Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2113
Peer-review started: January 2, 2024
First decision: January 10, 2024
Revised: January 19, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: May 15, 2024
Processing time: 128 Days and 3.1 Hours
Accumulating evidence has shown that adipose tissue-derived mesenchymal stem cells (ADSCs) are an effective therapeutic approach for managing coronavirus disease 2019 (COVID-19); however, further elucidation is required to determine their underlying immunomodulatory effect on the mRNA expression of T helper cell-related transcription factors (TFs) and cytokine release in peripheral blood mononuclear cells (PBMCs).
To investigate the impact of ADSCs on the mRNA expression of TFs and cytokine release in PBMCs from colorectal cancer (CRC) patients with severe COVID-19 (CRC+ patients).
PBMCs from CRC+ patients (PBMCs-C+) and age-matched CRC patients (PBMCs-C) were stimulated and cultured in the presence/absence of ADSCs. The mRNA levels of T-box TF TBX21 (T-bet), GATA binding protein 3 (GATA-3), RAR-related orphan receptor C (RORC), and forkhead box P3 (FoxP3) in the PBMCs were determined by reverse transcriptase-polymerase chain reaction. Culture supernatants were evaluated for levels of interferon gamma (IFN-γ), interleukin 4 (IL-4), IL-17A, and transforming growth factor beta 1 (TGF-β1) using an enzyme-linked immunosorbent assay.
Compared with PBMCs-C, PBMCs-C+ exhibited higher mRNA levels of T-bet and RORC, and increased levels of IFN-γ and IL-17A. Additionally, a significant decrease in FoxP3 mRNA and TGF-β1, as well as an increase in T-bet/GATA-3, RORC/FoxP3, IFN-γ/IL-4, and IL-17A/TGF-β1 ratios were observed in PBMCs-C+. Furthermore, ADSCs significantly induced a functional regulatory T cell (Treg) subset, as evidenced by an increase in FoxP3 mRNA and TGF-β1 release levels. This was accompanied by a significant decrease in the mRNA levels of T-bet and RORC, release of IFN-γ and IL-17A, and T-bet/GATA-3, RORC/FoxP3, IFN-γ/IL-4, and IL-17A/TGF-β1 ratios, compared with the PBMCs-C+alone.
The present in vitro studies showed that ADSCs contributed to the immunosuppressive effects on PBMCs-C+, favoring Treg responses. Thus, ADSC-based cell therapy could be a beneficial approach for patients with severe COVID-19 who fail to respond to conventional therapies.
Core Tip: In patients with colorectal cancer (CRC) who develop severe coronavirus disease 2019, peripheral blood mononuclear cells display a severe pro-inflammatory phenotype and corresponding functional cytokine profile upon deliberate in vitro stimulation. Adipose tissue-derived mesenchymal stem cells (ADSCs) can significantly induce a regulatory T cell-biased immunosuppressive response while concurrently restraining exaggerated T helper 1 (Th1)-predominant and Th17 pro-inflammatory responses. These results indicate the protective immunomodulatory activity of proactive ADSC therapy by manipulating Th cell polarization to create an anti-inflammatory environment against severe acute respiratory syndrome coronavirus 2-induced severe hyperinflammatory responses.
- Citation: Wang JF, Yang XX, Zhang J, Zheng Y, Zhang FQ, Shi XF, Wang YL. Immunomodulation of adipose-derived mesenchymal stem cells on peripheral blood mononuclear cells in colorectal cancer patients with COVID-19. World J Gastrointest Oncol 2024; 16(5): 2113-2122
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2113.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2113
The coronavirus disease 2019 (COVID-19) pandemic is fueled by the highly infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1,2]. The clinical manifestations of COVID-19 range from asymptomatic to severe/critical illness and death. The disease is often characterized by uncontrolled hyperactivation of the immune system, progressive lymphocyte dysfunction/depletion, cytokine/chemokine burst, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction[3]. As of November 19, 2023, over 772 million confirmed cases and 6 million deaths have been reported worldwide, with the numbers steadily rising[4]. However, no specific drugs are currently available for the trea
Mesenchymal stem cells (MSCs) constitute a multipotent mesoderm-derived progenitor cell population sourced from various tissues, including the bone marrow, umbilical cord (UC), and adipose tissues[5]. These cell populations exert extensive biological functions, including cell differentiation in multiple cell lines, tissue regeneration and repair, and broad immunomodulatory properties through cell-cell contact and paracrine effects[6]. Clinical trials are currently exploring the use of systemically infused MSCs as a cutting-edge and popular therapeutic approach for COVID-19 in contemporary medical research. As of November 2023, over 100 MSC-based clinical trials from phase I to III have already been registered in the www.clinicaltrials.gov/database. These trials, conducted following the initial therapeutic app
Colorectal cancer (CRC) is the third most common cancer worldwide[12] and the fourth leading cancer in China, with an increasing burden[13]. The management and treatment of patients with CRC had become challenging following the relaxation of COVID-19 restrictions in China due to the impact of SARS-CoV-2 infection. The interaction between MSCs and immune cells in COVID-19 patients has been considered a crucial mechanism through which MSCs improve severe COVID-19 outcomes by maintaining immune homeostasis[14]. ADSCs were employed in our experiments.
This study investigated the immunomodulatory effects of ADSCs on the mRNA expression of T helper (Th) cell-related transcription factors (TFs) and their related cytokine release in peripheral blood mononuclear cells (PBMCs) isolated from CRC patients with severe COVID-19 (CRC+ patients) in vitro.
The 20 novo CRC patients included in this study were admitted to Tianjin Medical University Cancer Hospital (Tianjin, China) from December 2022 to February 2023. Of the 20 patients with stage I–II CRC, 10 sex/age-matched patients were simultaneously diagnosed with CRC and severe COVID-19 caused by SARS-CoV-2. Severe COVID-19 was defined based on the guidelines for the diagnosis and treatment of new coronavirus pneumonia (version 9) issued by the National Health Commission of China on March 15, 2022. The inclusion criteria were based on the detection of SARS-CoV-2 by TaqMan quantitative polymerase chain reaction (qPCR) assays in oropharyngeal swab samples. The strains circulating during this period belonged to the Omicron sublineage. Patients (1) aged > 18 years; (2) who were definitively diagnosed with CRC; and (3) who underwent collection of peripheral blood samples prior to receiving standard treatments were evaluated. Table 1 shows the baseline characteristics of all study participants. This study was approved by the Research Ethics Committee of Tianjin Medical University Cancer Hospital and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
| Feature | CRC patients | CRC+ patients |
| Number | 10 | 10 |
| Age, median (range) | 62.4 (50-73) | 65.2 (51-75) |
| Sex, male:female | 5:5 | 5:5 |
| TNM stage | ||
| I | 6 | 6 |
| II | 4 | 4 |
| Degree of differentiation | ||
| High | 1 | 1 |
| Moderately | 9 | 9 |
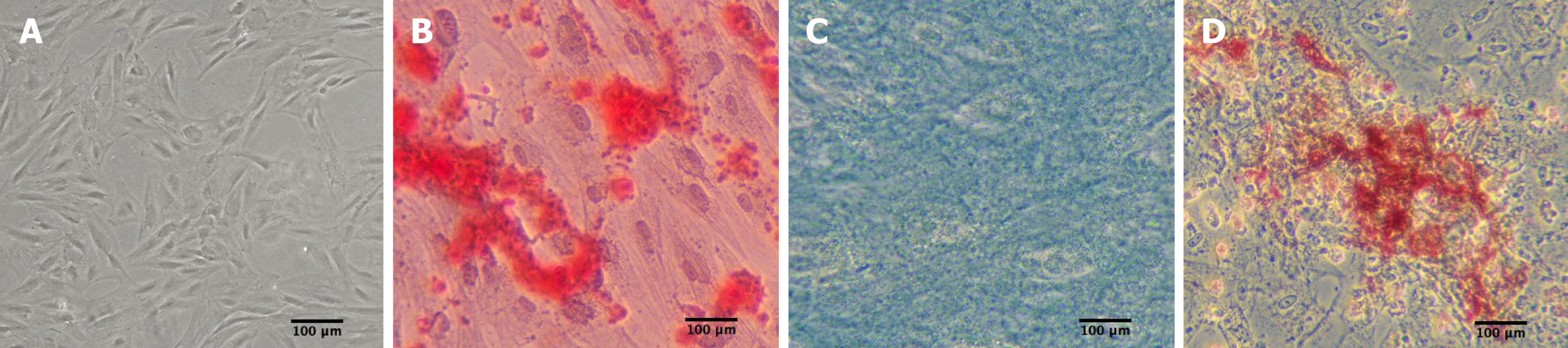
Abdominal subcutaneous adipose tissues were obtained from healthy donors who underwent abdominal liposuction. ADSCs were extracted from adipose tissues and cultured using a previously described standard method[15]. Cell surface markers [cluster of differentiation 14 (CD14), CD34, CD45, human leukocyte antigen-DR (HLA-DR), CD29, CD73, CD90, and CD105) (BD Pharmingen, Franklin Lakes, NJ, United States) were assessed using flow cytometry. Moreover, their multilineage differentiation potential into tissue-specific osteoblasts, chondrocytes, and adipocytes was induced using adipogenic and osteogenic differentiation medium (Sigma-Aldrich, St. Louis, MO, United States) and chondrogenic differentiation medium (Cyagen Co., Ltd., Guangzhou, China) in vitro[16]. After 2 wk, Oil red O staining was carried out to visualize the adipogenesis of ADSC under an inverted microscope. Approximately 3 wk later, Alizarin Red staining was employed to evaluate the formation of calcium deposits. After 4 wk, Alcian Blue staining was performed to quantify the glycosaminoglycans secreted by chondrocytes. All ADSCs for in vitro use were derived from passage 3.
Approximately 8 mL peripheral blood was collected into heparin tubes. Standard density gradient centrifugation on Ficoll-Paque Premium (density = 1.077) (GE Healthcare Bio-Sciences, Uppsala, Sweden) was performed as previously described to isolate PBMCs-C+ and PBMCs-C from CRC+ patients and CRC patients, respectively. PBMCs were resuspended in a freezing medium containing 90% fetal bovine serum (Gibco, Waltham, MA, United States) and 10% dimethyl sulfoxide (Sigma-Aldrich). Aliquots of PBMCs at a density of 5 × 106 cells/mL were cryopreserved in liquid nitrogen until use. One day prior to the experiment, PBMCs were thawed and incubated overnight at 37 °C with 5% carbon dioxide (CO2) to deplete adherent monocytes.
ADSCs were seeded in 12-well culture plates and allowed to attach for 24 h at 37 °C under 5% CO2. To assess for TF expression and cytokine release, 1 × 106/mL PBMCs were seeded into 12-well culture plates and stimulated with 10 μg/mL phytohaemagglutinin (PHA) (Sigma-Aldrich) and 100 U/mL IL-2 (Sihuan bio-pharmaceutical Co., Ltd., Beijing, China) and cultured in the presence/absence of adherent 1 × 105/mL ADSCs. PBMCs and supernatants from co-cultures were harvested after 5 d for subsequent examination using TaqMan qPCR or enzyme-linked immunosorbent assay (ELISA).
Total RNA was extracted from each PBMC sample using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). After digestion with DNase and sample clean-up, the RNA samples were reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s protocols. RNA samples without reverse transcription were used as templates for PCR to exclude possible genomic DNA contamination. PCR was performed using the ABI 7500 Real-Time PCR System (Applied Biosystems). The sequences of primers and FAM-labeled probes used were as follows: T-box TF TBX21 (T-bet) forward, 5′-CTTGGTGTGGACTGAGATTGC-3,’ reverse, 5’-ACTGGAAGGATAGGGGGACA-3’ and probe, 5’-FAM-ATTCAGGACTGGGCGAAGGAGACTCT-TAMRA-3’; GATA binding protein 3 (GATA-3) forward, 5′-AGACCACCACAACCACACTCT-3,’ reverse, 5’-GATGCCT
The Th1 [interferon gamma (IFN-γ)], Th2 [interleukin 4 (IL-4)], Th17 (IL-17A), and regulatory T cell (Treg) [transforming growth factor beta 1 (TGF-β1)] cytokine concentrations in the cell culture supernatants were determined by performing ELISA using ELISA kits (R & D Systems, Minneapolis, MN, United States).
Statistical analyses were performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, United States). All values are expressed as the means ± SD. The statistical significance of comparisons between the two groups was analyzed using the Student’s t-test. P < 0.05 was considered statistically significant.
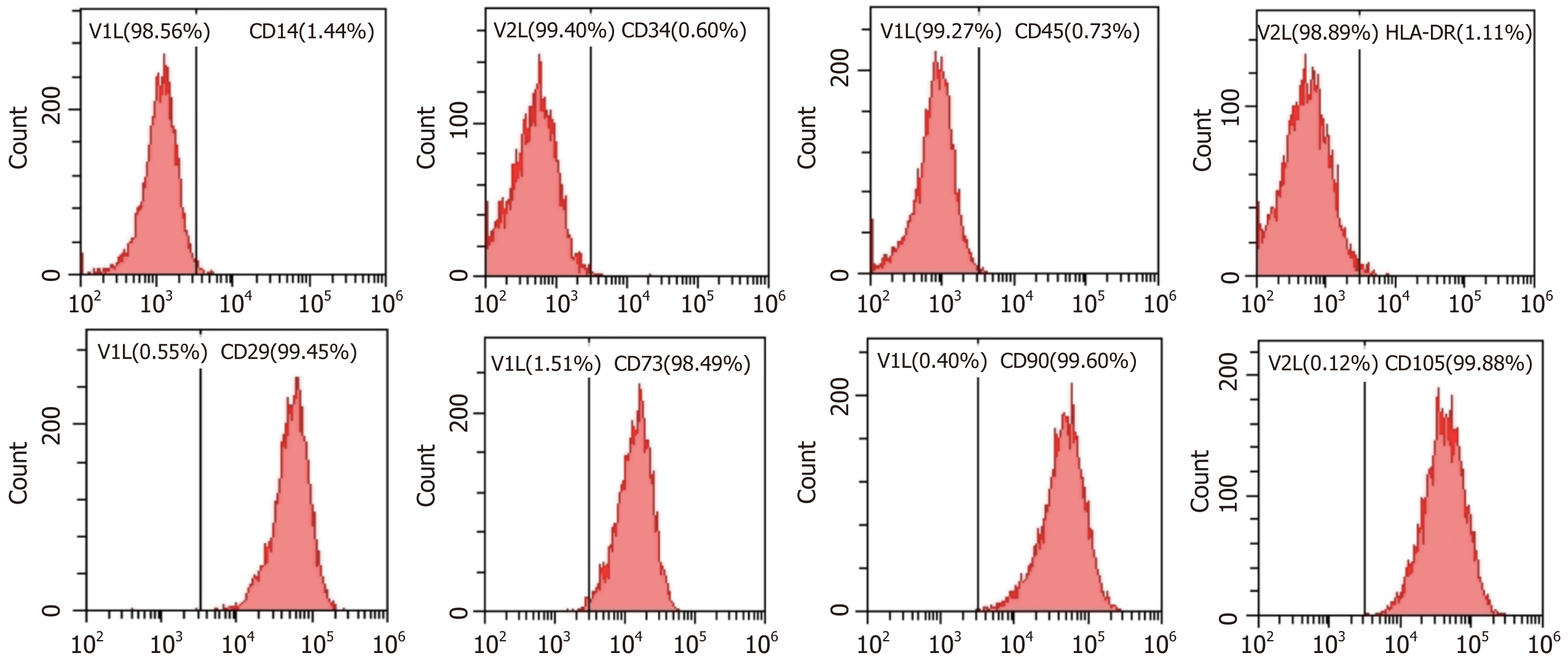
ADSCs showed an elongated fibroblastic morphology during the early passage. In accordance with the criteria set by the International Society for Cellular Therapy, the cell surface markers CD14 (a monocyte marker), CD34 (a hematopoietic stem cell and hematopoietic progenitor cell marker), CD45 (a pan-leukocyte marker), and HLA-DR (a molecule of major histocompatibility complex class II) demonstrated expression levels of 1.44%, 0.60%, 0.73%, and 1.11%, respectively. Meanwhile, the MSC markers CD29 (integrin β1), CD73 (ecto-5’-nucleotidase), CD90 (Thy-1), and CD105 (endoglin) showed expression levels of 99.45%, 98.49%, 99.60%, and 99.88%, respectively (Figure 1). As shown in Figure 2, ADSC exhibited multilineage differentiation potential toward osteoblasts, chondrocytes, and adipocytes.
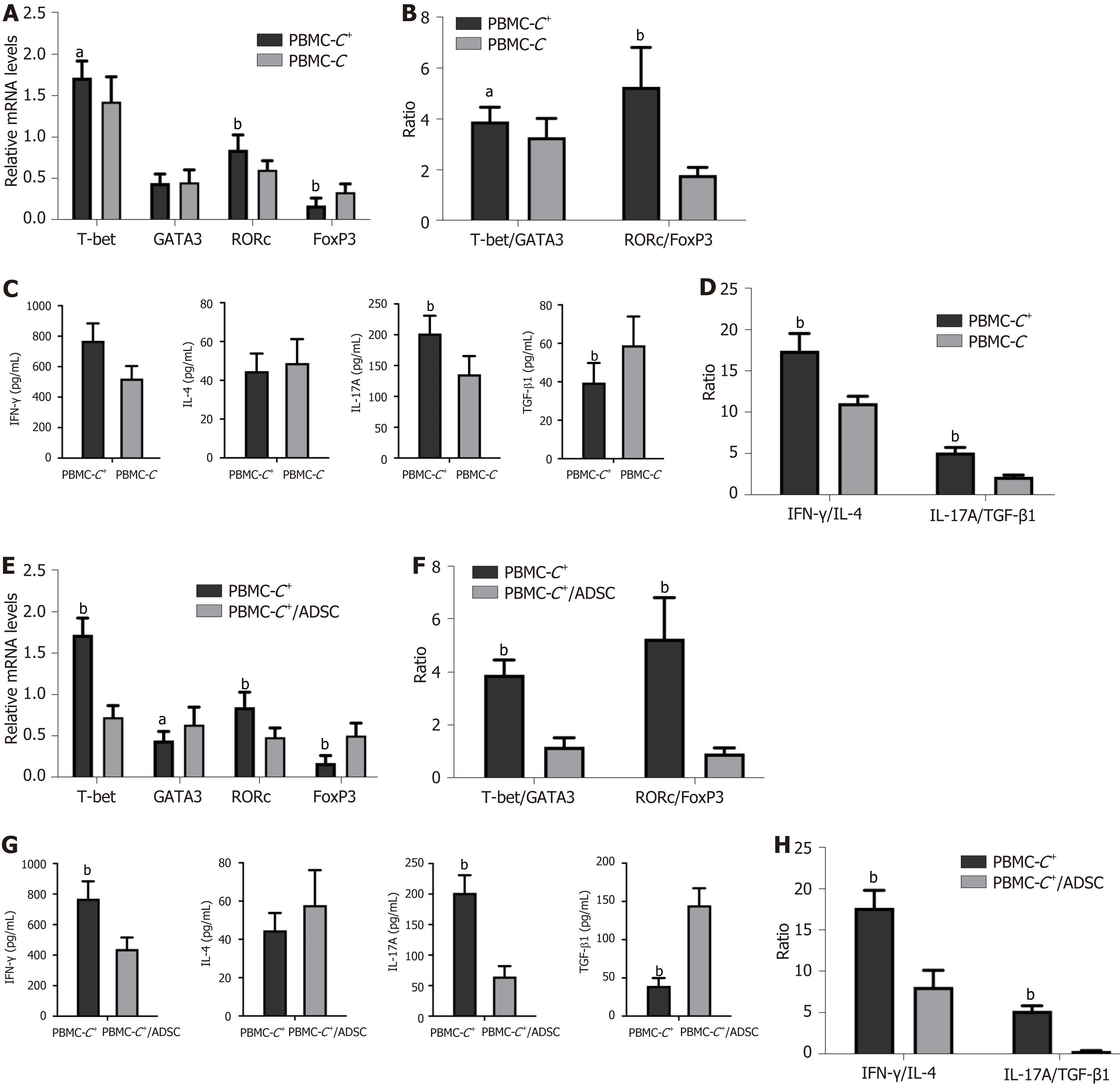
To simulate the in vitro activation of T lymphocytes in infected tissues in vivo, we initially assessed the expression of the studied TFs (T-bet, GATA-3, RORC, and FoxP3) associated with Th1, Th2, Th17, and Tregs in PBMCs-C+ and PBMCs-C stimulated with PHA/IL-2. Significantly higher levels of T-bet and RORC mRNA were observed in the PBMCs-C+ group than in the PBMCs-C (P < 0.05 and P < 0.01, respectively). Conversely, lower levels of FoxP3 mRNA were observed in the PBMCs-C+ group than in the PBMCs-C group (P < 0.01). No significant difference was found in the GATA-3 mRNA levels between the PBMCs-C+and PBMCs-C groups (Figure 3A). Compared with PBMCs-C cells, significantly higher T-bet/GATA-3 (Th1 vs Th2) and RORC/FoxP3 (Th17 vs Tregs) ratios were observed in PBMCs-C+ cells (P < 0.05 and P < 0.01, respectively; Figure 3B).
Cell culture supernatants obtained from the PBMCs-C+ group exhibited significantly elevated levels of Th1- and Th17-type cytokines (IFN-γ and IL-17A) but significantly reduced levels of the Treg tolerance cytokine TGF-β1, compared with the PBMCs-C group (P < 0.01). Although the levels were slightly increased, no statistical difference was found in Th2-type cytokine IL-4 levels between the PBMCs-C+ and PBMCs-C groups (P > 0.05; Figure 3C). Moreover, the IFN-γ/IL-4 (Th1 vs Th2) and Th17A/TGF-β1 (Th17 vs Tregs) ratios in the PBMCs-C+group were significantly increased compared with those in the PBMCs-C group (P < 0.01; Figure 3D).
To determine the immunomodulatory effects of ADSC on PBMCs isolated from CRC patients with severe COVID-19, activated PBMCs-C+ were added to the ADSC monolayer at a 10:1 ratio and co-cultured for 5 d. A slight upregulation of GATA-3 mRNA levels and significant upregulation of FoxP3 mRNA levels were observed in the PBMCs-C+/ADSC group compared with that in the PBMCs-C+ cultured alone group (P < 0.05 and P < 0.01, respectively). By contrast, the T-bet and RORC mRNA levels were significantly downregulated in the PBMCs-C+/ADSC group (P < 0.01; Figure 3E). Moreover, the T-bet/GATA-3 and RORC/FoxP3 ratios significantly decreased in the PBMCs-C+/ADSC group (P < 0.01; Figure 3F).
The levels of IFN-γ and IL-17A in the cell culture supernatants from the PBMCs-C+/ADSC group were significantly decreased (P < 0.01), while the TGF-β1 levels were significantly increased compared with those from the PBMCs-C+ cultured alone group (P < 0.01). The IL-4 release levels slightly increased in the co-cultured samples; however, this trend was not significant (P > 0.05; Figure 3G). ADSCs significantly inhibited the IFN-γ/IL-4 and Th17/Treg ratios (P < 0.01; Figure 3H).
To date, numerous clinical trials have contributed to a growing body of evidence and consensus affirming the safety, effi
Achieving an immunologically balanced homeostasis between Th1/Th2 and Th17/Tregs is crucial for maintaining normal immunological functions within the body. Conversely, imbalances in the differentiation of these T cell subsets are associated with various diseases such as cancer, autoimmune diseases, infectious diseases, and allograft rejection[19-22]. Numerous studies have highlighted that the imbalance of T cell subsets and the subsequent excessive release of cytokines contribute to both local and systemic cytokine storms, leading to immunopathological complications such as ARDS in patients with severe COVID-19[23,24]. In the present study, we evaluated the gene expression of TFs (T-bet, GATA-3, RORC, and FoxP3) associated with Th1, Th2, Th17, and Tregs in PHA/IL-2-activated PBMCs-C+ and the corresponding cytokines secreted by each Th cell profile in the cell culture supernatants. The results indicated a significant shift toward amplified Th1 and Th17 immunity. Specifically, we observed a significant upregulation in the production of T-bet (Th1) and RORC (Th17) mRNA and a significant downregulation in the production of FoxP3 (Tregs) mRNA in PBMCs-C+. Furthermore, we detected a significant increase in the secretion of pro-inflammatory Th1 and Th17 type cytokines (IFN-γ and IL-17A), and a significant decrease in the secretion of Tregs tolerance cytokine (TGF-β1) in the cell culture supernatants from the PBMCs-C+ group. Various studies related to COVID-19 and cytokine storms have reflected the known Th subset bias and distinctive cytokine profiles that correlate with the severity of the disease, thus influencing the course and outcome of COVID-19[25-27]. Our results agree with those of a recent study, which reported the transcriptional activity and functional phenotype of T cells and the relationship between pro- and anti-inflammatory cytokines and the clinical parameters of COVID-19 severity. The authors reported increased Th1 and Th17 transcriptional activity and a subsequent increase in the systemic concentration of proinflammatory cytokines in patients with severe COVID-19. Notably, the study identified IFN-γ, IL-17, IL-12, and IL-23 as crucial markers of COVID-19[28]. Chen et al[29] strongly suggested that COVID-19 plasma exosomes are a risk factor for intensifying COVID-19 by stimulating the proinflammatory response of peripheral blood immune cells[29]. Furthermore, our study demonstrated that the polarization of cellular immune response toward Th1 and Th17 was evident through an increase in T-bet/GATA-3 and RORC/FoxP3 mRNA ratio as well as IFN-γ/IL-4 and IL-17A/TGF-β1 ratio. These data suggest an uncontrolled and deleterious inflammatory status in severely ill COVID-19 patients, at least partially favoring Th1 and Th17 cellular and pro-inflammatory phenotypes. This phenomenon is accompanied by an increase in Th1- and Th17-type cytokine cascade, contributing to lung damage and ARDS.
Although the precise therapeutic mechanisms of MSCs require further investigation, MSC-based therapy has been explored for various diseases, including graft vs host disease, autoimmune diseases, pulmonary diseases, and allograft rejection. This approach has shown promise in attenuating inflammatory responses, reducing ischemic injuries, maintaining self-tolerance, and promoting immunomodulatory effects, and tissue repair and regeneration[30,31]. Ongoing clinical trials are actively assessing the safety and efficacy of MSCs in cellular immunotherapy[32,33]. Given their potent abilities, we directly treated the activated PBMCs-C+ group with ADSCs to investigate whether proactive ADSC therapy could suppress overly active Th cells during a cytokine storm in patients with COVID-19. Remarkably, our data demonstrated the significance of Th1/Th2 and Th17/Tregs ratios, as these ratios indicate the superiority of one Th cell subset over others. In the present study, the presence of ADSCs resulted in the downregulation of T-bet and RORC mRNA expression, along with the upregulation of GATA-3 and FoxP3 mRNA expression. This led to a reversal in T-bet/GATA-3 and RORC/FoxP3 ratios in co-cultures compared with activated PBMCs-C+ cultured alone. Similarly, these changes in TFs were accompanied by an increase in Tregs tolerance cytokine TGF-β1 release and a decrease in Th1- and Th17-type cytokine IFN-γ and IL-17A release. Recent studies have demonstrated that MSCs from the microenvironment can exhibit two functionally distinct phenotypes. The MSC1 phenotype displays pro-inflammatory properties when the immune system is under-activated. By contrast, the MSC2 phenotype exhibits anti-inflammatory properties to prevent a massive inflammatory cascade and self-attack during immune system overactivation[34,35]. Preconditioned ADSCs with pro-inflammatory factors increased the release of relevant bioactive factors, enhancing their efficacy to manipulate the microenvironment toward a favorable polarization of Tregs along with an increased TGF-β1 release[36,37]. Therefore, systemic MSC administration in COVID-19 patients may expose MSCs to a deleterious inflammatory environment upon entering the circulation, and the subsequent interaction between MSC and pro-inflammatory factors may lead to MSC activation. Our results are consistent with those of recent clinical trials evaluating the safety and efficacy of UC-derived MSC (UC-MSC) transplantations in patients with mild-to-moderate COVID-19-induced ARDS. These trials demonstrated that UC-MSC therapy could safely downregulate disease progression by decreasing the activation and expansion of Th1 and Th17 cells, along with an increase in the expression of anti-inflammatory T lymphocytes[38,39]. In summary, our findings suggest that ADSC can be activated when exposed to a highly inflammatory environment in vitro. They directly influence Th cell differentiation in favor of Tregs induction, intervening in the Th effector cell activation to restore the immunological balance through an immunological “braking” mechanism. Additionally, ADSCs induce Tregs tolerance cytokine production and inhibit Th1- and Th17-type cytokine release, thereby attenuating the progression of cytokine storms and tissue damage.
Our study had several limitations. First, our data collection was restricted to patients admitted from December 2022 to February 2023, due to the COVID-19 restrictions. However, despite this limitation, we presented preliminary evidence suggesting an association between severe COVID-19 and widespread inflammatory damage. Second, only a small sample size and early-stage patients with CRC and SARS-CoV-2 infection were investigated. The study did not encompass the assessments of patients with advanced disease stages, warranting confirmation by conducting large-scale studies. Notably, Guo et al[40] demonstrated that MSCs and MSC-secreted exosomes could exert beneficial effects on CRC by modulating immune responses and inducing anti-tumor responses. Thus, as a potential therapeutic agent, further clinical investigations are warranted to examine the promising effects of ADSC therapy on advanced stages of CRC and COVID-19.
In the context of countering the cytokine storm, the intervention involving ADSCs, as a living immunomodulatory agent, is a promising therapeutic approach. This strategy can promote an anti-inflammatory environment by restraining excessive inflammation reactions and driving Treg-biased tolerance immune responses. Consequently, ADSCs hold promise as a viable alternative treatment option for preventing the progression of severe COVID-19 progression.
Mesenchymal stem cells have emerged as viable and ideal therapeutic agents demonstrating efficacy in immunomodulation, antiviral activity, and tissue regeneration/repair after injury, as evidenced by preclinical studies and clinical trials. The ongoing coronavirus disease 2019 (COVID-19) pandemic, fueled by the highly infectious severe acute respiratory syndrome coronavirus 2, has prompted the exploration of potential treatments. Accumulating evidence supports the effectiveness of adipose tissue-derived mesenchymal stem cells (ADSCs) as an effective therapeutic approach for managing COVID-19. However, the underlying immunomodulatory effects on the mRNA expression of Th cell-related transcription factors (TFs) as well as cytokine release in peripheral blood mononuclear cells (PBMCs) need to be elu
This study investigated the effect of ADSC therapy on the mRNA expression of TFs and cytokine release in colorectal cancer (CRC) patients with severe COVID-19 (CRC+ patients).
This study investigated the immunomodulatory effect of ADSCs in CRC+ patients.
PBMCs from CRC+ patients (PBMCs-C+) and age-matched CRC patients (PBMCs-C) were stimulated and cultured in the presence/absence of ADSC. The mRNA levels of T-box TF TBX21 (T-bet), GATA binding protein 3 (GATA-3), RAR-related orphan receptor C (RORC), and forkhead box P3 (FoxP3) in the PBMCs were determined by real-time reverse transcriptase-polymerase chain reaction; the culture supernatants were examined to measure the levels of interferon gamma (IFN-γ), interleukin 4 (IL-4), IL-17A, and transforming growth factor beta one (TGF-β1) using an enzyme-linked immunosorbent assay.
Compared with the PBMCs-C group, the mRNA levels of TFs (T-bet and RORC) and the release of cytokines (IFN-γ and IL-17A) were higher in the PBMCs-C+group. Additionally, a significant decrease in FoxP3 mRNA and TGF-β1 Levels, coupled with an increase in T-bet/GATA-3, RORC/FoxP3, IFN-γ/IL-4, and IL-17A/TGF-β1 ratios were observed in the PBMCs-C+group. Further analysis revealed that ADSC therapy significantly induced functional regulatory T cell (Treg) subsets, as evidenced by an increase in the levels of FoxP3 mRNA and TGF-β1, and a decrease in the mRNA levels of TFs (T-bet and RORC), release of cytokines (IFN-γ and IL-17A), and T-bet/GATA-3, RORC/FoxP3, IFN-γ/IL-4, and IL-17A/TGF-β1 ratios, compared with the PBMCs-C+alone group.
ADSCs contributed to the immunosuppressive effects on PBMCs-C+.
Our study demonstrated that ADSC intervention, as a living immunomodulatory agent, is a promising therapeutic approach to create an anti-inflammatory environment by mitigating excessive inflammatory reactions and promoting Treg-biased tolerance immune responses. Thus, ADSCs are expected to be a viable alternative treatment option for preventing severe COVID-19 progression.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, United States S-Editor: Li L L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Wu Y, Long Y, Wang F, Liu W, Wang Y. Emergence of SARS-CoV-2 Omicron variant and strategies for tackling the infection. Immun Inflamm Dis. 2022;10:e733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Long Y, Wang F, Li C, Liu W. Characterization of SARS-CoV-2 recombinants and emerging Omicron sublineages. Int J Med Sci. 2023;20:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Scaramuzzo G, Nucera F, Asmundo A, Messina R, Mari M, Montanaro F, Johansen MD, Monaco F, Fadda G, Tuccari G, Hansbro NG, Hansbro PM, Hansel TT, Adcock IM, David A, Kirkham P, Caramori G, Volta CA, Spadaro S. Cellular and molecular features of COVID-19 associated ARDS: therapeutic relevance. J Inflamm (Lond). 2023;20:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. COVID-19 Epidemiological Update - 24 November 2023. Nov 24, 2023. [cited 3 January 2024]. Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-november-2023. |
| 5. | Yang G, Fan X, Liu Y, Jie P, Mazhar M, Dechsupa N, Wang L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev Rep. 2023;19:1214-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 6. | Harrell CR, Pavlovic D, Djonov V, Volarevic V. Therapeutic potential of mesenchymal stem cells in the treatment of acute liver failure. World J Gastroenterol. 2022;28:3627-3636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 8. | O'Kane CM, Matthay MA. Understanding the Role of Mesenchymal Stromal Cells in Treating COVID-19 Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2023;207:231-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Chen X, Wang F, Huang Z, Wu Y, Geng J, Wang Y. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: An Update and Concise Review. Int J Med Sci. 2021;18:2849-2870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Farhana S, Kai YC, Kadir R, Sulaiman WAW, Nordin NA, Nasir NAM. The fate of adipose tissue and adipose-derived stem cells in allograft. Cell Tissue Res. 2023;394:269-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Czerwiec K, Zawrzykraj M, Deptuła M, Skoniecka A, Tymińska A, Zieliński J, Kosiński A, Pikuła M. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 923] [Article Influence: 461.5] [Reference Citation Analysis (1)] |
| 13. | Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943-e955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 219] [Reference Citation Analysis (0)] |
| 14. | Alavi-Dana SMM, Gholami Y, Meghdadi M, Fadaei MS, Askari VR. Mesenchymal stem cell therapy for COVID-19 infection. Inflammopharmacology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Jin Y, Yang L, Zhang Y, Gao W, Yao Z, Song Y, Wang Y. Effects of age on biological and functional characterization of adiposederived stem cells from patients with endstage liver disease. Mol Med Rep. 2017;16:3510-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Liu T, Zhang Y, Shen Z, Zou X, Chen X, Chen L, Wang Y. Immunomodulatory effects of OX40Ig gene-modified adipose tissue-derived mesenchymal stem cells on rat kidney transplantation. Int J Mol Med. 2017;39:144-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kandula UR, Wake AD. Effectiveness of RCTs Pooling Evidence on Mesenchymal Stem Cell (MSC) Therapeutic Applications During COVID-19 Epidemic: A Systematic Review. Biologics. 2023;17:85-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Whittaker Brown SA, Iancu-Rubin C, Aboelela A, Abrahams A, Burke E, Drummond T, Grossman F, Itescu S, Lagdameo J, Lin JY, Mark A, Levine JE, Osman K. Mesenchymal stromal cell therapy for acute respiratory distress syndrome due to coronavirus disease 2019. Cytotherapy. 2022;24:835-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Lan YT, Fan XP, Fan YC, Zhao J, Wang K. Change in the Treg/Th17 cell imbalance in hepatocellular carcinoma patients and its clinical value. Medicine (Baltimore). 2017;96:e7704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Kuca-Warnawin E, Janicka I, Bonek K, Kontny E. Modulatory Impact of Adipose-Derived Mesenchymal Stem Cells of Ankylosing Spondylitis Patients on T Helper Cell Differentiation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Gupta A, Kumar A, Deepak RK, Arora U, Agrawal A, Soneja M, Garg P, Kumar S, Kumar P, Kanga U, V GK, Soni KD, Wig N. Th17 overexpression in severe COVID-19: a prospective observational study. Infect Disord Drug Targets. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Lee SK, Park MJ, Jhun JY, Beak JA, Choi JW, Rye JY, Jang JW, Bae SH, Yoon SK, Choi HJ, You YK, Cho ML, Choi JY. Combination Treatment With Metformin and Tacrolimus Improves Systemic Immune Cellular Homeostasis by Modulating Treg and Th17 Imbalance. Front Immunol. 2020;11:581728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Hawerkamp HC, Dyer AH, Patil ND, McElheron M, O'Dowd N, O'Doherty L, Mhaonaigh AU, George AM, O'Halloran AM, Reddy C, Kenny RA, Little MA, Martin-Loeches I, Bergin C, Kennelly SP, Donnelly SC, Bourke NM, Long A, Sui J, Doherty DG, Conlon N, Cheallaigh CN, Fallon PG. Characterisation of the pro-inflammatory cytokine signature in severe COVID-19. Front Immunol. 2023;14:1170012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 25. | Kalinina O, Golovkin A, Zaikova E, Aquino A, Bezrukikh V, Melnik O, Vasilieva E, Karonova T, Kudryavtsev I, Shlyakhto E. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Qudus MS, Tian M, Sirajuddin S, Liu S, Afaq U, Wali M, Liu J, Pan P, Luo Z, Zhang Q, Yang G, Wan P, Li Y, Wu J. The roles of critical pro-inflammatory cytokines in the drive of cytokine storm during SARS-CoV-2 infection. J Med Virol. 2023;95:e28751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 27. | Drake KA, Talantov D, Tong GJ, Lin JT, Verheijden S, Katz S, Leung JM, Yuen B, Krishna V, Wu MJ, Sutherland AM, Short SA, Kheradpour P, Mumbach MR, Franz KM, Trifonov V, Lucas MV, Merson J, Kim CC; PRESCO Study Group. Multi-omic profiling reveals early immunological indicators for identifying COVID-19 Progressors. Clin Immunol. 2023;256:109808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Jovanovic M, Sekulic S, Jocic M, Jurisevic M, Gajovic N, Jovanovic M, Arsenijevic N, Mijailovic M, Milosavljevic M, Jovanovic I. Increased Pro Th1 And Th17 Transcriptional Activity In Patients With Severe COVID-19. Int J Med Sci. 2023;20:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Chen L, Chen R, Yao M, Feng Z, Yuan G, Ye F, Nguyen K, Karn J, McComsey GA, McIntyre TM, Jin G. COVID-19 plasma exosomes promote proinflammatory immune responses in peripheral blood mononuclear cells. Sci Rep. 2022;12:21779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Lopez-Santalla M, Fernandez-Perez R, Garin MI. Mesenchymal Stem/Stromal Cells for Rheumatoid Arthritis Treatment: An Update on Clinical Applications. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Kouchakian MR, Baghban N, Moniri SF, Baghban M, Bakhshalizadeh S, Najafzadeh V, Safaei Z, Izanlou S, Khoradmehr A, Nabipour I, Shirazi R, Tamadon A. The Clinical Trials of Mesenchymal Stromal Cells Therapy. Stem Cells Int. 2021;2021:1634782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Kim KI, Lee MC, Lee JH, Moon YW, Lee WS, Lee HJ, Hwang SC, In Y, Shon OJ, Bae KC, Song SJ, Park KK, Kim JH. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am J Sports Med. 2023;51:2243-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 33. | Zarrabi M, Shahrbaf MA, Nouri M, Shekari F, Hosseini SE, Hashemian SR, Aliannejad R, Jamaati H, Khavandgar N, Alemi H, Madani H, Nazari A, Amini A, Hassani SN, Abbasi F, Jarooghi N, Fallah N, Taghiyar L, Ganjibakhsh M, Hajizadeh-Saffar E, Vosough M, Baharvand H. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res Ther. 2023;14:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 34. | Dabrowska S, Andrzejewska A, Janowski M, Lukomska B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front Immunol. 2020;11:591065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 35. | Zhang Z, Yang X, Meng Q, Long Y, Shi X, Wang Y. Adipose tissue-derived mesenchymal stromal cells attenuate acute lung injury induced by trauma and haemorrhagic shock. Immunobiology. 2023;228:152765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 36. | Wang P, Gu X, Zhang N, Zhang H, Shi S, Wang Y. Interferon-γ promotes immunomodulatory of adipose tissue-derived mesenchymal stem cells on peripheral blood lymphocytes. Tianjin Yiyao. 2016;44:683-686. [DOI] [Full Text] |
| 37. | Luan X, Chen P, Li Y, Yuan X, Miao L, Zhang P, Cao Q, Song X, Di G. TNF-α/IL-1β-licensed hADSCs alleviate cholestatic liver injury and fibrosis in mice via COX-2/PGE2 pathway. Stem Cell Res Ther. 2023;14:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Kaffash Farkhad N, Sedaghat A, Reihani H, Adhami Moghadam A, Bagheri Moghadam A, Khadem Ghaebi N, Khodadoust MA, Nikpoor AR, Tavakol Afshari J. Specific Clinical and Immunological Changes Following Mesenchymal Stem Cell Transplantation in COVID-19-induced Acute Respiratory Distress Syndrome Patients: A Phase-I Clinical Trial. Iran J Allergy Asthma Immunol. 2022;21:687-703. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Kaffash Farkhad N, Sedaghat A, Reihani H, Adhami Moghadam A, Bagheri Moghadam A, Khadem Ghaebi N, Khodadoust MA, Ganjali R, Tafreshian AR, Tavakol-Afshari J. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther. 2022;13:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Guo G, Tan Z, Liu Y, Shi F, She J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res Ther. 2022;13:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |