Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1861
Peer-review started: December 19, 2023
First decision: December 28, 2023
Revised: January 16, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: May 15, 2024
Processing time: 142 Days and 1.7 Hours
Sarcopenia is a progressively diminishing state characterized by the reduction of muscle mass and density, which is frequently observed in malignancies of solid organs.
To assess how sarcopenia affects the overall survival of individuals who have been diagnosed with metastatic gastric cancer.
The study retrospectively included individuals who had been diagnosed with metastatic gastric cancer between January 2008 and December 2020. Sarcopenia was identified through the calculation of the average Hounsfield units (HUAC) using computed tomography (CT) images taken at the time of diagnosis in patients.
A total of 118 patients with metastatic gastric cancer were evaluated. Sarcopenia was detected in 29 patients (24.6%). The median survival of all patients was 8 (1-43) mo. The median survival of patients with sarcopenia was 2 mo, while it was 10 mo for those without sarcopenia (P < 0.001). A significant relationship was found between sarcopenia and survival.
Sarcopenia has been observed to impact survival outcomes in various types of solid tumor cancers. Sarcopenic patients can be identified in a short time, easily and inexpensively, by HUAC measurements from CT images used for diagnosis, and survival could be promoted with nutritional support.
Core Tip: In our study, the median survival of patients with sarcopenia was 2 mo, while it was 10 mo for those without sarcopenia and it was a statistically significant difference. Sarcopenia has an impact on the survival rates of individuals with solid tumors. If we can quickly identify sarcopenic patients through the Hounsfield units’ measurement at the time of diagnosis, contribute to survival by furnishing these patients with the requisite nutritional assistance before treatment.
- Citation: Dogan O, Sahinli H, Duzkopru Y, Akdag T, Kocanoglu A. Is sarcopenia effective on survival in patients with metastatic gastric cancer? World J Gastrointest Oncol 2024; 16(5): 1861-1868
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1861.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1861
Gastric cancer is indeed the fifth most common cancer type in the world and ranks third among cancers in terms of mortality rate. It is a significant global health concern due to its prevalence and high mortality rate[1]. Especially in metastatic disease, survival rates are very low, and survival rates do not exceed 12 mo even in patients receiving cytotoxic chemotherapy[2,3]. Patients with good performance status (PS) and organ functions have better adherence and continuity of treatment and may provide more benefit from palliative chemotherapy.
One of the conditions reducing PS and thus adherence to chemotherapy in patients with advanced gastric cancer is the patient’s nutritional deficiency. Nutritional intake decreases due to both anorexia resulting from catabolism and inflammation in solid cancers, and especially in gastric cancer, the narrowing of passages due to tumor and gastric surgery[4-6].
Sarcopenia, an ongoing decrease in muscle mass, density, and quality, is an issue of increasing importance in recent years. The European Working Group on Sarcopenia in Older People (EWGSOP) consensus in 2010 highlighted that sarcopenia is a condition not limited to older individuals but can also affect cancer patients. This recognition underscores the importance of addressing sarcopenia in various patient populations, including those with cancer, due to its potential impact on health and outcomes[7]. Many studies in recent years have been conducted to explore the impact of insufficient nutritional intake and the development of sarcopenia on mortality and morbidity in individuals with gastric cancer. These studies aim to better understand the relationship between nutrition, sarcopenia, and health outcomes in gastric cancer patients, to improve patient care and management[8-12].
Sarcopenia is typically assessed by evaluating three key components: Muscle mass, muscle potency, and physical functionality. Assessment can be conducted using Whole-body dual-energy X-ray absorptiometry, computed tomo
In our study, our primary objective was to determine the prevalence of sarcopenia by the average Hounsfield units (HUAC) while measuring psoas muscle mass and density from CT images obtained at the time of diagnosis in patients who were being monitored for metastatic gastric cancer at our center. Additionally, we aimed to explore the impact of sarcopenia on the survival of these patients. This research aims to provide insights into the relationship between sarcopenia and the prognosis of individuals diagnosed with metastatic gastric cancer, potentially contributing to improved patient care and outcomes.
We performed a retrospective analysis by screening patients who had been diagnosed with metastatic gastric cancer at our oncology clinic from January 2008 to December 2020. A total of 118 patients over the age of 18, who were followed up with metastatic stomach cancer in our clinic and were part of the analysis, were included in the study. The demographic and clinicopathological characteristics of the patients, as well as CT images taken at the time of diagnosis of advanced gastric cancer, were collected and documented from both patient medical records and computer-based medical records. Patients lacking CT scan images and clinicopathological data in computerized medical records upon diagnosis were excluded from the study. Because the study was retrospective, informed consent could not be obtained from the patients. The study was carried out in adherence to the principles outlined in the Declaration of Helsinki. Prior approval was obtained from the Training and Research Hospital’s ethics committee to ensure compliance with ethical standards.
In our study, we assessed sarcopenia using the HUAC, which is a method that has been used in previous studies for the evaluation of sarcopenia and we determined the lowest sex-specific cutoff point for sarcopenia to be 25%, derived from the acquired results[14]. In our research, a sole radiologist assessed the CT scan images at the time of diagnosis.
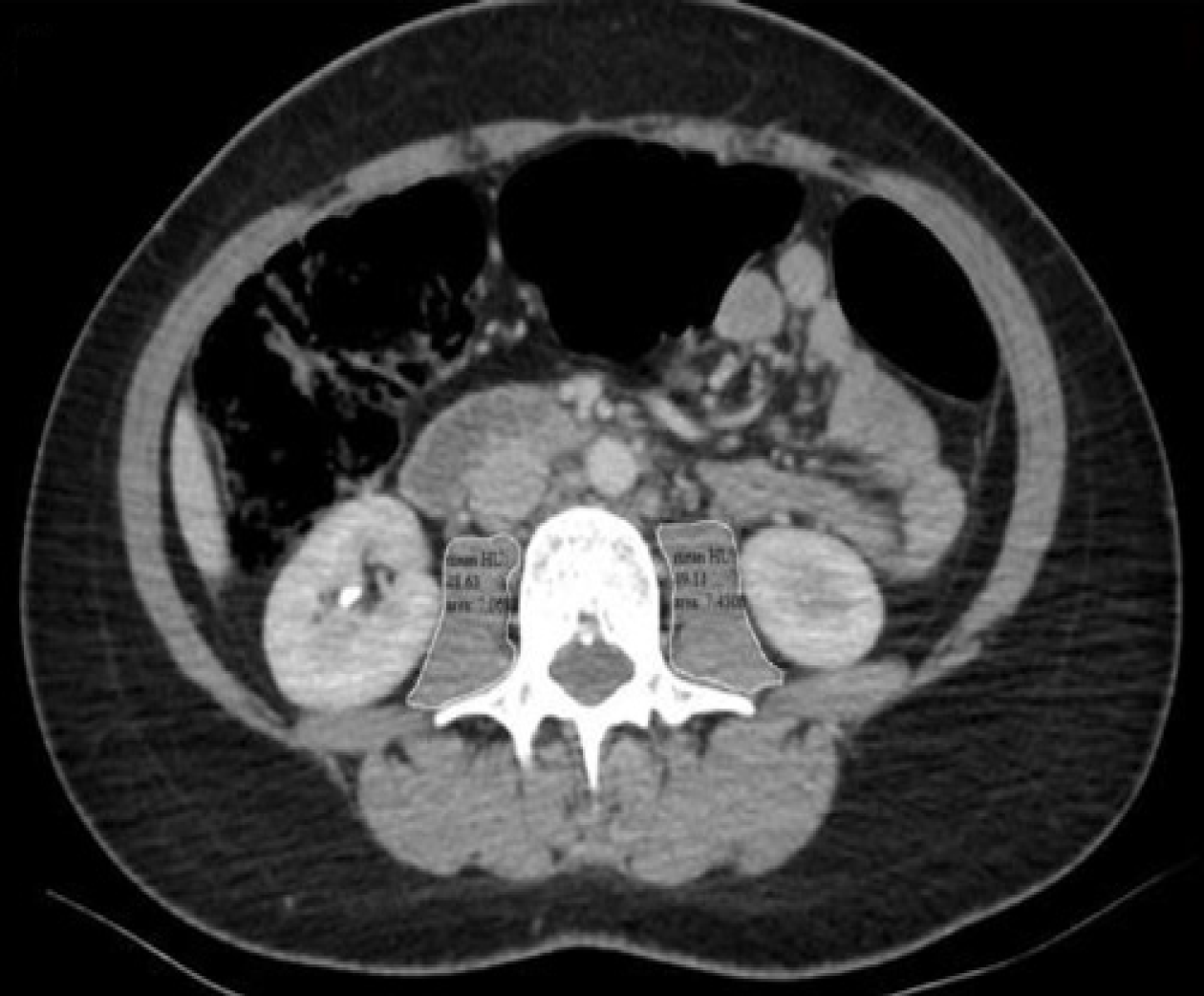
Hounsfield units (HU) are the radiation units utilized in the categorizing CT scan images based on tissue density. Accordingly, in our study, HUAC was calculated from this image by measuring each psoas muscle area (cm2) and density at the level of the L3 vertebra (Figure 1) HUAC was determined through a series of calculations involving both right and left measurements.
Right HUAC: (Hounsfield Unit right psoas × right psoas area)/total psoas area; HUAC: (Hounsfield Unit left psoas × left psoas area)/total psoas area; overall HUAC: (Right HUAC + left HUAC)/2.
Statistical analysis was conducted using the SPSS 20 software package for Windows (IBM Corp., Armonk, NY, United States). Chi-square and Fisher exact test were used for categorical variables. Normal distribution was calculated by the Kolmogorov-Smirnov test for continuous variables. Continuous variables were presented as mean ± SD or median (minimum-maximum). Univariate and multivariate analyses were utilized to examine the impacts of clinicopathological features and sarcopenia on survival by determining a cut-off value for HUAC as 25% percentile, the minimum threshold according to sex. P < 0.05 was deemed statistically significant.
In our study, we included a total of 118 patients, with 90 of them being male, accounting for 76.3% of the study population. The median age of the patients was 63 years, ranging from 27 years to 89 years. Low threshold value of 25.0% was found to be 9 for the diagnosis of sarcopenia with HUAC in females and 10.45 for males. Total psoas area and HUAC value were adjusted for sex. As a result, a total of 29 patients (24.6%) were found to be sarcopenic, including 22 (75.9%) male, 7 (24.1%) female. The median age of sarcopenic patients was 66.0 years ± 10.1 years while it was 64.0 years ± 13.1 years for those non-sarcopenic.
There were no statistically significant differences observed between sarcopenic and non-sarcopenic patients in terms of sex (P = 0.567), age (P = 0.288), PS (P = 0.193), number of metastatic sites (P = 0.513), carcinoembryonic antigen level (P = 0.295), and body mass index (BMI) (P = 0.427) (Table 1).
| Patient characteristics | Sarcopenic | Non-sarcopenic | P value |
| Sex | |||
| Female | 7 (24.1) | 21 (23.6) | 0.567 |
| Male | 22 (75.9) | 68 (76.4) | N/A |
| Age in yr | |||
| < 65 | 12 (41.4) | 48 (53.9) | 0.288 |
| ≥ 65 | 17 (58.6) | 41 (46.1) | N/A |
| PS score in points | 0.197 | ||
| 0 or 1 | 19 (65.5) | 69 (77.5) | N/A |
| 2 or 3 | 10 (34.5) | 20 (22.5) | N/A |
| Differentiation | 0.823 | ||
| Moderate or well | 11 (44.0) | 29 (41.4) | N/A |
| Poor | 14 (56.0) | 41 (58.6) | N/A |
| Smoking history | 0.193 | ||
| Yes | 19 (65.5) | 46 (51.7) | N/A |
| No | 10 (34.5) | 43 (48.3) | N/A |
| Number of metastatic sites | 0.513 | ||
| < 2 | 19 (65.5) | 64 (71.9) | N/A |
| ≥ 2 | 10 (34.5) | 25 (28.1) | N/A |
| CEA level | 0.295 | ||
| < 5 | 10 (35.7) | 40 (47.1) | N/A |
| ≥ 5 | 18 (64.3) | 45 (52.9) | N/A |
| BMI in kg/m2 | 0.427 | ||
| Underweight, < 18.5 | 1 (3.4) | 10 (11.2) | N/A |
| Normal, 18.5-24.9 | 18 (62.1) | 47 (52.8) | N/A |
| Overweight, 25.0-29.9 | 7 (24.1) | 27 (30.3) | N/A |
| Obese, > 30.0 | 3 (10.3) | 5 (5.6) | N/A |
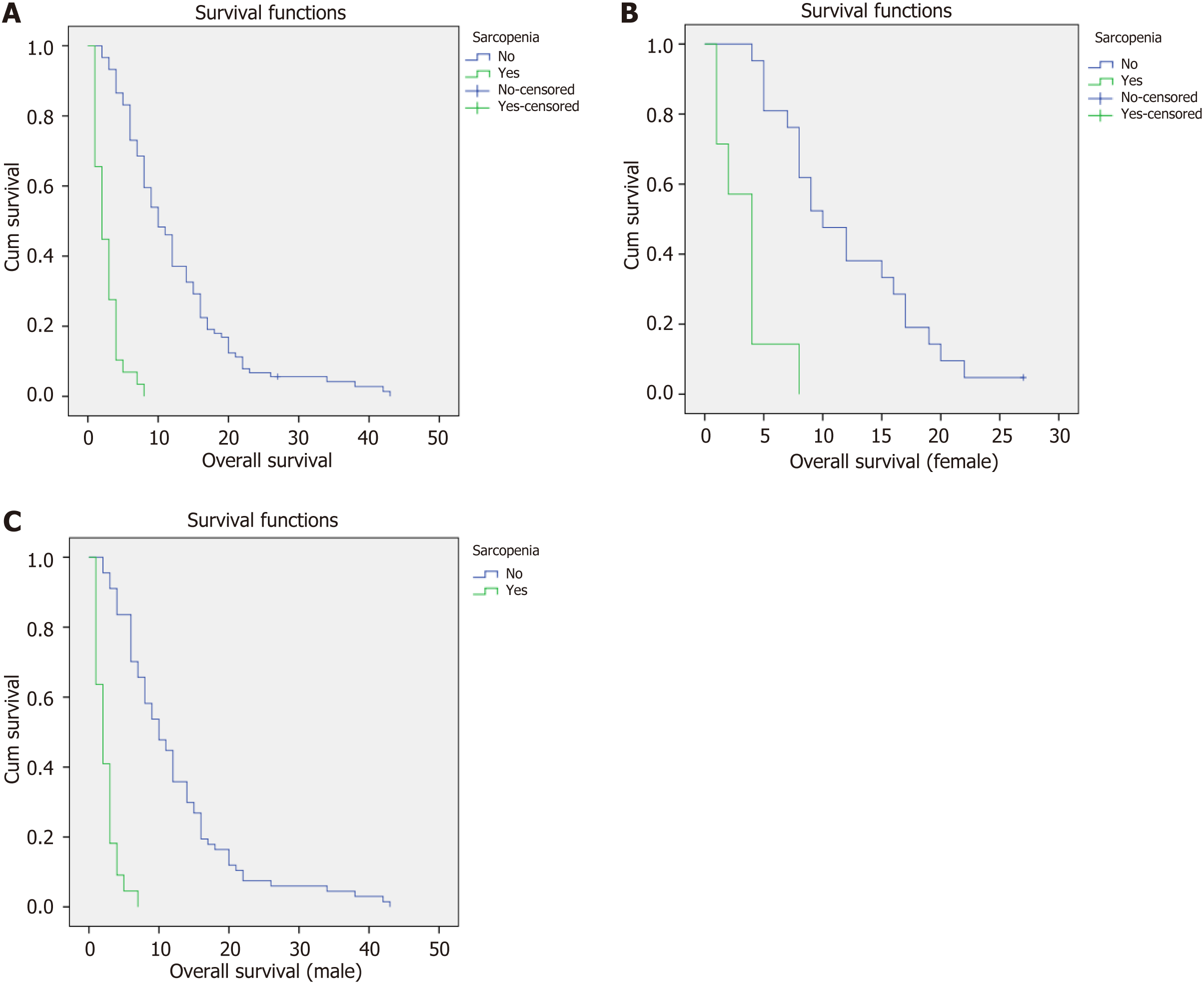
The median survival of all patients was 8 (1-43) mo. At the time of analysis, it was found that 117 out of the total study population, which represents 99.1% of the patients, had passed away. The median survival for sarcopenic patients was 2 mo, whereas patients without sarcopenia had a median survival of 10 mo (P < 0.001), indicating a statistically significant difference (Figure 2A).
The median survival of female patients was 8 mo. Among female patients, the median survival for sarcopenic individuals was 4 mo, while non-sarcopenic female patients had a median survival of 10 mo (P < 0.001), indicating a statistically significant difference (Figure 2B).
Among male patients, the median survival was 7 mo. Sarcopenic male patients had a median survival of 2 mo, whereas non-sarcopenic male patients had a median survival of 10 mo (P < 0.001), indicating a statistically significant difference (Figure 2C).
The impact of clinicopathological features on survival was assessed using univariate analysis, specifically considering age (< 65 years vs ≥ 65 years, P = 0.100), PS (0 or 1 points vs 2 or 3 points, P = 0.802), differentiation (good-moderate vs poor, P = 0.584), BMI (underweight vs normal vs overweight vs obese, P = 0.456), number of metastases (< 2 vs ≥ 2, P = 0.065), and smoking history (yes vs no, P = 0.634) revealing no significant effect on survival (Table 2). Multivariate analysis, performed with factors determined significant according to univariate analysis results, showed that sarcopenia remained to be statistically significant on survival.
| Clinicopathologic characteristics | Univariate analyses, HR (95%CI) | P value | Multivariate analyses, HR (95%CI) | P value |
| Age in yr, < 65 vs ≥ 65 | 1.366 (0.942-1.979) | 0.100 | N/A | N/A |
| Sex, female vs male | 0.963 (0.776-1.196) | 0.735 | N/A | N/A |
| PS score in points, 0 or 1 vs 2 or 3 | 1.142 (0.745-1.753) | 0.542 | N/A | N/A |
| Differentiation, moderate or well vs poor | 0.947 (0.622-1.441) | 0.800 | N/A | N/A |
| Number of metastases, < 2 vs ≥ 2 | 1.416 (0.947-2.118) | 0.090 | N/A | N/A |
| CEA level, < 5 vs ≥ 5 | 1.707 (1.144-2.548) | 0.009 | 1.650 (1.102-2.469) | 0.015 |
| Smoking history, yes vs no | 0.956 (0.794-1.151) | 0.634 | N/A | N/A |
| Presence of sarcopenia, yes vs no | 9.512 (5.489-16.484) | 0.000 | 9.542 (5.398-16.867) | 0.000 |
| BMI in kg/m2: underweight, < 18.5; normal, 18.5-24.9; overweight, 25.0-29.9; obese, > 30.0 | 0.914 (0.722-1.157) | 0.456 | N/A | N/A |
Gastric cancer is indeed a significant global health concern, and it is known to be the third leading cause of cancer-related deaths worldwide[1]. Both early satiety and loss of appetite developing due to the obstructive effect of tumor, and increased catabolism and inflammation in solid cancers, including gastric cancer, can indeed lead to the loss of muscle mass in patients[4,6]. As per the sarcopenia consensus released by EWGSOP in 2010, it is widely observed that cancer patients frequently experience sarcopenia, which is identified by a reduction in muscle mass, density, and quality[7]. In recent times, there have been many research investigations aimed at examining the influence of sarcopenia on survival results[8-11,13,14].
In our research, we specifically investigated how sarcopenia affects the survival of individuals who have been diagnosed with metastatic gastric cancer. Out of the total sample size, 29 patients (24.6%) were identified as sarcopenic, consisting of 22 (75.9%) male and 7 (24.1%) female patients. In previous studies, the sarcopenia rate in gastrointestinal cancers was found to be between 25%-60%, similar to our study[15,16]. The median survival for sarcopenic patients was 2 mo, while non-sarcopenic patients had a significantly longer median survival of 10 mo. This observed difference in survival between the two groups was statistically significant, indicating that sarcopenia has a substantial impact on survival outcomes in patients diagnosed with metastatic gastric cancer (P < 0.001). The median survival of sarcopenic and non-sarcopenic female patients was 4 and 10 mo, respectively. Similarly, the median survival in sarcopenic male patients was 2 mo, compared to 10 mo in non-sarcopenic males. While age, PS, tumor differentiation, BMI, number of metastases, and smoking history did not independently influence survival, the persistence of sarcopenia as a significant factor in our multivariate analysis underscores its unique role in predicting survival outcomes. This reinforces the notion that sarcopenia should be considered beyond conventional prognostic factors when planning treatment strategies for metastatic gastric cancer patients.
Our findings contribute to the growing body of evidence highlighting the significant impact of sarcopenia on survival outcomes in patients diagnosed with metastatic gastric cancer. The observed association between sarcopenia and reduced survival aligns with previous research in gastrointestinal cancers, emphasizing the need for early detection and inter
Similar to our study, Hayashi et al[11] found in their study that there was a relationship between low muscle density and low survival in patients with metastatic gastric cancer receiving chemotherapy. A meta-analysis published by Rinninella et al[17], indicated that low muscle mass measured at the L3 vertebra level was associated with poorer progression-free and overall survival in gastric cancer patients who were undergoing curative treatment, similar to our study.
In our study, sarcopenia was assessed using HUAC, a method that involves measuring both the psoas muscle area and density from CT scan images of the patients, similar to other studies[13]. HUAC is the measurement of spinal muscle mass and fat infiltration. Indeed, assessing both muscle area and density through HUAC can provide valuable insights into muscle health. Even if the muscle area appears normal, changes in muscle density can indicate the presence of fatty infiltration, which can be a sign of muscle quality deterioration. This comprehensive evaluation allows for a more nuanced understanding of muscle composition and can help identify subtle muscle changes that might not be apparent through muscle area measurement alone. It’s an important aspect of assessing sarcopenia and can lead to more accurate and informative results regarding muscle health and its impact on patients’ well-being and survival outcomes[18]. In the consensus stated by EWGSOP, psoas muscle area measurement for sarcopenia, by using CT scan and MRI images, were accepted as the golden standard[7]. CT scan, routinely used in the staging of metastatic gastric cancer, eliminates the additional cost allowing to measure in a short time and in a repeatable way.
In our study, overweight (BMI = 25.0-29.9 kg/m2) and obese (BMI ≥ 30.0 kg/m2) patients were classified together, and 10 patients determined as sarcopenic by HUAC were in the overweight/obese group. Despite a high BMI in sarcopenic obesity, muscle mass is decreased while adipose tissue increases[19]. It is therefore misleading to assess the nutritional status of patients with BMI.
Sarcopenia is more common in the elderly, and muscle mass decreases by 8%-10% in each decade after the age of 40[20,21]. Similarly, in our study, 58.6% of sarcopenic patients were over 65 years of age.
Our study acknowledges certain limitations, including its retrospective nature and the fact that it was conducted at a single center. These factors may introduce potential biases and limit the generalizability of the findings. Additionally, the relatively small sample size might impact the statistical power and precision of the results. It is important to consider these limitations when interpreting the study’s findings and to conduct larger, multicenter studies to further validate our results and provide more robust evidence. In addition to the limitations mentioned, it is important to note that sarcopenia is typically assessed by considering not only muscle mass but also muscle potency and physical functionality evaluation[7]. As this study was retrospective, data on muscle strength and physical performance could not be obtained. This narrow approach may overlook the comprehensive evaluation of sarcopenia and its influence on survival in patients with metastatic gastric cancer. Future studies should consider incorporating additional parameters, such as muscle strength and physical performance, to provide a more comprehensive assessment of sarcopenia in this context.
In summary, sarcopenia has an impact on the survival rates of individuals with solid tumors. Therefore, it is possible to detect sarcopenic patients quickly, conveniently, and cost-effectively through the measurement of HUAC using CT scan images acquired during the initial staging at the time of diagnosis. Providing the required nutritional support to these patients prior to treatment promotes the performance and adherence of patients as well as their survival by preventing sarcopenia. Further investigations with larger cohorts and a more comprehensive evaluation of sarcopenia are warranted to enhance the robustness of our findings and guide future clinical practices.
Sarcopenia is a progressively diminishing state characterized by the reduction of muscle mass and density, which is frequently observed in malignancies of solid organs. Identifying sarcopenia in gastric cancer may have an impact on survival.
To use supportive tools to predict and improve prognosis in metastatic gastric cancer.
To detect sarcopenia easily and inexpensively in metastatic stomach cancer and to investigate its effect on survival.
The study retrospectively included 118 patients who had been diagnosed with metastatic gastric cancer. Sarcopenia was identified through the calculation of the average Hounsfield units (HUAC) using computed tomography (CT) images taken at the time of diagnosis in patients.
The median survival of patients with sarcopenia was 2 mo, while it was 10 mo for those without sarcopenia (P < 0.001). A significant relationship was found between sarcopenia and survival.
Sarcopenic patients can be identified in a short time, easily and inexpensively, by HUAC measurements from CT images used for diagnosis, and survival could be promoted with nutritional support.
We think that survival in gastric cancer will increase by improving sarcopenia, which is easily and cheaply determined.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9919] [Article Influence: 4959.5] [Reference Citation Analysis (2)] |
| 2. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1467] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 3. | Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 359] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 4. | Stojcev Z, Matysiak K, Duszewski M, Banasiewicz T. The role of dietary nutrition in stomach cancer. Contemp Oncol (Pozn). 2013;17:343-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Takiguchi S, Takata A, Murakami K, Miyazaki Y, Yanagimoto Y, Kurokawa Y, Takahashi T, Mori M, Doki Y. Clinical application of ghrelin administration for gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2014;17:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Shizgal HM. Body composition of patients with malnutrition and cancer. Summary of methods of assessment. Cancer. 1985;55:250-253. [PubMed] [DOI] [Full Text] |
| 7. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8454] [Article Influence: 563.6] [Reference Citation Analysis (0)] |
| 8. | Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, Fujiwara T. Sarcopenia and Comorbidity in Gastric Cancer Surgery as a Useful Combined Factor to Predict Eventual Death from Other Causes. Ann Surg Oncol. 2018;25:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P, Cardellino GG, Nicoletti L, Fornaro L, Casadei-Gardini A, Aprile G. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. 2017;20:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, Tanikawa S, Maeda S, Uemura M, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Hayashi N, Ando Y, Gyawali B, Shimokata T, Maeda O, Fukaya M, Goto H, Nagino M, Kodera Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep. 2016;35:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Park HS, Kim HS, Beom SH, Rha SY, Chung HC, Kim JH, Chun YJ, Lee SW, Choe EA, Heo SJ, Noh SH, Hyung WJ, Cheong JH, Kim HI, Son T, Lim JS, Baek SE, Jung M. Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat After Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann Surg Oncol. 2018;25:3222-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, van Lanschot JJ. Sarcopenia/Muscle Mass is not a Prognostic Factor for Short- and Long-Term Outcome After Esophagectomy for Cancer. World J Surg. 2016;40:2698-2704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 774] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 15. | Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore). 2016;95:e3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 16. | Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, Tortora G, Gasbarrini A, Mele MC. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin Nutr. 2020;39:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: Current Concepts and Imaging Implications. AJR Am J Roentgenol. 2015;205:W255-W266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr. 2010;29:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1162] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 20. | Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772-M777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 586] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 21. | Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872-8; quiz 915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 311] [Article Influence: 15.6] [Reference Citation Analysis (1)] |