Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1756
Peer-review started: December 20, 2023
First decision: January 10, 2024
Revised: February 7, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 15, 2024
Processing time: 140 Days and 17.2 Hours
Pancreatic neuroendocrine tumors (PNETs) are relatively rare but rank as the second most common pancreatic neoplasm. They can be functional, causing early metabolic disturbances due to hormone secretion, or non-functional and diag
To evaluate poor prognostic factors in PNETs based on tumor size (> 2 cm or < 2 cm) in surgically treated patients.
This cohort study included 64 patients with PNETs who underwent surgical re
The presence of lymph node involvement, neural involvement, and lymphovascular invasion were all associated with an increased risk of mortality, with hazard ratios of 5.68 (95%CI: 1.26–25.61, P = 0.024), 6.44 (95%CI: 1.43–28.93, P = 0.015), and 24.87 (95%CI: 2.98–207.19, P = 0.003), respectively. Neural involvement and lym
Tumor size does not dictate prognosis; lymph node and lymphovascular involvement affect mortality, which high
Core Tip: Pancreatic neuroendocrine tumors (PNETs) are relatively uncommon but represent the second most frequent pancreatic neoplasm. This study, conducted in Colombia, challenges conventional size-based survival expectations for PNETs and advocates a surgical approach, even for tumors smaller than 2 cm. The findings underscore the importance of considering histological traits like lymphovascular and neural invasion, high mitotic index, and a Ki67 proliferation index exceeding 3%. These characteristics, traditionally associated with larger tumors and higher mortality rates, were also identified in a significant fraction of smaller tumors. More comprehensive studies are necessary to redefine clinical approaches and guidelines for managing PNETs.
- Citation: Hoyos S, Posada-Moreno P, Guzman-Arango N, Chanci-Drago R, Chavez J, Andrés-Duarte A, Salazar-Ochoa S. Pancreatic neuroendocrine tumors: Are tumors smaller than 2 cm truly indolent? World J Gastrointest Oncol 2024; 16(5): 1756-1762
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1756.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1756
Pancreatic neuroendocrine tumors (PNETs) are relatively rare tumors. Although they rank as the second most common neoplasm among pancreatic tumors, following pancreatic adenocarcinoma, they have an incidence of approximately 1 per 100000 individuals[1]. Nevertheless, post-mortem studies conducted in the general population have revealed that the actual incidence of PNETs varies from 1%–10%[1]. These tumors can be functional and result in metabolic manifestations that depend on the specific hormones they produce. In such cases, early diagnosis and surgical intervention are typically the preferred management approach. Conversely, non-functional PNETs are often diagnosed at a later stage, typically when symptoms become related to the tumor's size. It is worth noting that non-functional tumors are the most prevalent subtype within this pathology[2-4].
The widespread adoption of advanced imaging techniques has led to a significant increase in the diagnosis of PNETs, with reported growth ranging from four-fold to seven-fold in recent years[5]. This surge in diagnoses has led to debates regarding the best approach for managing these smaller tumors; the clinical outcomes of various studies have not pro
Recent studies have explored minimally invasive management strategies, such as endoscopic ultrasound-guided radiofrequency ablation[2]. However, it is important to note that the available evidence largely derives from case reports and small series; such investigations are considered less robust when it comes to establishing the effectiveness of these approaches[2]. It is therefore crucial to emphasize the ongoing importance of surgery in the management of PNETs, even for tumors smaller than 2 cm in diameter.
The primary objective of this study was to examine the histological and poor prognostic traits of PNETs within a patient cohort at a high-complexity hospital in Medellín, Colombia. The study sought to ascertain whether tumors with a diameter smaller than 2 cm, as observed in definitive pathological specimens, exhibited characteristics indicating a reduced likelihood of aggressiveness compared with larger tumors.
This cohort study included 64 patients with PNETs who underwent surgical resection between 2006 and 2019 in the hepatobiliary surgery department of a high-complexity reference hospital in Medellín, Colombia. To assess patient survival, quarterly follow-ups were conducted during the first year after surgery, followed by semi-annual consultations at the hospital's hepatobiliary surgery department.
Data collection was carried out using a prospective database maintained in the hepatobiliary surgery unit of the hospital. Any missing patient data necessary for the study were completed by referencing medical records. Patients for whom information necessary for the analysis could not be obtained were excluded from the study.
For the univariate analysis, qualitative variables were described using absolute and relative frequencies. Quantitative variables were expressed using measures of central tendency and their corresponding measures of dispersion. The normality of variables was assessed using the Kolmogorov-Smirnov test. The Kaplan-Meier method was used for survival analysis. Survival curves were compared among groups using the Log-Rank test, and associated variables were determined through Cox regression. Furthermore, hazard ratios (HR) were assessed to determine changes in the measure of associations.
For the bivariate analysis, the Log-Rank test was employed. P values less than 0.05 were presumed to indicate statistical significance.
From 2006 through 2019, a total of 420 pancreatic resections were carried out at Pablo Tobón Uribe Hospital. Of those resections, 64 were performed for PNETs; we focused on those procedures. The mean age of our patient cohort was 50 years, and females comprised 62.5% of the patient population. Laparoscopic procedures accounted for 32.2% of the resections; all of the laparoscopic procedures involved resections of the tail and body of the pancreas.
The majority of the pancreatic resections carried out (64.1%) were distal pancreatectomies involving resections of the pancreatic body or tail. Pancreatoduodenectomies, also known as the Whipple procedure, constituted 31.3% of the resections. Other types of pancreatic resections, including enucleations, accounted for 4.7% of the procedures. The overall 30-d postoperative mortality rate was 1.6%.
The majority of patients (65.6%) had PNETs larger than 2 cm in diameter; 34.3% had lesions smaller than 2 cm in diameter. Notably, lymph node involvement was documented in 30.8% of PNETs larger than 2 cm; it was not observed in smaller tumors. Disease recurrence was noted in 21.4% of patients with PNETs larger than 2 cm, and a slightly lower recurrence rate was observed in the group of patients with PNETs smaller than 2 cm (18.2%). There was no statistically significant difference in disease recurrence between the two groups (P = 0.98). The characteristics of the patients are detailed below and summarized in Table 1.
| Variable | PNET ≥ 2 cm, n = 42 | PNET < 2 cm, n = 22 | P value |
| Mean age (yr) | 49.1 | 52.4 | 0.32 |
| Sex (fraction of females) | 64.3 | 59.1 | - |
| Whipple vs DP | 31 vs 62 | 27 vs 68 | - |
| Recurrence rate | 21.4 | 18.2 | 0.98 |
| Mitosis > 3/10 HPF | 58.1 | 26.3 | 0.05 |
| Ki67 index > 3 | 51.5 | 31.6 | 0.79 |
| CK19 positive | 69.2 | 35.7 | 0.08 |
| Lymph node involvement | 30.8 | 0 | 0.01 |
| Perineural invasion | 16.7 | 9.1 | 0.65 |
| Lymphovascular invasion | 21.4 | 9.1 | 0.37 |
Bivariate analysis revealed that the presence of certain characteristics of PNETs larger than 2 cm in diameter and those smaller than 2 cm was associated with poorer outcomes and an increased risk of mortality. On the other hand, the multivariate analysis did not attain statistical significance due to the limitation in the limited size of the patient sample and the consequent lack of statistical power (Table 2).
| Variable | HR (raw) | Statistical significance (P value) | Multivariate analysis (P value) |
| PNET ≥ 2 cm | 0.878 | NS | NS |
| PNET < 2 cm | 1 | ||
| Mitosis > 3/10 HPF | 5.862 | NS | NS |
| Mitosis < 3/10 HPF | 1 | ||
| Ki67 index > 3% | 63.594 | NS | NS |
| Ki67 index < 3% | 1 | ||
| Lymph node involvement (+) | 5.688 | < 0.05 | NS |
| Lymph node involvement (-) | 1 | ||
| Perineural invasion (+) | 6.448 | < 0.05 | NS |
| Perineural invasion (-) | 1 | ||
| Lymphovascular invasion (+) | 24.875 | < 0.05 | NS |
| Lymphovascular invasion (-) | 1 |
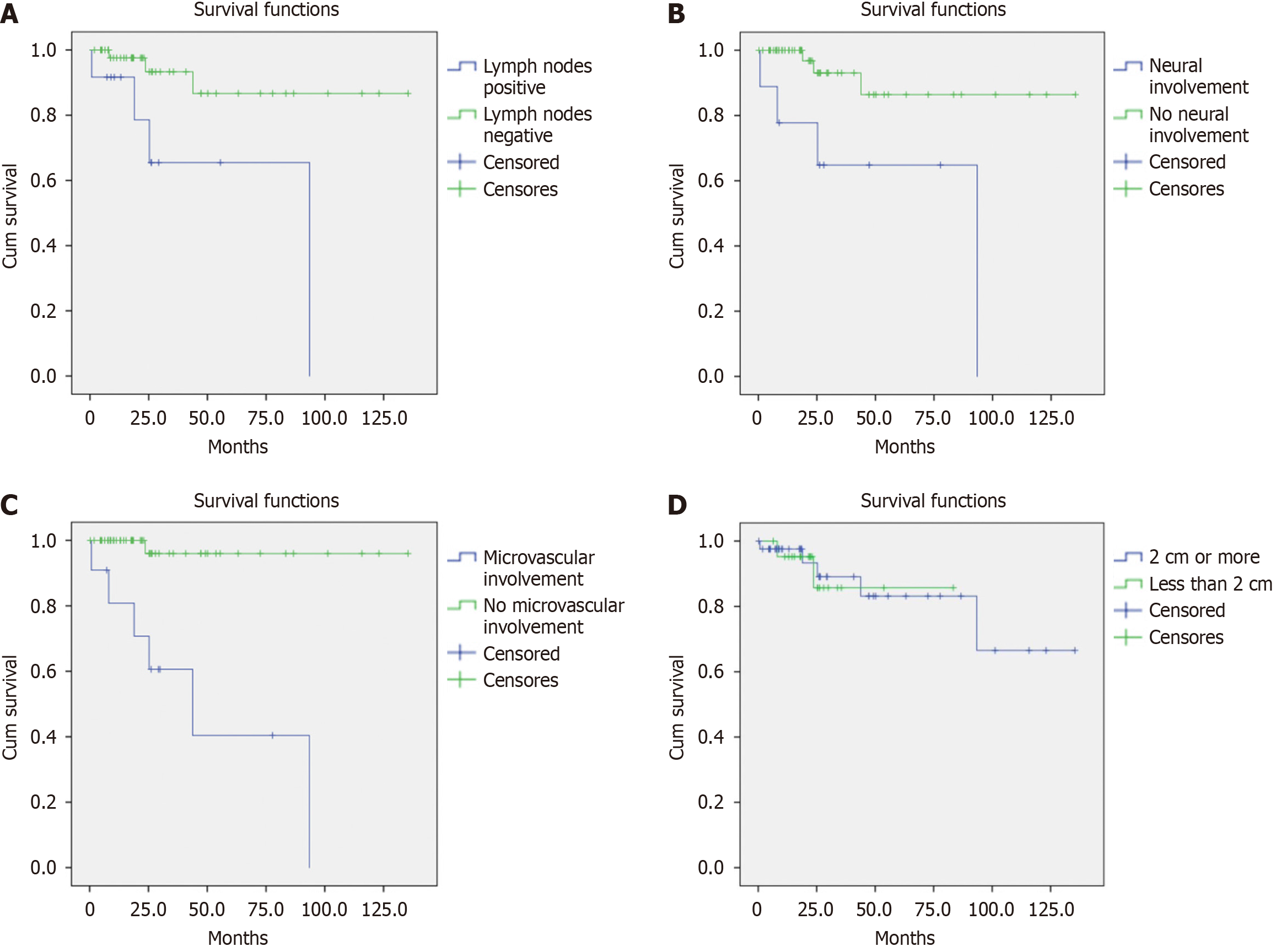
Lymph node involvement was significantly associated with a higher risk of mortality, with an HR of 5.68 (95%CI: 1.26–25.61, P = 0.024). This finding is illustrated in Figure 1A: Patients with lymph node involvement had a one-year survival rate of 91.7% and a five-year survival rate of 65.5%; patients without lymph node involvement had a one-year survival rate of 97.6% and a five-year survival rate of 86.7% (P = 0.011). A similar pattern was observed with neural involvement, which also increased the risk of mortality (HR: 6.44, 95%CI: 1.43–28.93, P = 0.015). Figure 1B shows that patients with neural involvement had one- and five-year survival rates of 77.8% and 64.8%, respectively; patients without perineural involvement had one- and five-year survival rates of 100% and 86.4%, respectively (P = 0.005). Furthermore, lymphovascular invasion was significantly associated with an increased risk of mortality, with an HR of 24.87 (95%CI: 2.98–207.19, P = 0.003). The data in Figure 1C reveal that patients with lymphovascular invasion had one- and five-year survival rates of 80.8% and 40.4%, respectively; patients without lymphovascular invasion had one- and five-year survival rates of 100% and 96.0%, respectively (P < 0.0001, Figure 1A).
One of the most remarkable findings of our study is illustrated in Figure 1D. The survival analysis demonstrates that there was no statistically significant difference in survival rates between patients with PNETs who had tumors larger than 2 cm in diameter and those with smaller tumors. The two patient populations were followed for, on average, 11.2 and 7.0 years, respectively, and they can be described by a P value of 0.88. This discovery has significant implications given that it challenges the conventional belief that tumor size alone is a definitive predictor of survival.
Pancreatic neuroendocrine tumors are rare entities, which can render their management complex. Currently, there is no clear consensus in the literature regarding the standard management PNETs, particularly for non-functional tumors with diameters below 2 cm discovered incidentally[9].
At the time of this study, there were no other investigations of PNETs in South America that focused on tumor size as a predictor of poor prognosis. It is important to emphasize that while tumors with diameters above 2 cm also exhibited a higher percentage of malignancy-related characteristics and were associated with a poorer prognosis, smaller tumors exhibited some of those same malignancy-related characteristics as well. For instance, up to 26.3% of smaller tumors had a mitotic rate of greater than 3/10 per high-power field, 31.6% had a Ki67 proliferation index above 3%, and 35.7% positively expressed the CK19 marker.
Similarly, neural and lymphovascular invasion, which are classically associated with malignancy and an increased risk of recurrence, were observed in up to 9.1% of tumors with diameters smaller than 2 cm[3,10]. Furthermore, neural and lymphovascular invasion were also correlated with shorter survival times and a higher risk of mortality: HR of 6.44 (95%CI: 1.43–28.93), P = 0.015 and HR of 24.87 (95%CI: 2.98–207.19), P = 0.003, respectively.
Other studies have reported that up to 33% of smaller tumors exhibit the presence of lymph node involvement[11]. However, this adverse prognostic feature was exclusively observed in tumors with diameters larger than 2 cm in our study. This discrepancy may be attributed, in part, to the relatively small sample size of this study.
Furthermore, the recurrence rate observed after tumor resection in this study was consistent with the rates reported in the literature[12]. But we found no statistically significant difference in recurrence rate as a function of tumor size: the recurrence rate for tumors smaller than 2 cm was 18.2%, and the recurrence rate for larger tumors was 21.4%. This finding raises questions about the potential biological aggressiveness of small tumors and prompts consideration of whether the 2 cm cutoff should be reconsidered as a defining threshold for a higher risk of malignancy. Regenet and colleagues emphasized that tumors with diameters larger than 1.7 cm were associated with a greater risk of malignancy[13]. More recently, Hsu et al[14] demonstrated that even among tumors ranging from 1 to 2 cm in size, 11% already had metastatic involvement at the time of diagnosis[14].
As noted above, our study revealed no statistically significant difference in survival rate for patients with tumors exceeding 2 cm in diameter and patients with smaller tumors. This observation carries profound implications for re
Studies like Sharpe et al[15] have demonstrated survival benefits for patients who underwent pancreatic resections for tumors smaller than 2 cm[15]. However, we are unable to provide a definitive recommendation for the optimal mana
It is important to acknowledge the limitations of our study, notably its retrospective nature, its single-center design, and its relatively small patient cohort. These limitations may reduce the overall validity and statistical power of our findings. To establish a robust association between the described characteristics in our study and the observed outcomes, a larger and more diverse patient sample would be essential. Further research is warranted to address these limitations comprehensively.
However, it is noteworthy that this study represents the largest reported series of Colombian patients with PNETs. Given the unique socio-economic context of Colombia (i.e., a country characterized by limited healthcare resources and accessibility challenges), surgical intervention may indeed be a viable and rational option. This perspective is further justified by the possibility that these tumors may exhibit histological characteristics associated with poor prognosis over time.
However, it is also crucial to acknowledge that, like any surgical procedure, pancreatic resections carry the inherent risk of complications. Notably, for small enucleations, morbidity rates can be as high as 28%[16]. Therefore, a balanced consideration of the potential benefits and risks of surgery, especially in the context of Colombia's healthcare landscape, is imperative when determining the most appropriate management strategy for PNETs.
Pancreatic neuroendocrine tumors with a diameter smaller than 2 cm share some of the characteristics associated with poor prognosis and poorer clinical outcomes with larger tumors. The presence of lymphovascular invasion, neural invasion, high mitotic index, and a Ki67 proliferation index above 3% are features that are more frequently observed in tumors with a diameter above 2 cm and are associated with a higher risk of mortality. It is crucial to underscore that the characteristics associated with unfavorable clinical outcomes were also detected in a noteworthy percentage of PNETs with a diameter below 2 cm. This finding implies that surgical resection of these small tumors might offer potential benefits to patients with this condition; these tumors may not be as benign, as previously suggested by earlier studies[7]. Nonetheless, it is imperative to carry out studies with a larger sample size and a prospective design to definitively ascertain whether this approach would indeed yield a significant clinical impact, thereby enabling concrete recommendations to be established.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A, Croatia S-Editor: A L-Editor: A P-Editor: Zheng XM
| 1. | Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D'Angelica MI, DeMatteo RP, Kingham TP, Groot Koerkamp B, Untch BR, Brennan MF, Jarnagin WR, Allen PJ. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol. 2016;23:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Imperatore N, de Nucci G, Mandelli ED, de Leone A, Zito FP, Lombardi G, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a systematic review of the literature. Endosc Int Open. 2020;8:E1759-E1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Pulvirenti A, Pea A, Chang DK, Jamieson NB. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front Med (Lausanne). 2020;7:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20:2815-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Bartolini I, Bencini L, Risaliti M, Ringressi MN, Moraldi L, Taddei A. Current Management of Pancreatic Neuroendocrine Tumors: From Demolitive Surgery to Observation. Gastroenterol Res Pract. 2018;2018:9647247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, Scarpa A, Falconi M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, Minter RM, Lairmore TC, Tseng JF, Zeh HJ, Libutti SK, Singh G, Lee JE, Hope TA, Kim MK, Menda Y, Halfdanarson TR, Chan JA, Pommier RF. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 10. | Dong DH, Zhang XF, Lopez-Aguiar AG, Poultsides G, Makris E, Rocha F, Kanji Z, Weber S, Fisher A, Fields R, Krasnick BA, Idrees K, Smith PM, Cho C, Beems M, Dillhoff M, Maithel SK, Pawlik TM. Resection of pancreatic neuroendocrine tumors: defining patterns and time course of recurrence. HPB (Oxford). 2020;22:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Pulvirenti A, Javed AA, Landoni L, Jamieson NB, Chou JF, Miotto M, He J, Gonen M, Pea A, Tang LH, Nessi C, Cingarlini S, D'Angelica MI, Gill AJ, Kingham TP, Scarpa A, Weiss MJ, Balachandran VP, Samra JS, Cameron JL, Jarnagin WR, Salvia R, Wolfgang CL, Allen PJ, Bassiy C. Multi-institutional Development and External Validation of a Nomogram to Predict Recurrence After Curative Resection of Pancreatic Neuroendocrine Tumors. Ann Surg. 2021;274:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Regenet N, Carrere N, Boulanger G, de Calan L, Humeau M, Arnault V, Kraimps JL, Mathonnet M, Pessaux P, Donatini G, Venara A, Christou N, Bachelier P, Hamy A, Mirallié E. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery. 2016;159:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Hsu D, Le S, Chang A, Spitzer A, Kazantsev G, Peng PD, Chang C, Truong TG. Should all pancreatic neuroendocrine tumors (PNET) over 1 cm be resected? J Clin Oncol. 2021;39:4108-4108. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19:117-23; discussion 123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, Hartwig W, Büchler MW, Werner J. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |