Canada, New Zealand and Israel | 110 patients with very low risk WT (defined as stage I favorable histology WT with nephrectomy weight < 550 g and age at diagnosis < 2 yr) | 11.5 months: 0.1 to 23 months | M = 80 months: 5 to 97 months | Multiplex Ligation-ependent Probe Amplification |
| Ref. | Selection | Comparability | Outcome | NOS score | Quality |
| Spreafico et al[27], 2013 | 4 | 1 | 1 | 6 | Moderate |
| Grundy et al[6], 2005 | 4 | 1 | 1 | 5 | Moderate |
| Chagtai et al[35], 2016 | 3 | 2 | 2 | 6 | High |
| Messahel et al[7], 2009 | 3 | 1 | 2 | 5 | High |
| Grundy et al[2], 1994 | 3 | 2 | 2 | 6 | High |
| Grundy et al[3], 1998 | 3 | 1 | 2 | 6 | High |
| Klamt et al[4], 1998 | 3 | 2 | 2 | 5 | Moderate |
| Skotnicka-Klonowicz et al[36], 2000 | 4 | 2 | 1 | 5 | Moderate |
| Zhang et al[37], 2002 | 3 | 2 | 2 | 6 | High |
| Kullendorff et al[38], 2003 | 3 | 3 | 1 | 5 | Moderate |
| Fernandez et al[22], 2017 | 3 | 1 | 2 | 6 | High |
A heterogeneity test was conducted on the 11 studies, resulting in an I2 value of 25% and a Q test P value of < 0.01, indicating no heterogeneity among the selected studies. A fixed-effects model was selected for the meta-analysis of the 11 studies, with the results depicted in Figure 2.
The meta-analysis, using a fixed-effects model, revealed that the survival time of the experimental group was 1.95 times higher than that of the control group, demonstrating statistical significance (P < 0.05; Figure 2).
A sensitivity analysis of the 11 studies revealed distinct groupings based on different efficacy judgments and varying sensitivities (Figure 3).
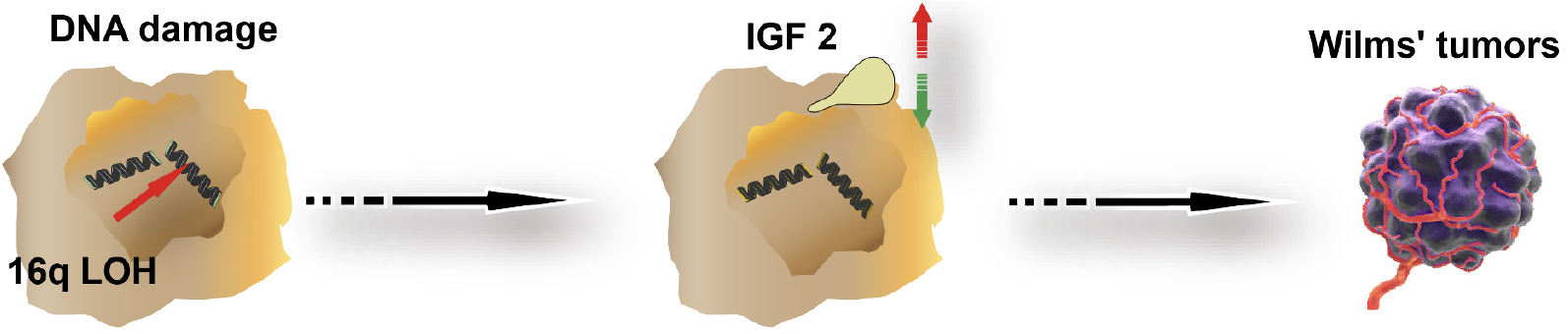
Drawing on the study results, a schematic figure depicting the 16q LOH mechanism causing WT was developed. The theoretical foundation of this study is based on the figure content (Figure 4).
Neoplasms continue to be a significant global health concern[12-14]. WT, as the most prevalent pediatric renal tumor, exhibits prognostic variability affected by factors such as tumor stage, histological type, age, and specific biomarkers. The NWTS, involving 232 patients with WT, initially hypothesized that LOH on chromosome 16q is a poor prognostic factor in patients with WT. However, the consensus on whether 16q LOH indeed signifies a poor prognosis remained contentious. Our previous study revealed an association between 16q LOH and an elevated risk of WT recurrence, prompting the current meta-analysis to elucidate the association between 16q LOH and survival in patients with WT.
LOH is the loss of one allele on a chromosome pair, leaving the paired chromosome intact[15]. Another related state is homozygous deletion, where both alleles on a chromosome pair are lost. This mechanism has been confirmed in diseases such as retinoblastoma, where the LOH of the RB1 allele contributes to pathogenesis. Notably, in patients with congenital iris deficiency (with an incidence rate of approximately 1.4%, also known as aniridia nephroblastoma syndrome), LOH of the RBI gene on chromosome 11 is frequently observed[16].
Dix et al[15] reported a 17% rate of LOH at 16q in patients with WT. The fifth WT research report indicated a worse prognosis, especially when both 16q LOH occurrences coincided, suggesting the potential presence of genes promoting WT formation or enhancing drug resistance in these regions. Evageliou et al[17] identified the minimum region of 1p LOH in WT (between DIS2694 and DIS244 sites), revealing decreased expression of TNFRSF9 and DFFA genes, which play roles in apoptosis regulation, indicating their involvement in WT occurrence. Some scholars suggested that 16q LOH occurrence is unrelated to WT histological typing but may be associated with its occurrence. Additionally, studies have observed that patients with LOH 16q in WT had tumor metastasis in later stages[16].
The Children’s Oncology Group (COG) reported a decrease in relapse-free survival with the presence of 16q LOH (91.2% without vs 82.5% with LOH)[18,19]. Notably, Fernandez et al[20] utilized fresh specimens for analysis, whereas our data rely on formalin-fixed paraffin-embedded sample analysis, possibly explaining some discrepancies. The incidence of 16q LOH ranges from 14%–20% in various reports[21], with some studies reporting 11.9%, marginally lower than the reported incidence[22]. Despite COG trials indicating higher relapse and death risk with 16q LOH, the Society of Pediatric Oncology renal tumor consortium failed to establish a significant effect on event-free survival/overall survival. Some studies reported differences in 16q rates between relapsed patients and survivors (19% vs 4.8%), albeit not statistically significant[23,24].
While LOH is universally recognized for its adverse prognostic effects across various factors, intriguing age-related trends have been documented. Previous studies from the NWTS group and an Egyptian study have noted a lower LOH frequency in younger children[19]. Similar trends have been observed in some other studies[25]. Concurrently, older age has been associated with an increased risk of relapse in WT by multiple research groups[2]. This correlation indicates that the heightened relapse risk with older age may be attributed to specific genetic changes. If translated into clinical practice, these findings could potentially guide therapy in selected patients[26].
Chromosomal segmental deletion is a common occurrence in various genetic syndromes and tumor tissues. Chromosome LOH is frequently linked to high-grade and low differentiation of tumors. Therefore, 16q LOH is not exclusive to WT but has been reported in other tumors such as neuroblastoma and gastrointestinal tumors[27]. The International Children’s Cancer Research Institute has acknowledged 1p or 16q LOH as a crucial reference standard for WT treatment and prognosis. Efforts have been made to identify a stable and simple LOH detection method, including the examination of the expression of four microsatellite foci in these two segments[28]. D16S303, located in LOH 16q, has exhibited a correlation with tumor grade[29]. Detecting 16q LOH in WT tissue through microsatellite focal amplification holds significant implications for understanding the patient’s condition and tailoring personalized treatment approaches[30]. Treatment for WT is multimodal, with surgery being the primary method. For patients with advanced tumors undergoing nephrectomy and extrarenal tumor resection, dialysis or kidney transplantation may not be necessary. However, in specific cases of bilateral nephrectomy without nephron preservation, absence of extrarenal disease, and patients opting out of treatment, transplantation may be considered.
This study distinguishes itself from previous research, such as that of Bu et al[31], by expanding the scope of research collection and conducting a more in-depth analysis of relevant content. Tumors with loss of chromosome heterozygosity play a critical role in tumor formation[32], as a multitude of missing chromosome segments contain cell cycle regulatory and cellular metabolic regulatory genes, including 1p36, 4 Lp36, cadherin 1616q22, and SLC family proteins[33]. The loss of expression of these proteins may affect renal function, and patients with 1p or 16q LOH have reported reduced sensitivity of tumor tissue to radiotherapy and chemotherapy[34]. Understanding gene polymorphism and chromosome LOH characteristics holds significant implications for developing individualized treatment plans. Consistent with our expectations, our study confirms that 16q LOH can serve as a reliable indicator for WT (RR = 1.95, 95%CI: 1.52–2.49, P < 0.001).
Despite the valuable insights provided by this study, several limitations must be acknowledged. Firstly, the inclusion of mostly randomized controlled trial (RCT) studies is a strength, but the limited number of RCT studies specifically focusing on 16q LOH in WT restricts the generalizability of our findings. The scarcity of RCTs dedicated to individual 16q LOH hampers the robustness of the study results. Additionally, the common co-occurrence of 16q LOH with 1p LOH poses a challenge, as individual 16q LOH may not have been extensively studied in RCTs, leading to a dearth of references and potentially incomplete research outcomes. Secondly, the references included in this study primarily originate from an earlier period. The reliance on older literature may not adequately reflect the current landscape of research, especially concerning new diagnostic technologies and methods. The lack of recent references creates a gap in the understanding of the contemporary diagnostic value of 16q LOH for WT.
In conclusion, this meta-analysis highlights the poorer prognosis and significantly shorter survival time associated with 16q LOH in patients with WT compared to those without 16q LOH. The significance of this study lies in its pioneering compilation of evidence, establishing 16q LOH as a positive prognostic factor for patients with WT. However, the limitations of this study, including a small sample size of original studies and the need for larger-scale mutation center trials, underscore the necessity for further research to comprehensively elucidate the prognostic value of 16q LOH in WT.
Wilms tumor (WT) is a prevalent childhood disease with variable treatment outcomes, primarily attributed to multi-gene mutations. Ongoing research has identified 16q loss of heterozygosity (LOH) as a site deletion linked to a poor prognosis.
The aim is to elucidate the relationship between 16q LOH and WT, providing effective guidance for the clinical treatment of this disease.
The primary objective is to determine whether 16q LOH can serve as a predictive marker for a dismal prognosis in patients with WT, utilizing statistical analysis.
The primary objective is to determine whether 16q LOH can serve as a predictive marker for a dismal prognosis in patients with WT, utilizing statistical analysis.
Eleven cohort studies were evaluated to estimate the relationship between event-free survival and 16q LOH in patients with WT (I2 = 25%, P < 0.001). As expected, 16q LOH emerged as a reliable predictor of event-free survival in patients with WT (risk ratio = 1.95, 95%CI: 1.52–2.49, P < 0.001).
In pediatric patients with WT, a discernible correlation exists between 16q LOH and an unfavorable treatment prognosis. The clinical detection of chromosome 16q LOH warrants increased attention to the patient’s prognosis.
Genetic mutations in the 16q LOH locus are indicative of a poor prognosis in patients with WT. However, to establish a clearer relationship between the two factors, conclusive results necessitate a larger sample size, incorporating randomized controlled trial research combined with meta-analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassan MA, Egypt S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Maw MA, Grundy PE, Millow LJ, Eccles MR, Dunn RS, Smith PJ, Feinberg AP, Law DJ, Paterson MC, Telzerow PE. A third Wilms' tumor locus on chromosome 16q. Cancer Res. 1992;52:3094-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Grundy PE, Telzerow PE, Breslow N, Moksness J, Huff V, Paterson MC. Loss of heterozygosity for chromosomes 16q and 1p in Wilms' tumors predicts an adverse outcome. Cancer Res. 1994;54:2331-2333. [PubMed] [DOI] [Full Text] |
| 3. | Grundy RG, Pritchard J, Scambler P, Cowell JK. Loss of heterozygosity on chromosome 16 in sporadic Wilms' tumour. Br J Cancer. 1998;78:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Klamt B, Schulze M, Thäte C, Mares J, Goetz P, Kodet R, Scheulen W, Weirich A, Graf N, Gessler M. Allele loss in Wilms tumors of chromosome arms 11q, 16q, and 22q correlate with clinicopathological parameters. Genes Chromosomes Cancer. 1998;22:287-294. [PubMed] [DOI] [Full Text] |
| 5. | Wittmann S, Zirn B, Alkassar M, Ambros P, Graf N, Gessler M. Loss of 11q and 16q in Wilms tumors is associated with anaplasia, tumor recurrence, and poor prognosis. Genes Chromosomes Cancer. 2007;46:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Grundy PE, Breslow NE, Li S, Perlman E, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D'Angio GJ, Donaldson M, Coppes MJ, Malogolowkin M, Shearer P, Thomas PR, Macklis R, Tomlinson G, Huff V, Green DM; National Wilms Tumor Study Group. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312-7321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Messahel B, Williams R, Ridolfi A, A'hern R, Warren W, Tinworth L, Hobson R, Al-Saadi R, Whyman G, Brundler MA, Kelsey A, Sebire N, Jones C, Vujanic G, Pritchard-Jones K; Children's Cancer and Leukaemia Group (CCLG). Allele loss at 16q defines poorer prognosis Wilms tumour irrespective of treatment approach in the UKW1-3 clinical trials: a Children's Cancer and Leukaemia Group (CCLG) Study. Eur J Cancer. 2009;45:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Bown N, Cotterill SJ, Roberts P, Griffiths M, Larkins S, Hibbert S, Middleton H, Kelsey A, Tritton D, Mitchell C. Cytogenetic abnormalities and clinical outcome in Wilms tumor: a study by the U.K. cancer cytogenetics group and the U.K. Children's Cancer Study Group. Med Pediatr Oncol. 2002;38:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Li T, Providencia R, Mu N, Yin Y, Chen M, Wang Y, Liu M, Yu L, Gu C, Ma H. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Zhang F, Wang K, Du P, Yang W, He Y, Li T, Mei Z. Risk of Stroke in Cancer Survivors: A Meta-analysis of Population-Based Cohort Studies. Neurology. 2021;96:e513-e526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of Metabolic Syndrome and Its Components With Risk of Stroke Recurrence and Mortality: A Meta-analysis. Neurology. 2021;97:e695-e705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Li T, Yang Z, Jiang S, Di W, Ma Z, Hu W, Chen F, Reiter RJ, Yang Y. Melatonin: does it have utility in the treatment of haematological neoplasms? Br J Pharmacol. 2018;175:3251-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Ma Z, Fan C, Yang Y, Di S, Hu W, Li T, Zhu Y, Han J, Xin Z, Wu G, Zhao J, Li X, Yan X. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci Rep. 2016;6:35196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Sun M, Liu X, Xia L, Chen Y, Kuang L, Gu X, Li T. A nine-lncRNA signature predicts distant relapse-free survival of HER2-negative breast cancer patients receiving taxane and anthracycline-based neoadjuvant chemotherapy. Biochem Pharmacol. 2021;189:114285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Dix DB, Fernandez CV, Chi YY, Mullen EA, Geller JI, Gratias EJ, Khanna G, Kalapurakal JA, Perlman EJ, Seibel NL, Ehrlich PF, Malogolowkin M, Anderson J, Gastier-Foster J, Shamberger RC, Kim Y, Grundy PE, Dome JS; AREN0532 and AREN0533 study committees. Augmentation of Therapy for Combined Loss of Heterozygosity 1p and 16q in Favorable Histology Wilms Tumor: A Children's Oncology Group AREN0532 and AREN0533 Study Report. J Clin Oncol. 2019;37:2769-2777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Dix DB, Seibel NL, Chi YY, Khanna G, Gratias E, Anderson JR, Mullen EA, Geller JI, Kalapurakal JA, Paulino AC, Perlman EJ, Ehrlich PF, Malogolowkin M, Gastier-Foster JM, Wagner E, Grundy PE, Fernandez CV, Dome JS. Treatment of Stage IV Favorable Histology Wilms Tumor With Lung Metastases: A Report From the Children's Oncology Group AREN0533 Study. J Clin Oncol. 2018;36:1564-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Evageliou N, Renfro LA, Geller J, Perlman E, Kalapurakal J, Paulino A, Dix D, Eklund MJ, Murphy AJ, Romao RLP, Ehrlich PF, Varela CR, Vallance K, Fernandez CV, Dome JS, Mullen EA. Prognostic impact of lymph node involvement and loss of heterozygosity of 1p or 16q in stage III favorable histology Wilms tumor: A report from Children's Oncology Group Studies AREN03B2 and AREN0532. Cancer. 2024;130:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Fernandez CV, Mullen EA, Chi YY, Ehrlich PF, Perlman EJ, Kalapurakal JA, Khanna G, Paulino AC, Hamilton TE, Gow KW, Tochner Z, Hoffer FA, Withycombe JS, Shamberger RC, Kim Y, Geller JI, Anderson JR, Grundy PE, Dome JS. Outcome and Prognostic Factors in Stage III Favorable-Histology Wilms Tumor: A Report From the Children's Oncology Group Study AREN0532. J Clin Oncol. 2018;36:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Gratias EJ, Jennings LJ, Anderson JR, Dome J, Grundy PE, and Perlman E. Prognostic implications of gain of 1q in favorable histology Wilms tumor: A report from the Children's Oncology Group. J Clin Oncol. 2013;31. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Fernandez CV, Mullen EA, Ehrlich PF, Kalapurakal JA, Khanna G, Chi YY, Perlman E, Paulino A, Hamilton TE, Gow KW, Ferrer FA, Tochner Z, An Q, Hoffer FA, Withycombe J, Shamberger RC, Geller JI, Anderson JR, Grundy PE, and Dome JS. Outcome and prognostic factors in stage III favorable histology Wilms tumor (FHWT): A report from the Children's Oncology Group (COG) study AREN0532. J Clin Oncol. 2015;33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Agarwal S, Iyer VK, Agarwala S, Mathur SR, Aron M, Datta Gupta S, Verma K. Apoptotic protein expression in favorable-histology Wilms tumor correlates with tumor recurrence. Pediatr Surg Int. 2011;27:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Fernandez CV, Perlman EJ, Mullen EA, Chi YY, Hamilton TE, Gow KW, Ferrer FA, Barnhart DC, Ehrlich PF, Khanna G, Kalapurakal JA, Bocking T, Huff V, Tian J, Geller JI, Grundy PE, Anderson JR, Dome JS, Shamberger RC. Clinical Outcome and Biological Predictors of Relapse After Nephrectomy Only for Very Low-risk Wilms Tumor: A Report From Children's Oncology Group AREN0532. Ann Surg. 2017;265:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Gratias EJ, Dome JS, Jennings LJ, Chi YY, Tian J, Anderson J, Grundy P, Mullen EA, Geller JI, Fernandez CV, Perlman EJ. Association of Chromosome 1q Gain With Inferior Survival in Favorable-Histology Wilms Tumor: A Report From the Children's Oncology Group. J Clin Oncol. 2016;34:3189-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Vujanić GM, Gessler M, Ooms AHAG, Collini P, Coulomb-l'Hermine A, D'Hooghe E, de Krijger RR, Perotti D, Pritchard-Jones K, Vokuhl C, van den Heuvel-Eibrink MM, Graf N; International Society of Paediatric Oncology–Renal Tumour Study Group (SIOP–RTSG). The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat Rev Urol. 2018;15:693-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 25. | Gratias EJ, Jennings LJ, Anderson JR, Dome JS, Grundy P, Perlman EJ. Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: a report from the Children's Oncology Group. Cancer. 2013;119:3887-3894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Kitamura E, Cowell JK, Chang CS, Hawthorn L. Variant profiles of genes mapping to chromosome 16q loss in Wilms tumors reveals link to cilia-related genes and pathways. Genes Cancer. 2020;11:137-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Spreafico F, Gamba B, Mariani L, Collini P, D'Angelo P, Pession A, Di Cataldo A, Indolfi P, Nantron M, Terenziani M, Morosi C, Radice P, Perotti D; AIEOP Wilms Tumor Working Group. Loss of heterozygosity analysis at different chromosome regions in Wilms tumor confirms 1p allelic loss as a marker of worse prognosis: a study from the Italian Association of Pediatric Hematology and Oncology. J Urol. 2013;189:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Sredni ST, Gadd S, Huang CC, Breslow N, Grundy P, Green DM, Dome JS, Shamberger RC, Beckwith JB, Perlman EJ; Renal Tumor Committee of the Children's Oncology Group. Subsets of very low risk Wilms tumor show distinctive gene expression, histologic, and clinical features. Clin Cancer Res. 2009;15:6800-6809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Madanat-Harjuoja LM, Renfro LA, Klega K, Tornwall B, Thorner AR, Nag A, Dix D, Dome JS, Diller LR, Fernandez CV, Mullen EA, Crompton BD. Circulating Tumor DNA as a Biomarker in Patients With Stage III and IV Wilms Tumor: Analysis From a Children's Oncology Group Trial, AREN0533. J Clin Oncol. 2022;40:3047-3056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Natrajan R, Little SE, Reis-Filho JS, Hing L, Messahel B, Grundy PE, Dome JS, Schneider T, Vujanic GM, Pritchard-Jones K, Jones C. Amplification and overexpression of CACNA1E correlates with relapse in favorable histology Wilms' tumors. Clin Cancer Res. 2006;12:7284-7293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Bu Q, He H, Fan D, Lyu J, Pan Z, You H. Association between loss of heterozygosity of chromosome 16q and survival in Wilms' tumor: A meta-analysis. Pathol Res Pract. 2018;214:1772-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Ohshima J, Haruta M, Fujiwara Y, Watanabe N, Arai Y, Ariga T, Okita H, Koshinaga T, Oue T, Hinotsu S, Nakadate H, Horie H, Fukuzawa M, Kaneko Y. Methylation of the RASSF1A promoter is predictive of poor outcome among patients with Wilms tumor. Pediatr Blood Cancer. 2012;59:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Park JE, Noh OK, Lee Y, Choi HS, Han JW, Hahn SM, Lyu CJ, Lee JW, Yoo KH, Koo HH, Jeong SY, Sung KW. Loss of Heterozygosity at Chromosome 16q Is a Negative Prognostic Factor in Korean Pediatric Patients with Favorable Histology Wilms Tumor: A Report of the Korean Pediatric Hematology Oncology Group (K-PHOG). Cancer Res Treat. 2020;52:438-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Rahiman EA, Singh M, Bhatia P, Kakkar N, Trehan A, Bansal D. Copy Number Variations in Wilms Tumor: A Pilot Study From India. J Pediatr Hematol Oncol. 2020;42:e299-e304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Chagtai T, Zill C, Dainese L, Wegert J, Savola S, Popov S, Mifsud W, Vujanić G, Sebire N, Le Bouc Y, Ambros PF, Kager L, O'Sullivan MJ, Blaise A, Bergeron C, Mengelbier LH, Gisselsson D, Kool M, Tytgat GA, van den Heuvel-Eibrink MM, Graf N, van Tinteren H, Coulomb A, Gessler M, Williams RD, Pritchard-Jones K. Gain of 1q As a Prognostic Biomarker in Wilms Tumors (WTs) Treated With Preoperative Chemotherapy in the International Society of Paediatric Oncology (SIOP) WT 2001 Trial: A SIOP Renal Tumours Biology Consortium Study. J Clin Oncol. 2016;34:3195-3203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Skotnicka-Klonowicz G, Rieske P, Bartkowiak J, Szymik-Kantorowicz S, Daszkiewicz P, Debiec-Rychter M. 16q heterozygosity loss in Wilms' tumour in children and its clinical importance. Eur J Surg Oncol. 2000;26:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Zhang L, Gao JC, Chen L, Shen ZZ, and Luo JM. Loss of 16q heterozygosity in Wilms’ tumor and its clinical importance. Zhonghua Xiaoer Waihe Zazhi. 2002;23:202-204. |
| 38. | Kullendorff CM, Soller M, Wiebe T, Mertens F. Cytogenetic findings and clinical course in a consecutive series of Wilms tumors. Cancer Genet Cytogenet. 2003;140:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |