Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1773
Peer-review started: January 7, 2024
First decision: January 30, 2024
Revised: February 4, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: May 15, 2024
Processing time: 123 Days and 1.9 Hours
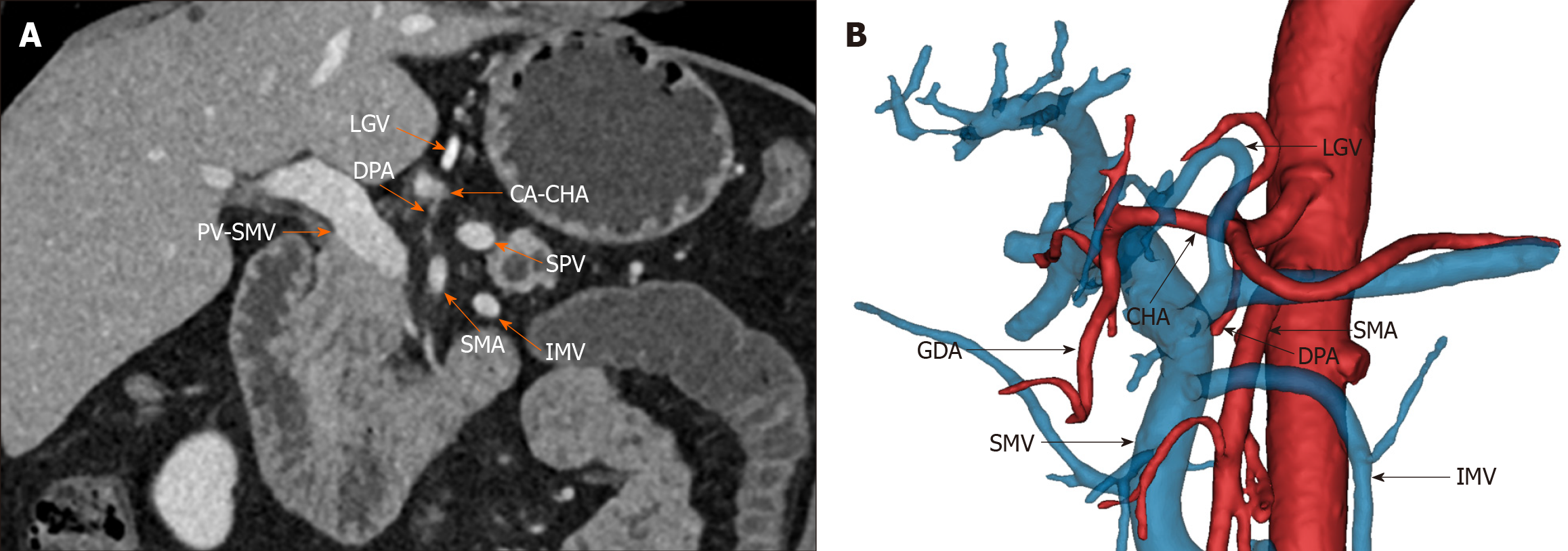
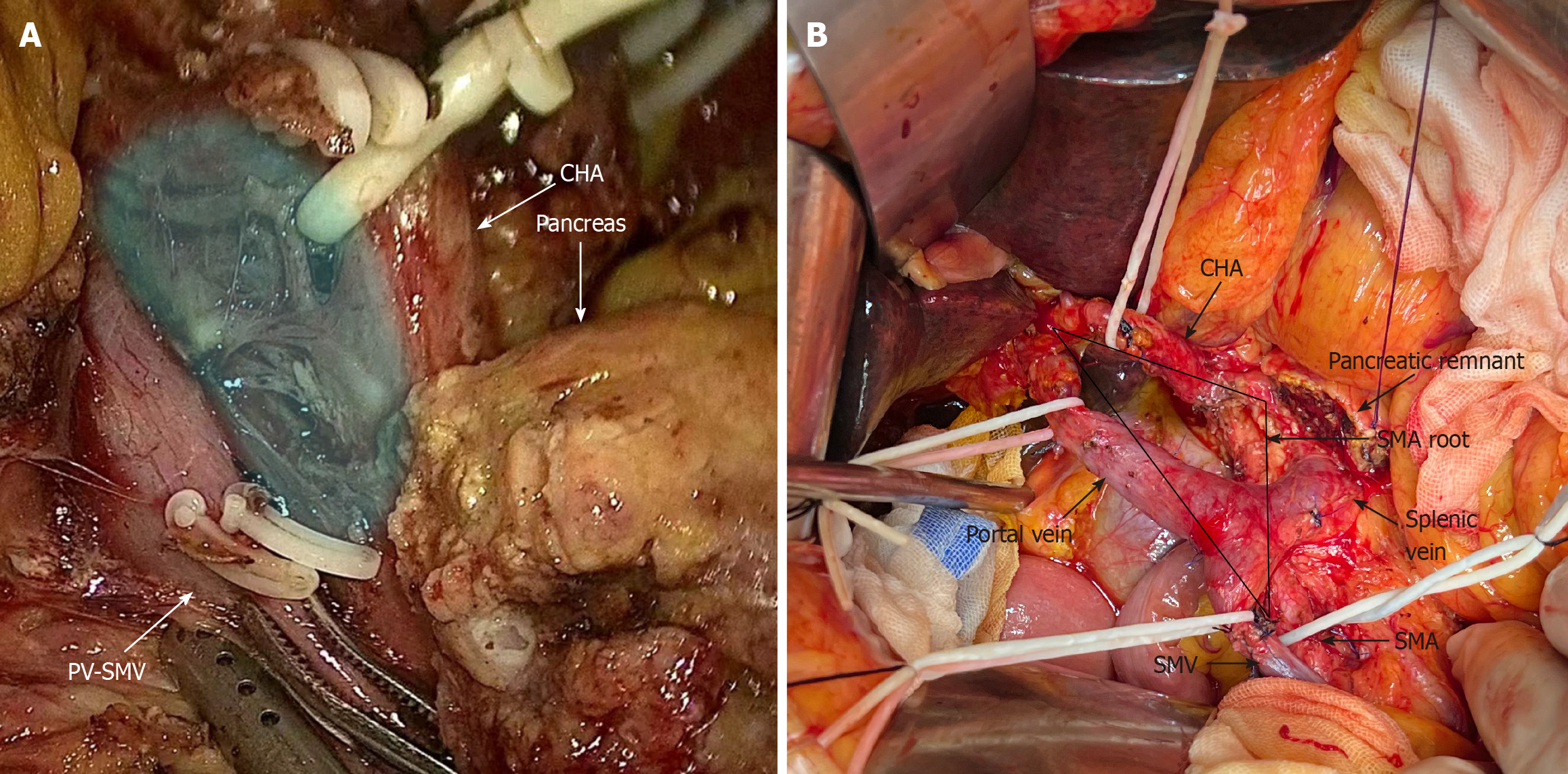
The TRIANGLE operation involves the removal of all tissues within the triangle bounded by the portal vein-superior mesenteric vein, celiac axis-common hepatic artery, and superior mesenteric artery to improve patient prognosis. Although previously promising in patients with locally advanced pancreatic ductal adeno
To evaluate the safety of the TRIANGLE operation during PD and the prognosis in patients with resectable PDAC.
This retrospective cohort study included patients who underwent PD for pan
The PDTRIANGLE and PDnon-TRIANGLE groups included 52 and 55 patients, respectively. There were no significant diffe
The TRIANGLE operation is safe for PDAC patients undergoing PD. Moreover, it reduces the local recurrence rate of PDAC and may improve survival in patients who receive adequate adjuvant chemotherapy.
Core Tip: Although the TRIANGLE operation has shown efficacy in treating locally advanced pancreatic ductal adenocarcinoma (PDAC) patients, long-term oncological data from patients with resectable PDAC who underwent pancreaticoduodenectomy (PD) are limited. This study demonstrated the safety and efficacy of the TRIANGLE operation in reducing local recurrence of resectable PDAC and suggested survival benefits for patients receiving adequate adjuvant chemotherapy.
- Citation: Chen JH, Zhu LY, Cai ZW, Hu X, Ahmed AA, Ge JQ, Tang XY, Li CJ, Pu YL, Jiang CY. TRIANGLE operation, combined with adequate adjuvant chemotherapy, can improve the prognosis of pancreatic head cancer: A retrospective study. World J Gastrointest Oncol 2024; 16(5): 1773-1786
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1773.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1773
Pancreatic ductal adenocarcinoma (PDAC) is one of the most common fatal malignancies and has a low 5-year survival rate of 11%[1]. While approximately 20% of pancreatic cancer patients are eligible for curative resection, local recurrence and metastasis are common, particularly in patients with ductal adenocarcinoma of the pancreatic head[2]. In addition to the TNM stage, the status of the surgical margin is closely related to overall survival (OS) and is a prognostic factor[3]. However, the rate of positive surgical margins remains high, with the retroperitoneal margin being the most commonly affected site. Local recurrence typically occurs after pancreaticoduodenectomy (PD)[4-7]. Therefore, it is crucial to meticulously dissect this area to improve patient prognosis.
Gockel et al[8] first proposed the concept of the mesopancreas based on the mesorectum, emphasizing that resection of the mesopancreas could achieve a negative resection margin of the retroperitoneum. Adham and Singhirunnusorn provided further knowledge about the mesopancreas by proposing anatomical boundaries such as the celiac axis (CA)/common hepatic artery (CHA), superior mesenteric artery (SMA) and portal vein (PV)/superior mesenteric vein (SMV)[9]. These authors also introduced total mesopancreas excision (TMpE), which improves posterior clearance and achieves R0 resection. Similarly, Hackert et al[10] proposed the TRIANGLE operation and applied it to patients with locally advanced pancreatic cancer. This procedure is performed on patients who are in stable condition after induction therapy and who have no viable tumor cells in the tissue surrounding the artery, as confirmed by frozen sectioning. The TRIANGLE operation aims to reduce the percentage of patients with a positive resection margin. It involves dissection between the SMA, SMV/PV, and CA/CHA, as well as the location of pancreatic head nerve plexus I (PLphI) and panc
While initial findings from a pilot study involving 15 patients with locally advanced PDAC suggest acceptable morbidity associated with the TRIANGLE procedure[10], comprehensive data from a larger cohort need to be established. Moreover, while the safety of the TRIANGLE operation has been the subject of several studies[13,14], research focusing on the impact of combining this procedure with PD on survival outcomes in patients with resectable pancreatic head cancer is still limited.
The aim of the present study was to assess the safety of the TRIANGLE procedure and its impact on the survival of patients with resectable ductal adenocarcinoma of the pancreatic head in a single-center cohort.
This retrospective cohort study received approval from the institutional review board of Huadong Hospital affiliated with Fudan University, and the protocol was approved and registered on ClinicalTrials.gov (ID: NCT05703581) before statistical analysis (January 30, 2023). This study was also approved by the Ethics Committee of Huadong Hospital Affiliated to Fudan University (No. 20170014), and all patients provided informed consent before the operation. This study adhered to the STROBE guidelines.
This study included adult patients diagnosed with pancreatic head cancer who underwent PD between January 2017 and April 2023 at the Department of General Surgery, Huadong Hospital affiliated with Fudan University. All patients had complete clinicopathological and prognostic data and were followed up for a minimum of 6 months. The exclusion criteria were as follows: (1) Died due to non-neoplastic causes; (2) Incomplete follow-up data; and (3) Received chemo
The adjuvant chemotherapy regimens included gemcitabine-based chemotherapy and 5-fluorouracil-based chemo
Our institution successfully performed PD using a standard approach. Following the removal of the pancreatic head, we employed a modified version of Child’s method to accomplish intestinal reconstruction. The reconstruction procedure involved performing a pancreatojejunostomy based on the Blumgart method, utilizing a duct-to-mucosa, end-to-side technique, and placing a pancreatic drainage tube. A continuous suture technique was used for biliary-enteric anastomosis. Gastrojejunostomy was achieved using interrupted 3-0 polypropylene monofilament sutures.
The PDnon-TRIANGLE group underwent standard lymphadenectomy during the PD operation according to the ISGPS consensus[15], which included the removal of suprapyloric and infrapyloric lymph nodes (No. 5, 6), the anterior superior region of the CHA (No. 8a), the anterior and posterior pancreatoduodenal lymph nodes (No. 13a, 13b, 17a, 17b), the right side of the hepatoduodenal ligament (12b1, 12b2, 12c), and the right side of the SMA from its origin to the inferior pancreaticoduodenal artery (14a, 14b). However, Heidelberg triangle dissection was not performed in this group.
The PDTRIANGLE group, on the other hand, underwent standard lymphadenectomy along with Heidelberg triangle dis
The following baseline data were obtained from the hospital laboratory information system: age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, main pancreatic duct size, carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), albumin, and serum bilirubin. The following pathological data were obtained from the pathology database: tumor grade, PNI, perivascular invasion (PVI), number of examined lymph nodes, and margin status. R0 resection was confirmed microscopically when tumor cells were absent from the margin within a 1 mm distance, and R1 resection was identified when tumor cells were present within the 1 mm boundary. The TNM stage was determined according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system[17]. Postoperative complications, such as postoperative pancreatic fistula (POPF)[18], bile leakage[19], chyle leak[20], delayed gastric emptying (DGE)[21], and postpancreatectomy hemorrhage (PPH)[22], were classified according to the ISGPS definitions. Clinically relevant complications (grades B and C according to ISGPS) were also recorded. Diarrhea was defined as having more than three bowel movements per day and receiving regular use of antidiarrheal medication during the second postoperative week[23]. The occurrence of diarrhea during both the postoperative in-hospital period and the subsequent follow-up period was recorded. Recurrence-free survival (RFS) was calculated from the date of treatment to the first radiographic evidence of recurrence, and postoperative imaging was obtained as per the National Comprehensive Cancer Network (NCCN) guidelines[24]. OS was calculated from the date of treatment to the date of death or the last follow-up. Locoregional recurrence was defined as radiographic or pathological evidence of recurrent disease in the remnant pancreas and retroperitoneum along the SMV/PV, SMA, or CA/CHA. Distant recurrence was defined as a tumor that spread outside the locoregional area, including but not limited to the peritoneum, lungs, and liver. Simultaneous local and distant recurrence was defined as the presence of both local and distant recurrences at the same time. According to the radiological resectability criteria of the NCCN guidelines, all pancreatic head cancer patients enrolled in our study were classified as resectable[24].
Continuous variables with a normal distribution are presented as the mean ± SD and were analyzed using Student’s t test. Continuous variables that were not normally distributed are presented as medians with interquartile ranges and were analyzed using the Mann-Whitney U test. Categorical variables were analyzed by the chi-square test or Fisher’s exact test, depending on the situation.
For survival analyses, patients who were lost to follow-up were censored at the time of the last normal imaging or clinical visit for RFS and the last interaction within the institution’s medical record for OS. Kaplan-Meier survival curves were generated for RFS and OS, and survival was compared using the log-rank test. Univariate and multivariate analyses with Cox proportional hazard models were performed to evaluate significant predictors of RFS. Variables included in the multivariable model were selected based on their significance in the univariate comparison of the two groups. The HR and its 95%CI were calculated using Cox proportional hazard analysis. All tests were two-tailed, and the statistical significance was set at 0.05. Statistical analyses were performed using SPSS V.26 software (IBM, United States), and Kaplan-Meier survival curves were generated using the survival and survminer package in R statistical software (v. 4.2.2).
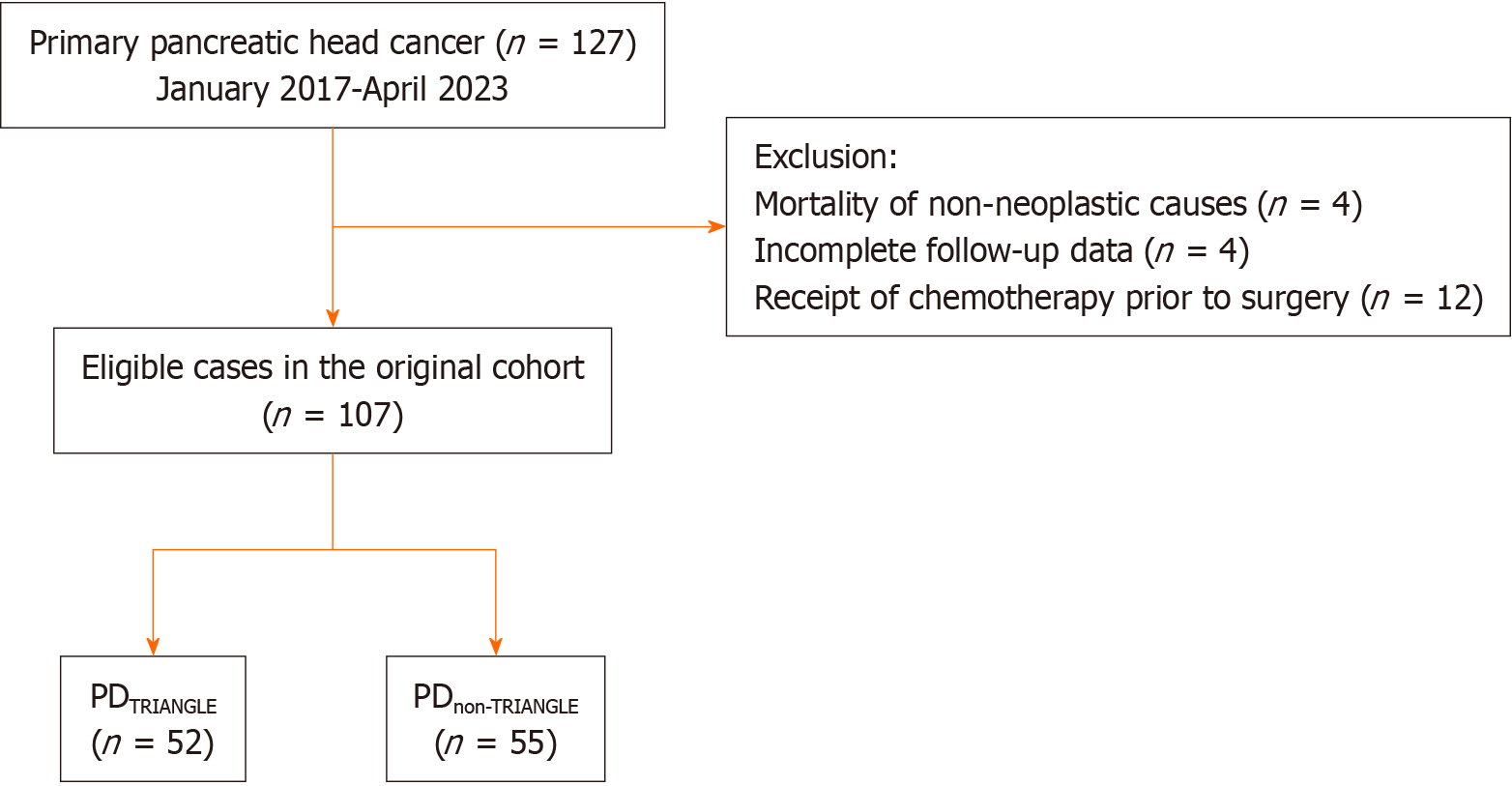
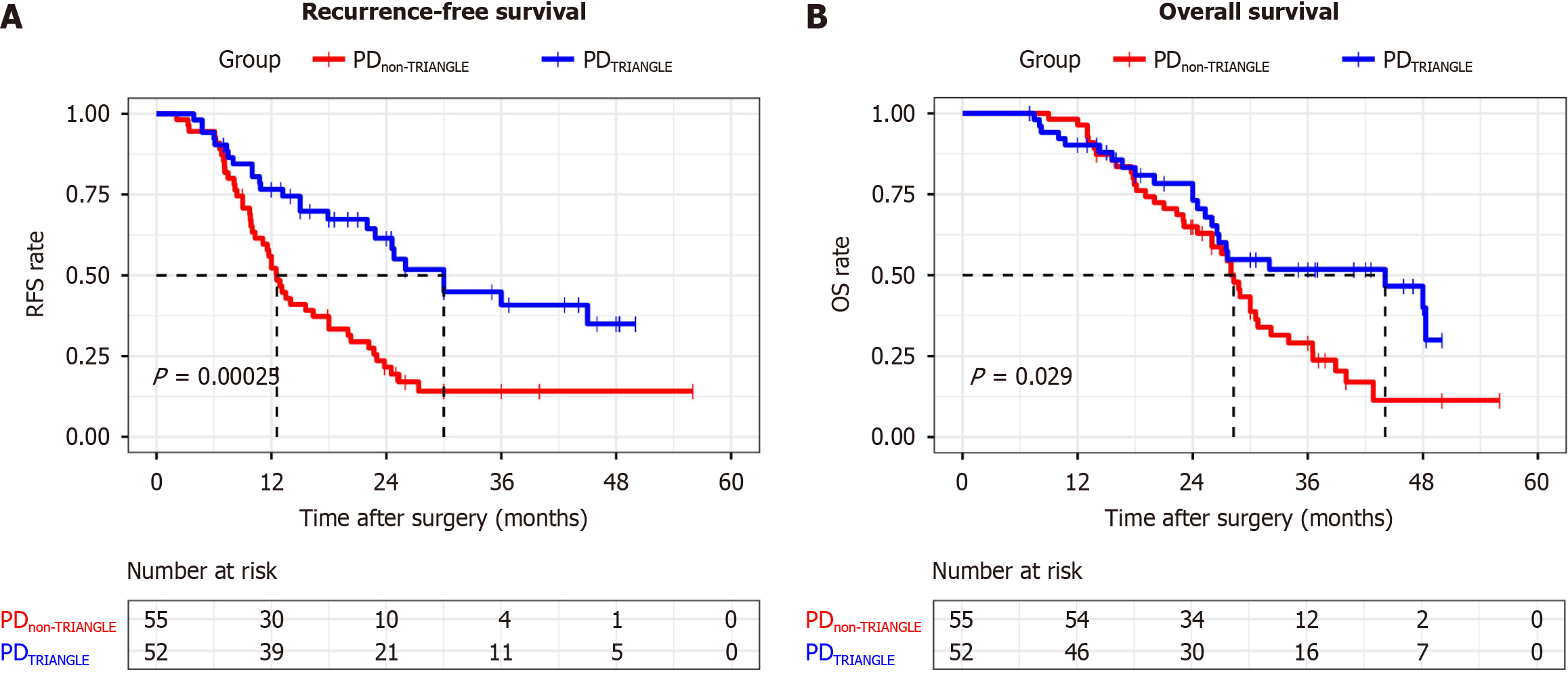
We retrospectively analyzed 127 patients with pancreatic head ductal adenocarcinoma who underwent PD between January 2017 and April 2023 at the Department of General Surgery, Huadong Hospital, Fudan University. All operations were performed by the same surgical team. We excluded four patients who died from nonsurgical causes, four patients with incomplete follow-up data and twelve patients who received chemotherapy prior to surgery. The final analysis included 107 patients with PDAC who met the inclusion criteria. These patients were divided into the PDTRIANGLE (n = 52) and PDnon-TRIANGLE (n = 55) groups based on whether they underwent Heidelberg triangle clearance (Figure 3). There were no statistically significant differences in the baseline data, such as age, sex, BMI, ASA score, CA19-9, or CEA, between the two groups. Similarly, there were no statistically significant differences in clinicopathological indexes, including the PNI, PVI, TNM stage, or resection margin status, between the two groups. The longest follow-up period was 56 months, with median follow-up times of 37.0 months and 40.0 months for the PDTRIANGLE group and the PDnon-TRIANGLE group, respectively. Notably, the median number of examined lymph nodes was significantly greater in the PDTRIANGLE group than in the PDnon-TRIANGLE group (20 vs 14, P = 0.001), indicating that Heidelberg triangle dissection enabled the acquisition of a greater number of lymph nodes (Table 1).
| Variables | PDTRIANGLE (n = 52) | PDnon-TRIANGLE (n = 55) | P value |
| Age, median (IQR; yr) | 69 (60-74) | 67 (61-74) | 0.589 |
| Sex | 0.154 | ||
| Male | 26 (50.0) | 35 (63.6) | |
| Female | 26 (50.0) | 20 (36.4) | |
| BMI (mean ± SD, kg/m²) | 22.86 ± 3.19 | 22.15 ± 2.79 | 0.224 |
| ASA score | 0.387 | ||
| 1 | 10 (19.6) | 15 (27.3) | |
| 2 | 37 (72.6) | 33 (60.0) | |
| 3 | 4 (7.8) | 7 (12.7) | |
| Main pancreatic duct size (mm) | 0.208 | ||
| ≤ 3 | 18 (34.6) | 19 (34.5) | |
| > 3 | 34 (65.4) | 36 (65.5) | |
| CA19-9, median (IQR; IU/mL) | 94.4 (37.3-358.5) | 108.0 (57.6-213.2) | 0.711 |
| CEA, median (IQR; ng/mL) | 3.4 (2.1-6.5) | 3.9 (2.3-5.7) | 0.521 |
| Albumin (mean ± SD, g/L) | 39.7 ± 3.9 | 40.7 ± 4.5 | 0.227 |
| Serum bilirubin, median (IQR; μmol/L) | 29.3 (8.3-186.4) | 18.8 (9.4-114.9) | 0.660 |
| AJCC stage (8th ed) | 0.291 | ||
| I/IIa | 28 (53.8) | 24 (43.6) | |
| IIb/III | 24 (46.2) | 31 (56.4) | |
| Grading | 0.385 | ||
| 1 | 2 (3.9) | 0 (0.0) | |
| 2 | 39 (75.0) | 45 (81.8) | |
| 3 | 11 (21.1) | 10 (18.2) | |
| PNI | 44 (84.6) | 44 (80.0) | 0.532 |
| PVI | 28 (53.9) | 27 (49.1) | 0.623 |
| Number of ELN, median (IQR) | 20 (14-26) | 14 (8-20) | 0.001a |
| Margin status | 0.579 | ||
| R0 | 42 (80.8) | 42 (76.4) | |
| R1 | 10 (19.2) | 13 (23.6) |
There were no significant differences in operative time (386 minutes vs 390 minutes, P = 0.527) or intraoperative bleeding volume (300 mL vs 300 mL, P = 0.863) between the two groups, suggesting that Heidelberg triangle dissection was safe in PD. The postoperative length of stay (14 d vs 15 d, P = 0.177) and the proportion of patients requiring ICU admission for ≥ 2 d (25.0% vs 30.9%, P = 0.496) were similar between the two groups. Although extended lymphadenectomy was involved in Heidelberg triangle dissection, there was no significant difference in complication rates, including POPF (B/C), bile leakage (B/C), chyle leak (B/C), DGE (B/C), PPH (B/C), intra-abdominal infection, or diarrhea, between the two groups. Moreover, there was no significant difference in the proportion of patients requiring reoperation between the two groups (1.9% vs 5.5%, P = 0.651; Table 2).
| Variables | PDTRIANGLE (n = 52) | PDnon-TRIANGLE (n = 55) | P value |
| Operative time, median (IQR; min) | 386 (324-423) | 390 (345-425) | 0.527 |
| Intraoperative blood loss, median (IQR; mL) | 300 (200-500) | 300 (200-500) | 0.863 |
| LOS, median (IQR) | 14 (11-19) | 15 (12-22) | 0.177 |
| ICU stay ≥ 2 d | 13 (25.0) | 17 (30.9) | 0.496 |
| POPF (B/C) | 9 (17.3) | 13 (23.6) | 0.418 |
| Bile leakage (B/C) | 0 (0.0) | 3 (5.5) | 0.262 |
| Chyle leak (B/C) | 0 (0.0) | 2 (3.6) | 0.496 |
| DGE (B/C) | 3 (5.8) | 5 (9.1) | 0.775 |
| PPH (B/C) | 0 (0.0) | 2 (3.6) | 0.496 |
| Intra-abdominal infection | 10 (19.2) | 9 (16.4) | 0.698 |
| Diarrhea | 14 (26.9) | 9 (16.4) | 0.184 |
| Reoperation | 1 (1.9) | 3 (5.5) | 0.651 |
| 90-d mortality | 0 (0.0) | 0 (0.0) | 1.000 |
| Adjuvant chemotherapy, ≥ 6 months | 30 (57.7) | 27 (49.1) | 0.373 |
| Chemotherapy regimens1 | 0.887 | ||
| Gemcitabine-based | 24 (80.0) | 22 (81.5) | |
| 5-fluorouracil-based | 6 (20.0) | 5 (18.5) | |
| Recurrence | < 0.001a | ||
| Yes | 25 (48.1) | 45 (81.8) | |
| No | 27 (51.9) | 10 (18.2) |
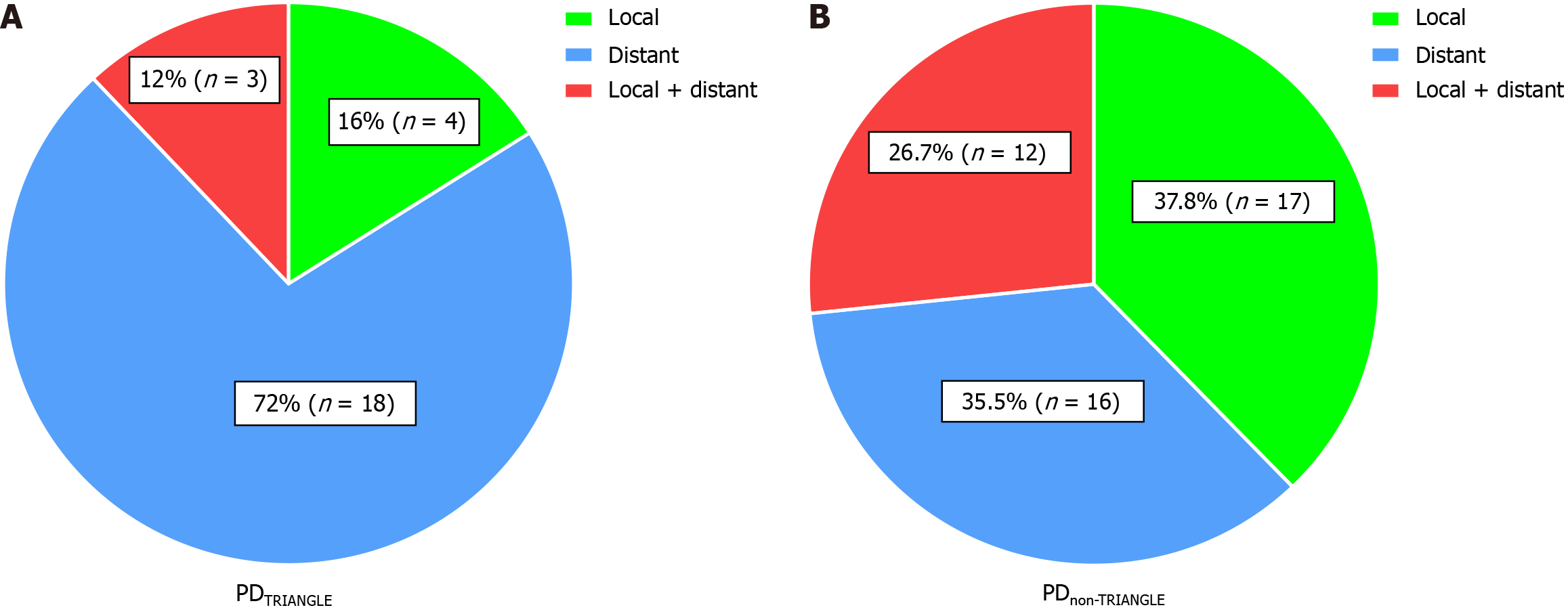
Additionally, while the two groups were balanced in terms of receiving adjuvant chemotherapy longer than 6 months (57.7% vs 49.1%, P = 0.373) and chemotherapy regimens (P = 0.887), the recurrence rate was lower after Heidelberg triangle dissection (48.1% vs 81.8%, P < 0.001). Notably, clearance of the Heidelberg triangle significantly reduced the local recurrence rate of PDAC patients from 37.8% to 16.0% and reduced the combined local and distant recurrence rates from 26.7% to 12.0% (Figure 4).
The median RFS was 30.0 months in the PDTRIANGLE group and 12.6 months in the PDnon-TRIANGLE group (P < 0.001). Similar results were observed for the median OS of both groups (44.1 months vs 28.3 months, P = 0.029; Figure 5). To identify independent risk factors for postoperative recurrence, Cox univariate and multivariate regression analyses were performed for each variable. Multivariate Cox regression analysis revealed that PDTRIANGLE (HR = 0.424; 95%CI: 0.256-0.702; P = 0.001), adequate adjuvant chemotherapy ≥ 6 months (HR = 0.370; 95%CI: 0.222-0.618; P < 0.001) and margin status (HR = 2.255; 95%CI: 1.252-4.064; P = 0.007) were found to be independent factors associated with the recurrence rate (Table 3). Further analysis was also conducted to explore the factors affecting the recurrence pattern. Moreover, the PDTRIANGLE (HR = 0.175; 95%CI: 0.059-0.521; P = 0.002) was found to be an independent risk factor for a lower local recurrence rate. Moreover, adequate adjuvant chemotherapy ≥ 6 months (HR = 0.332; 95%CI: 0.164-0.672; P = 0.002) and CA19-9 > 37.0 (HR = 5.684; 95%CI: 1.354-23.868; P = 0.018) were found to be independent factors for the distant recurrence rate. Finally, both the PDTRIANGLE (HR=0.188; 95%CI: 0.052-0.683; P = 0.011) and the AJCC stage (8th ed; HR = 3.608; 95%CI: 1.057-12.308; P = 0.040) were found to be independent factors for simultaneous local and distant recurrence (Table 3).
| Variables | Total (n = 70) | Local (n = 21) | Distant (n = 34) | Local + distant (n = 15) | ||||||||
| P1 | HR (95%CI) | P2 | P1 | HR (95%CI) | P2 | P1 | HR (95%CI) | P2 | P1 | HR (95%CI) | P2 | |
| Age (yr), > 70/≤ 70 | 0.206 | - | 0.092 | - | 0.864 | - | 0.470 | - | ||||
| Sex, male/female | 0.572 | - | 0.304 | - | 0.866 | - | 0.707 | - | ||||
| PDTRIANGLE, +/- | < 0.001c | 0.424 (0.256-0.702) | 0.001b | 0.002b | 0.175 (0.059-0.521) | 0.002b | 0.649 | - | 0.011a | 0.188 (0.052-0.683) | 0.011a | |
| Grading, 2-3/1 | ||||||||||||
| 2 | 0.663 | - | 0.997 | - | 0.997 | - | 0.240 | - | ||||
| 3 | 0.813 | - | 0.997 | - | 0.997 | - | 0.292 | - | ||||
| Margin status, R1/R0 | 0.031a | 2.255 (1.252-4.064) | 0.007b | 0.249 | - | 0.346 | - | 0.066 | - | |||
| AJCC stage (8th ed), I-IIa/IIb-III | 0.057 | - | 0.801 | - | 0.077 | - | 0.038a | 3.608 (1.057-12.308) | 0.040a | |||
| PNI, +/- | 0.427 | - | 0.887 | - | 0.478 | - | 0.512 | - | ||||
| PVI, +/- | 0.064 | - | 0.694 | - | 0.036a | 1.862 (0.940-3.691) | 0.075 | 0.833 | ||||
| CA19-9, > 37.0/≤ 37.0 | 0.098 | - | 0.141 | - | 0.019a | 5.684 (1.354-23.868) | 0.018a | 0.152 | - | |||
| CEA, > 5.0/≤ 5.0 | 0.823 | - | 0.068 | - | 0.289 | - | 0.612 | - | ||||
| Adjuvant chemotherapy, ≥ 6 months/< 6 months | 0.001b | 0.370 (0.222-0.618) | < 0.001c | 0.527 | - | 0.002b | 0.332 (0.164-0.672) | 0.002b | 0.127 | - | ||
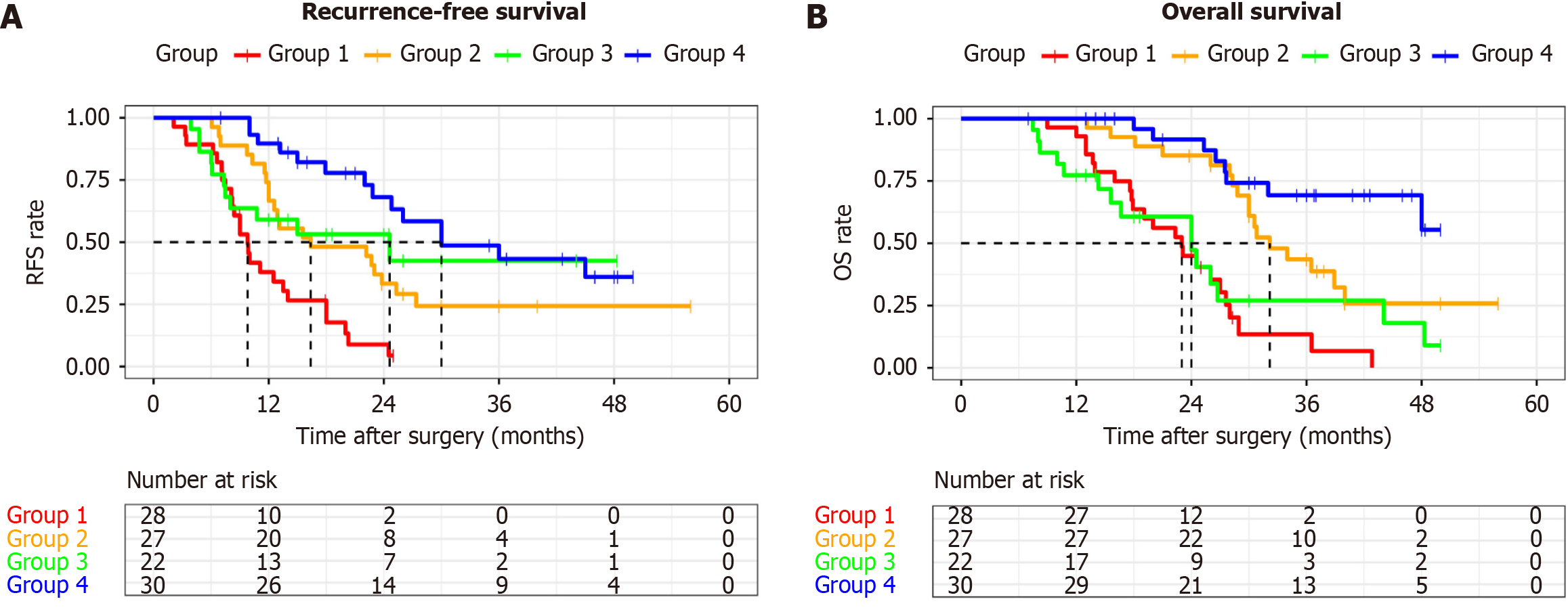
The data were divided into four groups based on the two independent prognostic factors derived from the Cox analysis: Group 1: No clearance of the Heidelberg triangle and no adjuvant chemotherapy; Group 2: No clearance of the Heidel
The extent of lymphadenectomy in PD patients remains a topic of debate. Previous studies have suggested that there is no significant difference in the long-term prognosis between standard lymphadenectomy and extended lymphadenectomy for PDAC, although the latter may result in longer operation times and increased intraoperative bleeding[25-29]. In 2017, Hackert et al[10] introduced the TRIANGLE operation for locally advanced PDAC, which is a modified form of extended lymphadenectomy encompassing the same range as the TMpE proposed by Adham and Singhirunnusorn[9] in 2012. This procedure has been shown to improve the margin status of PDAC without increasing the complication rate[23]. However, studies on the long-term prognosis of the TRIANGLE operation are currently lacking. The objective of this study was to investigate the effect of combining PD with the TRIANGLE operation on the long-term prognosis of PDAC patients.
Previous studies examining the early postoperative outcomes of the TRIANGLE operation have demonstrated that it yields a greater number of examined lymph nodes than standard lymphadenectomy does, with a lower rate of R1 (direct) resection, although no significant difference has been observed in R0 rates[23]. Similarly, our study revealed that the PDTRIANGLE group had a greater lymph node yield than the PDnon-TRIANGLE group did, while the R0 rate did not significantly differ. Additionally, we observed that the TRIANGLE operation did not increase the operative time, intraoperative bleeding volume, or postoperative complication rate, suggesting that it is safer than extended lymphadenectomy, as discussed in previous studies[25-29]. Regarding postoperative quality of life, extended lymphadenectomy has been associated with increased rates of postoperative diarrhea[30]. Our results revealed a greater incidence of diarrhea in the TRIANGLE operation group (26.9% vs 16.4%, P = 0.184), but this difference was not statistically significant. This increase in diarrhea rates might be attributed to the resection of autonomic nerves around the SMA and CA. Furthermore, in the present study, the TRIANGLE operation involved dissection of only the right 180° of the SMA while preserving the nerves on the left 180°; hence, the incidence of diarrhea was not very high[25]. Notably, patients who experienced diarrhea responded well to oral antidiarrheal medication, implying a limited impact on overall quality of life.
Previous studies investigating factors influencing postoperative recurrence in patients with PDAC have shown the PNI to be an independent prognostic factor for OS and disease-free survival (DFS), while adjuvant chemotherapy has been shown to reduce the rate of local and distant recurrence after surgery, resulting in an improvement in OS[31-33]. Despite both study groups receiving equal proportions of adjuvant chemotherapy, our findings showed a lower recurrence rate when the Heidelberg triangle was cleared. Notably, in patients who experienced recurrence, clearing the Heidelberg triangle reduced the local recurrence rate of PDAC from 37.8% to 16.0% and the combined local and distant rate from 26.7% to 12.0%. These findings suggested that the TRIANGLE operation reduces the local recurrence rate and alters the recurrence pattern of PDAC. Cox regression analysis of the recurrence pattern further confirmed this phenomenon. The PLphI and PLphII, as mentioned in the JPS guidelines, are located within the Heidelberg triangle[11], and the TRIANGLE operation involves clearance of the corresponding nerve plexus, thereby reducing the local recurrence rate. This may provide a plausible explanation for the observed phenomenon. A recent clinical study demonstrated that selective extended dissection, which involves removal of the extrapancreatic nerve plexus that may be invaded by tumors, in
Previous studies have demonstrated that the completeness of adjuvant chemotherapy is more critical than the timing of chemotherapy initiation[35]. Complete adjuvant chemotherapy (> 6 cycles) has been shown to improve OS and DFS after curative surgery for PDAC, while early termination of chemotherapy has been associated with shorter OS[36-38]. Typically, chemotherapy regimens for pancreatic cancer that last 6 cycles span 6 months or longer[38]. Thus, in this study, we used 6 months rather than 6 cycles as the criterion for complete chemotherapy. Through multivariate Cox regression analysis, we identified adjuvant chemotherapy lasting ≥ 6 months as an independent factor associated with lower rates of distant recurrence. The results of the ESPAC-3 study showed that completion of all six cycles of planned adjuvant chemotherapy rather than early initiation was an independent prognostic factor after resection for pancreatic adenocarcinoma[38]. Similarly, a previous study by Hirono et al[16] revealed that noncompletion of postoperative adjuvant therapy was a risk factor for distant recurrence in resectable PDAC patients who underwent PD. These findings emphasize the importance of completing an adequate course of adjuvant chemotherapy. Subgroup analysis based on the combination of the TRIANGLE operation and chemotherapy lasting ≥ 6 months revealed that for patients who underwent a sufficient course of adjuvant chemotherapy, previous implementation of the TRIANGLE operation significantly prolonged mRFS. In addition, not only may the TRIANGLE operation itself prolong RFS, but this effect is also more pronounced when accompanied by an adequate course of adjuvant chemotherapy. The results indicate that the TRIANGLE operation extends mRFS but should be combined with an adequate course of adjuvant chemotherapy. Furthermore, sufficient courses of adjuvant chemotherapy were found to significantly prolong OS in the PDTRIANGLE and PDnon-TRIANGLE groups. The results highlight the importance of combining an appropriate extent of lymphadenectomy with an adequate course of adjuvant chemotherapy, consistent with the concept of comprehensive treatment of pancreatic cancer.
This study has several limitations. First, the study spans a long period, during which definitions of some clinicopathological indicators changed, and chemotherapy regimens evolved based on yearly guidelines, which may introduce confounding results. Second, this study was conducted at a single center, which may limit the generalizability of the findings. Future multicenter studies are necessary to validate the results.
The results of this study suggest that the TRIANGLE operation is a safe and feasible approach for treating PDAC during PD. More importantly, the TRIANGLE operation reduces the local recurrence rate of PDAC and may also prolong RFS and OS when combined with adequate postoperative adjuvant chemotherapy. However, additional studies with larger cohorts are needed to confirm the conclusions drawn from this study.
Data on the long-term oncological outcomes of the TRIANGLE operation in resectable pancreatic ductal adenocarcinoma (PDAC) patients undergoing pancreaticoduodenectomy (PD) are limited.
The TRIANGLE operation shows efficacy postneoadjuvant therapy in locally advanced PDAC, but its role in resectable PDAC is unclear. This study explored the safety and prognostic impact of this approach in patients with resectable PDAC.
To assess the safety of the TRIANGLE operation during PD and its prognostic relevance for resectable PDAC recurrence and survival.
This retrospective cohort study included patients who underwent PD for pancreatic head cancer between January 2017 and April 2023, with or without the TRIANGLE operation. Patients were divided into the PDTRIANGLE and PDnon-TRIANGLE groups. Surgical and survival outcomes were compared between the two groups. Adequate adjuvant chemotherapy was defined as adjuvant chemotherapy ≥ 6 months.
The study included 52 patients in the PDTRIANGLE group and 55 in the PDnon-TRIANGLE group, with no significant differences in baseline or perioperative outcomes. The PDTRIANGLE group had a lower recurrence rate (48.1% vs 81.8%, P < 0.001) and a decrease in local PDAC recurrence from 37.8% to 16.0%. Multivariate Cox regression analysis revealed that PDTRIANGLE, adequate adjuvant chemotherapy (≥ 6 months), and margin status were independent predictors of the recurrence rate.
The TRIANGLE operation during PD is safe, reduces local recurrence of PDAC, and potentially enhances recurrence-free survival and overall survival with sufficient adjuvant chemotherapy.
Further research with a larger cohort is essential to validate the survival outcomes of the TRIANGLE operation.
The authors appreciate all the team members for their help. We appreciate Hekai Shi for his help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania S-Editor: Lin C L-Editor: A P-Editor: Zheng XM
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11428] [Article Influence: 3809.3] [Reference Citation Analysis (4)] |
| 2. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1670] [Article Influence: 334.0] [Reference Citation Analysis (1)] |
| 3. | Tummers WS, Groen JV, Sibinga Mulder BG, Farina-Sarasqueta A, Morreau J, Putter H, van de Velde CJ, Vahrmeijer AL, Bonsing BA, Mieog JS, Swijnenburg RJ. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg. 2019;106:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, Jäger D, Schirmacher P, Hackert T, Büchler MW. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg. 2017;265:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Honselmann KC, Pergolini I, Castillo CF, Deshpande V, Ting D, Taylor MS, Bolm L, Qadan M, Wellner U, Sandini M, Bausch D, Warshaw AL, Lillemoe KD, Keck T, Ferrone CR. Timing But Not Patterns of Recurrence Is Different Between Node-negative and Node-positive Resected Pancreatic Cancer. Ann Surg. 2020;272:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | McIntyre CA, Zambirinis CP, Pulvirenti A, Chou JF, Gonen M, Balachandran VP, Kingham TP, D'Angelica MI, Brennan MF, Drebin JA, Jarnagin WR, Allen PJ. Detailed Analysis of Margin Positivity and the Site of Local Recurrence After Pancreaticoduodenectomy. Ann Surg Oncol. 2021;28:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Fernandes ESM, Strobel O, Girão C, Moraes-Junior JMA, Torres OJM. What do surgeons need to know about the mesopancreas. Langenbecks Arch Surg. 2021;406:2621-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Gockel I, Domeyer M, Wolloscheck T, Konerding MA, Junginger T. Resection of the mesopancreas (RMP): a new surgical classification of a known anatomical space. World J Surg Oncol. 2007;5:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Adham M, Singhirunnusorn J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur J Surg Oncol. 2012;38:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, Berchtold C, Ulrich A, Büchler MW. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford). 2017;19:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 11. | Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, Furuse J, Hanada K, Okazaki K; Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas. 2020;49:326-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 12. | Wang J, Chen Y, Li X, Zou X. Perineural Invasion and Associated Pain Transmission in Pancreatic Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Study Group of Minimally Invasive Treatment for Pancreatic Cancer in China Anti-Cancer Association; Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. [Chinese expert consensus on minimally invasive radical surgery for pancreatic ductal adenocarcinoma (version 2022)]. Zhonghua Wai Ke Za Zhi. 2023;61:187-195. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Kauffmann EF, Napoli N, Ginesini M, Gianfaldoni C, Asta F, Salamone A, Amorese G, Vistoli F, Boggi U. Feasibility of "cold" triangle robotic pancreatoduodenectomy. Surg Endosc. 2022;36:9424-9434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M, Büchler MW, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM; International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 16. | Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Kobayashi R, Hayami S, Ueno M, Yamaue H. Complete circumferential lymphadenectomy around the superior mesenteric artery with preservation of nerve plexus reduces locoregional recurrence after pancreatoduodenectomy for resectable pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2021;47:2586-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | van Roessel S, Kasumova GG, Verheij J, Najarian RM, Maggino L, de Pastena M, Malleo G, Marchegiani G, Salvia R, Ng SC, de Geus SW, Lof S, Giovinazzo F, van Dam JL, Kent TS, Busch OR, van Eijck CH, Koerkamp BG, Abu Hilal M, Bassi C, Tseng JF, Besselink MG. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg. 2018;153:e183617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 18. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2957] [Article Influence: 369.6] [Reference Citation Analysis (35)] |
| 19. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1413] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 20. | Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, Asbun HJ, Bockhorn M, Strobel O, Büchler MW, Busch OR, Charnley RM, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Izbicki JR, Lillemoe KD, Neoptolemos JP, Sarr MG, Shrikhande SV, Sitarz R, Vollmer CM, Yeo CJ, Hartwig W, Wolfgang CL, Gouma DJ; International Study Group on Pancreatic Surgery. Definition and classification of chyle leak after pancreatic operation: A consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2327] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 22. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1945] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 23. | Klotz R, Hackert T, Heger P, Probst P, Hinz U, Loos M, Berchtold C, Mehrabi A, Schneider M, Müller-Stich BP, Strobel O, Diener MK, Mihaljevic AL, Büchler MW. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB (Oxford). 2022;24:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Pancreatic adenocarcinoma (version 1.2024). [cited 3 January 2024]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. |
| 25. | Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, Kim SG, Kim SW. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Jang JY, Kang JS, Han Y, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Park JS, Yu HC, Kang KJ, Kim SG, Lee H, Kwon W, Yoon YS, Han HS, Kim SW. Long-term outcomes and recurrence patterns of standard versus extended pancreatectomy for pancreatic head cancer: a multicenter prospective randomized controlled study. J Hepatobiliary Pancreat Sci. 2017;24:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Ignjatovic I, Knezevic S, Knezevic D, Dugalic V, Micev M, Matic S, Ostojic S, Bogdanovic M, Pavlovic I, Jurisic V. Standard versus extended lymphadenectomy in radical surgical treatment for pancreatic head carcinoma. J BUON. 2017;22:232-238. [PubMed] |
| 28. | Staerkle RF, Vuille-Dit-Bille RN, Soll C, Troller R, Samra J, Puhan MA, Breitenstein S. Extended lymph node resection versus standard resection for pancreatic and periampullary adenocarcinoma. Cochrane Database Syst Rev. 2021;1:CD011490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Wang W, Lou W, Xu Z, Chen H, Shen Z, Deng X, Peng C, Liu Y, Shen B. Long-term outcomes of standard versus extended lymphadenectomy in pancreatoduodenectomy for pancreatic ductal adenocarcinoma: A Chinese multi-center prospective randomized controlled trial. J Adv Res. 2023;49:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Farnell MB, Aranha GV, Nimura Y, Michelassi F. The role of extended lymphadenectomy for adenocarcinoma of the head of the pancreas: strength of the evidence. J Gastrointest Surg. 2008;12:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Crippa S, Pergolini I, Javed AA, Honselmann KC, Weiss MJ, Di Salvo F, Burkhart R, Zamboni G, Belfiori G, Ferrone CR, Rubini C, Yu J, Gasparini G, Qadan M, He J, Lillemoe KD, Castillo CF, Wolfgang CL, Falconi M. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann Surg. 2022;276:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 32. | Parikh AA, Maiga A, Bentrem D, Squires MH 3rd, Kooby DA, Maithel SK, Weber SM, Cho CS, Katz M, Martin RC, Scoggins CR, Sutton J, Ahmad SA, Abbott DE, Carr J, Kim HJ, Yakoub D, Idrees K, Merchant N. Adjuvant Therapy in Pancreas Cancer: Does It Influence Patterns of Recurrence? J Am Coll Surg. 2016;222:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Schorn S, Demir IE, Haller B, Scheufele F, Reyes CM, Tieftrunk E, Sargut M, Goess R, Friess H, Ceyhan GO. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Surg Oncol. 2017;26:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | Guo X, Song Y, Xu P, Zhu W, Wang H, Zhou Y, Huang C, Hao J, Gao S. Selective extended dissection for pancreaticoduodenectomy is associated with better survival in pancreatic cancer patients: retrospective cohort study. Int J Surg. 2023;109:1852-1862. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Lee W, Yoon YS, Han HS, Jang JY, Cho JY, Jung W, Kwon W, Choi Y, Kim SW. Prognostic Relevance of the Timing of Initiating and the Completion of Adjuvant Therapy in Patients with Resected Pancreatic Ductal Adenocarcinoma. World J Surg. 2017;41:562-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Chikhladze S, Lederer AK, Kousoulas L, Reinmuth M, Sick O, Fichtner-Feigl S, Wittel UA. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: retrospective real-life data. World J Surg Oncol. 2019;17:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Epelboym I, Zenati MS, Hamad A, Steve J, Lee KK, Bahary N, Hogg ME, Zeh HJ, Zureikat AH. Analysis of Perioperative Chemotherapy in Resected Pancreatic Cancer: Identifying the Number and Sequence of Chemotherapy Cycles Needed to Optimize Survival. Ann Surg Oncol. 2017;24:2744-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, Rawcliffe CL, Bassi C, Stocken DD, Cunningham D, O'Reilly D, Goldstein D, Robinson BA, Karapetis C, Scarfe A, Lacaine F, Sand J, Izbicki JR, Mayerle J, Dervenis C, Oláh A, Butturini G, Lind PA, Middleton MR, Anthoney A, Sumpter K, Carter R, Büchler MW. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |