Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1878
Peer-review started: November 17, 2023
First decision: December 12, 2023
Revised: December 14, 2023
Accepted: March 26, 2024
Article in press: March 26, 2024
Published online: May 15, 2024
Processing time: 174 Days and 1 Hours
Colorectal cancer (CRC) is a prevalent global malignancy with complex pro
We aimed to establish a simplified protocol for quantifying M2-like TAMs and explore their correlation with clinicopathological factors.
A cross-sectional study included histopathological assessment of paraffin-embedded tissue blocks obtained from 43 CRC patients. Using CD68 and CD163 immunohistochemistry, we quantified TAMs in tumor stroma and front, focusing on M2 proportion. Demographic, histopathological, and clinical parameters were collected.
TAM density was significantly higher at the tumor front, with the M2 proportion three times greater in both zones. The tumor front had a higher M2 proportion, which correlated significantly with advanced tumor stage (P = 0.04), pathological nodal involvement (P = 0.04), and lymphovascular invasion (LVI, P = 0.01). However, no significant association was found between the M2 proportion in the tumor stroma and clinicopathological factors.
Our study introduces a simplified protocol for quantifying M2-like TAMs in CRC tissue samples. We demonstrated a significant correlation between an increased M2 proportion at the tumor front and advanced tumor stage, nodal involvement, and LVI. This suggests that M2-like TAMs might serve as potential indicators of disease progression in CRC, warranting further investigation and potential clinical application.
Core Tip: Colorectal cancer is a highly prevalent type of cancer worldwide. Its prognosis depends on several factors including pathological features. Novel prognostic factors with improved predictive and prognostic potential are being sought. Tumor-associated macrophages have been investigated as a novel biomarker for improved prognosis and theragnosis.
- Citation: Fazal F, Khan MA, Shawana S, Rashid R, Mubarak M. Correlation of tumor-associated macrophage density and proportion of M2 subtypes with the pathological stage of colorectal cancer. World J Gastrointest Oncol 2024; 16(5): 1878-1889
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1878.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1878
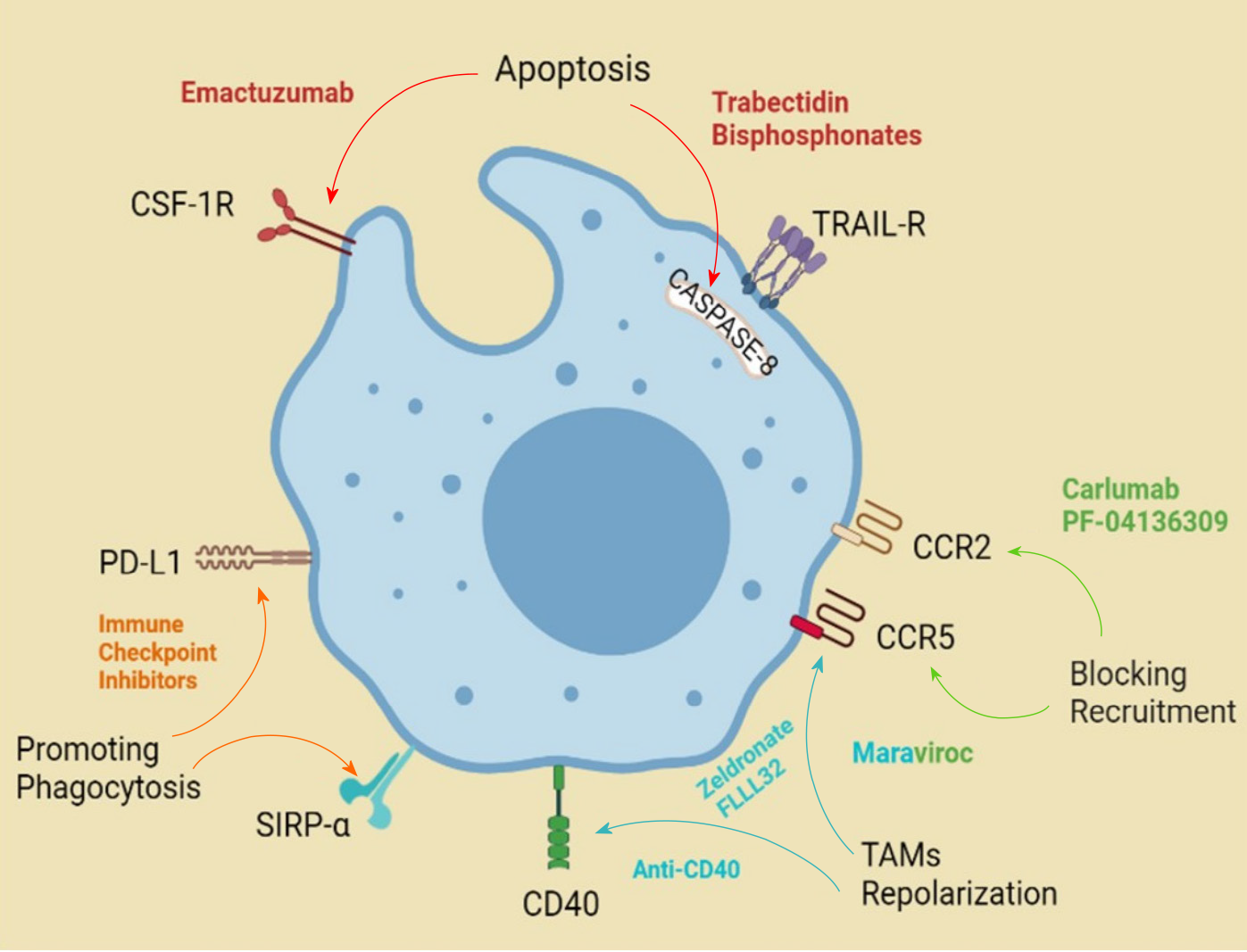
Colorectal cancer (CRC) is the third most common malignancy worldwide[1] and the second most common among men in Pakistan[2]. The pathological staging of resected CRC specimens correlates with the prognosis[3]. With increasing focus on precision medicine and related personalized chemotherapy, the focus has shifted to finding a fine print in pathological assessment and reporting of cancers[4], that has a bearing on the treatment and prognosis of patients with CRC as well[5]. During the last decade, significant research has been done to establish tumor-associated macrophages (TAMs) as an additional pathological marker to enable further therapeutic targets and improve cancer therapy for different malignancies including CRC[6,7], as shown in Figure 1.
Decreased patient survival is associated with an increased density of TAMs, for instance, in breast, melanoma, and kidney malignancy patients, based on several studies[8]. Paradoxically, in patients with CRC, studies demonstrate better survival in cases with an increased infiltration of TAMs. This was especially true if the high TAM density was localized to the tumor front as opposed to the tumor stroma[8]. A possible explanation of this paradox was theorized to be a higher concentration of antitumoral M1 polarized macrophages compared with protumoral M2 macrophages in those tumors[9]. Others have demonstrated that proportional distribution with a high M1:M2 ratio, correlated with better prognosis[10]. Mechanistically, this may relate to the secretion of transforming growth factor beta (TGF-β) by TAMs, which has a tumor suppressor role in the early stages of cancer[11-15]. This effect is lost and paradoxically turned to the tumor-progressor function of TGF-β, with the loss or mutation of SMAD-4 in the nucleus of the tumor cells[12,16-20]. This stage has also demonstrated a higher propensity for metastasis. In summary, in CRC, a high density of TAMs represents a good prognostic factor, but when the proportion of M2-like TAMs predominates, likely under the influence of an altered tumor microenvironment, the prognosis worsens[21-25].
Histologically, we can determine the M2 phenotype with immunohistochemistry (IHC) using antibodies directed against CD68+ and CD163+ cells. CD68 is a pan-macrophage marker and CD163 is specific for M2-like macrophages[26-30]. Hence, we propose a simplified protocol for assessment of the percentage of M2-like TAMs among all TAMs, as opposed to the M1:M2 ratio, along with the assessment of TAM density in tumor stroma and tumor front as a practical prognostic tool for CRC. The rationale of this assessment is that this differentiation to M2 may represent an early marker for advanced disease, followed by decreased TAM density as an additional marker representing poor prognosis. A simplified protocol would ensure easier adaptability for clinical application. This study aimed to establish a simplified protocol for quantifying M2-like TAMs and explore their correlation with clinicopathological factors in patients with CRC.
This cross-sectional study received ethical approval from the Ethics Review Committee of Bahria University Medical and Dental College, Karachi, Pakistan. Paraffin-embedded tissue blocks were obtained from patients who underwent resection of colorectal adenocarcinoma, and a specific histological evaluation was performed. Endoscopic biopsy specimens, poorly fixed tissues, and samples from patients under 18 years of age or with metastatic disease were excluded. A sample size of 43 was determined to be adequate based on the incidence of CRC in Pakistan, which is 6.9%, with an alpha of 5% and a 95% confidence interval (OpenEpi, Version 3). The study was conducted without direct interaction with patients, and all relevant clinical information was obtained from the histology reports. General parameters noted included patient age and sex, tumor site, size, differentiation, presence of perineural and lymphovascular invasion (LVI), and the pathological TNM stage. This approach allowed for a comprehensive evaluation of the histological and clinical features of the cohort.
Study-specific IHC evaluation was then performed. Hematoxylin and eosin-stained slides of selected CRC specimens were retrieved and reviewed by a senior histopathologist and the required tissue blocks were retrieved. Formalin-fixed paraffin-embedded blocks were then cut into 4 μm thick sections using a microtome and placed on poly L-lysine coated slides. Slides were fixed in an oven at 80 °C for 20-25 min. Deparaffinization and rehydration were performed with xylene and ethanol. After deparaffinization, endogenous peroxidase activity was blocked by treating the sections with H2O2 for 5 min. Antigen retrieval was then performed using a pressure cooker and boiling in pH 6.0 citrate buffer for 7 min at 98 °C. Following that, sections were allowed to cool down for 25 min and then washed with pH 7.2 phosphate buffered saline (PBS) solution. Next, sections were incubated with prediluted mouse monoclonal CD68 (KP1) and monospecific mouse monoclonal CD163 (ZM29) antibodies for 1 h at room temperature. After thorough washed with PBS, the primary antibody was visualized using Zeta max HRP polymer detection kits. Finally, the sections were counterstained with hematoxylin and mounted. The positive control tissues were lymph nodes for CD68 was and liver for CD163. The controls were run with the initial few slides and were treated similarly as the sample specimens. This was adopted from an earlier protocol[9].
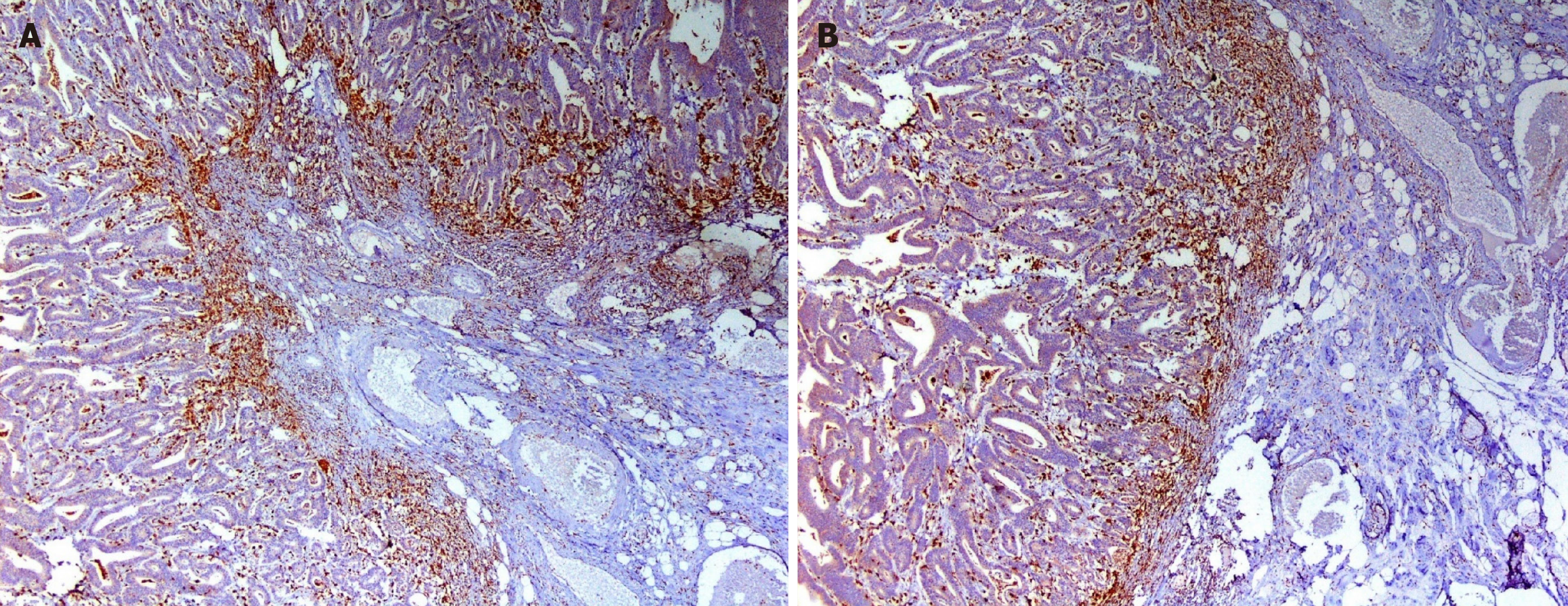
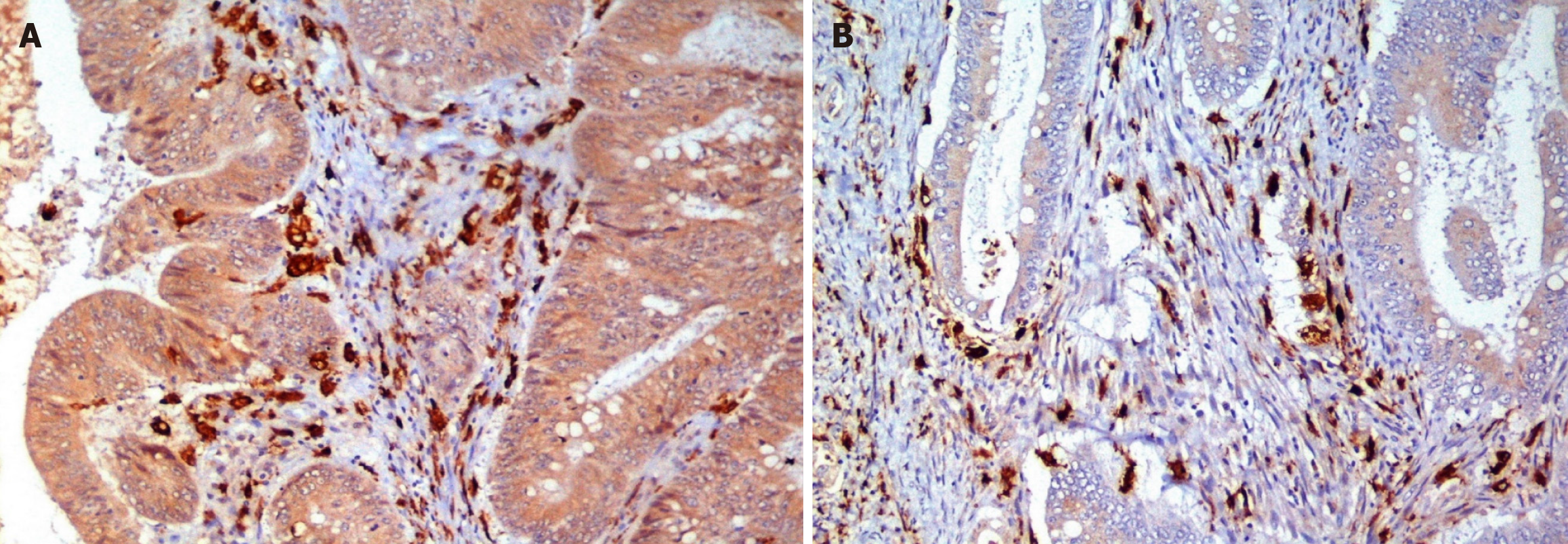
The TAM enumeration protocol was then followed. IHC slides were studied by light microscopy in low-power fields (× 40 and × 100), using a Leica DM 1000 microscope. Tumor stroma and tumor front were defined. Cells immunoreactive to CD68/ CD163 antibodies and exhibiting macrophage-like morphology were considered to be M1 and M2 macrophages as shown in the photomicrographs in Figures 2-4. The total number of CD68+ and CD163+ macrophages were counted in a continuous line, from left to right, in 5 × 400 high-power fields (HPFs). The counting was done along the tumor front and in the tumor stroma for both the antibodies under supervision of a senior pathologist. The mean number of CD68+ and CD163+ macrophages, per HPF (5 hotspots), for each slide (tumor front and stroma) was defined as the TAM and M2 densities. TAM density was further divided into high and low after calculating the median value for both tumor front and stroma. M2 proportion was calculated as described earlier and was further divided into high and low after calculating the median value for both the tumor front and stroma. This is based on previously reported study protocols[13-15].
All collected data were entered into a purposely designed data sheet in SPSS version 23.0 (IBM Corp., Armonk, NY, United States). Descriptive statistics were generated for the baseline characteristics including patient demographics, tumor pathological characteristics, and differential counts of TAM density and M2 proportion. Measures of central tendency and dispersion were reported as means and standard deviation for continuous variables. Categorical data were reported as frequency and percentage. We also assessed medians in cases where the distribution plots demonstrated significant outliers and skewed tails. Further analysis was performed in two steps. First, a cross-tabulation of main variables was performed to determine significant differentiation of subgroups utilizing Pearson’s chi-square test across all variables. Based on the data and distribution characteristics, other specified statistical tests (Wilcoxon signed-rank test for median difference in a nonparametric distribution, McNemar test for difference between paired proportions, or Friedman’s two-way analysis of variance by ranks to compare matched samples in multiple groups) were applied. Only statistically significant differences was reported in such cases. P-values of < 0.05 were considered statistically significant. The determination of association with independent risk factors with the M2 proportion in tumor front and stroma was made using odds ratios and Forrest plots. Risk factors were selected for known association with prognosis in CRCs based on prior studies. In the next step, a logistic regression analysis was performed to determine a significant correlation of TAM density and M2 proportion in the tumor stroma and the tumor front with the main outcome variable, viz, pathological tumor stage.
Histopathology specimens from 43 patients were carefully selected based on the predefined criteria and sample size calculation. Related clinical information was recorded in the study proforma, and subsequently entered into the electronic database. Focusing on age, sex, tumor location, gross and histological characteristics, as well as pathological stage, the data analysis outlined the baseline characteristics of the patients and tissue specimens detailed in the following section and Table 1.
| Characteristic | n (%) |
| Demographics | |
| Age in yr, mean (± SD) | 51.02 (16.9) |
| Sex | |
| Male | 30 (69.8) |
| Female | 13 (30.2) |
| F:M ratio | 1:2.3 |
| Specimen details | |
| Procedure | |
| Right hemicolectomy | 18 (41.9) |
| Left hemicolectomy | 7 (16.3) |
| Sigmoidectomy | 7 (16.3) |
| Rectal resection (APR or LAR) | 11 (25.5) |
| Tumor site | |
| Cecum (including ileocecal valve) | 11 (25.5) |
| Right colon (including hepatic flexure) | 6 (13.9) |
| Transverse colon | 1 (2.3) |
| Left colon (including splenic flexure) | 3 (7.0) |
| Sigmoid colon | 10 (23.3) |
| Rectosigmoid | 4 (9.3) |
| Rectum | 8 (18.6) |
| Tumor size, mean (± SD) | |
| Greatest dimension, cm | 6.18 (4.8) |
| Additional dimension, cm | 3.9 (2.1) |
| Pathology | |
| Histologic type | |
| Adenocarcinoma | 36 (83.7) |
| Mucinous adenocarcinoma | 6 (14.0) |
| Signet ring cell adenocarcinoma | 1 (2.3) |
| Histologic grade | |
| G1: well differentiated | 21 (48.8) |
| G2: moderately differentiated | 14 (32.5) |
| G3: poorly differentiated | 8 (18.6) |
| Perforation | |
| Not identified | 40 (90.3) |
| Present | 3 (7.0) |
| Lymphovascular invasion | |
| Not identified | 19 (44.2) |
| Present | 24 (55.8) |
| Perineural invasion | |
| Not identified | 34 (79.1) |
| Present | 9 (20.9) |
| TNM | |
| Primary tumor | |
| pT1 | 3 (7.0) |
| pT2 | 8 (18.6) |
| pT3 | 26 (60.5) |
| pT4 | 6 (14.0) |
| Regional lymph nodes | |
| pN0 | 17 (39.5) |
| pN1 | 23 (53.3) |
| pN2 | 3 (7.0) |
| Distant metastasis | |
| pMX | 43 (100) |
| pM1 | 0 (0) |
Two-thirds of the enrolled patients were male (n = 30, 69.8%) and the median age was 54 (range: 18-80) years. Most of the samples were colonic resection specimens, while a quarter originated from rectal or rectosigmoid resection (n = 11, 25.5%). Tumors were predominantly located in the cecum, sigmoid colon, and rectum (combined n = 31, 72%), with an overall tendency towards the left side of the colon (n = 24, 55%). The median size of the tumors was 4.5 cm in the greatest dimension. Additional details are provided in Table 1.
The tumor characteristics revealed well differentiated adenocarcinoma (n = 36, 83.7%) as the predominant histology, with most cases staged as pT3 (n = 26, 60.5%) and pN1 (n = 23, 53.3%). No specimens showed histologically proven tumor metastasis. A small proportion of tumors exhibited tumor perforation (n = 3, 7%) and perineural invasion (n = 9, 20.9%) and more than half of the specimens exhibited LVI (n = 24, 55.8%). TNM stage III was the most prevalent (n = 26, 60.5%). Further details are presented in Table 1.
The study revealed noteworthy variation of TAM density between the tumor front and tumor stroma, as well as variation in the distribution and proportion of M1 and M2 subsets within the two assessment zones, as detailed in Table 2. Specifically, the median TAM density was significantly higher at the tumor front, and this trend was observed for each of the M1 and M2 subsets as well. Notably, the proportion of M2 macrophages was approximately three times higher in both zones, with a relatively higher proportion observed in the stromal area. This observation was consistent when comparing the distribution of M1 to M2 macrophages.
| Population | Tumor stroma | Tumor front | P value1 |
| Densities, median (IQR) | |||
| TAM (anti-CD68+) | 32 (22-32) | 40 (24-53) | 0.00031 |
| M2 (anti-CD163+) | 21 (15-30) | 29 (19-38) | 0.00031 |
| M1 (calculated) | 7 (4-10) | 8 (4-13) | 0.0381 |
| Proportion, % | |||
| M1 | 25 | 29 | 0.00012 |
| M2 | 75 | 71 | |
| Ratio, median | |||
| M1 | 9.21 (± 8.98) | 10.91 (± 9.36) | 0.000013 |
| M2 | 22.56 (± 9.7) | 30 (± 14.86) | |
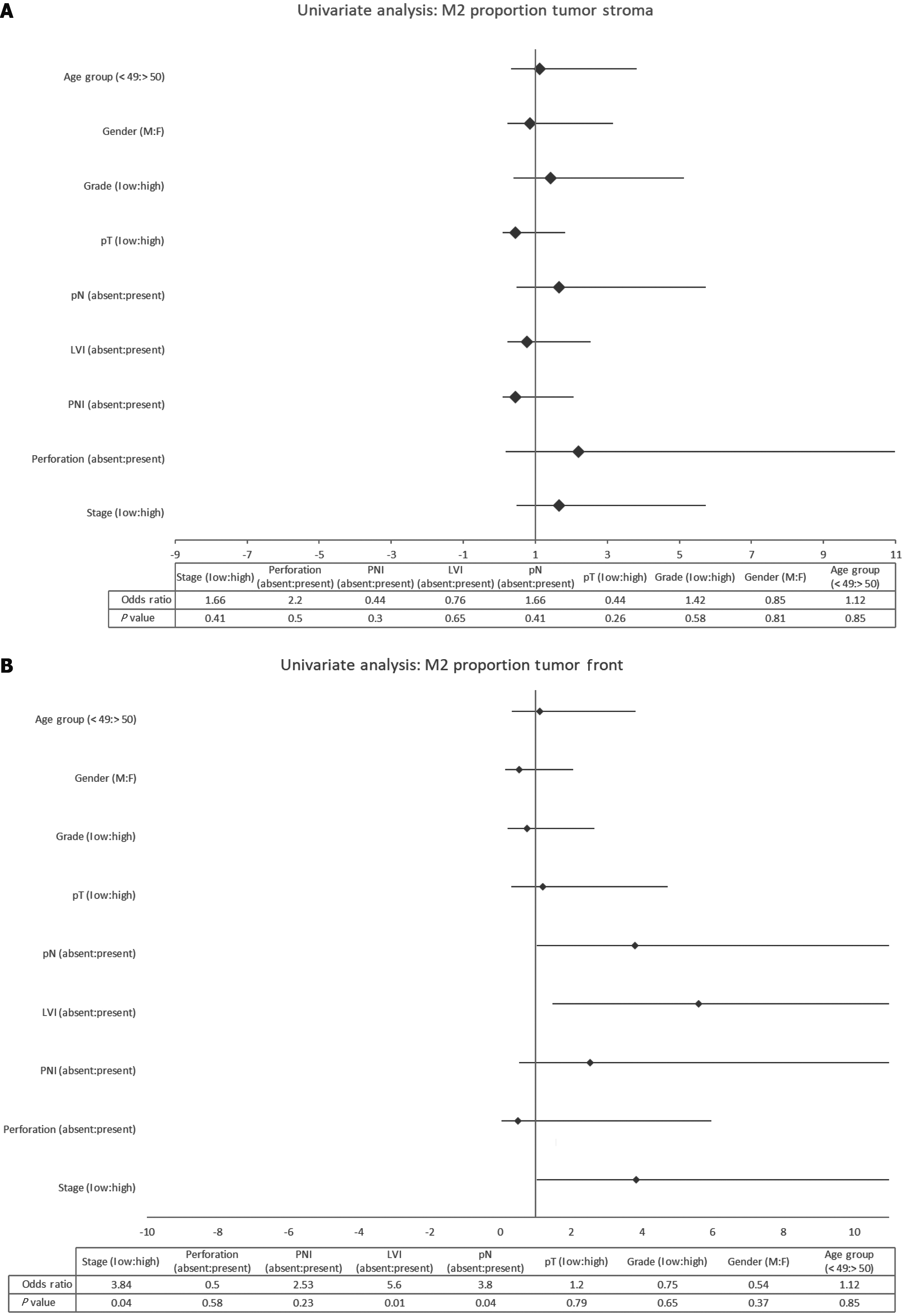
Univariate analysis was performed for selected demographic and tumor characteristics of the M2 proportions in the tumor front and the tumor stroma, as shown in Figure 5. A significant association was determined for tumor stage (P = 0.04), pathological nodal involvement (P = 0.04), and LVI (P = 0.01) with odds ratio of 3.84, 3.8, and 5.6 respectively, with the M2 proportion in the tumor front. The Forrest plot also demonstrated this trend, as well as a similar trend for perineural invasion with an odds ratio of 2.53, but it did not reach statistical significance (P = 0.23). Interestingly, none of these factors was significantly associated with the M2 proportion in the tumor stroma.
Logistic regression analysis was conducted to ascertain the meaningful correlations of TAM density with the proportion of M2 macrophages within both the tumor stroma and the tumor front. These correlations were then assessed in relation to the primary outcome variable, namely, the pathological tumor stage. The results of the logistic regression are detailed in Table 3.
| Model | Observed | Predicted | ||||
| Without independent predictors | Stage type | Percentage correct | ||||
| Early | Late | |||||
| Model 1 (TAM density + M2 proportion) | Step 0 | Stage type: Early or late | Early | 0 | 17 | 0 |
| Late | 0 | 26 | 100 | |||
| Overall percentage | 60.5 | |||||
| Step 1 | Stage type: Early or late | Early | 6 | 11 | 35.31 | |
| Late | 6 | 20 | 76.92 | |||
| Overall percentage | 60.53 | |||||
| Model 2 (TAM density + M2 proportion + tumor grade + lymphovascular invasion) | Step 0 | Stage type: Early or late | Early | 0 | 17 | 0 |
| Late | 0 | 26 | 100 | |||
| Overall percentage | 60.5 | |||||
| Step 1 | Stage type: Early or late | Early | 13 | 4 | 76.51 | |
| Late | 4 | 22 | 84.62 | |||
| Overall percentage | 81.43 | |||||
This model was not statistically significant (P = 0.49). It only correctly classified 60.5% of the cases, similar to what could be predicted without using this model. The sensitivity was 76.9% and the specificity was 35.3%. We then added the factors determined to be significantly different among comparison groups from the prior discussion, viz, lymphovascular infiltration and pathological nodal involvement. This is referred to as model 2 in subsequent discussion. Interestingly, adding these factors remarkably improved the test’s predictive ability. Chi-square tests for all these factors showed that the model was statistically significant (P = 0.024). It correctly classified 81.4% of the cases. The test sensitivity was calculated to be 84.6% with a specificity of 76.5%.
TAMs, as already alluded to in the introduction, hold promise as a prognostic biomarker and a possible therapeutic target for improving outcomes in the management of CRC[31-34]. Our study focused on the procedural aspects of simplifying TAM enumeration while defining associated demographic and tumor traits from resected specimens with the statistical relationship of these to the TAM subtypes, in our patient population. To simplify the enumeration protocol, we excluded M1 subtypes from formal IHC staining and counting. We instead stained and counted the entire TAM population with the CD68+ IHC method and the M2 subtype with CD163+ IHC staining. We then deducted the number of M2 subtypes from the total number of TAMs to determine the M1 subpopulation. The discussion will focus on these aspects, i.e. highlighting the demographic distinctions of the study group to larger reported national and international data, reviewing the distribution, with the statistically determined associations of TAMs and subtypes, and characterizing the merits and demerits of our methodology.
Our study sample was largely consistent with the reported national[2], regional[35], and international data. Most patients were more than 50 years of age with a higher ratio of men compared with women. There was notably a significant proportion of younger adults, as has been a worldwide pattern over the last decade, with an even higher propensity for these in low- and middle-income countries. Our study sample had a higher proportion of colonic resection specimens compared with rectal cancers (about 3:1). In large part, this represents a higher propensity for resectability rather than relative population distribution as reported by national registries in Pakistan[2]. These likely relate to the fact that endoscopic screening is not readily available and there are social patterns[36] that lead to delayed presentation of rectal cancers, which are less amenable to operative resection.
Investigating the distribution of TAMs and their M1 and M2 subtypes within the tumor front and stroma, overall we found a higher distribution of TAMs in the tumor front, with a three times higher proportion of M2 subtype in both the tumor front and the tumor stroma. We also related significant odds of M2 macrophages with higher tumor pathological stage. For drawing comparisons with prior research to identify consistent patterns, it is important to be mindful of the fact that our data were based on pathological reports, so we did not relate any direct association to patient survival data as in most other studies. We instead propose a comparison based on the pathological tumor stage in place of survival data, for this analysis.
A seminal study by Forssell et al[37] (2007) demonstrated a correlation between TAM density assessed by CD-68 and cancer-specific survival. Although focusing on correlating TAM density and M2 proportion with tumor stage as opposed to survival, the similarity in clinicopathological correlations is notable. We speculate that the disparity of outcomes may be attributed to our quantitative approach vs their semiquantitative grading method. Recent studies by Waniczek et al[7] (2017), Yang et al[10] (2019), and Pinto et al[9] (2019) further explored the influence of TAMs on patient survival and clinicopathological variables, revealing varying degrees of correlation. Waniczek et al[7] reported a positive correlation between M2 TAMs and regulatory T cells (T-regs), leading to poorer survival. Yang et al[10] highlighted the significance of the CD163/CD68 ratio at the tumor front for recurrence-free and overall survival, while Pinto et al[9] delved into CD80 (M1) and CD163 (M2) subtype distribution, linking them with tumor stage and survival. Our study found low M1 counts, potentially limiting correlation analysis.
Additionally, our study uniquely emphasizes the association between M2 proportion in the tumor front and regional lymph node involvement, LVI, tumor stage, and perineural invasion. While some prior studies touched upon aspects of macrophage subtypes and their distribution, none of them directly addressed these specific correlations in the context of M2 proportion within the tumor microenvironment. In our opinion, these specific pathological constructs are known prognostic indicators that influence subsequent treatment decisions following tumor resection, and the association with the M2 cellular subtype not only provides added prognostic information but potential target for therapeutic interventions. Therefore, translating TAMs assessment for clinical application has merits.
Among the strengths of our study, we employed a quantitative approach for assessing M2 proportion in the tumor front and stroma, allowing for a more precise evaluation compared with some prior studies that used semiquantitative or ratio-based methods. Another distinction stems from the fact that while other studies explored the overall distribution of TAMs or examined ratios of different macrophage subtypes, our study specifically investigated the correlation of the M2 proportion with clinicopathological factors, providing targeted insights into the role of M2 macrophages. Moreover, we observed significant associations between M2 proportion in the tumor front and clinic-pathological factors such as tumor stage, regional lymph node involvement, and LVI. This contributes to a better understanding of M2 macrophages' potential role in disease progression. Finally, including LVI in our diagnostic model enhanced its sensitivity and specificity. This adjustment aligns with existing evidence and recommendations for LVI assessment in CRC prognosis.
The major limitation of this study relates to the smaller sample size that might have restricted the statistical power, making it challenging to detect subtle associations or correlations, particularly in subgroup analyses. Furthermore, the limited M1 subtype representation in our samples hindered comprehensive analysis and comparison with the M2 proportion. This limitation may have impacted the ability to draw robust conclusions regarding the M1-M2 balance.
Each study employs varying methods of TAM assessment, subtype identification, and data analysis. For instance, unlike some prior studies that directly correlated TAMs or subtype ratios with patient survival, our focus on clinicopathological factors, particularly tumor stage, limited the direct comparison of our results to survival outcomes seen in other studies. Thus, a lack of follow-up data is also a major limitation of this study. We also did not look for TAMs or subtypes in normal or paracancerous tissues, which would have yielded a control group for comparison.
In summary, the strengths of our study design lie in its quantitative assessment and focus on the M2 proportion's correlation with clinicopathological factors. However, limitations in terms of sample size, M1 counts, lack of survival analysis, and methodological variations among studies hinder direct comparison and comprehensive interpretation of findings. Despite these limitations, our study provides valuable insights into the complex interplay of macrophage subtypes and clinicopathological factors in CRC.
Our study introduces a simplified protocol for quantifying M2-like TAMs in CRC tissue samples. We demonstrated a significant correlation between higher M2 proportion at the tumor front and advanced tumor stage, nodal involvement, and lymphovascular invasion. This suggests that M2-like TAMs might serve as potential indicators of disease progression in CRC, warranting further investigation and potential clinical application.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo W, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Zheng XM
| 1. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1068] [Article Influence: 178.0] [Reference Citation Analysis (1)] |
| 2. | Yousaf A, Mahmood S, Faraz R, Quader A, Asif H, Adna A, Nadeem L, Parveen N, Tanveer R, Hussain A, Badar F. Annual Cancer Registry Report-2018, of the Shaukat Khanum Memorial Cancer Hospital & Research Center, Pakistan. 2018. [cited 14 December 2023]. Available from: https://shaukatkhanum.org.pk/wp-content/uploads/2019/07/acrr-2018.pdf. |
| 3. | Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 274] [Reference Citation Analysis (0)] |
| 4. | Tran NH, Cavalcante LL, Lubner SJ, Mulkerin DL, LoConte NK, Clipson L, Matkowskyj KA, Deming DA. Precision medicine in colorectal cancer: the molecular profile alters treatment strategies. Ther Adv Med Oncol. 2015;7:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Yau TO. Precision treatment in colorectal cancer: Now and the future. JGH Open. 2019;3:361-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 796] [Cited by in RCA: 762] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 7. | Waniczek D, Lorenc Z, Śnietura M, Wesecki M, Kopec A, Muc-Wierzgoń M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch Immunol Ther Exp (Warsz). 2017;65:445-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 9. | Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, Barbosa MA, Carneiro F, Oliveira MJ. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front Immunol. 2019;10:1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Yang C, Wei C, Wang S, Shi D, Zhang C, Lin X, Dou R, Xiong B. Elevated CD163(+)/CD68(+) Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int J Biol Sci. 2019;15:984-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Itatani Y, Kawada K, Sakai Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 12. | Cai J, Xia L, Li J, Ni S, Song H, Wu X. Tumor-Associated Macrophages Derived TGF-β‒Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3-4/Snail Signaling Pathway. Cancer Res Treat. 2019;51:252-266. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Salmi S, Siiskonen H, Sironen R, Tyynelä-Korhonen K, Hirschovits-Gerz B, Valkonen M, Auvinen P, Pasonen-Seppänen S. The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. 2019;29:237-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Morita Y, Zhang R, Leslie M, Adhikari S, Hasan N, Chervoneva I, Rui H, Tanaka T. Pathologic evaluation of tumor-associated macrophage density and vessel inflammation in invasive breast carcinomas. Oncol Lett. 2017;14:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, Cui XB, Yang L, Zhao J, Shen XH, Jiang JF, Liang WH, Yuan XL, Li F. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8:21526-21538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Anwar N, Badar F, Yusuf MA. Profile of patients with colorectal cancer at a tertiary care cancer hospital in Pakistan. Ann N Y Acad Sci. 2008;1138:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21 Suppl 7:vii89-vii92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Ranković B, Zidar N, Žlajpah M, Boštjančič E. Epithelial-Mesenchymal Transition-Related MicroRNAs and Their Target Genes in Colorectal Cancerogenesis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Su S, Liu Q, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 579] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 20. | Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 21. | Minami K, Hiwatashi K, Sakoda M, Iino S, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Natsugoe S. The expression of CD163+ and CD68+ macrophage in hepatocellular carcinoma. Hpb. 2018;20 Suppl 2:S386. [DOI] [Full Text] |
| 22. | Idrees R, Fatima S, Abdul-Ghafar J, Raheem A, Ahmad Z. Cancer prevalence in Pakistan: meta-analysis of various published studies to determine variation in cancer figures resulting from marked population heterogeneity in different parts of the country. World J Surg Oncol. 2018;16:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 981] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 24. | Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 25. | Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 531] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 26. | Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J Breast Cancer. 2019;22:38-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 27. | Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, Zhang H, Wang W, Ma X, Gao X, Zhang S. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8:30576-30586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 28. | Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. Prognostic significance of CD68, CD163 and Folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15:4465-4476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Huang YK, Wang M, Sun Y, Di Costanzo N, Mitchell C, Achuthan A, Hamilton JA, Busuttil RA, Boussioutas A. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat Commun. 2019;10:3928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Yan Y, Yang Y, Wang L, Li M, Wang J, Liu X, Duan X. High Infiltration of Tumor-Associated Macrophages Influences Poor Prognosis in Human Gastric Cancer Patients, Associates With the Phenomenon of EMT. Medicine (Baltimore). 2016;95:e2636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One. 2017;12:e0170042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Gulubova M, Ananiev J, Yovchev Y, Julianov A, Karashmalakov A, Vlaykova T. The density of macrophages in colorectal cancer is inversely correlated to TGF-β1 expression and patients' survival. J Mol Histol. 2013;44:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber EP, Fesl C, Greil R; Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 34. | Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch'ng ES. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol. 2019;9:1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 35. | Deo SVS, Kumar S, Bhoriwal S, Shukla NK, Sharma A, Thulkar S, Das P, Bhagat P, Dhall K, Pathy S, Mohanti BK. Colorectal Cancers in Low- and Middle-Income Countries-Demographic Pattern and Clinical Profile of 970 Patients Treated at a Tertiary Care Cancer Center in India. JCO Glob Oncol. 2021;7:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Hasan F, Mahmood Shah SM, Munaf M, Khan MR, Marsia S, Haaris SM, Shaikh MH, Abdur Rahim I, Anwar MS, Qureshi KS, Iqbal M, Qazi S, Kasi BA, Tahir M, Ur Rehman SI, Fatima K. Barriers to Colorectal Cancer Screening in Pakistan. Cureus. 2017;9:e1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |