Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1796
Peer-review started: December 12, 2023
First decision: January 2, 2024
Revised: January 15, 2024
Accepted: February 29, 2024
Article in press: February 29, 2024
Published online: May 15, 2024
Processing time: 149 Days and 5.2 Hours
Rectal carcinoma (RC), one of the most common malignancies globally, presents an increasing incidence and mortality year by year, especially among young people, which seriously affects the prognosis and quality of life of patients. At present, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters and serum carbohydrate antigen 19-9 (CA19-9) and CA125 Levels have been used in clinical practice to evaluate the T stage and differentiation of RC. However, the accuracy of these evaluation modalities still needs further re
To analyze the diagnostic performance of DCE-MRI parameters combined with serum tumor markers (TMs) in assessing pathological processes and prognosis of RC patients.
A retrospective analysis was performed on 104 RC patients treated at Yantai Yuhuangding Hospital from May 2018 to January 2022. Patients were categorized into stages T1, T2, T3, and T4, depending on their T stage and differentiation degree. In addition, they were assigned to low (L group) and moderate-high differentiation (M + H group) groups based on their differentiation degree. The levels of DCE-MRI parameters and serum CA19-9 and CA125 in different groups of patients were compared. In addition, the value of DCE-MRI parameters [volume transfer constant (Ktrans), rate constant (Kep), and extravascular extracellular volume fraction (Ve) in assessing the differentiation and T staging of RC patients was discussed. Furthermore, the usefulness of DCE-MRI parameters combined with serum CA19-9 and CA125 Levels in the evaluation of RC differentiation and T staging was analyzed.
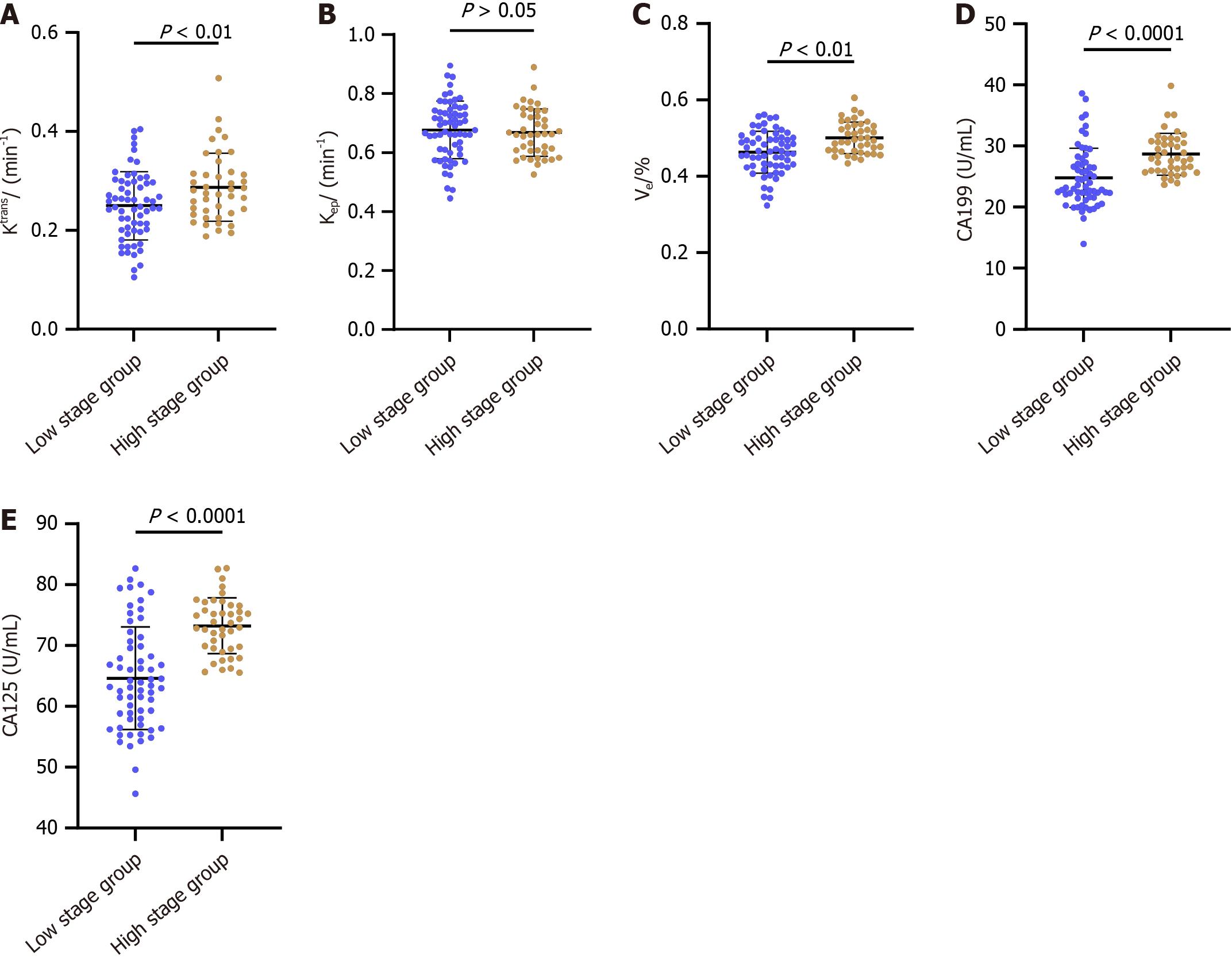
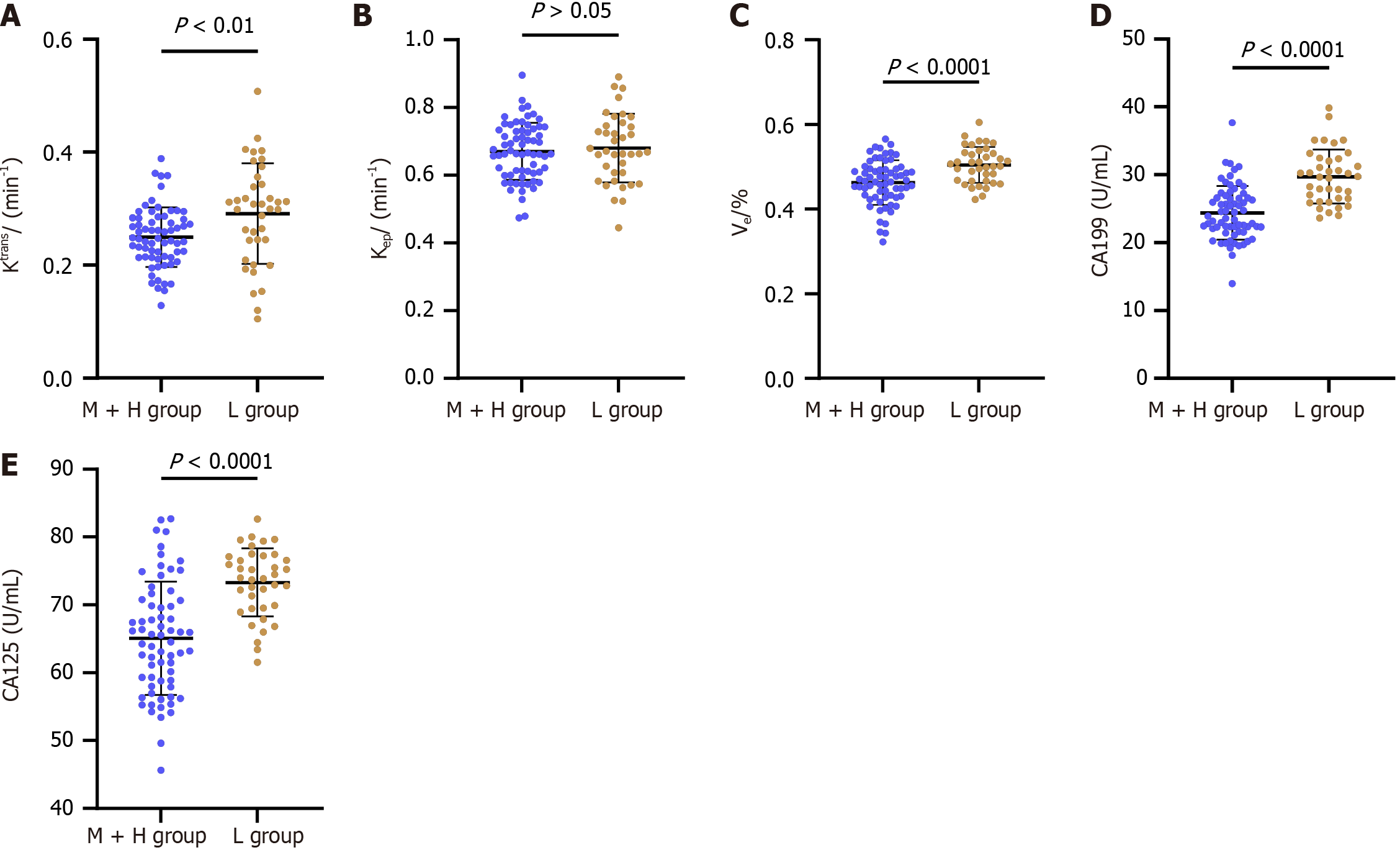
Ktrans, Ve, CA19-9 and CA125 were higher in the high-stage group and L group than in the low-stage group and M + H Group, respectively (P < 0.05). The areas under the curve (AUCs) of the Ktran and Ve parameters were 0.638 and 0.694 in the diagnosis of high and low stages, respectively, and 0.672 and 0.725 in diagnosing moderate-high and low differentiation, respectively. The AUC of DCE-MRI parameters (Ktrans + Ve) in the diagnosis of high and low stages was 0.742, and the AUC in diagnosing moderate-high and low differentiation was 0.769. The AUCs of CA19-9 and CA-125 were 0.773 and 0.802 in the diagnosis of high and low stages, respectively, and 0.834 and 0.796 in diagnosing moderate-high and low differentiation, respectively. Then, we combined DCE-MRI (Ktrans + Ve) parameters with CA19-9 and CA-125 and found that the AUC of DCE-MRI parameters plus serum TMs was 0.836 in the diagnosis of high and low stages and 0.946 in the diagnosis of moderate-high and low differentiation. According to the Delong test, the AUC of DCE-MRI parameters plus serum TMs increased significantly compared with serum TMs alone in the diagnosis of T stage and differentiation degree (P < 0.001).
The levels of the DCE-MRI parameters Ktrans and Ve and the serum TMs CA19-9 and CA125 all increase with in
Core Tip: This study explored the application and value of dynamic contrast-enhanced magnetic resonance imaging para
- Citation: Mu RQ, Lv JW, Ma CY, Ma XH, Xing D, Ma HS. Diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging parameters and serum tumor markers in rectal carcinoma prognosis. World J Gastrointest Oncol 2024; 16(5): 1796-1807
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1796.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1796
The latest statistics from the World Health Organization (WHO) reveal that there were approximately 19.3 million new cancer cases diagnosed globally in 2020, of which colorectal carcinoma (CRC) accounted for more than 1.9 million, indicating that one out of every ten new cancer cases was CRC[1]. By 2021, the incidence and mortality of CRC have climbed to third and second place in the global cancer rankings, respectively, and it is worth noting that rectal carcinoma (RC) cases account for approximately 40% of the total CRC cases[2]. In China, the prevalence of RC is rising constantly, while the age of onset is gradually decreasing[3]. Early-stage RC is often atypical and difficult to detect, and the prognosis of patients is usually grim when there are middle and late symptoms such as hematochezia and abdominal pain[4]. Early detection, diagnosis and treatment of RC is therefore key to improving survival and quality of life.
Doctors will choose appropriate treatment strategies, such as local lesion excision, radical surgical resection, and preoperative neoadjuvant chemoradiotherapy, depending on the stages and grades of the patients' tumors[5]. According to the National Comprehensive Cancer Network clinical practice guidelines for RC and National Guidelines for the Diagnosis and Treatment of Colorectal Cancer in China (2020 Edition)[6], early RC (T1-T2N0) is usually treated with endoscopic resection, local resection or radical surgical resection. For those with locally advanced T3-T4 RC and/or N+ resectable RC, neoadjuvant chemoradiotherapy is recommended to shrink the focus and downgrade the tumor before radical surgery. In addition, RC can be classified into high-, moderate-, and low-differentiation as well as undifferentiated, according to cancer cell differentiation. These different histopathological differentiation degrees have significant variations in biological characteristics, which may be characterized by slow growth or widespread metastasis and rapid growth[7]. Currently, patients' pathological grades are mostly determined by biopsy. However, when diagnosed, many of them miss out on the best chance of treatment. Hence, early diagnosis, determination of pathological grades as soon as possible, and selection of appropriate treatment strategies are of great importance for improving survival and prognosis[8,9]. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can display blood perfusion at the tissue capillary level, enable in vivo evaluation of tumor microcirculation perfusion, and be used to quantitatively evaluate the benign and malignant degree of tumors[10,11]. Tumor markers (TMs) are biochemical substances secreted by malignant tumor cells in the process of proliferation that have certain biological characteristics and are commonly used in early diagnosis and prognosis prediction. Research[4] shows that serum carbohydrate antigen (CA) 19-9 and CA125 Levels can reflect the characteristics of primary tumors to a certain extent and play an important role in tumor cell metastasis and invasion[12]. However, there are limited studies on using DCE-MRI parameters combined with serum CA19-9 and CA125 Levels to evaluate the differentiation degree and T stage of RC patients, although it is an area worthy of in-depth discussion.
Accordingly, 129 RC patients with different degrees of tumor differentiation were selected and subjected to DCE-MRI and serum CA19-9 and CA125 detection to explore the evaluation value of these indices on the degree of tumor differentiation.
This retrospective study comprised 184 RC patients who were enrolled between May 2018 and January 2022.
The eligibility criteria were as follows: (1) Meeting the relevant diagnostic criteria for RC with confirmed diagnosis after pathological examination, including histopathology, gross pathological specimens and immunohistochemistry; (2) receiving preoperative examination by magnetic resonance imaging (MRI); and (3) normal language communication skills and active cooperation with the study.
The exclusion criteria were as follows: (1) Serious diseases of the heart, kidney, liver, lungs or other vital organs; (2) hematological diseases; (3) use of tumor-related treatment, such as radiotherapy and chemotherapy, before presentation; (4) patients whose largest tumor diameter was less than 1 cm or whose rectal peristalsis amplitude was too large to be accurately measured; (5) poor DCE-MRI image quality or artifacts affecting data measurement; or (6) pregnant and lactating patients.
A total of 104 eligible patients were screened according to the above eligibility and exclusion criteria. Based on T staging, patients with T1/2 and T3/4 RC were assigned to the low stage group and high stage group, respectively. In addition, they were divided into low (L group) and moderate-high (M + H group) differentiation groups based on their differentiation degree.
In this study, a Siemens (Germany) Skyra 3-T MRI scanner and abdominal phased-array coil were used for inspection. All patients abstained from food and water for 4 h prior to examination. MRI scans included conventional axial T1-weighted imaging (T1WI), axial, coronal and sagittal T2-weighted imaging (T2WI), and axial diffusion-weighted imaging. DCE scanning involved a T1 three-dimensional volume interpolated breath-hold examination sequence with the same scanning planes as the T2WI scans. The parameters of DCE-MRI scanning included a field of view of 260 mm, repetition time of 5.08 ms, echo time of 1.77 ms, matrix of 154 × 192, layer thickness of 3.5 mm, flip angle of 15°, and number of excitations of 1. The contrast agent was Gd-DTPA, which was injected through the elbow vein at a flow rate of 3 mL/s with a dose of 0.1 mmol/kg. Then, 35 consecutive phases were scanned, each with a scanning time of 8 s, allowing the patient to breathe freely. The contrast agent was injected at the third phase with a total dose of 15-20 mL, covering the whole tumor, and the scanning time was 280 s. After contrast injection, 20 mL of normal saline was flushed down the pipeline at the same flow rate. After that, all the raw images were transmitted to the Siemens postprocessing workstation, and DCE-MRI data were analyzed using Tissue 4D software.
Two MRI diagnosticians with more than 5 years of experience read the DCE-MRI images without understanding the pathological findings of the patients. Three circular or elliptical regions of interest (ROIs) were selected in the areas with obvious tumor parenchyma enhancement. The ROIs were as uniform in size as possible, covering the parenchyma of the lesion, with an area greater than 1/3 of the lesion parenchyma, and avoiding the necrotic area and cystic change area of the tumor. Parameters such as the volume transfer constant (Ktrans), rate constant (Kep), and extravascular extracellular volume fraction (Ve) were analyzed using a three-compartment pharmacokinetic model (Tofts model).
For TM detection, 4 mL of fasting venous blood was taken from all patients in the morning and centrifuged for 10 minutes (3000 r/min) to separate CA19-9 and CA125 measurements with a Liaison XL type 2210 automatic electrochemiluminescence analyzer from DiaSorin Company in Italy. The operation was performed according to the kit instructions.
The levels of DCE-MRI parameters and the expression of serum CA19-9 and CA125 were comparatively analyzed. The values of DCE-MRI parameters (Ktrans, Kep, and Ve) in the diagnosis of differentiation degree and T staging of RC patients were analyzed. The performance of DCE-MRI parameters combined with serum CA19-9 and CA125 Levels for the diagnosis of differentiation and T-staging of RC patients was discussed.
This study employed SPSS 26.0 for data processing. Continuous variables, represented by means, were analyzed with the t test. Categorical variables were expressed by n (%) and tested by χ2. The role of DCE-MRI parameters and serum CA19-9 and CA125 Levels in the evaluation of differentiation and T staging of RC patients was analyzed using receiver operating characteristic (ROC) curves. The difference in the ROC curves of these indicators was analyzed using the Delong test.
In this part of the study, we conducted a comprehensive comparison of clinical data among patients. Our analysis en
| Factors | Low stage group (n = 62) | High stage group (n = 42) | P value |
| Age (yr) | 0.474 | ||
| ≥ 60 | 34 | 26 | |
| < 60 | 28 | 16 | |
| Sex | 0.636 | ||
| Male | 34 | 25 | |
| Female | 28 | 17 | |
| BMI (kg/m2) | 0.755 | ||
| ≥ 25 | 16 | 12 | |
| < 25 | 46 | 30 | |
| Tumor site | 0.991 | ||
| Upper | 21 | 14 | |
| Middle | 17 | 12 | |
| Lower | 24 | 16 | |
| Tumor type | 0.854 | ||
| Protruded | 8 | 7 | |
| Ulcerative | 44 | 29 | |
| Infiltrating | 10 | 6 | |
| Differentiation degree | 0.786 | ||
| Moderate-high differentiation | 40 | 26 | |
| Low differentiation | 22 | 16 |
| Factors | M + H group (n = 66) | L group (n = 38) | P value |
| Age (yr) | 0.703 | ||
| ≥ 60 | 39 | 21 | |
| < 60 | 27 | 17 | |
| Sex | 0.855 | ||
| Male | 37 | 22 | |
| Female | 29 | 16 | |
| BMI (kg/m2) | 0.572 | ||
| ≥ 25 | 19 | 9 | |
| < 25 | 47 | 29 | |
| Tumor site | 1.069 | ||
| Upper | 23 | 12 | |
| Middle | 20 | 9 | |
| Lower | 23 | 17 | |
| Tumor type | 0.797 | ||
| Protruded | 10 | 5 | |
| Ulcerative | 47 | 26 | |
| Infiltrating | 9 | 7 | |
| T staging | 0.786 | ||
| T1-T2 | 40 | 22 | |
| T3-T4 | 26 | 16 |
Our study further explored the levels of DCE-MRI parameters and TMs – specifically Ktrans, Ve, CA19-9, and CA125 – between the low and high tumor stage groups. Notably, Ktrans, Ve, CA19-9, and CA125 levels were significantly higher in patients with advanced tumor stages (P < 0.05; Figure 1). This finding was intriguing, as it suggests these markers' potential in indicating tumor stage. However, Kep levels did not show a significant difference between these groups (P > 0.05; Figure 1), indicating its limited utility in distinguishing between low and high tumor stages.
In this section, we compared the same DCE-MRI parameters and TMs between the L and M + H groups. The results were somewhat unexpected: Ktrans, Ve, CA19-9, and CA125 Levels were higher in the L group compared to the M + H group (P < 0.05; Figure 2). The similarity in Kep levels between these groups (P > 0.05; Figure 2) mirrored the findings from the tumor stage comparison, reinforcing Kep's limited differentiating ability.
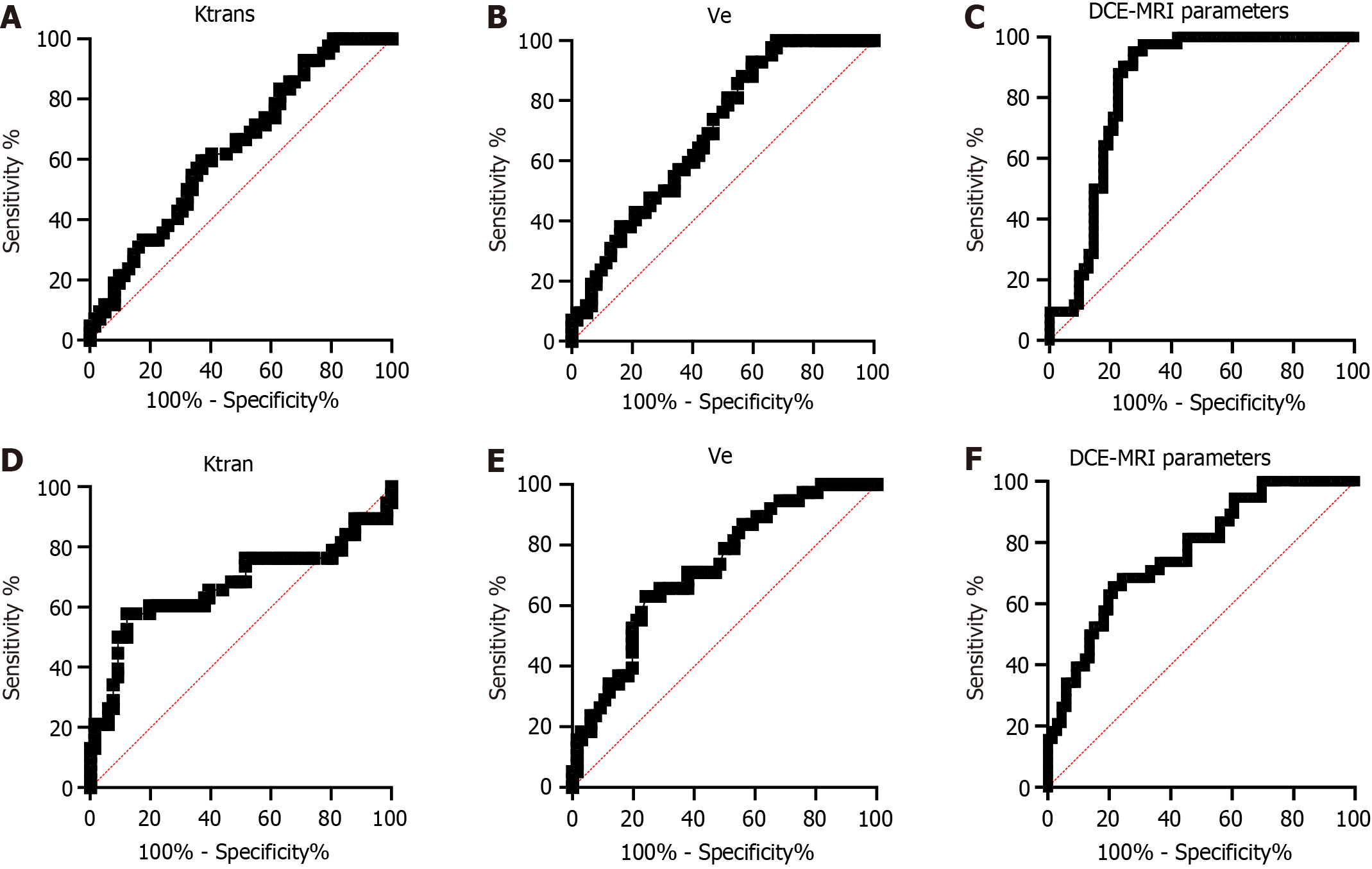
In the above study, we confirmed a connection between the DCE-MRI parameters Ktran and Ve and the T stage and differentiation degree in RC patients. To determine the diagnostic value of Ktran and Ve in T staging and differentiation degree, we analyzed their ROC curves. The areas under the curve (AUCs) of Ktran and Ve for diagnosing high and low tumor stages were 0.638 and 0.694, respectively, and those for diagnosing moderate-high and low differentiation were 0.672 and 0.725, respectively. In addition, we combined Ktran and Ve for diagnosis. Through plotting ROC curves, it was found that the AUC of Ktrans + Ve was 0.742 for diagnosing high and low stages and 0.769 for diagnosing moderate-high and low differentiation (Figure 3 and Table 3).
| Diagnostic variable | AUC | 95%CI | Cut-off | Sensitivity | Specificity | Youden index |
| T stage | ||||||
| Ktrans | 0.638 | 0.532-0.745 | 0.26 | 62.90% | 59.52% | 22.43% |
| Ve | 0.695 | 0.595-0.794 | 0.45 | 40.32% | 92.86% | 33.18% |
| DCE-MRI parameters | 0.742 | 0.646-0.837 | 0.68 | 53.23% | 92.86% | 46.08% |
| Differentiation degree | ||||||
| Ktrans | 0.672 | 0.549-0.795 | 0.29 | 87.88% | 57.90% | 45.77% |
| Ve | 0.725 | 0.626-0.824 | 0.49 | 75.76% | 63.16% | 38.92% |
| DCE-MRI parameters | 0.769 | 0.677-0.861 | 0.35 | 78.79% | 65.79% | 44.58% |
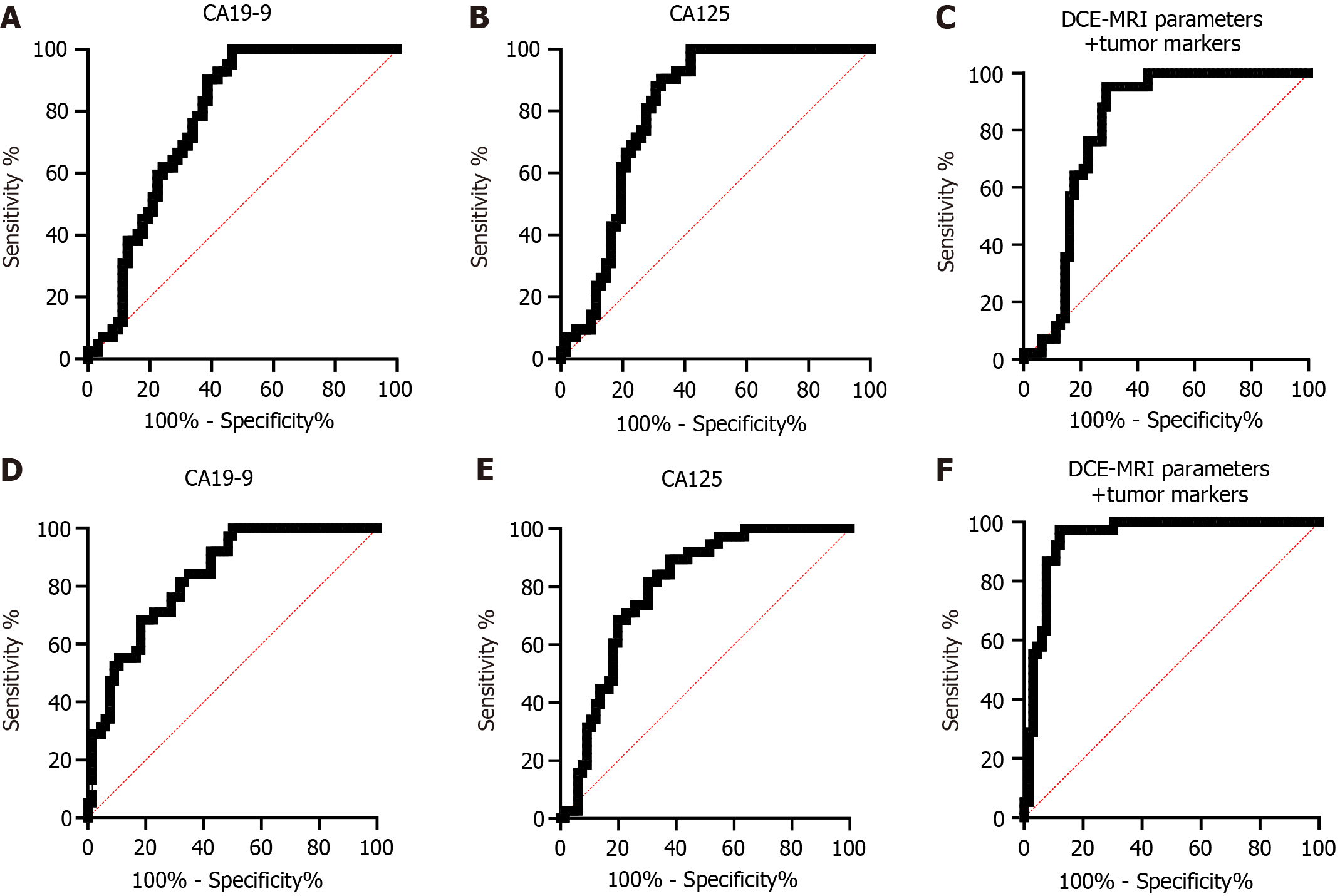
In this section, we studied the diagnostic performance of TMs for T stage and differentiation. Through analysis, it was found that the AUCs of CA19-9 and CA-125 in the diagnosis of high and low stages were 0.773 and 0.802, respectively, while those in the diagnosis of moderate-high differentiation and low differentiation were 0.834 and 0.796, respectively. Then, we combined DCE-MRI parameters (Ktrans + Ve) with CA19-9 and CA-125 for detection and found that the AUC of DCE-MRI parameters plus TMs was 0.836 in the diagnosis of high and low stages and 0.946 in diagnosing moderate-high differentiation and low differentiation (Figure 4 and Table 4). In addition, through the Delong test analysis, it was found that the AUC of DCE-MRI parameters + TMs increased significantly compared with TMs alone in the diagnosis of T stage and differentiation degree (P < 0.001; Table 5).
| Diagnostic variable | AUC | 95%CI | Cut-off | Sensitivity | Specificity | Youden index |
| T stage | ||||||
| CA19-9 | 0.773 | 0.684-0.863 | 23.63 | 53.23% | 100.00% | 53.23% |
| CA125 | 0.802 | 0.716-0.888 | 66.90 | 67.74% | 90.48% | 58.22% |
| DCE-MRI parameters + tumor markers | 0.836 | 0.756-0.916 | 0.93 | 72.58% | 95.24% | 67.82% |
| Differentiation degree | ||||||
| CA19-9 | 0.834 | 0.758-0.909 | 27.49 | 81.82% | 68.42% | 50.24% |
| CA125 | 0.796 | 0.711-0.881 | 66.82 | 62.12% | 89.47% | 51.60% |
| DCE-MRI parameters + tumor markers | 0.946 | 0.903-0.990 | 0.76 | 87.88% | 97.37% | 85.25% |
| Test results | Z value | P value | Difference in AUC | Standard error difference | 95%CI | |
| Lower bound | Upper bound | |||||
| T stage | ||||||
| CA199-CA125 | -1.452 | 0.146 | -0.029 | 0.293 | -0.068 | 0.01 |
| CA199 - DCE-MRI parameters + tumor markers | 7.616 | < 0.001 | 0.609 | 0.299 | 0.453 | 0.766 |
| CA125- DCE-MRI parameters + tumor markers | 7.856 | < 0.001 | 0.638 | 0.296 | 0.479 | 0.797 |
| Differentiation degree | ||||||
| CA199-CA125 | 0.617 | 0.537 | 0.038 | 0.287 | -0.082 | 0.157 |
| CA199- DCE-MRI parameters + tumor markers | 14.913 | < 0.001 | 0.780 | 0.248 | 0.677 | 0.882 |
| CA125- DCE-MRI parameters + tumor markers | 13.382 | < 0.001 | 0.742 | 0.257 | 0.633 | 0.851 |
RC is the third most prevalent digestive system malignancy worldwide and the fourth most deadly of all malignancies[13]. It shows increasing morbidity and mortality and a rising incidence at younger ages, thereby having an enormous negative impact on people's prognosis and quality of life[14]. Tumor angiogenesis, the growth basis of RC, is also related to tumor pathological differentiation. The lower the tumor differentiation degree, the higher the heterogeneity, the more common mitotic figures and pathological mitotic figures, and the higher the degree of malignancy. Due to the low accuracy of the early diagnosis of RC, most patients have reached the advanced stage at the time of diagnosis, leading to poor prognoses[15]. In addition, according to the 2011 National Comprehensive Cancer Network guidelines, all patients with stage T3 or lymph node-positive RC of any T stage should receive preoperative neoadjuvant therapy[16]. Therefore, accurate evaluation of the tumor focus of RC, including T stage and differentiation degree, can provide an objective basis for clinicians to choose treatment plans and evaluate the prognosis of patients.
DCE-MRI is an imaging modality superior to conventional modalities, which enables accurate and quantitative assessment of the T stage and differentiation of RC prior to surgery by combining morphological and hemodynamic changes[17]. One of the methods commonly used in early diagnosis involves detection of TMs, among which CA19-9 and CA125 are often used in the diagnosis of digestive system malignancies. The abnormal expression of these markers can reflect the degree of pathological changes and malignant transformation of cells, which can be used for tumor differentiation evaluation[18]. To better guide clinical work, this study involved using a joint assessment of DCE-MRI parameters and serum CA19-9 and CA125 Levels to assess the T stage and differentiation degree of RC. In the study, Ktrans and Ve values were found to be lower in patients in the M + H group than in the L group, suggesting that DCE-MRI parameters can reflect microcirculation differences in RC. DCE-MRI quantitative analysis can be used to quantify the contrast agent exchange between intercellular spaces and blood vessels in tissues, which reflects tissue perfusion and vascular endothelial integrity[19,20]. Ktrans represents the contrast dose per unit volume of tissue from blood into the extravascular extracellular space and is therefore related to the angiogenic activity and aggressiveness of the tumor; its magnitude depends on the surface area of the vascular endothelium, permeability, and the amount of blood passing through the tissue[21,22]. The Ve value represents the volume of extravascular extracellular space per unit volume of tissue and reflects the degree of histocellular cellularization and tissue necrosis of ROIs. The Ve value is positively associated with the volume of extracellular space, indicating a greater degree of tissue necrosis or a lower degree of cellularization[22]. Kep represents the contrast dose that penetrates into the blood vessels from the extravascular extracellular space per unit time, and its magnitude depends mainly on the permeability of the capillaries, reflecting the growth state of the tumor microvessels[22]. We believe that the malignant degree of RC gradually increases as the differentiation degree of the patient's tumor decreases and the T-stage increases; along with enhanced local neovascularization and blood perfusion, stimulation of vascular endothelial growth factor on blood vessels increases, as does vascular permeability, making it easier for plasma contrast agents to be extravasated, triggering an increase in Ktrans value, promoting the change of extracellular matrix and the increase of extracellular space, and leading to an increase in Ve value. Kep is related to the components in the extracellular space of tissues. Although the local microvascular per
We also observed lower serum CA19-9 and CA125 Levels in the M + H group than in the L group and also in the low-stage group compared to the high-stage group, suggesting that as the T stage of the tumor increases and the degree of differentiation decreases, the levels of these TMs increase accordingly. TMs are specific substances produced and released by tumor cells. The lower the degree of tumor differentiation, the higher the malignancy degree and the higher the proliferative activity of tumor cells, resulting in a corresponding increase in the levels of TMs. CA19-9 is a mucin-type carbohydrate protein extracted from human colon cancer cells. As a nonspecific tumor-associated antigen, it is mainly present in the fetal gastrointestinal tract and pancreatic epithelial tissue. CA19-9 is expressed at a very low level in normal humans, and its high levels may indicate a high degree of malignancy, so it is often used as an auxiliary diagnostic indicator for malignancy[25,26]. CA125 is a high molecular weight glycoprotein, a mucin-like glycoprotein complex, presenting lower concentrations in serum in healthy adults but significantly elevated levels in gastrointestinal malignancies[27]. The above results indicate that serum CA19-9 and CA125 levels can be used to judge the malignant degree of RC, and the tumor malignant degree is closely related to T stage and differentiation degree. Therefore, it is inferred that serum CA125 expression is obviously different in RCs with different T stages and differentiation degrees and increases with increasing T stage and decreasing differentiation degree.
Finally, we evaluated the diagnostic value of TMs and DCE-MRI parameters in T staging and differentiation. The TMs CA19-9 and CA125 were analyzed first. Through ROC curve analysis, it was found that the sensitivity of the two was low when they were used alone in evaluating the T stage and differentiation of RC, but when combined with DCE-MRI parameters, the sensitivity of CA19-9 and CA125 reached 72.58% and 87.88%, respectively. This shows that combined detection can significantly improve the sensitivity of TMs in evaluating the differentiation degree of RC, which is helpful for assessing RC at an early stage and guiding clinical treatment. We believe that this is because DCE-MRI parameters can help clinicians understand the differentiation degree of RC from the imaging point of view, while serum CA19-9 and CA125 reflect the differentiation degree of RC from the perspective of biochemistry. They complement each other and using them can reduce missed diagnoses and misdiagnoses.
However, there are some limitations in this study. First, as a retrospective study, there is inevitable selection bias. Second, the image acquisition and postprocessing are not standardized and involve some subjective factors, which are easily biased and cannot truly reflect the blood flow situation of the tumor parenchyma. For example, the results may vary depending on the ROIs chosen. It is hoped that future research on the quantitative parameters of DCE-MRI will achieve gratifying results so that the diagnosis and treatment of RC will enter a new era.
The DCE-MRI parameters Ktrans and Ve as well as the serum levels of the TMs CA19-9 and CA125 all increased with increasing T stage and decreasing differentiation degree of RC. These indices can be used as important markers to evaluate the differentiation degree of RC, especially when they are jointly evaluated, playing a superior role with more obvious clinical value. These indices have substantial guiding importance for clinical diagnosis and treatment selection.
Rectal carcinoma (RC) is a globally prevalent fatal tumor, and its early diagnosis and staging are crucial. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters and serum levels of carbohydrate antigen 19-9 (CA19-9) and CA125 have shown potential diagnostic value in many diseases, but their roles in the assessment of T stage and degree of differentiation in RC remain unclear.
The motivation of this study is to clarify the effectiveness of DCE-MRI parameters and serum levels of CA19-9 and CA125 in assessing the T stage and degree of differentiation in RC. We hope that through this study, we can provide clinicians with a reliable tool for more accurate diagnosis and treatment of RC.
The objective of this study was to investigate the application of DCE-MRI parameters and serum levels of CA19-9 and CA125 in evaluating the T stage and degree of differentiation of RC to improve the diagnostic accuracy and treatment effect of RC.
We conducted a retrospective study on eligible patients with RC and collected and analyzed the DCE-MRI parameters and serum levels of CA19-9 and CA125. We then evaluated their predictive ability for T stage and degree of differentiation of RC using statistical models.
Our research results show that DCE-MRI parameters and serum levels of CA19-9 and CA125 have significant predictive abilities and may improve RC-related diagnostic accuracy.
DCE-MRI parameters and serum levels of CA19-9 and CA125 have significant value in assessing the T stage and degree of differentiation of RC and can be used to help physicians provide more personalized treatment plans, improving patient prognosis.
Future research might further explore the application of these tools in a broader cohort of patients with RC as well as how they could be combined with other potential biomarkers and imaging parameters to provide a more comprehensive framework for RC assessment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine & medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mimori K, Japan S-Editor: Wang JL L-Editor: A P-Editor: Zhao S
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64721] [Article Influence: 16180.3] [Reference Citation Analysis (177)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1803] [Article Influence: 450.8] [Reference Citation Analysis (1)] |
| 3. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 4. | Wilkinson N. Management of Rectal Cancer. Surg Clin North Am. 2020;100:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 6. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 410] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 7. | Ma X, Shen F, Jia Y, Xia Y, Li Q, Lu J. MRI-based radiomics of rectal cancer: preoperative assessment of the pathological features. BMC Med Imaging. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Siregar GA, Anshari F. Absolute Neutrophil Count Levels among Degree of Differentiation and Tumor Location in Colorectal Cancer Patients in Medan. Open Access Maced J Med Sci. 2019;7:3472-3474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Siregar GA, Sibarani H. Comparison of Carcinoembryonic Antigen Levels Among Degree of Differentiation and Colorectal Cancer's Location in Medan. Open Access Maced J Med Sci. 2019;7:3447-3450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Li Z, Huang H, Wang C, Zhao Z, Ma W, Wang D, Mao H, Liu F, Yang Y, Pan W, Lu Z. DCE-MRI radiomics models predicting the expression of radioresistant-related factors of LRP-1 and survivin in locally advanced rectal cancer. Front Oncol. 2022;12:881341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Fusco R, Granata V, Sansone M, Rega D, Delrio P, Tatangelo F, Romano C, Avallone A, Pupo D, Giordano M, Grassi R, Ravo V, Pecori B, Petrillo A. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol Med. 2021;126:1044-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Liu T, Li X, Liu D, Liu S, Dong M. Increased serum CA125 II, but not CEA, CA19-9, AFP or CA72-4 in colon cancer compared to rectal cancer. Br J Biomed Sci. 2021;78:218-220. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 13. | Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol. 2022;43:101739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Massihnia D, Pizzutilo EG, Amatu A, Tosi F, Ghezzi S, Bencardino K, Di Masi P, Righetti E, Patelli G, Scaglione F, Vanzulli A, Siena S, Sartore-Bianchi A. Liquid biopsy for rectal cancer: A systematic review. Cancer Treat Rev. 2019;79:101893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin Colorectal Cancer. 2022;21:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Mazzeo E, Triggiani L, Frassinelli L, Guarneri A, Bartoncini S, Antognoni P, Gottardo S, Greco D, Borghesi S, Nanni S, Bruni A, Ingrosso G, D'Angelillo RM, Detti B, Francolini G, Magli A, Guerini AE, Arcangeli S, Spiazzi L, Ricardi U, Lohr F, Magrini SM. How Has Prostate Cancer Radiotherapy Changed in Italy between 2004 and 2011? An Analysis of the National Patterns-Of-Practice (POP) Database by the Uro-Oncology Study Group of the Italian Society of Radiotherapy and Clinical Oncology (AIRO). Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Yang X, Wen Z, Liu Y, Lu B, Yu S, Xiao X. Association between high-resolution MRI-detected extramural vascular invasion and tumour microcirculation estimated by dynamic contrast-enhanced MRI in rectal cancer: preliminary results. BMC Cancer. 2019;19:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Deng L, Guo S, Li H, You X, Song Y, Su H. CA125, CEA, CA19-9, and Heteroploid Cells in Ascites Fluid May Help Diagnose Peritoneal Carcinomatosis in Patients with Gastrointestinal and Ovarian Malignancies. Cancer Manag Res. 2020;12:10479-10489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Petrillo A, Fusco R, Petrillo M, Granata V, Bianco F, Di Marzo M, Delrio P, Tatangelo F, Botti G, Pecori B, Avallone A. DCE-MRI time-intensity curve visual inspection to assess pathological response after neoadjuvant therapy in locally advanced rectal cancer. Jpn J Radiol. 2018;36:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Bakke KM, Grøvik E, Meltzer S, Negård A, Holmedal SH, Mikalsen LTG, Lyckander LG, Ree AH, Gjesdal KI, Redalen KR, Bjørnerud A. Comparison of Intravoxel incoherent motion imaging and multiecho dynamic contrast-based MRI in rectal cancer. J Magn Reson Imaging. 2019;50:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Weber JD, Spiro JE, Scheffler M, Wolf J, Nogova L, Tittgemeyer M, Maintz D, Laue H, Persigehl T. Reproducibility of dynamic contrast enhanced MRI derived transfer coefficient Ktrans in lung cancer. PLoS One. 2022;17:e0265056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ya G, Wen F, Xing-Ru L, Zhuan-Zhuan G, Jun-Qiang L. Difference of DCE-MRI Parameters at Different Time Points and Their Predictive Value for Axillary Lymph Node Metastasis of Breast Cancer. Acad Radiol. 2022;29 Suppl 1:S79-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Sun D, Wu X, Wang L, Li G, Huang J, Li Y. Distinguishing T1-2 and T3a tumors of rectal cancer with texture analysis and functional MRI parameters. Diagn Interv Radiol. 2022;28:200-207. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Kim YE, Lim JS, Choi J, Kim D, Myoung S, Kim MJ, Kim KW. Perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging in patients with rectal cancer: correlation with microvascular density and vascular endothelial growth factor expression. Korean J Radiol. 2013;14:878-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Björkman K, Mustonen H, Kaprio T, Kekki H, Pettersson K, Haglund C, Böckelman C. CA125: A superior prognostic biomarker for colorectal cancer compared to CEA, CA19-9 or CA242. Tumour Biol. 2021;43:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Rokhgireh S, Mehdizadeh Kashi A, Chaichian S, Delbandi AA, Allahqoli L, Ahmadi-Pishkuhi M, Khodaverdi S, Alkatout I. The Diagnostic Accuracy of Combined Enolase/Cr, CA125, and CA19-9 in the Detection of Endometriosis. Biomed Res Int. 2020;2020:5208279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Lertkhachonsuk AA, Buranawongtrakoon S, Lekskul N, Rermluk N, Wee-Stekly WW, Charakorn C. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J Obstet Gynaecol Res. 2020;46:2287-2291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |