Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1787
Peer-review started: January 14, 2024
First decision: January 30, 2024
Revised: February 19, 2024
Accepted: March 26, 2024
Article in press: March 26, 2024
Published online: May 15, 2024
Processing time: 116 Days and 8.9 Hours
Individuals diagnosed with gastrointestinal tumors are at an increased risk of developing cardiovascular diseases. Among which, ventricular arrhythmia is a prevalent clinical concern. This suggests that ventricular arrhythmias may have predictive value in the prognosis of patients with gastrointestinal tumors.
To explore the prognostic value of ventricular arrhythmias in patients with gastrointestinal tumors receiving surgery.
We retrospectively analyzed data from 130 patients undergoing gastrointestinal tumor resection. These patients were evaluated by a 24-h ambulatory electrocardiogram (ECG) at the Sixth Affiliated Hospital of Sun Yat-sen University from January 2018 to June 2020. Additionally, 41 general healthy age-matched and sex-matched controls were included. Patients were categorized into survival and non-survival groups. The primary endpoint was all-cause mortality, and secondary endpoints included major adverse cardiovascular events (MACEs).
Colorectal tumors comprised 90% of cases. Preoperative ambulatory ECG monitoring revealed that among the 130 patients with gastrointestinal tumors, 100 (76.92%) exhibited varying degrees of premature ventricular contractions (PVCs). Ten patients (7.69%) manifested non-sustained ventricular tachycardia (NSVT). The patients with gastrointestinal tumors exhibited higher PVCs compared to the healthy controls on both conventional ECG [27 (21.3) vs 1 (2.5), P = 0.012] and 24-h ambulatory ECG [14 (1.0, 405) vs 1 (0, 6.5), P < 0.001]. Non-survivors had a higher PVC count than survivors [150.50 (7.25, 1690.50) vs 9 (0, 229.25), P = 0.020]. During the follow-up period, 24 patients died and 11 patients experienced MACEs. Univariate analysis linked PVC > 35/24 h to all-cause mortality, and NSVT was associated with MACE. However, neither PVC burden nor NSVT independently predicted outcomes according to multivariate analysis.
Patients with gastrointestinal tumors exhibited elevated PVCs. PVCs > 35/24 h and NSVT detected by 24-h ambulatory ECG were prognostically significant but were not found to be independent predictors.
Core Tip: We retrospectively analyzed data from 130 patients undergoing gastrointestinal tumor resection who were evaluated by a 24-h ambulatory electrocardiogram to determine the prognostic value of ventricular arrhythmias in these types of patients. Additionally, 41 age-matched and sex-matched general healthy controls were evaluated. In a long-term follow-up, we found that patients with gastrointestinal tumors exhibited elevated premature ventricular contractions. Premature ventricular contractions > 35/24 h and non-sustained ventricular tachycardia were prognostically significant but were not found to be independent predictors.
- Citation: Xue JJ, Hu ST, Wang CC, Chen ZC, Cheng SY, Yu SQ, Peng HJ, Zhang YT, Zeng WJ. Prognostic relevance of ventricular arrhythmias in surgical patients with gastrointestinal tumors. World J Gastrointest Oncol 2024; 16(5): 1787-1795
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1787.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1787
Gastrointestinal tumors are a major global health concern[1], posing a significant threat to human life. Currently, surgery is the preferred treatment option for complete tumor removal[2]. However, patients undergoing surgical removal of gastrointestinal tumors often face critical and at times fatal challenges due to the occurrence of cardiovascular events[3,4]. An underlying link between cardiovascular disease and gastrointestinal tumors has been proposed. Cardiovascular risk factors are associated with an increased risk of colorectal cancer[5,6], and patients with colorectal cancer face a higher risk of cardiovascular death, especially within the 1st year after diagnosis[7]. Moreover, various anticancer treatments, including anthracyclines, trastuzumab, and cyclophosphamide, can induce cardiotoxicity in up to half of cancer patients. Even in cancer patients not subjected to chemotherapy, there is a risk of cardiovascular function deterioration[8]. Therefore, it is essential to identify patients with gastrointestinal tumor at risk of cardiovascular issues before, during, and after treatment to implement preventive measures effectively.
Ventricular arrhythmia, a common disorder with an estimated prevalence ranging from 1%-4% by routine electrocardiogram (ECG), is a significant clinical concern[9]. Ambulatory ECG reveals an even higher prevalence, with as many as 40%-75% of patients exhibiting premature ventricular contractions (PVCs)[10,11]. However, the prognostic significance of PVCs remains unclear. While PVCs are known to increase the risk of mortality and cardiovascular events in patients with structural heart disease or apparent heart disease[12-17], one study suggested that there was no direct correlation between higher PVC counts and increased mortality, particularly in patients with structural heart disease[18]. The question of whether ventricular arrhythmias have prognostic value for patients with gastrointestinal tumors has drawn clinical attention. Studies have shown that non-sustained ventricular tachycardia (NSVT) ≥ 4 heartbeats and PVCs ≥ 20/24 h measured by 24-h ambulatory ECG have prognostic value for cancer patients. NSVT and PVC burdens are crucial predictors of mortality, independent of other prognostic factors[19].
Unfortunately, these studies are limited by small sample sizes of patients with colorectal cancer and the exclusion of important indicators like cardiac troponin I (cTnI), which is an independent risk factor for patients with gastrointestinal tumors[20]. Additionally, UCG parameters were not included in the multivariate analyses of these studies, and there was a lack of major adverse cardiovascular events (MACEs) analysis. Moreover, the heterogeneous etiology might lead to inconsistent results. For example, cTnI demonstrated significant predictive value in patients with colorectal cancer patients, but its effectiveness was limited to patients undergoing orthopedic surgery or patients with head and neck squamous cell carcinoma[21,22]. Therefore, further research must evaluate the prognostic impact of ventricular arrhythmia in patients with specific etiologies, such as gastrointestinal tumors. The present study explored the prognostic value of ventricular arrhythmias in patients with gastrointestinal tumors through 24-h ambulatory ECG monitoring.
The study was a single-center retrospective cohort study. We included patients with gastrointestinal tumors scheduled for surgery between January 2018 and June 2020. The inclusion criteria were major abdominal surgery under general anesthesia, an age of 18 years or older, high-sensitivity cTnI (hs-cTnI) testing at least 7 d prior to surgery, and preoperative ambulatory ECG examination. Exclusion criteria included emergent surgery, failure to perform surgery, or clinical evidence of unstable coronary artery disease (cardiac chest pain with or without ischemic ECG changes) according to the medical records at the preoperative evaluation. Patients with left ventricular ejection fractions < 45% were also excluded.
In total, 130 patients were included in our study to further analyze the prognostic value of ventricular arrhythmias in patients undergoing gastrointestinal tumor resection. Additionally, we included 41 age-matched and sex-matched general healthy controls from the same period. The control group was free of significant cardiovascular events and cancer, except for well-controlled hypertension. The study was approved by the local ethics committee of the Sixth Affiliated Hospital of Sun Yat-sen University. The study methods were compliant with the STROBE checklist.
The primary endpoint of the study was all-cause mortality during the follow-up period. Secondary combined endpoints included MACEs, such as myocardial infarction, congestive heart failure, sudden cardiac death, ischemic stroke, and other related outcomes.
The diagnosis of myocardial infarction was made according to the universal definition of myocardial infarction[23]. A history of coronary artery disease was defined as prior bypass surgery, coronary intervention, myocardial infarction, or compliance with the guideline definition[24]. The Lee index (revised cardiac index) was calculated as described previously[25]. Briefly, one point was assigned to each of the following factors: Coronary artery disease history; a history of cerebrovascular disease, heart failure, insulin-dependent diabetes mellitus, impaired renal function; and a high-risk surgery[25]. Staging was performed according to the tumor, node, and metastasis (TNM) staging system developed by the 8th edition of the American Joint Committee on Cancer Staging Manual. In our study, TNM stage III or higher was defined as advanced disease. PVC referred to a ventricular beat produced in advance by ectopic pacemakers below the atrioventricular node. NSVT was defined as runs of beats arising from the ventricles with a duration between 3 beats and 30 s and with a cycle length of < 600 ms (> 100 beats per minute).
Clinical, laboratory, ECG parameters, medication, and surgery were collected from medical records during the initial assessment. Follow-up data were obtained through telephone interviews with patients and a review of their records. Hs-cTnI was measured by a high-sensitivity electrochemiluminescence immunoassay on an automatic analyzer (Architect i1000SR; Abbott Core Laboratory Systems, Lake Forest, IL, United States). UCG images were obtained following the guidelines outlined by the American Society of Echocardiography[26] and were stored using a digital ultrasound system (Vivid E9; GE HealthCare, Chicago, IL, United States). The ambulatory ECG recording was obtained using a portable Holter machine and conducted for 24 h by professional technicians.
Continuous variables were compared using the independent t-test or nonparametric test (Mann-Whitney U) and expressed as mean ± SD or median (25%, 75%) as appropriate. Categorical variables were compared using the χ2 test or Fisher’s exact test where appropriate and reported as frequencies (percentage). The optimal cutoff value for the PVCs was determined using the receiver operating characteristic curve. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Postoperative long-term survival was calculated from the date of discharge after the initial tumor resection until the time of death or MACE. Patients who did not reach the endpoints during the follow-up period were censored on November 1, 2021 or the date of loss to follow-up.
To identify independent predictors of all-cause death and MACE in the population, Cox multivariate regression analysis was conducted. The multivariate model was constructed using forward stepwise selection, with candidate variables included if they met the entry criterion of P < 0.05 in the univariate analysis. Hazard ratios and 95% confidence intervals were estimated using Cox regression models. Statistical analyses were performed using IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY, United States), and significance was set at P < 0.05. The study was reviewed by our expert biostatistician, Li-Shuo Shi, MD.
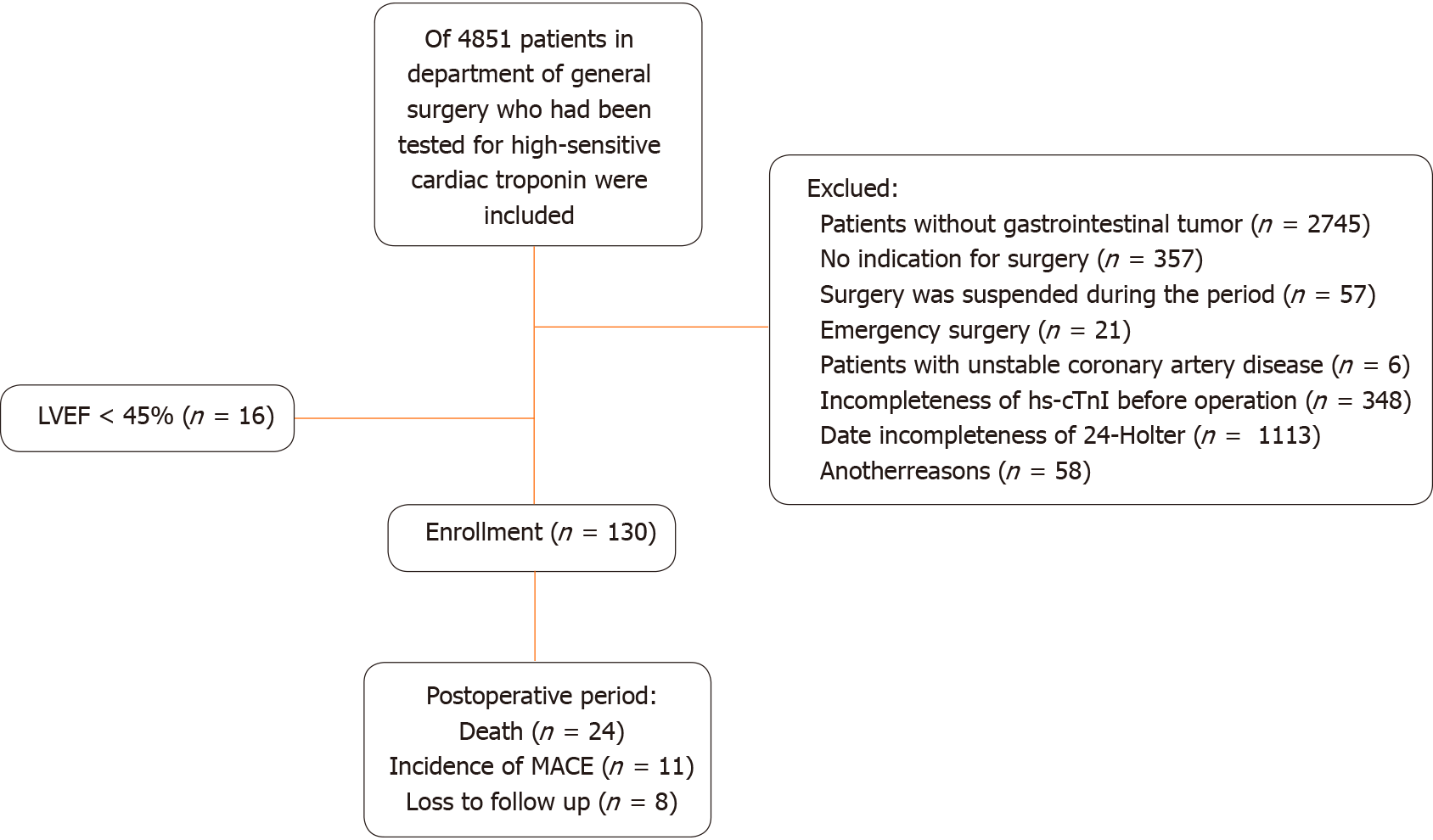
Our study included 130 patients with gastrointestinal tumors and 41 controls. The study flow diagram is shown in Figure 1. The baseline characteristics of all patients are shown in Supplementary Table 1. Among these patients, 117 primary tumors (90.0%) were in the colorectal region, 12 tumors (9.2%) in the stomach, and 1 tumor (0.8%) in the small intestine. Advanced TNM stages were present in 57 patients (43.8%). Preoperative ambulatory ECG monitoring revealed that 100 patients (76.9%) exhibited varying degrees of PVCs, while 10 patients (7.7%) exhibited NSVT. Patients with gastrointestinal tumors exhibited higher PVCs compared to the control group in both conventional and 24-h ambulatory ECG recordings. The incidence of NSVT did not significantly differ between patients with gastrointestinal tumors and the controls.
A comparison between survivors and non-survivors indicated that non-survivors were characterized by advanced age and elevated levels of hs-cTnI. Additionally, non-survivors demonstrated a thicker interventricular septum and left ventricular posterior wall. Non-survivors exhibited a Lee index > 2, a higher tumor burden, and higher PVC counts compared to survivors [150.50 (7.25, 1690.50) vs 9.0 (0, 229.25), respectively, P = 0.020]. Furthermore, non-survivors had lower diastolic blood pressure, reduced hemoglobin levels, and lower levels of low-density lipoprotein cholesterol. There were no significant differences in comorbidities and medication history between the survivor and non-survivor groups.
Over a median follow-up period of 36 mo, 24 patients died, with 7 deaths attributed to cardiovascular events, 13 to tumor-related causes, and 4 to other causes such as infections. Additionally, 11 patients with gastrointestinal tumors experienced MACE, including 2 cases of myocardial infarction, 2 cases of heart failure, 4 cases of sudden cardiac death, 1 case of ischemic stroke, and 2 cases due to other reasons.
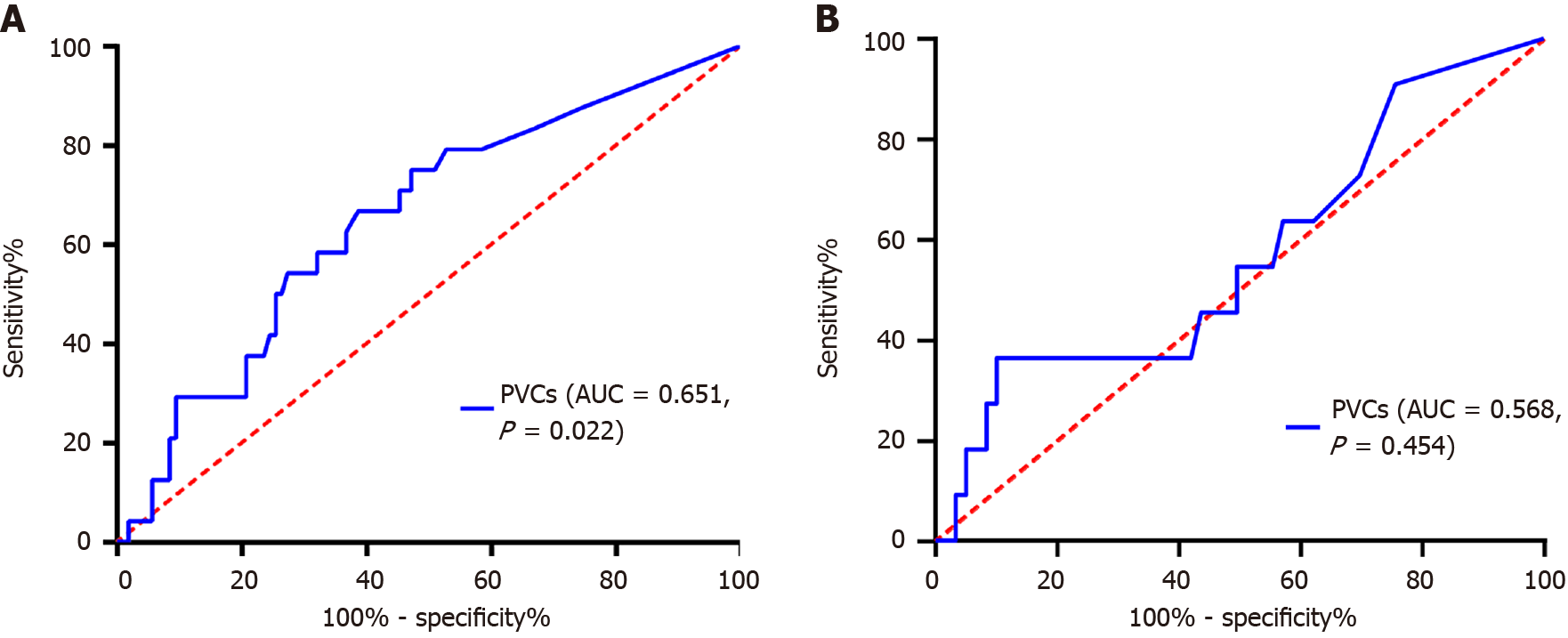
Receiver operating characteristic analysis identified a PVC count of 35 as the optimal cutoff value for predicting all-cause mortality. The sensitivity and specificity of a PVC count of 35/24 h were 66.7% and 61.3%, respectively. The area under the curve (c-statistics) for the PVC count was 0.651 (95% confidence interval: 0.530-0.771, P = 0.022) (Figure 2A). However, when predicting MACE, there was no significant difference in PVC counts (P = 0.454) (Figure 2B).
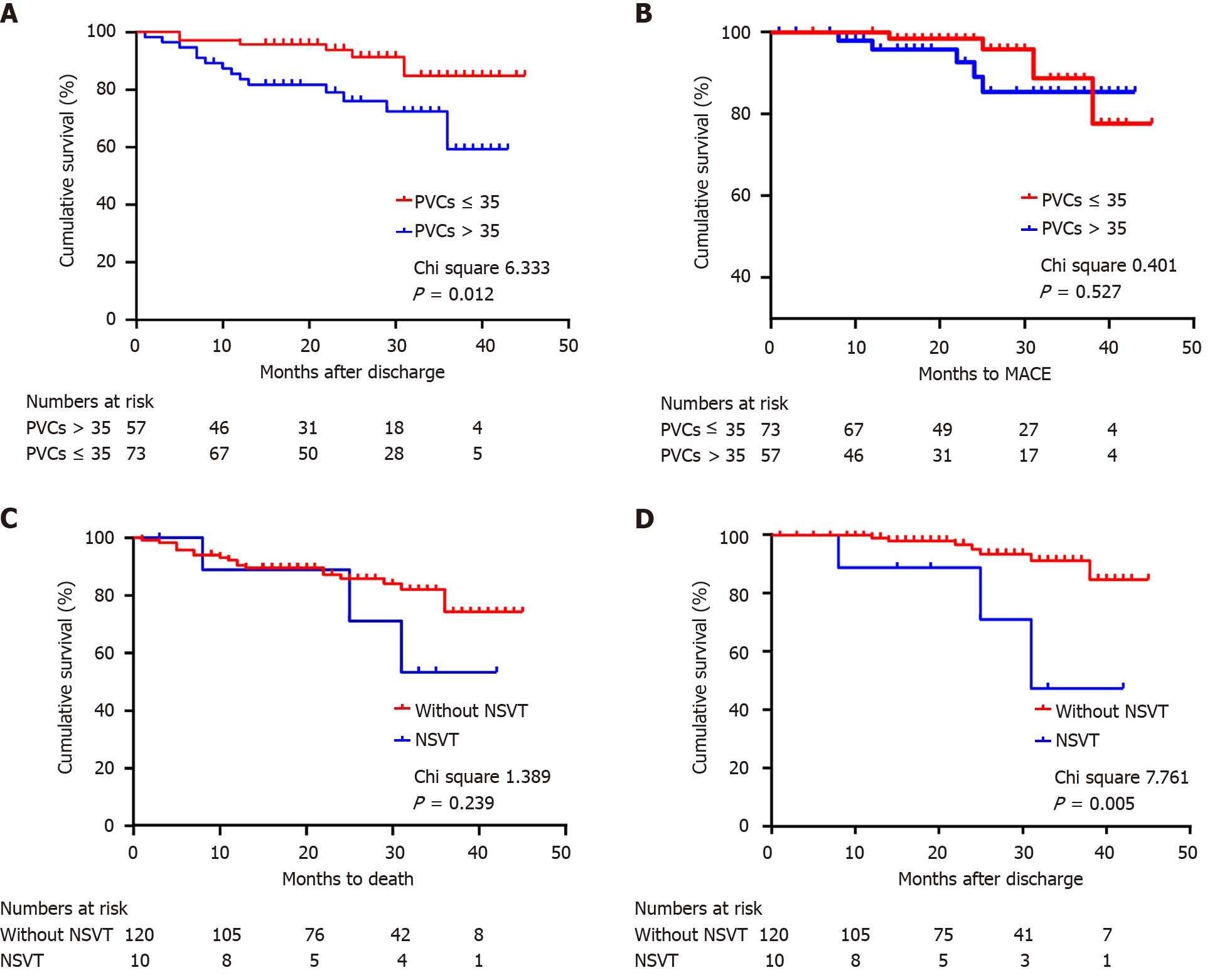
Patients with PVC counts > 35/24 h had a significantly higher mortality rate (P = 0.012) (Figure 3A). However, no significant differences were detected in the occurrence of MACE between patients with PVC counts ≤ 35/24 h and those with higher PVC burdens (P = 0.527) (Figure 3B).
Notably, the presence of NSVT was associated with an increased risk of MACE compared to patients without NSVT during follow-up (P = 0.005) (Figure 3C). However, no significant differences were detected in the occurrence of all-cause death between these two groups (P = 0.239) (Figure 3D).
Univariate analysis identified significant associations between mortality and various factors, including age, diastolic blood pressure, TnI levels, hemoglobin count, carcinoembryonic antigen levels, carbohydrate antigen 125 levels, low-density lipoprotein cholesterol levels, more advanced tumor stage, history of radical operation, Lee score ≥ 2, increased left ventricular posterior wall thickness, and PVC > 35/24 h (Table 1). Similarly, age, creatinine levels, TnI levels, and the incidence of NSVT were significantly associated with the occurrence of MACE (Table 2). However, in the multivariate Cox regression analyses, neither high PVC burdens nor the presence of NSVT emerged as independent predictors for all-cause death or the occurrence of MACE.
| Risk factors | Univariable Cox regression | Multivariable Cox regression | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age as per 1 yr increase | 1.088 (1.039-1.140) | < 0.001 | 1.067 (1.015-1.121) | 0.011 |
| Diastolic blood pressure as per 1 mmHg increase | 0.954 (0.917-0.993) | 0.020 | 0.935 (0.887-0.985) | 0.011 |
| Hemoglobin as per 1 g/L increase | 0.980 (0.965-0.995) | 0.011 | ||
| Troponin I > 0.028 as per 1 μg/L increase | 6.377 (2.841-14.311) | 0.001 | 6.576 (2.586-16.727) | < 0.001 |
| Carcinoembryonic antigen as per 1 ng/mL increase | 1.009 (1.005-1.013) | < 0.001 | 1.009 (1.004-1.015) | < 0.001 |
| Carbohydrate antigen 125 as per 1 ng/mL increase | 1.015 (1.004-1.026) | 0.008 | ||
| LVPW as per 1 mm increase | 1.375 (1.112-1.701) | 0.003 | ||
| LDL-C as per 1 mmol/L increase | 0.570 (0.328-0.989) | 0.046 | ||
| Tumor stage, TNM 3-4 vs 1-2 | 3.211 (1.329-7.762) | 0.010 | ||
| Radical operation history as yes vs no | 0.295 (0.100-0.868) | 0.027 | ||
| Lee score ≥ 2 as yes vs no | 6.201 (1.424-27.003) | 0.015 | ||
| Premature ventricular contractions > 35 as yes vs no | 2.764 (1.181-6.468) | 0.019 | ||
| NSVT as yes vs no | 1.836 (0.547-6.163) | 0.326 | ||
| Risk factors | Univariable Cox regression | Multivariable Cox regression | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age as per 1 yr increase | 1.161 (1.066-1.264) | 0.001 | 1.150 (1.058-1.251) | 0.001 |
| Creatinine as per 1 μmol/L increase | 1.021 (1.006-1.036) | 0.006 | ||
| Troponin I > 0.028 as yes vs no | 4.981 (1.445-17.167) | 0.011 | 4.208 (1.098-16.127) | 0.036 |
| Premature ventricular contractions > 35 as yes vs no | 1.222 (0.372-4.010) | 0.741 | ||
| NSVT as yes vs no | 4.775 (1.266-18.013) | 0.021 | ||
Our study found that patients with gastrointestinal tumors have a higher burden of PVCs than healthy controls. Patients exhibiting PVCs exceeding 35 per 24 h by 24-h ambulatory ECG were at a higher risk of all-cause mortality. Additionally, patients with the presence of NSVT had an increased risk of MACE. Nevertheless, neither high PVC burdens nor the presence of NSVT emerged as independent predictors for all-cause death or the occurrence of MACE.
Our findings underscored that the incidence of PVCs in patients with gastrointestinal tumors surpassed that in the healthy population. These results were consistent with the findings reported by Anker et al[19]. The underlying mechanisms contributing to arrhythmias in patients with tumors are intricate and diverse, involving factors such as electrolyte imbalances, inflammatory reactions, autonomic nervous system dysregulation, cachexia, and the cardiotoxic effects of anticancer treatments[27-33]. Our findings highlight the significance and rationale for dynamic monitoring in patients with gastrointestinal tumors.
Notably, our study observed that patients with gastrointestinal tumors with PVC > 35/24 h demonstrated a higher risk for all-cause mortality. A prospective study conducted by Anker et al[19], involving patients with non-small lung, colon, and pancreatic cancers, demonstrated a significant rise in overall mortality among individuals experiencing PVCs at a frequency of ≥ 50 per day. Likewise, Albrecht et al[34] observed that PVCs ≥ 20 per day were indicative of a poor prognosis in cancer patients. Our findings align with these prior studies. However, it is crucial to note the variation in PVC cutoff values across these studies. When interpreting PVC burden, daily fluctuations are a vital consideration. Mullis et al[35] discovered that in a 14-d study involving 59 patients the median of absolute changes in PVC/24 h was 9.9% (interquartile range: 5.4%-14.5%). Remarkably, 72.9% of patients experienced shifts across at least two categories of PVC burdens based on the 24-h period under consideration.
Our study demonstrated that PVC burden was not an independent risk factor for all-cause mortality. However, a meta-analysis conducted by Ataklte et al[36] demonstrated a significant association between frequent PVCs in apparently healthy individuals and an elevated risk of all-cause death and cardiac-related mortality. Our study results deviate from those observed in previous studies focused on populations with cancer[19,34]. The inconsistency may be attributed to etiological heterogeneity. Anker et al[19] highlighted the differential prognostic impact of PVC burdens, emphasizing its greater significance in colorectal and pancreatic cancer patients compared to those with non-small cell lung cancer. Consequently, these results cannot be directly extrapolated to patients with gastrointestinal tumors.
Our study exclusively focused on patients with gastrointestinal tumors, avoiding the potential confounding effects introduced by disease heterogeneity present in previous studies. Additionally, our study had the advantage of including more measures that could affect prognosis compared with previous studies. We confirmed that hs-cTnI is an independent prognostic factor in colorectal cancer[37]. Previous research indicates that patients with colorectal tumors may experience impaired cardiac function[8], and elevated cTnI levels are closely associated with impaired cardiac function[38]. Our results suggest that in the prognostic assessment of patients with gastrointestinal tumors cTnI has more advantages compared to PVC measurements.
Interestingly, we found that NSVT rather than a PVC burden was associated with an increased risk of MACE in patients with gastrointestinal tumors. This aligns with prior research indicating that the presence of NSVT is associated with an increased likelihood of cardiovascular events, encompassing outcomes like cardiovascular mortality, acute myocardial infarction, coronary revascularization, or stroke, particularly in individuals lacking apparent or manifest structural heart disease[39]. Our research expands on this foundation, underscoring the significance of vigilant NSVT monitoring specifically in patients with tumors.
Nevertheless, it is crucial to acknowledge the limitations of our study. This research is a single-center retrospective study. Despite having the largest sample size for evaluating the prognostic significance of ventricular arrhythmias in patients with gastrointestinal tumors, a prospective, multicenter study is essential to gain more comprehensive insights. This approach will provide a broader perspective and enhance the robustness of our findings.
Our study found that patients with gastrointestinal tumors had a higher burden of PVC than healthy controls. The presence of PVC > 35/24 h and NSVT observed hold prognostic significance. However, they are not independent predictors, indicating the complexity of factors influencing postoperative outcomes in this patient population.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin TT, Türkiye S-Editor: Wang JJ L-Editor: A P-Editor: Li X
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1355] [Article Influence: 338.8] [Reference Citation Analysis (5)] |
| 2. | Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, Weiss HJ, Zareba W, Brown MW, Liang CS, Lichstein E, Little WC, Gillespie JA, Van Voorhees L, Krone RJ, Bodenheimer MM, Hochman J, Dwyer EM Jr, Arora R, Marcus FI, Watelet LF, Case RB. Thrombogenic factors and recurrent coronary events. Circulation. 1999;99:2517-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Sanaiha Y, Juo YY, Aguayo E, Seo YJ, Dobaria V, Ziaeian B, Benharash P. Incidence and trends of cardiac complications in major abdominal surgery. Surgery. 2018;164:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Devereaux PJ, Sessler DI. Cardiac Complications in Patients Undergoing Major Noncardiac Surgery. N Engl J Med. 2015;373:2258-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | Zhang C, Cheng Y, Luo D, Wang J, Liu J, Luo Y, Zhou W, Zhuo Z, Guo K, Zeng R, Yang J, Sha W, Chen H. Association between cardiovascular risk factors and colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine. 2021;34:100794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Niederseer D, Stadlmayr A, Huber-Schönauer U, Plöderl M, Schmied C, Lederer D, Patsch W, Aigner E, Datz C. Cardiovascular Risk and Known Coronary Artery Disease Are Associated With Colorectal Adenoma and Advanced Neoplasia. J Am Coll Cardiol. 2017;69:2348-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Gaitanidis A, Spathakis M, Tsalikidis C, Alevizakos M, Tsaroucha A, Pitiakoudis M. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population-based study. Int J Clin Oncol. 2019;24:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol. 2014;64:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Raftery EB, Cashman PM. Long-term recording of the electrocardiogram in a normal population. Postgrad Med J. 1976;52 Suppl 7:32-38. [PubMed] |
| 10. | Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 377] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Bikkina M, Larson MG, Levy D. Asymptomatic ventricular arrhythmias and mortality risk in subjects with left ventricular hypertrophy. J Am Coll Cardiol. 1993;22:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Shapiro S. Ventricular premature beats and mortality after myocardial infarction. N Engl J Med. 1977;297:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 460] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol. 2015;66:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Lee V, Hemingway H, Harb R, Crake T, Lambiase P. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: a meta-analysis and systematic review. Heart. 2012;98:1290-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Lin CY, Chang SL, Lin YJ, Chen YY, Lo LW, Hu YF, Tuan TC, Chao TF, Chung FP, Liao JN, Chang YT, Lin CH, Walia R, Te ALD, Yamada S, Chiou CW, Tsao HM, Chen SA. An observational study on the effect of premature ventricular complex burden on long-term outcome. Medicine (Baltimore). 2017;96:e5476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am J Cardiol. 2006;97:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Parreira L, Marinheiro R, Amador P, Mesquita D, Farinha J, Lopes A, Fonseca M, Chambel D, Venancio J, Lopes C, Caria R. Frequent premature ventricular contractions. Association of burden and complexity with prognosis according to the presence of structural heart disease. Ann Noninvasive Electrocardiol. 2021;26:e12800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Anker MS, von Haehling S, Coats AJS, Riess H, Eucker J, Porthun J, Butler J, Karakas M, Haverkamp W, Landmesser U, Anker SD. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur J Heart Fail. 2021;23:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Huang Z, Hu S, Si J, Cheng S, Chen Z, Xue J, Lou X, Peng H, Li Z, Ouyang M, Gao X, Zeng W. The association of preoperative high-sensitivity cardiac troponin i and long-term outcomes in colorectal cancer patients received tumor resection surgery. Cardiooncology. 2023;9:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Chong CP, van Gaal WJ, Ryan JE, Burrell LM, Savige J, Lim WK. Troponin I and NT-proBNP (N-terminal pro-brain natriuretic peptide) do not predict 6-month mortality in frail older patients undergoing orthopedic surgery. J Am Med Dir Assoc. 2010;11:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | de Oliveira KG, Thebit MM, Andrade TU, Campos FV, Podestá JRV, Zeidler SLVV, Bissoli NS, Gouvea SA. Prognostic value of pretreatment cardiovascular biomarkers in head and neck squamous cell carcinoma. Oral Dis. 2021;27:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 2495] [Article Influence: 356.4] [Reference Citation Analysis (1)] |
| 24. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 4508] [Article Influence: 901.6] [Reference Citation Analysis (0)] |
| 25. | Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2439] [Cited by in RCA: 2146] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 26. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9342] [Article Influence: 934.2] [Reference Citation Analysis (0)] |
| 27. | Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Romitan DM, Rădulescu D, Berindan-Neagoe I, Stoicescu L, Grosu A, Rădulescu L, Gulei D, Ciuleanu TE. Cardiomyopathies and Arrhythmias Induced by Cancer Therapies. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Nevruz O, Yokusoglu M, Uzun M, Demirkol S, Avcu F, Baysan O, Koz C, Cetin T, Sag C, Ural AU, Isik E. Cardiac autonomic functions are altered in patients with acute leukemia, assessed by heart rate variability. Tohoku J Exp Med. 2007;211:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Coumbe BGT, Groarke JD. Cardiovascular Autonomic Dysfunction in Patients with Cancer. Curr Cardiol Rep. 2018;20:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Noor B, Akhavan S, Leuchter M, Yang EH, Ajijola OA. Quantitative assessment of cardiovascular autonomic impairment in cancer survivors: a single center case series. Cardiooncology. 2020;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1409] [Cited by in RCA: 1498] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 33. | Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Albrecht A, Porthun J, Eucker J, Coats AJS, von Haehling S, Pezzutto A, Karakas M, Riess H, Keller U, Landmesser U, Haverkamp W, Anker SD, Anker MS. Spontaneous Non-Sustained Ventricular Tachycardia and Premature Ventricular Contractions and Their Prognostic Relevance in Patients with Cancer in Routine Care. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Mullis AH, Ayoub K, Shah J, Butt M, Suffredini J, Czarapata M, Delisle B, Ogunbayo GO, Darrat Y, Elayi CS. Fluctuations in premature ventricular contraction burden can affect medical assessment and management. Heart Rhythm. 2019;16:1570-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol. 2013;112:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Xue J, Zhou L, Si J, Cheng S, Cheng K, Yu S, Ouyang M, Chen Z, Chen D, Zeng W. The predictive value of high-sensitive troponin I for perioperative risk in patients undergoing gastrointestinal tumor surgery. EClinicalMedicine. 2021;40:101128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 38. | Yu S, Cheng S, Si J, Peng H, Wan J, Xue J, Chen Z, Hu S, Zhou L, Zhang Y, Zeng W. Risk factors of preoperative myocardial injury in patients with gastrointestinal tumors. BMC Cardiovasc Disord. 2023;23:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 39. | Sadjadieh G, Sajadieh A. Prognosis After Finding Incidental Ventricular Tachycardia on Ambulatory Electrocardiogram-recording. Am J Cardiol. 2021;150:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |