Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1808
Peer-review started: November 10, 2023
First decision: January 30, 2024
Revised: February 2, 2024
Accepted: March 12, 2024
Article in press: March 12, 2024
Published online: May 15, 2024
Processing time: 181 Days and 10.3 Hours
Vessels encapsulating tumor clusters (VETC) represent a recently discovered vascular pattern associated with novel metastasis mechanisms in hepatocellular carcinoma (HCC). However, it seems that no one have focused on predicting VETC status in small HCC (sHCC). This study aimed to develop a new nomo
To construct a nomogram that combines preoperative clinical parameters and image features to predict patterns of VETC and evaluate the prognosis of sHCC patients.
A total of 309 patients with sHCC, who underwent segmental resection and had their VETC status confirmed, were included in the study. These patients were recruited from three different hospitals: Hospital 1 contributed 177 patients for the training set, Hospital 2 provided 78 patients for the test set, and Hospital 3 provided 54 patients for the validation set. Independent predictors of VETC were identified through univariate and multivariate logistic analyses. These inde
Alpha-fetoprotein_lg10, carbohydrate antigen 199, irregular shape, non-smooth margin, and arterial peritumoral enhancement were identified as independent predictors of VETC. The model incorporating these predictors demonstrated strong predictive performance. The AUC was 0.811 for the training set, 0.800 for the test set, and 0.791 for the validation set. The calibration curve indicated that the predicted probability was consistent with the actual VETC status in all three sets. Furthermore, the decision curve analysis demonstrated the clinical benefits of our model for patients with sHCC. Finally, early recurrence was more likely to occur in the VETC-positive group compared to the VETC-negative group, regardless of whether considering the actual or predicted VETC status.
Our novel prediction model demonstrates strong performance in predicting VETC positivity in sHCC (≤ 3 cm) patients, and it holds potential for predicting early recurrence. This model equips clinicians with valuable information to make informed clinical treatment decisions.
Core Tip: Our research objective is to construct a nomogram that combines preoperative clinical parameters and image features to predict patterns of vessels encapsulating tumor clusters and evaluate the prognosis of small hepatocellular carcinoma patients. The nomogram has undergone extensive validation across multiple patient centers, demonstrating robust predictive performance and promising implications for postoperative recurrence prediction.
- Citation: Chen HL, He RL, Gu MT, Zhao XY, Song KR, Zou WJ, Jia NY, Liu WM. Nomogram prediction of vessels encapsulating tumor clusters in small hepatocellular carcinoma ≤ 3 cm based on enhanced magnetic resonance imaging. World J Gastrointest Oncol 2024; 16(5): 1808-1820
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1808.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1808
Primary liver cancer stands as a significant global health concern, ranking as the sixth most prevalent malignancy worldwide and the third leading cause of cancer-related deaths[1]. Among the various types of primary liver cancer, hepatocellular carcinoma (HCC) is the most common pathological subtype, with nearly 800000 new cases diagnosed each year[2]. Typically, segmental resection is a widely employed treatment for HCC patients. However, despite surgical intervention, tumor recurrence remains a major challenge, with recurrence rates reaching as high as 70%[3].
The high recurrence rate in HCC patients is largely attributed to early metastasis, with the theory of epithelial-mesenchymal transition (EMT) being recognized as the conventional mechanism underlying metastasis[4]. However, recent studies have identified vessels encapsulating tumor clusters (VETC) as a novel risk factor for liver cancer metastasis. VETC represent a vascular pattern in which tumor vascular endothelial cells (CD34+) encircle clusters of HCC cells, forming a distinctive spiderweb-like network[5]. In contrast to EMT-dependent metastasis, the spread of HCC with VETC relies on specific vascular patterns, enabling the entire endothelial-encased tumor cluster to be released directly into the bloodstream[6]. Consequently, VETC not only serves as a robust predictor of aggressive HCC but also represents a significant adverse prognostic factor following liver cancer resection. Previous studies have shown that HCC patients with VETC positivity experience shorter recurrence-free survival and overall survival time[7].
Small HCC (sHCC) is a specific subtype of HCC characterized by tumors with a diameter of ≤ 3 cm[8]. Typically, patients with sHCC have a more favorable prognosis compared to those with larger tumors[9]. Nevertheless, even after undergoing surgery, patients with sHCC still face the risk of intrahepatic cancer cell metastasis and recurrence, with VETC potentially playing a significant role[10]. While radiomics models, utilizing features extracted from gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI), have been developed to predict VETC and prognosis in HCC patients[11], no specialized study currently exists for the preoperative prediction and prognosis of VETC in sHCC patients. Given that the current diagnosis of VETC relies on CD34 vascular staining results, there is a critical need to explore a simple and non-invasive preoperative prediction method for VETC in sHCC patients[12].
In this study, our objective was to construct a nomogram that incorporates preoperative clinical parameters and image features to predict the presence of VETC and assess the prognosis of sHCC[13]. By using this preoperative prediction tool, we aimed to provide patients with precise diagnoses and effective treatment strategies[14].
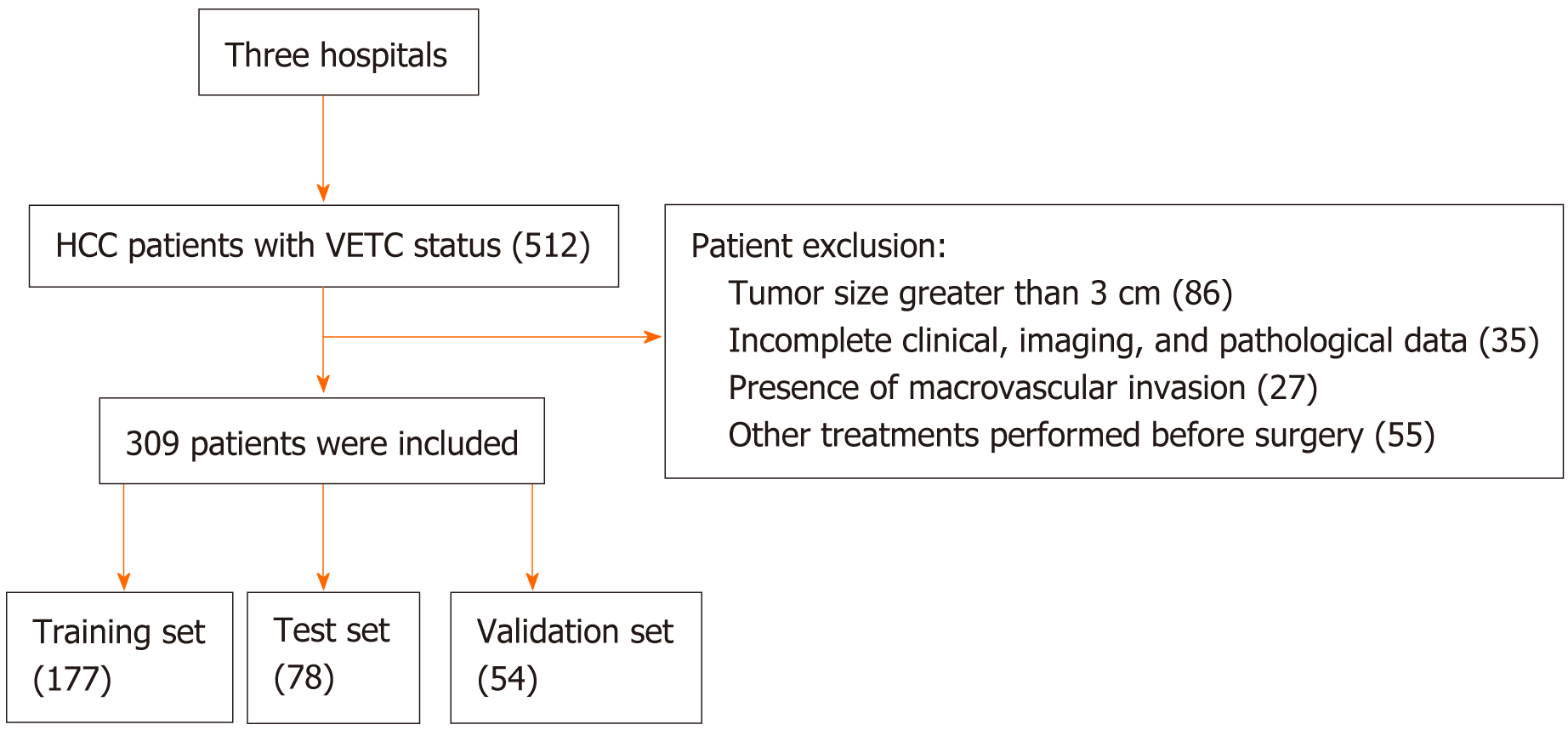
This retrospective study was approved by the Institutional Review Committee, and the requirement for written informed consent was waived. The study involved data collected from three hospitals. Hospital 1 contributed data from 177 patients at the Third Affiliated Hospital of Naval Medical University (Yangpu Campus) from August 2016 to June 2019 for the training set, Hospital 2 provided data from 78 patients at the Third Affiliated Hospital of Naval Medical University (Anting Campus) from April 2016 to September 2019 for the testing set, and Hospital 3 provided data from 54 patients at Tongji University Affiliated Tongji Hospital from January 2020 to December 2021 for the validation set.
The inclusion criteria were as follows: (1) Patients who underwent segmental resection and had an enhanced MRI examination within 1 month prior to the procedure; (2) confirmation of VETC status through histopathology; and (3) the absence of large vessel invasion or distant metastasis.
The exclusion criteria included: (1) Patients who had received other anti-tumor treatments prior to segmental resection; (2) the presence of other malignant tumors; (3) the number of lesions greater than 3 or a lesion size exceeding 3 cm; and (4) incomplete clinical, imaging, or pathological data. The patient inclusion process is illustrated in Figure 1.
Before the scan, patients fasted for 4-6 h. The scan encompassed the entire liver, from the top to the bottom edge. A high-pressure syringe was used to inject 0.1 mmol/kg of gadopentetate dimeglumine into the patient’s median vein at the elbow, with an injection rate of 2.0 mL/s, followed by a 20 mL saline rinse. Following the injection of the contrast agent, the scanning time for the arterial phase (AP), portal vein phase (PVP), and delayed phase (DP) was 20-35 s, 55-75 s, and 120-180 s, respectively. Additionally, the acquisition included diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC) maps, T1-weighted imaging (T1WI), and fat-suppressed T2WI. Detailed information regarding the MRI procedures at each hospital in this study can be found in Supplementary Tables 1-3.
Two radiologists, each with over 5 years of experience in abdominal MRI, conducted a blinded review of MRI images for all patients. In cases of disagreement, a third radiologist with over 10 years of experience in liver MRI was consulted to make a final decision.
The MRI features evaluated in this study, in accordance with LI-RADS version 2018, include: (1) Tumor diameter, defined as the largest outer-edge-to-outer-edge dimension of an observation, with the measurement including “capsule” and select clear phases and clear layers[15]; (2) shape, categorized as regular when round or oval and irregular when lobulated, star-like, or needle-like; (3) margin, smooth for simple nodular tumors with smooth contours, and non-smooth for multinodular confluent or nodular with extranodular growth[16]; (4) restricted diffusion, identified by hyperintensity on DWI with a b-value of 600 and hypointensity on ADC[17]; (5) radiological capsule enhancement, categorized as complete capsule for smooth, uniform, and sharp boundaries with complete enhanced edges visible in PVP and DP, absent capsule when the enhanced edge capsule is not visible, and incomplete capsule when the enhanced edge capsule is mostly visible[18]; (6) non-rim arterial phase hyperenhancement (APHE), i.e., the intensity of the tumor enhancement part must be higher than that of the liver during the AP[17]; (7) rim APHE, a spatially defined subtype of APHE, characterized by the most pronounced AP enhancement in the periphery of the observation[19]; (8) arterial peritumoral enhancement, defined as enhancement outside the tumor boundary in the late stage of the AP or early stage of the PVP with extensive contact with the tumor edge, which becomes isointense during the DP[16]; (9) nonperipheral “washout”; and (10) enhancement pattern. In cases of multiple tumors, the largest tumor was selected for the evaluation of these MRI features[16]. Further details are available in Supplementary Tables 4 and 5.
Two pathologists, each with over 5 years of experience in pathological diagnosis, independently evaluated all HCC tissue samples. In cases of disagreement, a third pathologist with more than 10 years of experience in pathological diagnosis was consulted to make the final decision.
Tissue microarrays: Small cores, each with a diameter of 1-2 mm, were used to assess phenotypic marker expression in the surgical specimens. A total of six tumoral cores (three from central areas and three from peripheral areas) were included, along with three spots from the surrounding liver parenchyma for additional analysis.
VETC assessment: VETC are characterized by tumor vascular endothelial cells (CD34+) encapsulating HCC cell clusters, forming a cobweb-like network[20]. The extent of VETC was semi-quantitatively evaluated on a scale from 0% to 100% in 5% increments[7]. HCC patients were categorized into VETC-positive and VETC-negative sets based on a cutoff value of 55%, indicating the presence or absence of VETC pattern, respectively[21].
During the initial two years following resection, recurrence was monitored every 3 months using alpha-fetoprotein (AFP) levels, contrast computed tomography (CT), or MRI. Subsequent follow-ups were conducted every 6 months, and the final review date was September 1, 2023. The study’s endpoint was early recurrence, which was defined as recurrence or metastasis within two years after segmental resection[22]. Recurrence-free survival (RFS) was defined as the duration from the date of resection to the occurrence of the first recurrence, metastasis, or the final follow-up[23].
Statistical analyses were performed utilizing SPSS 25.0 and R software (version 4.3.1). Continuous variables are reported as the mean with standard deviation or median with interquartile range, while categorical variables are presented as numbers and percentages. Interobserver agreement for qualitative features was assessed using the kappa test, and for quantitative imaging features, the intraclass correlation coefficient was employed. Variables with a consistency coefficient of ≥ 0.8 were retained for subsequent analysis. Univariate logistic regression analysis was performed to identify risk factors, with those having a significance level of P < 0.05 included in the multivariate analysis. We established a multivariate logistic model through backward stepwise selection to identify independent risk factors related to VETC patterns. R software was used to construct a nomogram, which underwent internal validation with 1000 bootstrapping resamples. Receiver operating characteristic (ROC) curves were employed to assess the predictive accuracy of the nomogram, and calibration curves were used to evaluate consistency. Decision curve analysis was conducted to determine the clinical utility of the nomogram. Kaplan-Meier (Log-rank) tests were applied to compare early recurrence differences between VETC-positive and VETC-negative cases. Statistical significance was determined with a two-sided P value < 0.05.
The training set comprised 177 patients with a median age of 55 years (range: 25-80 years), including 141 men and 36 women. Among them, 86 patients were VETC-positive, and 91 were VETC-negative. The test set consisted of 78 patients with a median age of 57 years (range: 27-74 years), comprising 68 men and 10 women. Of these, 41 patients were VETC-positive, and 37 were VETC-negative. In the validation set, there were 54 patients with a median age of 58 years (range: 38-78 years), including 44 men and 10 women. This set comprised 28 VETC-positive patients and 26 VETC-negative patients (Table 1; Supplementary Table 6).
| Characteristic | Training set (n = 177) | Test set (n = 78) | Validation set (n = 54) | ||||
| VETC negative (n = 91) | VETC positive (n = 86) | P value | VETC negative (n = 37) | VETC positive (n = 41) | VETC negative (n = 26) | VETC positive (n = 28) | |
| Clinical features | |||||||
| Age | 55.960 ± 10.102 | 54.350 ± 11.541 | 0.323 | 54.590 ± 9.494 | 58.510 ± 12.386 | 56.270 ± 10.294 | 58.930 ± 8.675 |
| Gender | 0.578 | ||||||
| Male | 71 (40.1) | 70 (39.5) | 32 (41.0) | 36 (46.2) | 22 (40.7) | 22 (40.7) | |
| Female | 20 (11.3) | 16 (9.0) | 5 (6.4) | 5 (6.4) | 4 (7.4) | 6 (11.1) | |
| Liver disease | 0.750 | ||||||
| HAV | 0 (0.0) | 1 (0.6) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| HBV | 77 (43.5) | 75 (42.4) | 35 (44.9) | 37 (47.4) | 19 (35.2) | 25 (46.3) | |
| HCV | 3 (1.7) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 2 (3.7) | 0 (0.0) | |
| None | 11 (6.2) | 8 (4.5) | 2 (2.6) | 4 (5.1) | 5 (9.3) | 3 (5.6) | |
| AFP-L3 | 0.886 | ||||||
| Negative | 69 (39.0) | 66 (37.3) | 28 (35.9) | 34 (43.6) | 20 (37.0) | 21 (38.9) | |
| Positive | 22 (12.4) | 20 (11.3) | 9 (11.5) | 7 (9.0) | 6 (11.1) | 7 (13.0) | |
| AFP_lg10 | 1.07 (0.49-2.05) | 1.48 (0.92-2.36) | 0.009 | 1.10 (0.64-1.85) | 1.47 (0.85-2.45) | 1.23 (0.69-1.90) | 1.95 (0.90-2.64) |
| PIVKA-II (mAU/mL) | 50 (27.00-194.05) | 83 (28.00-213.75) | 0.635 | 41 (24.5-135.5) | 88 (31.00-193.52) | 58 (27.75-321.5) | 60 (23.00-295.75) |
| CA199 (U/mL) | 12.6 (7.6-21.7) | 17.85 (7.4-32.5) | 0.003 | 16.7 (10.6-27.6) | 19.6 (10.4-28.9) | 18.66 (9.4-29.4) | 28.3 (13.6-49.4) |
| HBsAg | 0.621 | ||||||
| Negative | 14 (7.9) | 11 (6.2) | 2 (2.6) | 6 (7.7) | 7 (13.0) | 4 (7.4) | |
| Positive | 77 (43.5) | 75 (42.4) | 35 (44.9) | 35 (44.9) | 19 (35.2) | 24 (44.4) | |
| HBV/C-DNA, IU/mL | 0.083 | ||||||
| < 50 | 55 (31.1) | 37 (20.9) | 20 (25.6) | 23 (29.5) | 15 (27.8) | 17 (31.5) | |
| 50-103 | 10 (5.6) | 17 (9.6) | 8 (10.3) | 7 (9.0) | 6 (11.1) | 3 (5.6) | |
| 103-105 | 14 (7.9) | 13 (7.3) | 4 (5.1) | 5 (6.4) | 3 (5.6) | 4 (7.4) | |
| > 105 | 12 (6.8) | 19 (10.7) | 5 (6.4) | 6 (7.7) | 2 (3.7) | 3 (5.6) | |
| MRI features | |||||||
| Tumor diameter (cm) | 2.151 ± 0.590 | 2.185 ± 0.560 | 0.689 | 2.089 ± 0.590 | 2.083 ± 0.620 | 2.371 ± 0.600 | 2.086 ± 0.600 |
| Tumor number | 0.894 | ||||||
| Solitary | 82 (46.3) | 78 (44.1) | 33 (42.3) | 38 (48.7) | 26 (48.1) | 25 (46.3) | |
| Multiple | 9 (5.1) | 8 (4.5) | 4 (5.1) | 3 (3.8) | 0 (0.0) | 3 (5.6) | |
| Shape | 0.004 | ||||||
| Regular | 54 (30.5) | 32 (18.1) | 17 (21.8) | 15 (19.2) | 22 (40.7) | 15 (27.8) | |
| Irregular | 37 (20.9) | 54 (30.5) | 20 (25.6) | 26 (33.3) | 4 (7.4) | 13 (24.1) | |
| Margin | 0.003 | ||||||
| Smooth | 52 (29.4) | 30 (16.9) | 22 (28.2) | 8 (10.3) | 21 (38.9) | 15 (27.8) | |
| Non-smooth | 39 (22.0) | 56 (31.6) | 15 (19.2) | 33 (42.3) | 5 (9.3) | 13 (24.1) | |
| Radiological capsule enhancement | 0.231 | ||||||
| Complete | 25 (14.1) | 33 (18.6) | 11 (14.1) | 6 (7.7) | 17 (31.5) | 13 (24.1) | |
| Incomplete | 49 (27.7) | 36 (20.3) | 16 (20.5) | 27 (34.6) | 4 (7.4) | 9 (16.7) | |
| Absent | 17 (9.6) | 17 (9.6) | 10 (12.8) | 8 (10.3) | 5 (9.3) | 6 (11.1) | |
| Restricted diffusion | 0.276 | ||||||
| Present | 88 (49.7) | 80 (45.2) | 36 (46.2) | 35 (44.9) | 25 (46.3) | 28 (51.9) | |
| Absent | 3 (1.7) | 6 (3.4) | 1 (1.3) | 6 (7.7) | 1 (1.9) | 0 (0.0) | |
| Non-rim APHE | 0.689 | ||||||
| Present | 67 (37.9) | 61 (34.5) | 23 (29.5) | 28 (35.9) | 24 (44.4) | 24 (44.4) | |
| Absent | 24 (13.6) | 25 (14.1) | 14 (17.9) | 13 (16.7) | 2 (3.7) | 4 (7.4) | |
| Rim APHE | 0.037 | ||||||
| Absent | 27 (15.3) | 14 (7.9) | 24 (30.8) | 32 (41.0) | 24 (44.4) | 22 (40.7) | |
| Present | 64 (36.2) | 72 (40.7) | Present | 13 (16.7) | 9 (11.5) | 2 (3.7) | 6 (11.1) |
| Arterial peritumoral enhancement | < 0.001 | ||||||
| Absent | 83 (46.9) | 52 (29.4) | 34 (43.6) | 27 (34.6) | 22 (40.7) | 15 (27.8) | |
| Present | 8 (4.5) | 34 (19.2) | 3 (3.8) | 14 (17.9) | 4 (7.4) | 13 (24.1) | |
| Nonperipheral “washout” | 0.823 | ||||||
| Present | 61 (34.5) | 59 (33.3) | 21 (26.9) | 29 (37.2) | 19 (35.2) | 22 (40.7) | |
| Absent | 30 (16.9) | 27 (15.3) | 16 (20.5) | 12 (15.4) | 7 (13.0) | 6 (11.1) | |
| Enhancement pattern | 0.832 | ||||||
| Typical | 60 (33.9) | 58 (32.8) | 20 (25.6) | 28 (35.9) | 19 (35.2) | 22 (40.7) | |
| Atypical | 31 (17.5) | 28 (15.8) | 17 (21.8) | 13 (16.7) | 7 (13.0) | 6 (11.1) | |
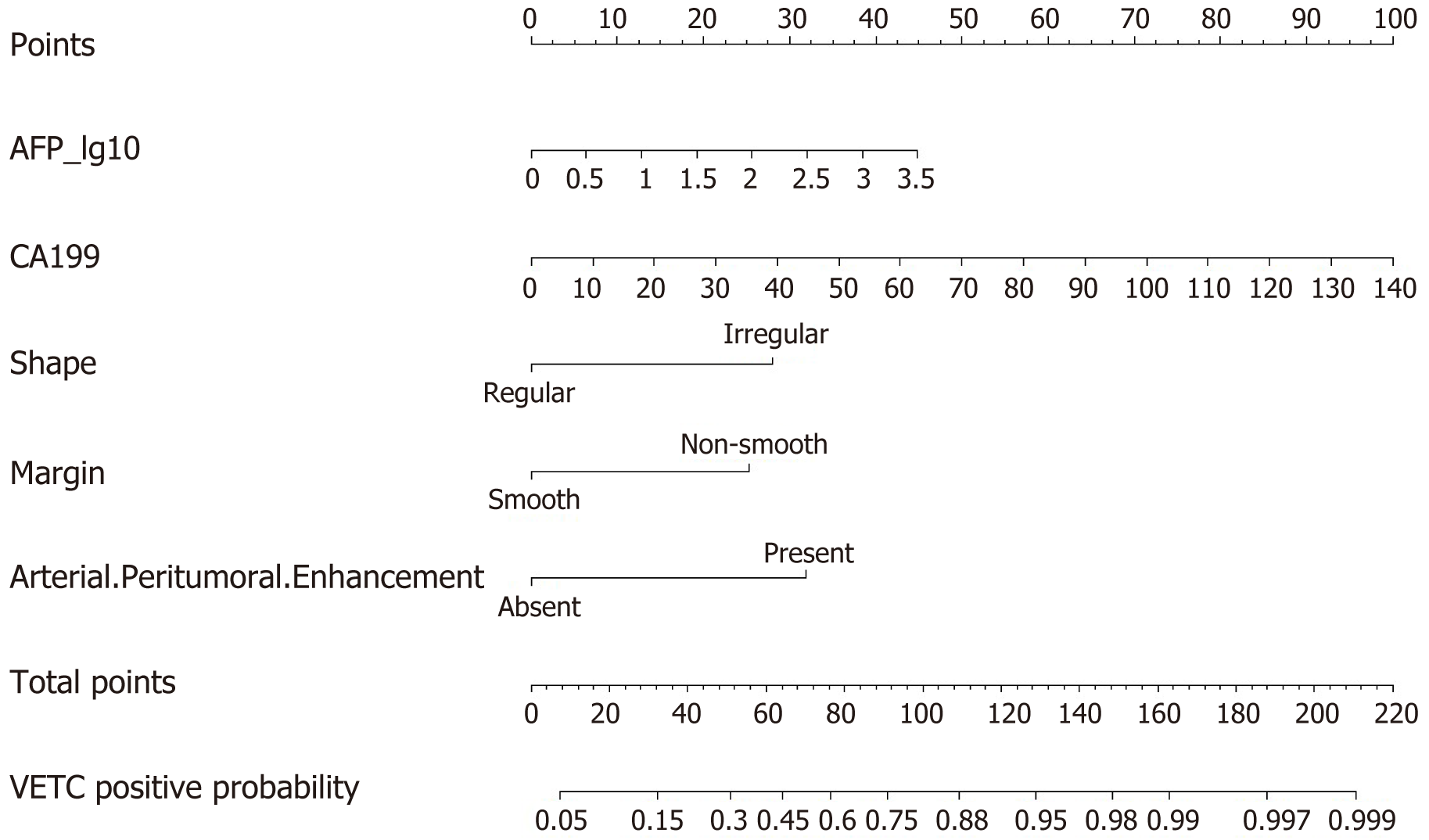
Univariate and multivariate logistic regression analyses were only conducted on the training set, with a total of 177 patients, including 86 VETC-positive patients. Regarding MRI features, the rim APHE feature was excluded from analysis due to a Kappa consistency value below the threshold of 0.85. Univariate logistic regression analysis, based on a P value threshold of < 0.05, indicated that quantitation of hepatitis B virus genomic DNA (HBV/C-DNA) level [50-103: odds ratio (OR): 2.527, P = 0.04; 103-105: OR: 2.567, P = 0.040], AFP_lg10 (OR: 1.607, P = 0.009), carbohydrate antigen 199 (CA199) (OR: 1.033, P = 0.003), irregular shape (OR: 2.463, P = 0.004), non-smooth margin (OR: 2.489, P = 0.003), and arterial peritumoral enhancement (OR: 6.784, P < 0.001) were significantly different between the VETC-positive and -negative groups (Table 2). We included these potential predictive factors in the multivariate logistic regression and found that AFP_lg10 (OR: 1.857, P = 0.004), CA199 (OR: 1.035, P = 0.006), irregular shape (OR: 3.881, P = 0.001), non-smooth margin (OR: 3.409, P = 0.003), and arterial peritumoral enhancement (OR: 4.679, P < 0.001) were independent predictors of VETC positivity (Table 2).
| Variable | Univariable logistic regression | Multivariable logistic regression | ||||
| OR | 95%CI | P value | OR | 95%CI | P valuea | |
| HBV/C-DNA (IU/mL) | 0.083 | |||||
| 50-103 | 2.527 | 1.043, 6.125 | 0.040 | |||
| 103-105 | 0.464 | 0.583, 3.269 | 0.464 | |||
| > 105 | 2.354 | 1.022, 5.421 | 0.044 | |||
| AFP_Lg10 | 1.607 | 1.126, 2.293 | 0.009 | 1.857 | 1.212, 2.845 | 0.004 |
| CA199 | 1.033 | 1.011, 1.055 | 0.003 | 1.035 | 1.010, 1.061 | 0.006 |
| Shape (Irregular) | 2.463 | 1.345, 4.511 | 0.004 | 3.881 | 1.750, 8.609 | 0.001 |
| Margin (non-smooth) | 2.489 | 1.356, 4.569 | 0.003 | 3.409 | 1.526, 7.616 | 0.003 |
| Arterial peritumoral enhancement (present) | 6.784 | 2.915, 15.786 | < 0.001 | 4.679 | 1.847, 11.853 | 0.001 |
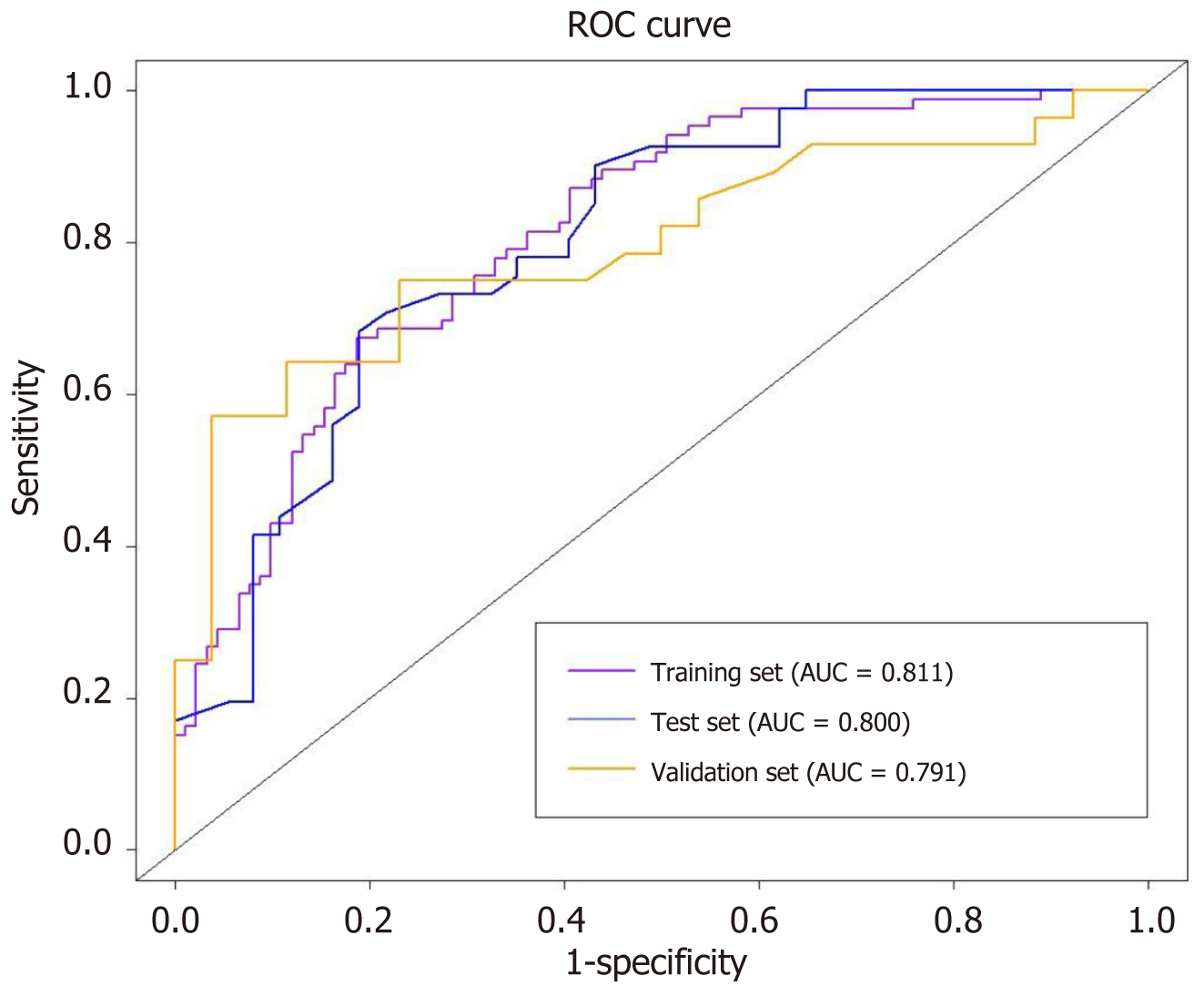
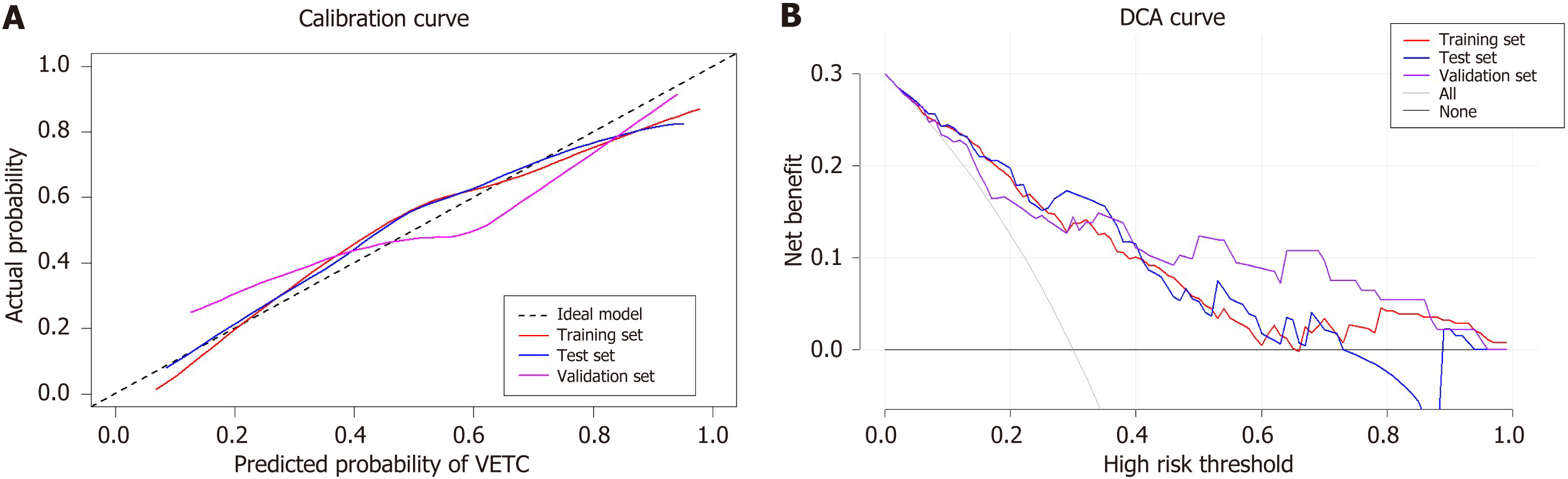
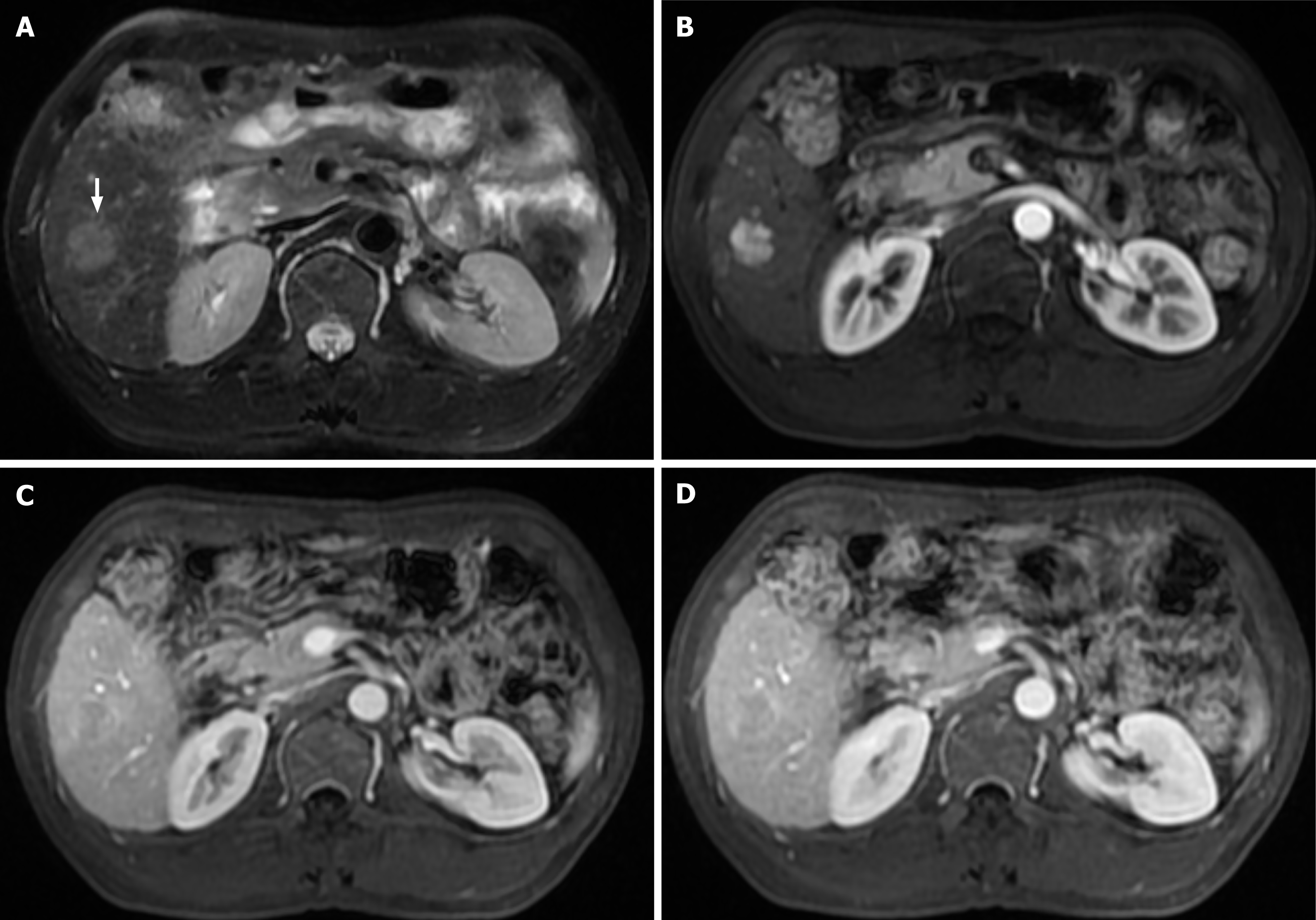
We created a predictive nomogram using the independent predictive factors mentioned above for the preoperative identification of VETC-positive/negative cases (Figure 2). Subsequently, we assessed the predictive performance of the nomogram by plotting ROC curves and calculating the area under the curve (AUC). In the training set, the AUC was 0.811 [95% confidence interval (CI): 0.749, 0.873], with an accuracy of 0.734, specificity of 0.813, and sensitivity of 0.674. In the test set, the AUC was 0.800 (95%CI: 0.702, 0.898), with an accuracy of 0.705, specificity of 0.811, and sensitivity of 0.683. For the validation set, the AUC was 0.791 (95%CI: 0.669, 0.914), with an accuracy of 0.722, specificity of 0.769, and sensitivity of 0.750 (Table 3). Based on the AUC values, our model effectively predicted VETC patterns, demonstrating good prediction performance in the training set, validation set, and external validation set (Figure 3). Calibration curves indicated that the predicted probabilities aligned well with the actual VETC status in all the three sets, and decision curve analysis highlighted the clinical advantages of our model for sHCC patients (Figure 4). Representative images for early recurrence in HCC patients with actual and predicted VETC positivity are presented in Figure 5.
| Training | Test | Validation | |
| AUC | 0.811 (0.749, 0.873) | 0.800 (0.702, 0.898) | 0.791 (0.669, 0.914) |
| Accuracy | 0.734 | 0.705 | 0.722 |
| Sensitivity | 0.674 | 0.683 | 0.750 |
| Specificity | 0.813 | 0.811 | 0.769 |
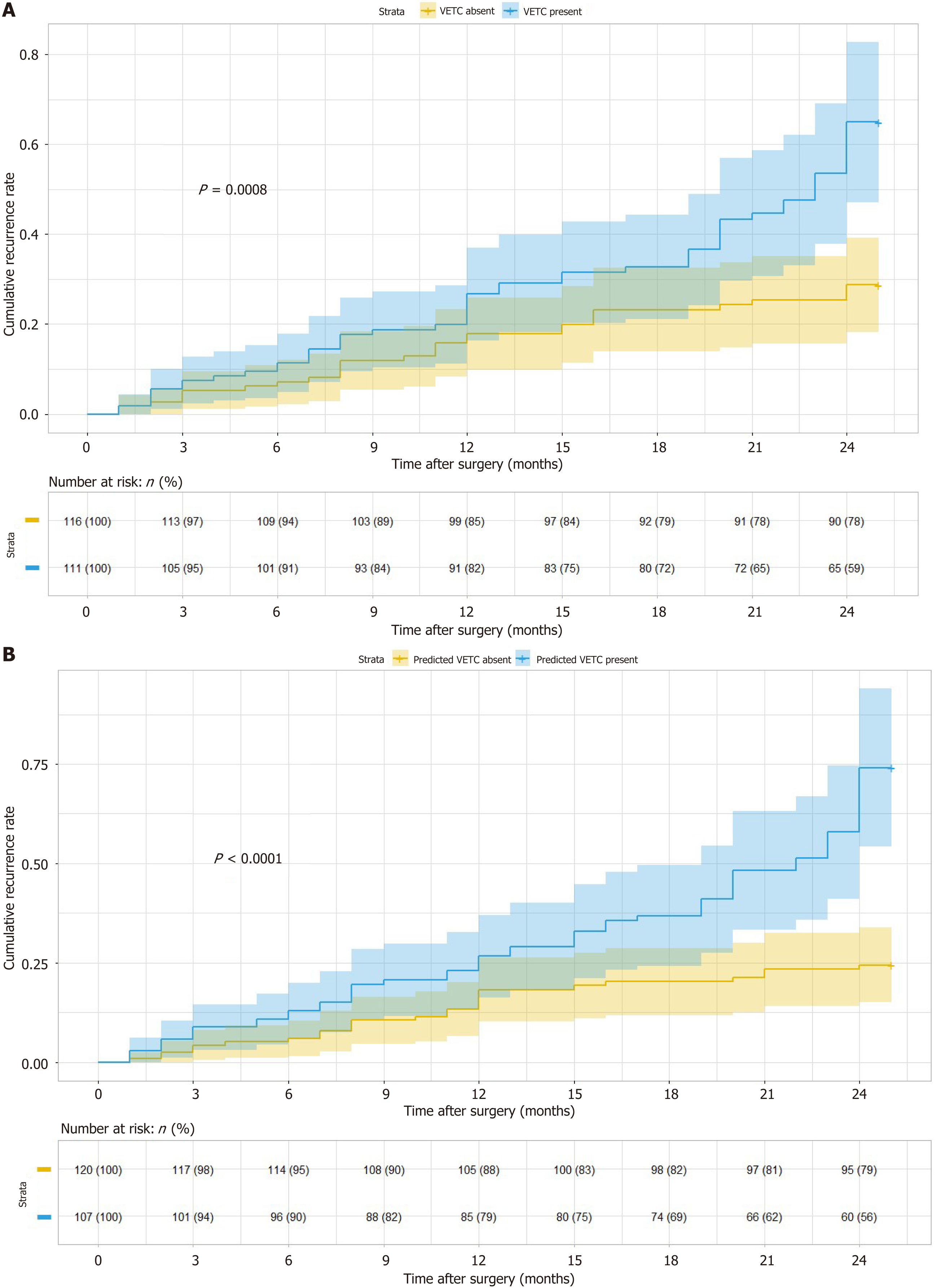
Out of the 227 patients across the three hospitals who underwent follow-up assessments, 111 (48.9%) were identified as VETC-positive. Among these VETC-positive patients, 53 (47.7%) experienced early recurrence, with an average recurrence time of 14 months. On the other hand, 116 patients (51.1%) were classified as VETC-negative, and 29 (25.0%) of them had early recurrence, with an average recurrence time of 11 months.
Kaplan-Meier survival analysis indicated that VETC-positive patients had a significantly higher risk of early recurrence compared to VETC-negative patients (P = 0.0008). Moreover, patients predicted as VETC-positive by the nomogram also exhibited a substantially higher risk of early recurrence than those predicted as VETC-negative by the nomogram (P < 0.0001; Figure 6).
The discovery of VETC as a distinct vascular pattern has significant implications for the metastasis and recurrence of HCC[4,24,25]. VETC can only be definitively diagnosed through histological examination, making it crucial to develop a nomogram for predicting VETC based on preoperative characteristics of HCC patients, especially those with tumors less than 3 cm. Such a tool can offer valuable insights for personalized treatment and prognosis. In our study, we identified AFP_lg10, CA199, irregular shape, non-smooth margin, and arterial peritumoral enhancement on MRI as independent predictors of the VETC pattern. Subsequently, we successfully developed and validated a nomogram that combines these features to provide non-invasive prediction of VETC patterns before surgery. Importantly, this nomogram also serves as an independent preoperative predictor of early recurrence following surgical resection, offering critical information for clinical treatment decisions.
Previous research has already indicated a strong correlation between elevated AFP levels and VETC positivity in HCC patients[22,26,27]. Furthermore, univariate regression analysis conducted by Chen et al[26] showed significant associations between non-smooth margin, arterial peritumoral enhancement, and VETC positivity. This is consistent with our research results, even though our study specifically focused on sHCC. In addition, Chen et al[26] used their nomogram to diagnose the VETC pattern in HCC (< 3 cm) patients, which showed a low sensitivity (33.3%) and high specificity (96.8%). In contrast, our nomogram demonstrated a significantly improved sensitivity (68.3%) and specificity (81.1%) in predicting VETC patterns among sHCC patients in the test set, which was confirmed in the validation set, with a sensitivity of 75.0% and specificity of 76.9%. These results not only build upon prior research findings but also highlight the robustness and broad applicability of our model, which has been rigorously validated across multiple centers.
HCC is usually a tumor with abundant blood vessels, and VETC have been described as sinusoidal tumor angiogenesis, and the arachnoid pattern of blood vessels isolates and envelops a single tumor cluster[28]. The metastasis of VETC-positive tumors can be achieved by releasing the entire tumor cluster into the bloodstream, which is different from the metastasis mechanism dependent on the EMT[4]. The recurrence of HCC is usually associated with its metastasis. In recent years, VETC have been proven to be an independent predictor of recurrence and overall survival in HCC patients undergoing liver resection[21,29-31]. Radiological markers related to VETC can also predict the prognosis of HCC patients[32-34]. In addition, VETC can also guide clinical treatment by predicting responses to transcatheter arterial chemoembolization (TACE) and conventional TACE[10,35]. Unfortunately, there has been a dearth of research on predicting VETC in sHCC. Our study further establishes that the likelihood of early postoperative recurrence is higher in VETC-positive sHCC patients compared to VETC-negative patients, with our nomogram effectively differentiating high-risk VETC patients from low-risk ones in terms of early recurrence.
Our study had several limitations. First, our study only encompasses sHCC patients who underwent surgical resection, and the VETC diagnosis remains histopathology-dependent, potentially limiting the generalizability to all HCC cases; Second, being a retrospective study, bias is inherent, although conducting a multicenter study aimed to minimize this bias; Third, there is currently no consensus on the optimal threshold for determining the VETC pattern; Fourth, our evaluation of prognostic factors is focused on early recurrence in HCC patients who underwent surgical resection; Lastly, our study primarily comprises patients from China, necessitating further research in diverse ethnic populations.
Our study underscores the significance of AFP_lg10, CA199, irregular shape, non-smooth margin, and arterial peritumoral enhancement as independent risk factors for predicting VETC. Through the development of a nomogram that utilizes these clinical and imaging features, we can non-invasively predict VETC in sHCC. The nomogram has undergone extensive validation across multiple patient centers, demonstrating robust predictive performance and promising implications for postoperative recurrence prediction.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, and HCC patients have a poor prognosis. Vessels encapsulating tumor clusters (VETC) are a vascular pattern associated with a novel metastasis mechanism and have been proven to be an independent poor prognostic factor for early HCC patients.
It seems that no one has focused on predicting the VETC pattern of small HCC (sHCC; ≤ 3 cm) patients in multicenter studies.
To construct a nomogram that combines preoperative clinical parameters and image features to predict patterns of VETC and evaluate the prognosis of sHCC patients.
We collected patients with VETC and HCC status from three hospitals. Data from one hospital were used as a training set to train the prediction model, while those from the other two hospitals were used as test and validation sets, respectively. Univariate and multivariate logistic regression analyses were used to screen the independent predictive factors associated with VETC, and these factors were included to construct a model for predicting the pattern of VETC in sHCC patients. The performance of the model was evaluated using area under curve (AUC), decision curve analysis (DCA), and calibration curve. Kaplan-Meier survival analysis was performed to confirm whether the VETC status predicted by the model was associated with early recurrence in sHCC patients.
The independent predictive factors that we identified include alpha-fetoprotein_lg10, carbohydrate antigen 199 (CA199), irregular shape, non-smooth margin, and arterial peritumoral enhancement. The model for predicting VETC status, which incorporates these factors, showed good results under the evaluation of AUC, DCA, and calibration curves in the three sets. Finally, Kaplan-Meier survival analysis confirmed that the VETC pattern was associated with early recurrence in sHCC patients.
The nomogram constructed by incorporating preoperative clinical parameters and imaging features has undergone extensive validation across multiple patient centers, demonstrating strong predictive performance and having good significance for predicting postoperative recurrence.
Improving the predictive performance of VETC status in preoperative prediction of sHCC patients also requires the combination of radiomics and artificial intelligence, in order to better provide assistance for clinical treatment decision-making.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine & medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Li J, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11436] [Article Influence: 3812.0] [Reference Citation Analysis (4)] |
| 3. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1232] [Article Influence: 410.7] [Reference Citation Analysis (41)] |
| 4. | Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, Zheng L, Yuan Y, Guo RP, Jia WH, Yun JP, Chen MS, Zhang Y, Zhuang SM. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62:452-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Guan R, Lin W, Zou J, Mei J, Wen Y, Lu L, Guo R. Development and Validation of a Novel Nomogram for Predicting Vessels that Encapsulate Tumor Cluster in Hepatocellular Carcinoma. Cancer Control. 2022;29:10732748221102820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Huang CW, Lin SE, Huang SF, Yu MC, Tang JH, Tsai CN, Hsu HY. The Vessels That Encapsulate Tumor Clusters (VETC) Pattern Is a Poor Prognosis Factor in Patients with Hepatocellular Carcinoma: An Analysis of Microvessel Density. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, Viganò L, Akiba J, Lee HS, Rhee H, Park YN, Roncalli M, Di Tommaso L. Vessels Encapsulating Tumor Clusters (VETC) Is a Powerful Predictor of Aggressive Hepatocellular Carcinoma. Hepatology. 2020;71:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 8. | Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, Sun H, Zhou J, Ji Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Lin C, He Y, Liu M, Wu A, Zhang J, Li S, Cao Q, Liu F. Vessels That Encapsulate Tumor Clusters (VETC) Predict cTACE Response in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Yu Y, Fan Y, Wang X, Zhu M, Hu M, Shi C, Hu C. Gd-EOB-DTPA-enhanced MRI radiomics to predict vessels encapsulating tumor clusters (VETC) and patient prognosis in hepatocellular carcinoma. Eur Radiol. 2022;32:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Dong X, Yang J, Zhang B, Li Y, Wang G, Chen J, Wei Y, Zhang H, Chen Q, Jin S, Wang L, He H, Gan M, Ji W. Deep Learning Radiomics Model of Dynamic Contrast-Enhanced MRI for Evaluating Vessels Encapsulating Tumor Clusters and Prognosis in Hepatocellular Carcinoma. J Magn Reson Imaging. 2024;59:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Wang R, Xu H, Chen W, Jin L, Ma Z, Wen L, Wang H, Cao K, Du X, Li M. Gadoxetic acid-enhanced MRI with a focus on LI-RADS v2018 imaging features predicts the prognosis after radiofrequency ablation in small hepatocellular carcinoma. Front Oncol. 2023;13:975216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Liu B, Gao S, Guo J, Kou F, Liu S, Zhang X, Wang X, Cao G, Chen H, Liu P, Xu H, Gao Q, Yang R, Zhu X. A Novel Nomogram for Predicting the Overall Survival in Patients with Unresectable HCC after TACE plus Hepatic Arterial Infusion Chemotherapy. Transl Oncol. 2023;34:101705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Galgano SJ, Smith EN. LI-RADS Version 2018 for MRI and CT: Interreader Agreement in Real-World Practice. Radiology. 2023;307:e231212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Lee S, Kim KW, Jeong WK, Kim MJ, Choi GH, Choi JS, Song GW, Lee SG. Gadoxetic acid-enhanced MRI as a predictor of recurrence of HCC after liver transplantation. Eur Radiol. 2020;30:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Zhou C, Lu X, Wang Y, Qian X, Yang C, Zeng M. Histopathological components correlated with MRI features and prognosis in combined hepatocellular carcinoma-cholangiocarcinoma. Eur Radiol. 2022;32:6702-6711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Yan M, Zhang X, Zhang B, Geng Z, Xie C, Yang W, Zhang S, Qi Z, Lin T, Ke Q, Li X, Wang S, Quan X. Deep learning nomogram based on Gd-EOB-DTPA MRI for predicting early recurrence in hepatocellular carcinoma after hepatectomy. Eur Radiol. 2023;33:4949-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Tang M, Zhou Q, Huang M, Sun K, Wu T, Li X, Liao B, Chen L, Liao J, Peng S, Chen S, Feng ST. Nomogram development and validation to predict hepatocellular carcinoma tumor behavior by preoperative gadoxetic acid-enhanced MRI. Eur Radiol. 2021;31:8615-8627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Fang JH, Xu L, Shang LR, Pan CZ, Ding J, Tang YQ, Liu H, Liu CX, Zheng JL, Zhang YJ, Zhou ZG, Xu J, Zheng L, Chen MS, Zhuang SM. Vessels That Encapsulate Tumor Clusters (VETC) Pattern Is a Predictor of Sorafenib Benefit in Patients with Hepatocellular Carcinoma. Hepatology. 2019;70:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 21. | Wang YY, Dong K, Wang K, Sun Y, Xing BC. Effect of vessels that encapsulate tumor clusters (VETC) on the prognosis of different stages of hepatocellular carcinoma after hepatectomy. Dig Liver Dis. 2023;55:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Yang J, Dong X, Wang G, Chen J, Zhang B, Pan W, Zhang H, Jin S, Ji W. Preoperative MRI features for characterization of vessels encapsulating tumor clusters and microvascular invasion in hepatocellular carcinoma. Abdom Radiol (NY). 2023;48:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 23. | Xia TY, Zhou ZH, Meng XP, Zha JH, Yu Q, Wang WL, Song Y, Wang YC, Tang TY, Xu J, Zhang T, Long XY, Liang Y, Xiao WB, Ju SH. Predicting Microvascular Invasion in Hepatocellular Carcinoma Using CT-based Radiomics Model. Radiology. 2023;307:e222729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 103] [Reference Citation Analysis (1)] |
| 24. | Dennis C, Prince DS, Moayed-Alaei L, Remash D, Carr-Boyd E, Bowen DG, Strasser SI, Crawford M, Pulitano C, Kench J, McCaughan GW, McKenzie C, Liu K. Association between vessels that encapsulate tumour clusters vascular pattern and hepatocellular carcinoma recurrence following liver transplantation. Front Oncol. 2022;12:997093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Liu K, Dennis C, Prince DS, Marsh-Wakefield F, Santhakumar C, Gamble JR, Strasser SI, McCaughan GW. Vessels that encapsulate tumour clusters vascular pattern in hepatocellular carcinoma. JHEP Rep. 2023;5:100792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Chen FM, Du M, Qi X, Bian L, Wu D, Zhang SL, Wang J, Zhou Y, Zhu X. Nomogram Estimating Vessels Encapsulating Tumor Clusters in Hepatocellular Carcinoma From Preoperative Gadoxetate Disodium-Enhanced MRI. J Magn Reson Imaging. 2023;57:1893-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 27. | Fan Y, Yu Y, Wang X, Hu M, Du M, Guo L, Sun S, Hu C. Texture Analysis Based on Gd-EOB-DTPA-Enhanced MRI for Identifying Vessels Encapsulating Tumor Clusters (VETC)-Positive Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Zhou HC, Liu CX, Pan WD, Shang LR, Zheng JL, Huang BY, Chen JY, Zheng L, Fang JH, Zhuang SM. Dual and opposing roles of the androgen receptor in VETC-dependent and invasion-dependent metastasis of hepatocellular carcinoma. J Hepatol. 2021;75:900-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Lin WP, Xing KL, Fu JC, Ling YH, Li SH, Yu WS, Zhang YF, Zhong C, Wang JH, Chen ZY, Lu LH, Wei W, Guo RP. Development and Validation of a Model Including Distinct Vascular Patterns to Estimate Survival in Hepatocellular Carcinoma. JAMA Netw Open. 2021;4:e2125055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 30. | He C, Zhou Z, Jiang H, Yin Z, Meng S, Zhang J, Huang P, Xu K, Bian L, Xiao Z, Wang J. Epithelial-Mesenchymal Transition is Superior to Vessels-Encapsulate Tumor Cluster in Promoting Metastasis of Hepatocellular Carcinoma: a Morphological Evidence. J Cancer. 2017;8:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 31. | Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, Qian CN, Zheng L. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878-4889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Feng Z, Li H, Zhao H, Jiang Y, Liu Q, Chen Q, Wang W, Rong P. Preoperative CT for Characterization of Aggressive Macrotrabecular-Massive Subtype and Vessels That Encapsulate Tumor Clusters Pattern in Hepatocellular Carcinoma. Radiology. 2021;300:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 33. | Rhee H, An C, Kim HY, Yoo JE, Park YN, Kim MJ. Hepatocellular Carcinoma with Irregular Rim-Like Arterial Phase Hyperenhancement: More Aggressive Pathologic Features. Liver Cancer. 2019;8:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Chu T, Zhao C, Zhang J, Duan K, Li M, Zhang T, Lv S, Liu H, Wei F. Application of a Convolutional Neural Network for Multitask Learning to Simultaneously Predict Microvascular Invasion and Vessels that Encapsulate Tumor Clusters in Hepatocellular Carcinoma. Ann Surg Oncol. 2022;29:6774-6783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 35. | Wang JH, Li XS, Tang HS, Fang RY, Song JJ, Feng YL, Guan TP, Ruan Q, Wang J, Cui SZ. Vessels that encapsulate tumor clusters (VETC) pattern predicts the efficacy of adjuvant TACE in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149:4163-4172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |