Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1319
Peer-review started: October 23, 2023
First decision: December 12, 2023
Revised: December 20, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: April 15, 2024
Processing time: 170 Days and 12.2 Hours
Cholangiocarcinoma (CCA) is a highly malignant biliary tract cancer with poor prognosis. Previous studies have implicated the gut microbiota in CCA, but evidence for causal mechanisms is lacking.
To investigate the causal relationship between gut microbiota and CCA risk.
We performed a two-sample mendelian randomization study to evaluate potential causal associations between gut microbiota and CCA risk using genome-wide association study summary statistics for 196 gut microbial taxa and CCA. Genetic variants were used as instrumental variables. Multiple sensitivity analyses assessed result robustness.
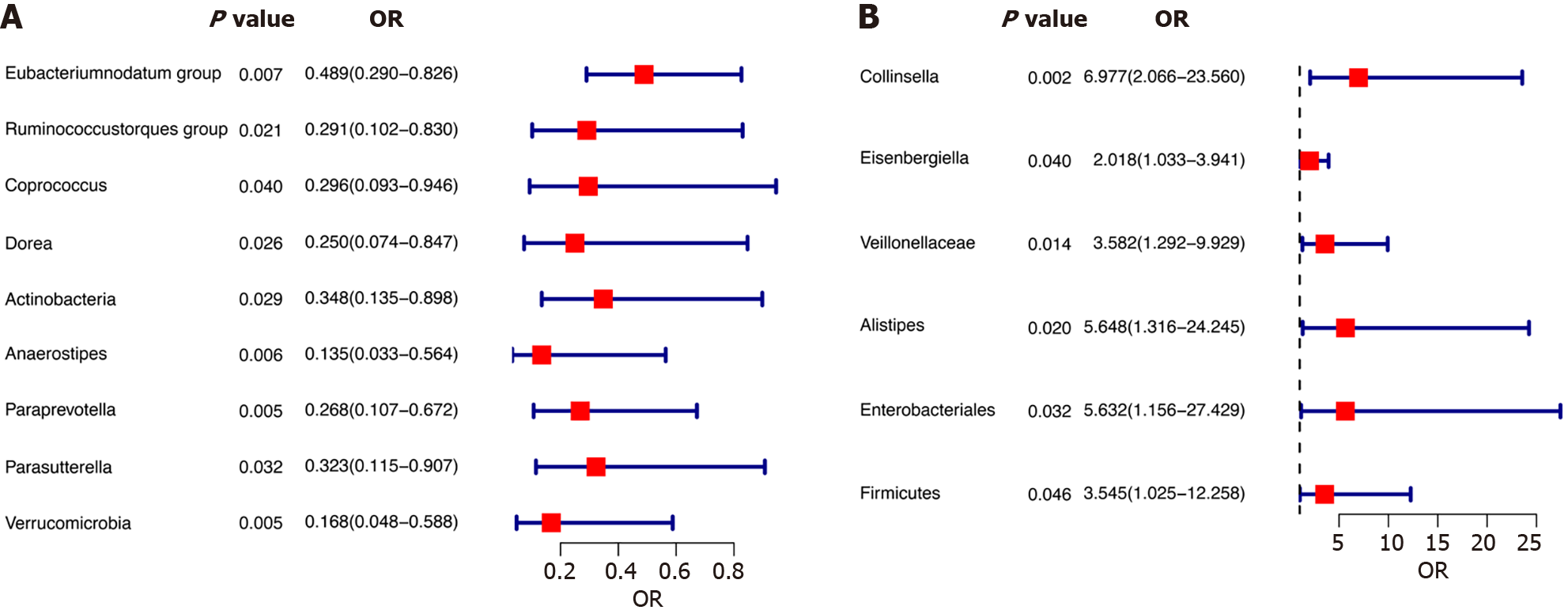
Fifteen gut microbial taxa showed significant causal associations with CCA risk. Higher genetically predicted abundance of genus Eubacteriumnodatum group, genus Ruminococcustorques group, genus Coprococcus, genus Dorea, and phylum Actinobacteria were associated with reduced risk of gallbladder cancer and extrahepatic CCA. Increased intrahepatic CCA risk was associated with higher abundance of family Veillonellaceae, genus Alistipes, order Enterobacteriales, and phylum Firmicutes. Protective effects against CCA were suggested for genus Collinsella, genus Eisenbergiella, genus Anaerostipes, genus Paraprevotella, genus Parasutterella, and phylum Verrucomicrobia. Sensitivity analyses indicated these findings were reliable without pleiotropy.
This pioneering study provides novel evidence that specific gut microbiota may play causal roles in CCA risk. Further experimental validation of these candidate microbes is warranted to consolidate causality and mechanisms.
Core Tip: Cholangiocarcinoma (CCA) is a highly malignant biliary tract cancer with poor prognosis. Emerging evidence suggests the gut microbiota may play a causal role in CCA pathogenesis, but robust genetic evidence is still lacking. Using genome-wide association study summary statistics, our study provides novel evidence that 15 gut microbial taxa may confer either protective or detrimental causal effects on CCA risk.
- Citation: Chen ZT, Ding CC, Chen KL, Gu YJ, Lu CC, Li QY. Causal roles of gut microbiota in cholangiocarcinoma etiology suggested by genetic study. World J Gastrointest Oncol 2024; 16(4): 1319-1333
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1319.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1319
Cholangiocarcinoma (CCA) originates from the biliary epithelium and is among the most prevalent malignancies due to its significant malignancy potential[1,2]. Based on anatomical site of origin, CCA manifests as three distinct subtypes: Intrahepatic CCA (iCCA), extrahepatic CCA (eCCA), and gallbladder cancer (GC)[1]. Established risk factors for CCA include fluke infections, inflammatory bowel disease, intrahepatic bile duct stones, choledochal cysts, and primary sclerosing cholangitis (PSC)[3,4]. Despite recent progress in diagnosis and therapy, CCA prognosis remains poor with 5-year survival below 5% for advanced disease[5-7]. Further elucidation of CCA pathogenesis at the molecular, epigenetic and genomic levels is therefore critical to enable novel treatment approaches.
In recent years, the gut microbiota has emerged as a key factor governing health[8,9]. Microbiota dysbiosis can impact immune function, metabolism and physiology, contributing to diseases like obesity, diabetes, non-alcoholic fatty liver disease and cancer[10-12]. Anatomically and physiologically, the hepatobiliary duct and gastrointestinal tract comprise a “gut-liver axis” that regulates liver pathology and intrahepatic/systemic immunity[13]. The microbiota likely contributes to diverse hepatobiliary conditions including cancer, PSC, choledocholithiasis and cholelithiasis[14-17]. Previous research has revealed that the gut microbiota plays a pivotal role in the diagnosis and treatment of CCA[18,19]. In-depth investigations into the role of the gut microbiota in CCA have significantly improved the prognostic outlook for individuals affected by this disease. However, the causal relationship between the gut microbiota and CCA remains unclear. Elucidating such mechanisms would enable microbiome modulation as an early preventative approach aligning with precancer interception paradigms.
Establishing causality is challenged by limited clinical trial follow-up and potential confounding in observational studies. Mendelian randomization (MR) helps address this by using genetic variants as instrumental variables (IVs)[20]. The present study represents a pioneering effort in employing a two-sample MR approach to discern a potential causal link between particular gut microbiota taxa and CCA, thereby offering valuable insights for subsequent mechanistic inquiries.
This research adheres to the STROBE-MR Guidelines, and all data employed in this study are openly available and appropriately cited[21]. Consequently, our study did not require additional ethics committee approval.
Figure 1 presents the directed acyclic graph guiding the design of the current MR study. In this framework, the gut microbiota represents the exposure variables, while CCA constitutes the outcome variable. Genetic variants associated with gut microbiota taxa were leveraged as IVs to evaluate potential causal associations of gut microbiota composition with CCA risk, thereby minimizing issues of confounding.
A large-scale genome-wide association study (GWAS) encompassed 18340 participants drawn from 24 diverse cohorts spanning multiple countries, examining 122110 Loci of genetic variation. This study provided summary statistics for gut microbiota based on 16S rRNA gene sequencing data obtained from the MiBioGen (https://mibiogen.gcc.rug.nl/) database[22]. Among the participants, a significant majority, 13266 individuals or 72.3%, were of European ancestry. The study encompassed a broad spectrum of 211 traits, which included members from 131 genera, 35 families, 20 orders, 16 classes, and 9 phyla. In the current MR study, 15 unidentified taxa were notably excluded, resulting in the analysis incorporating 196 taxonomic units, spanning 9 phyla, 16 classes, 20 orders, 32 families, and 119 genera. Jiang et al[23] conducted analyses of summary statistics for malignant neoplasms of the gallbladder and extrahepatic bile ducts (195 European ancestry cases, 456153 European ancestry controls) as well as intrahepatic CCA (104 European ancestry cases, 456244 European ancestry controls), which were provided by the GWAS Catalog (https://www.ebi.ac.uk/gwas/).
MR analyses utilized IVs, primarily single nucleotide polymorphisms (SNPs), as mediators to explore causality between exposures and outcomes. The foundational assumption in MR necessitates that all SNPs robustly and independently predict the exposure variable at the genome-wide significance level. In present research, we utilized robust SNPs associated with gut microbiota as IVs for the exposure variable. However, applying a stringent threshold of 5 × 10-8 would have excluded the majority of these SNPs. Consequently, we opted for a relatively lenient yet still statistically significant threshold of 1 × 10-05, as supported by prior studies[24,25]. This threshold was set to encompass most gut microbiota-associated SNPs, ensuring that those with an R2 < 0.001 and a physical distance (kb) of 10000 were included, thus mitigating linkage disequilibrium (LD). The F statistic was employed to assess the strength of the correlation between IVs and exposures, with an F statistic exceeding 10 typically indicating a substantial correlation. These screening criteria serve to establish the reliability of the findings in present MR study.
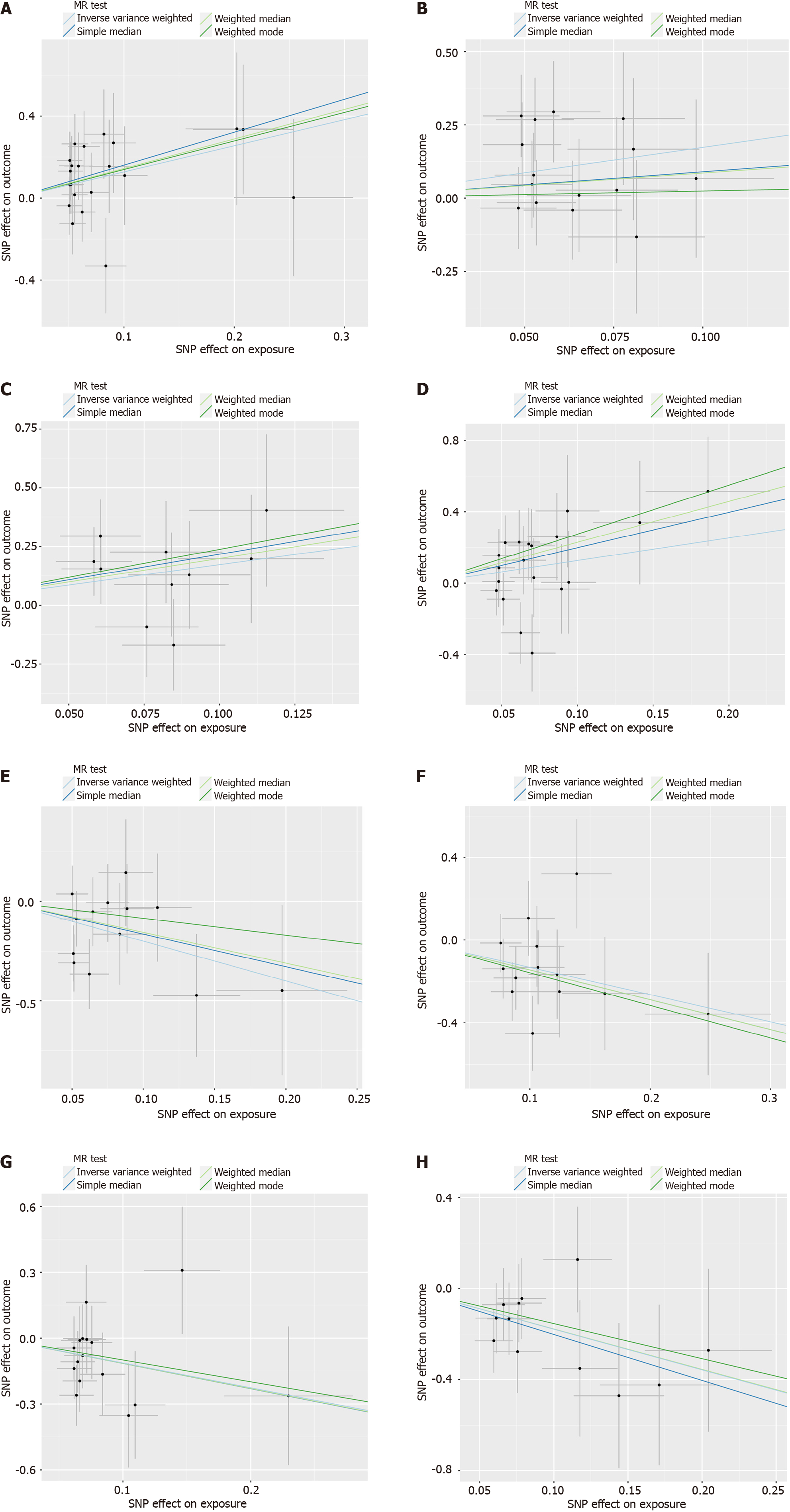
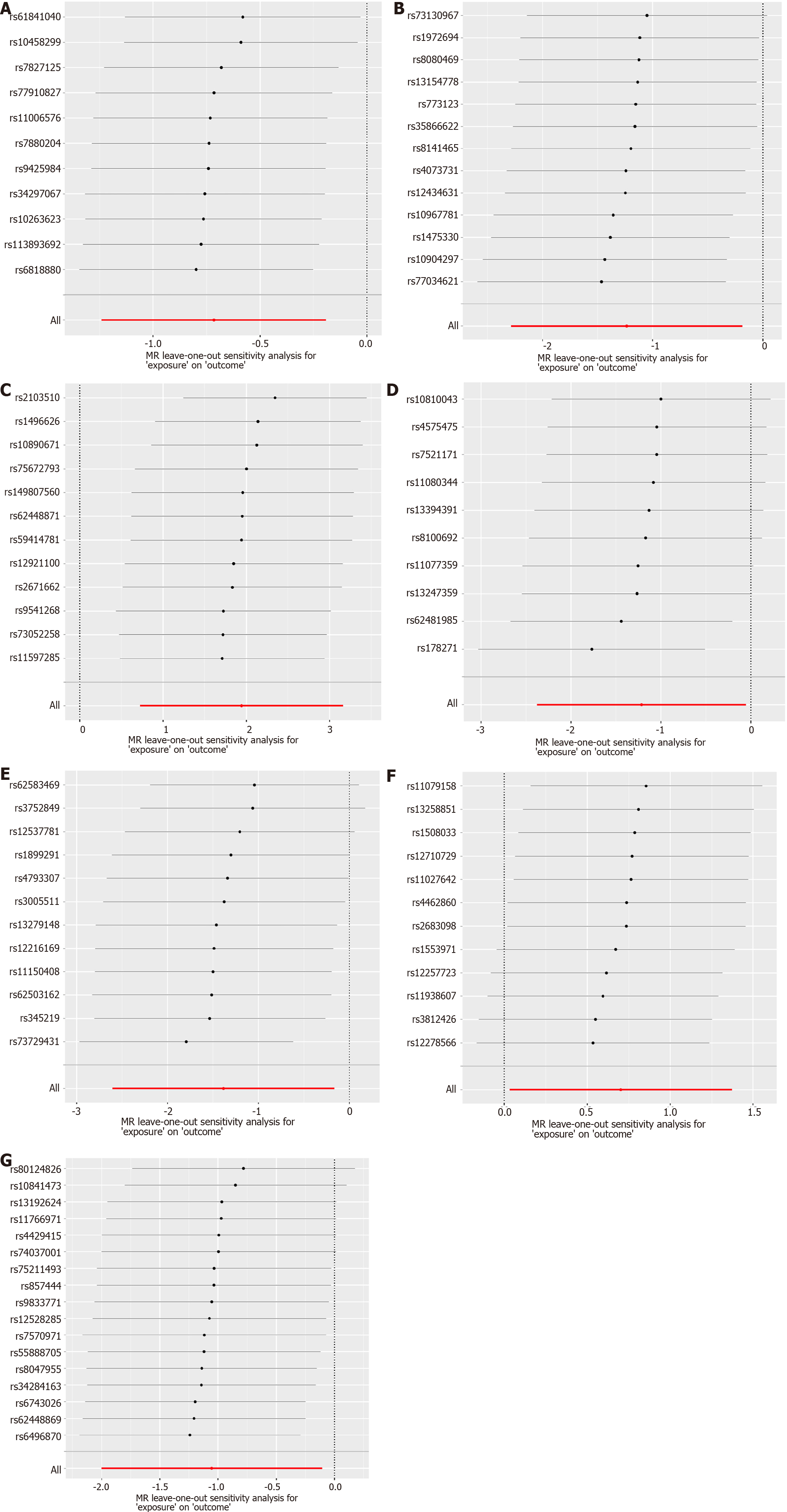
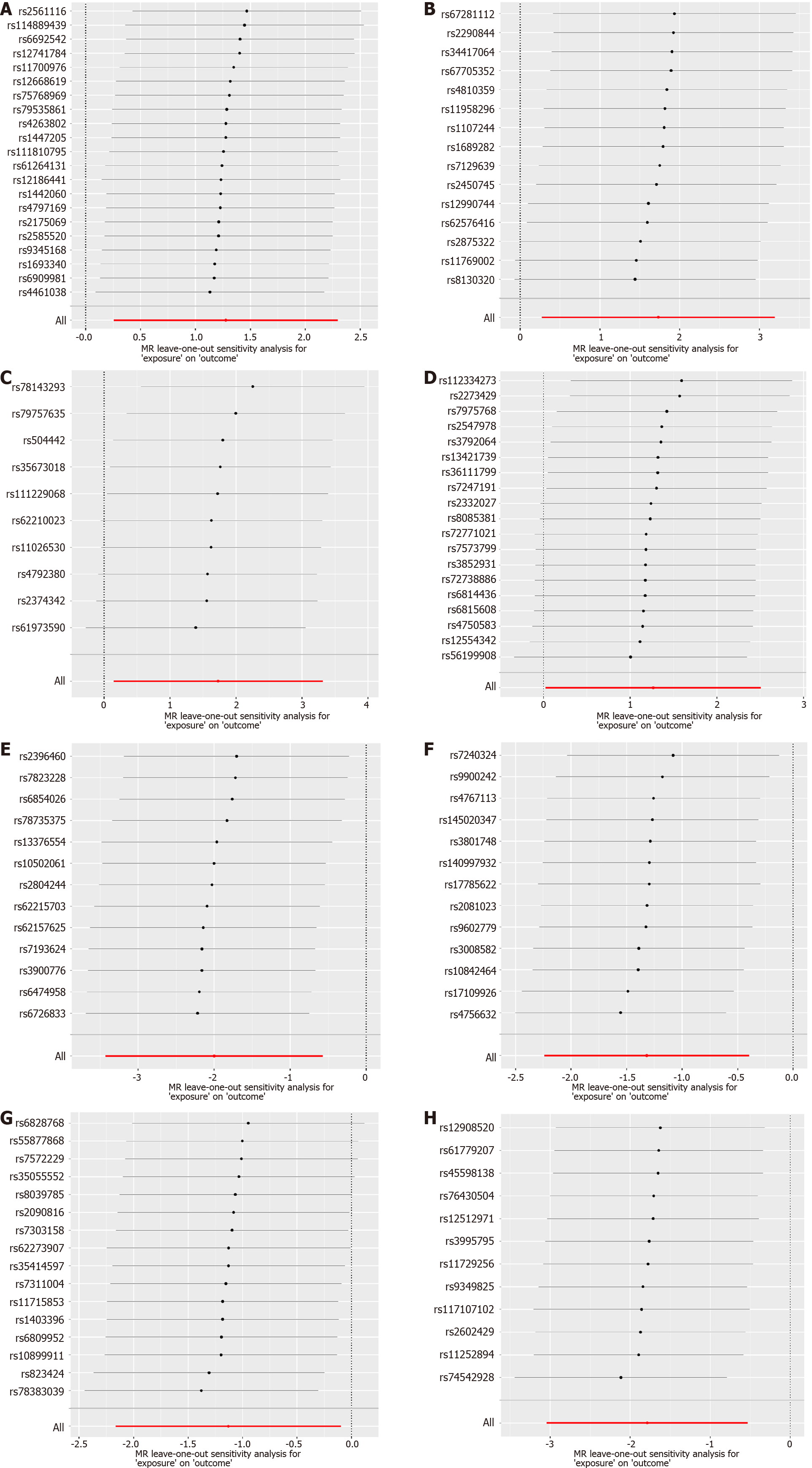
The analyses were conducted in RStudio (Version: 2023.06.1 + 524) using the TwoSampleMR package (version 0.5.7) and MRPRESSO package. In the context of a global-level test, a two-sided P value of 0.05 was considered statistically significant. In present study, we utilized a comprehensive approach, incorporating MR-Egger, weighted median, inverse variance weighted (IVW), simple mode, and weighted mode methodologies for MR analysis, enabling a thorough assessment of the causal relationship between gut microbiota and CCA. In cases where pleiotropy among IVs is absent, IVW is selected as the primary analytical method due to its superior statistical power[26]. To evaluate the reliability of our findings, we conducted a set of sensitivity analyses, including Cochran’s Q test, MR-Egger intercept test, and MR-PRESSO global test. Both the Cochrane’s Q test and MR-Egger intercept test were employed to assess the presence of SNP-associated heterogeneity and horizontal pleiotropy for each gut microbiota trait. The outcomes revealed P values exceeding 0.05, indicating the absence of heterogeneity and horizontal pleiotropy. Outliers were identified through the application of MR-PRESSO analysis. Additionally, we also employed funnel plots and conducted leave-one-out sensitivity tests to assess heterogeneity. The leave-one-out analysis was utilized to identify potential pleiotropic effects originating from individual SNPs. Scatter plots, forest plots, funnel plots, and leave-one-out sensitivity tests serve as valuable tools for visualizing MR results in a comprehensive manner.
Initially, we identified 122100 SNPs associated with gut microbiota traits through the MiBioGen Consortium dataset. Following a rigorous sequence of quality control procedures based on locus-wide statistical significance (P < 1 × 10-5) and the LD threshold (R2 < 0.001, with a clumping distance of 10000 kb), 2236 SNPs associated with 196 gut microbiota trials were selected as IVs. Notably, all IVs exhibited F-statistics exceeding 10, thereby indicating the absence of evidence for weak instrument bias (Supplementary Table 1). Based on these SNPs, we have extracted corresponding pieces of information from the outcome variable dataset (Supplementary Tables 2 and 3).
According to the results of the IVW method, the higher genetically predicted abundance of genus Eubacteriumnodatum group [odds ratio (OR) = 0.489, standard error (SE) = 0.267, 95% confidence interval (CI): 0.290-0.826, P = 0.007], genus Ruminococcustorques group (OR = 0.291, SE = 0.535, 95%CI: 0.102-0.830, P = 0.021), genus Coprococcus (OR = 0.296, SE = 0.593, 95%CI: 0.093-0.946, P = 0.040), genus Dorea (OR = 0.250, SE = 0.622, 95%CI: 0.074-0.847, P = 0.026), phylum Actinobacteria (OR = 0.348, SE = 0.483, 95%CI: 0.135-0.898, P = 0.029) were associated with a reduced risk of GC and eCCA (Figure 2A). In contrast, genetically predicted abundance of genus Collinsella (OR = 6.977, SE = 0.621, 95%CI: 2.006-23.560, P = 0.002), genus Eisenbergiella (OR = 2.018, SE = 0.342, 95%CI: 1.033-3.941, P = 0.040) was positively related to GC and eCCA risk (Figure 2B). The weighted median, simple mode, and weighted mode exhibited the same directional impact as IVW, although the P values were not consistently statistically significant (Supplementary Table 4, Figure 3).
Employing the IVW method, our study found suggestive evidence of a potential causal link between genetically predicted increases in the family Veillonellaceae (P = 0.014, 95%CI: 1.292-9.929, OR = 3.582, SE = 0.520), order Enterobacteriales/family Enterobacteriaceae (P = 0.032, 95%CI: 1.156-27.429, OR = 5.632, SE = 0.808), genus Alistipes (P = 0.020, 95%CI: 1.316-24.245, OR = 5.648, SE = 0.743), and phylum Firmicutes (P = 0.046, 95%CI: 1.025-12.258, OR = 3.545, SE = 0.633) with an increased risk of iCCA (Figure 2B). From the earlier mentioned traits, it was noted that both the order Enterobacteriales and the family Enterobacteriaceae fall under the same bacterial category and share identical IVs. Furthermore, our findings suggest that genetically predicted increases in the genus Anaerostipes (P = 0.006, 95%CI: 0.033-0.564, OR = 0.135, SE = 0.728), the genus Parasutterella (P = 0.032, 95%CI: 0.115-0.907, OR = 0.323, SE = 0.527), the genus Paraprevotella (P = 0.005, 95%CI: 0.107-0.672, OR = 0.268, SE = 0.470), and the phylum Verrucomicrobia (P = 0.005, 95%CI: 0.048-0.588, OR = 0.168, SE = 0.640) are associated with protective effects against iCCA (Figure 3A). Additionally, the causal effect estimates derived from the weighted median, simple mode, and weighted mode methods showed similar magnitudes and directions as those obtained with the previously mentioned IVW method (Supplementary Table 4, Figure 4).
Subsequently, a comprehensive sensitivity analysis was carried out to assess the stability and reliability of the inferred causal relationship between gut microbiota and CCA. The detailed description is summarized in Table 1. Cochran’s Q statistics revealed the absence of significant heterogeneity among the selected IVs, with P values exceeding 0.05 in both IVW and MR-Egger methods. Furthermore, our analysis, employing the MR-Egger intercept method, did not reveal any indications of a horizontal pleiotropic effect (P > 0.05). The outcomes from the MR-PRESSO trial indicated the absence of any horizontal pleiotropic outliers. The leave-one-out analysis results demonstrated that none of the SNPs were influential outliers (Figures 5 and 6). Additionally, the use of funnel and forest plots to depict a symmetrical pattern serves to visually affirm the reliability of the study’s results (Supplementary Figures 1-4).
| Exposure | Outcome | Method | Heterogeneity | Horizontal pleiotropy | MR-PRESSO | ||
| Q | Q, P value | Egger intercept | P value | P value | |||
| Verrucomicrobia | iCCA | MRE | 4.795 | 0.904 | -0.048 | 0.762 | 0.951 |
| IVW | 4.892 | 0.936 | |||||
| Firmicutes | iCCA | MRE | 16.151 | 0.513 | -0.117 | 0.342 | 0.527 |
| IVW | 17.108 | 0.516 | |||||
| Enterobacteriales/Enterobacteriaceae | iCCA | MRE | 5.600 | 0.692 | 0.231 | 0.433 | 0.725 |
| IVW | 6.282 | 0.711 | |||||
| Parasutterella | iCCA | MRE | 9.901 | 0.769 | -0.062 | 0.638 | 0.805 |
| IVW | 10.133 | 0.811 | |||||
| Paraprevotella | iCCA | MRE | 10.402 | 0.495 | -0.057 | 0.740 | 0.619 |
| IVW | 10.518 | 0.571 | |||||
| Anaerostipes | iCCA | MRE | 9.312 | 0.593 | -0.095 | 0.526 | 0.679 |
| IVW | 9.740 | 0.639 | |||||
| Alistipes | iCCA | MRE | 8.587 | 0.803 | 0.203 | 0.373 | 0.808 |
| IVW | 9.437 | 0.802 | |||||
| Veillonellaceae | iCCA | MRE | 13.399 | 0.818 | 0.037 | 0.672 | 0.850 |
| IVW | 13.584 | 0.851 | |||||
| Eubacteriumnodatum group | GC and eCCA | MRE | 6.622 | 0.676 | 0.106 | 0.555 | 0.755 |
| IVW | 6.997 | 0.726 | |||||
| Ruminococcustorques group | GC and eCCA | MRE | 6.527 | 0.836 | -0.113 | 0.309 | 0.812 |
| IVW | 7.665 | 0.811 | |||||
| Collinsella | GC and eCCA | MRE | 14.609 | 0.147 | 0.024 | 0.894 | 0.229 |
| IVW | 14.636 | 0.200 | |||||
| Coprococcus | GC and eCCA | MRE | 4.421 | 0.817 | -0.311 | 0.068 | 0.442 |
| IVW | 8.877 | 0.449 | |||||
| Dorea | GC and eCCA | MRE | 13.383 | 0.203 | -0.031 | 0.820 | 0.304 |
| IVW | 13.456 | 0.265 | |||||
| Eisenbergiella | GC and eCCA | MRE | 10.563 | 0.393 | -0.052 | 0.857 | 0.493 |
| IVW | 10.599 | 0.477 | |||||
| Actinobacteria | GC and eCCA | MRE | 14.593 | 0.481 | 0.182 | 0.153 | 0.406 |
| IVW | 16.855 | 0.395 | |||||
CCA is a highly diverse form of cancer, with its global incidence steadily on the rise[27]. With surgical resection being the exclusive curative treatment modality, the prognosis for individuals afflicted with CCA remains bleak[28]. In recent years, the prevention of tumor initiation and the inhibition of tumor progression have emerged as pivotal milestones in cancer management within the field of oncology. Changes in the composition of gut microbiota are closely associated with the initiation and progression of cancer[8,29]. This study represents the inaugural attempt to evaluate the causal link between gut microbiota and CCA while also endeavoring to identify particular causative microbial taxa through two-sample MR analyses based on GWAS summary statistics.
The gut microbiota constitutes a complex and dynamically evolving assembly of ecological microbial communities that reside within the human gastrointestinal tract, often referred to as a “neglected organ”[30-32]. These microorganisms assume a pivotal role in maintaining the homeostasis of the digestive system, exerting multifaceted metabolic, immunological, and protective functions that contribute to the overall health of the host[32]. Although gut microbiota plays a crucial role in facilitating various essential and advantageous physiological processes, such as the digestion of macronutrients and the synthesis of certain vitamins, a wealth of empirical data underscores their potential involvement in the emergence of detrimental phenotypes[33,34]. Notably, discernible alterations in both the structure and function of the microbial community have been linked to numerous disease states, including cancer[31]. Due to the bidirectional communication between the gastrointestinal tract and the biliary system, the liver excretes bile acids and other biologically active components via the bile duct to interface with the intestine. Simultaneously, the gut microbiota and its metabolites are transported to the liver through the bile duct. Therefore, the gut microbiota plays a pivotal role in the pathogenesis and progression CCA[18,35]. Hence, there is a compelling need for additional investigations to elucidate the causal connection between gut microbiota and CCA, thereby establishing a novel theoretical foundation for the prevention and treatment of CCA.
The impact of the gut microbiota in the field of oncology is a double-edged sword, and our research has equally substantiated this perspective from a genetic standpoint. The gut microbiota actively fosters the development of extraintestinal cancers by facilitating bacterial translocation and the generation of bioactive molecules within the biliary tract. Numerous research investigations have demonstrated notable distinctions in gut microbiota composition between individuals with extraintestinal cancers and those without the disease[32,35]. Bacteroides and Ruminococcaceae have been shown to potentially contribute to the pathogenesis of hepatocellular carcinoma by exacerbating hepatic inflammation, accumulating toxic compounds, and inducing liver steatosis[36]. Zhang et al[35] observed a depletion of Saccharomyces cerevisiae (S. cerevisiae) in iCCA. Significantly, past studies have shown that S. cerevisiae has the capacity to impede the growth of colorectal tumors. It achieves this by inducing apoptosis in epithelial cells, modulating intestinal immunity, and altering the composition of the gut microbiota[37]. The microbiota can indirectly influence tumor progression by generating and metabolizing bioactive molecules, which, when carried through systemic circulation, such as bacterial LPS entering the bloodstream, can impact tumor formation in distant tissues from the gastrointestinal tract[38]. Lactic acid bacteria and Bifidobacterium play a role in the regulation of pH and bile acid processes[39]. Furthermore, their enzymatic capacity to degrade potential carcinogens and their metabolites, including heterocyclic amines, nitrosamines, and aflatoxins, contributes to the inhibition of the development of various cancers, such as gastric and liver cancers[40].
In this MR study, we determined that 15 microbial taxa are causally associated with CCA. Elevated genetic predisposition towards higher abundance of the genus Eubacteriumnodatum group, genus Ruminococcustorques group, genus Coprococcus, genus Dorea, phylum Actinobacteria, family Veillonellaceae, genus Alistipes, order Enterobacteriales/family Enterobacteriaceae, and phylum Firmicutes were found to be associated with a decreased risk of CCA. Conversely, a genetically predicted increase in the abundance of the genus Collinsella, genus Eisenbergiella, genus Anaerostipes, genus Paraprevotella, genus Parasutterella, and phylum Verrucomicrobia exhibited a positive correlation with the risk of CCA. The abundance of the genus Eubacteriumnodatum group was found significantly reduced in colorectal cancer patients, and its functionality appeared to be associated with processes related to protein digestion and absorption, as well as the renin-angiotensin system pathway[41]. The genus Ruminococcustorques group and phylum Actinobacteria also demonstrated an association with an elevated risk of bladder cancer[42]. Genus Coprococcus is a bacterium known for producing butyrate, and its presence may be associated with a reduction in the effectiveness of neoadjuvant chemoradiation therapy for rectal cancer[43]. Elevated abundance of the gut genus Dorea has the potential to serve as a predictive factor for farnesoid X receptor deactivation, which is recognized as a risk factor for metabolic dysfunction-associated steatotic liver disease[44]. The aforementioned findings indicate that certain protective or risky gut microbiota identified in this MR analysis are consistent with previous research and are likely to play significant roles as reference points in future clinical studies. Furthermore, the causal relationship between gut microbiota and CCA warrant further investigation through clinical and in vivo experiments to enhance our understanding of the “gut-liver axis” theories.
One major strength of this study is the utilization of the MR method, which assists in mitigating the impact of confounding variables, thereby enhancing the persuasiveness of the findings compared to observational research. Nevertheless, our analysis has several limitations that warrant consideration. Firstly, it is important to note that MR analyses were conducted at the bacterial genus level, as opposed to a more specific species level, due to the limited resolution provided by 16S rRNA sequencing. Secondly, the significance threshold for exposure IVs was set at 1 × 10-05 due to the inadequacy of IVs reaching genome-wide significance. However, IVs with F-statistics below 10 were excluded to mitigate the potential bias associated with weak instruments.
In summary, this two-sample MR study offers new insights, suggesting a potential causal link between certain gut microbiota taxa and CCA. By utilizing genetic variants as IVs, we identified 15 microbial taxa that may confer either protective or detrimental effects on CCA risk. This study sheds new light on the intricate gut-liver axis interactions and microbiota-mediated mechanisms underlying CCA. Further experimental validations are warranted to consolidate the causality, delineate the molecular events, and exploit the clinical values of these candidate microbes.
Cholangiocarcinoma (CCA) is a highly malignant biliary tract cancer with poor prognosis. Previous studies have implicated the gut microbiota in CCA, but evidence for causal mechanisms is lacking.
To investigate the causal relationship between gut microbiota and CCA risk.
To investigate the causal relationship between gut microbiota and CCA risk.
We performed a two-sample mendelian randomization study to evaluate potential causal associations between gut microbiota and CCA risk using genome-wide association study summary statistics for 196 gut microbial taxa and CCA. Genetic variants were used as instrumental variables. Multiple sensitivity analyses assessed result robustness.
Fifteen gut microbial taxa showed significant causal associations with CCA risk. Higher genetically predicted abundance of genus Eubacteriumnodatum group, Genus Ruminococcustorques group, Coprococcus, Dorea, and Actinobacteria were associated with reduced risk of gallbladder cancer and extrahepatic CCA. Increased intrahepatic CCA risk was associated with higher abundance of Veillonellaceae, Alistipes, Enterobacteriales, and Firmicutes. Protective effects against CCA were suggested for Collinsella, Eisenbergiella, Anaerostipes, Paraprevotella, Parasutterella, and Verrucomicrobia. Sensitivity analyses indicated these findings were reliable without pleiotropy.
This pioneering study provides novel evidence that specific gut microbiota may play causal roles in CCA risk. Further experimental validation of these candidate microbes is warranted to consolidate causality and mechanisms.
Experimental validation of the candidate microbes identified to be causally associated with CCA risk. Further in vitro and in vivo studies could be conducted to consolidate the causal effects and explore the underlying molecular mechanisms. Analysis of species-level resolution of gut microbiota through metagenomic shotgun sequencing or other techniques. The current study was limited to genus-level associations due to 16S rRNA gene sequencing. A more detailed characterization at the species level could provide further insights.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee H, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 2. | Rodrigues PM, Olaizola P, Paiva NA, Olaizola I, Agirre-Lizaso A, Landa A, Bujanda L, Perugorria MJ, Banales JM. Pathogenesis of Cholangiocarcinoma. Annu Rev Pathol. 2021;16:433-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer. 2019;19:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 5. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 6. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1539] [Article Influence: 307.8] [Reference Citation Analysis (0)] |
| 7. | Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27:100737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 812] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 9. | Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22:703-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 149] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 10. | Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 429] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 11. | Zhou CB, Zhou YL, Fang JY. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer. 2021;7:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, Li Q. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 13. | Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 330] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, Yu J. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 541] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 15. | Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, Zhu Z, Hong Z, Zhang T, Du G, Xiang J, Wu D, Bai W, Yang B, Li Y, Huang J, Li H, Safadi R, Lu Y. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology. 2020;71:893-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 16. | Lemoinne S, Kemgang A, Ben Belkacem K, Straube M, Jegou S, Corpechot C; Saint-Antoine IBD Network, Chazouillères O, Housset C, Sokol H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut. 2020;69:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 17. | Philips CA, Phadke N, Ganesan K, Rajesh S, Padsalgi G, Ahamed R, John SK, Valiathan GC, Augustine P. Gut Microbiota in Alcoholic Hepatitis is Disparate from Those in Acute Alcoholic Pancreatitis and Biliary Disease. J Clin Exp Hepatol. 2019;9:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhang T, Zhang S, Jin C, Lin Z, Deng T, Xie X, Deng L, Li X, Ma J, Ding X, Liu Y, Shan Y, Yu Z, Wang Y, Chen G, Li J. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients With Cholangiocarcinoma. Front Cell Infect Microbiol. 2021;11:751795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Herraez E, Romero MR, Macias RIR, Monte MJ, Marin JJG. Clinical relevance of the relationship between changes in gut microbiota and bile acid metabolism in patients with intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2020;9:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2788] [Cited by in RCA: 3868] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 21. | Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 2066] [Article Influence: 516.5] [Reference Citation Analysis (0)] |
| 22. | Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, Le Roy CI, Raygoza Garay JA, Finnicum CT, Liu X, Zhernakova DV, Bonder MJ, Hansen TH, Frost F, Rühlemann MC, Turpin W, Moon JY, Kim HN, Lüll K, Barkan E, Shah SA, Fornage M, Szopinska-Tokov J, Wallen ZD, Borisevich D, Agreus L, Andreasson A, Bang C, Bedrani L, Bell JT, Bisgaard H, Boehnke M, Boomsma DI, Burk RD, Claringbould A, Croitoru K, Davies GE, van Duijn CM, Duijts L, Falony G, Fu J, van der Graaf A, Hansen T, Homuth G, Hughes DA, Ijzerman RG, Jackson MA, Jaddoe VWV, Joossens M, Jørgensen T, Keszthelyi D, Knight R, Laakso M, Laudes M, Launer LJ, Lieb W, Lusis AJ, Masclee AAM, Moll HA, Mujagic Z, Qibin Q, Rothschild D, Shin H, Sørensen SJ, Steves CJ, Thorsen J, Timpson NJ, Tito RY, Vieira-Silva S, Völker U, Völzke H, Võsa U, Wade KH, Walter S, Watanabe K, Weiss S, Weiss FU, Weissbrod O, Westra HJ, Willemsen G, Payami H, Jonkers DMAE, Arias Vasquez A, de Geus EJC, Meyer KA, Stokholm J, Segal E, Org E, Wijmenga C, Kim HL, Kaplan RC, Spector TD, Uitterlinden AG, Rivadeneira F, Franke A, Lerch MM, Franke L, Sanna S, D'Amato M, Pedersen O, Paterson AD, Kraaij R, Raes J, Zhernakova A. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 1040] [Article Influence: 260.0] [Reference Citation Analysis (0)] |
| 23. | Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. 2021;53:1616-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 346] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 24. | Chen S, Zhou G, Han H, Jin J, Li Z. Causal effects of specific gut microbiota on bone mineral density: a two-sample Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1178831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Li Z, Chen Y, Ke H. Investigating the Causal Relationship Between Gut Microbiota and Crohn's Disease: A Mendelian Randomization Study. Gastroenterology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Kutalik Z, Holmes MV, Minelli C, Morrison JV, Pan W, Relton CL, Theodoratou E. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 451] [Article Influence: 225.5] [Reference Citation Analysis (0)] |
| 27. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 28. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 29. | Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, Coker OO, Lau HCH, Chan AWH, Sung JJY, Yu J. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology. 2022;162:135-149.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 290] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 30. | Mohr AE, Jäger R, Carpenter KC, Kerksick CM, Purpura M, Townsend JR, West NP, Black K, Gleeson M, Pyne DB, Wells SD, Arent SM, Kreider RB, Campbell BI, Bannock L, Scheiman J, Wissent CJ, Pane M, Kalman DS, Pugh JN, Ortega-Santos CP, Ter Haar JA, Arciero PJ, Antonio J. The athletic gut microbiota. J Int Soc Sports Nutr. 2020;17:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 31. | Muscolino P, Granata B, Omero F, De Pasquale C, Campana S, Calabrò A, D'Anna F, Drommi F, Pezzino G, Cavaliere R, Ferlazzo G, Silvestris N, Speranza D. Potential predictive role of gut microbiota to immunotherapy in HCC patients: a brief review. Front Oncol. 2023;13:1247614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 32. | Xue X, Li R, Chen Z, Li G, Liu B, Guo S, Yue Q, Yang S, Xie L, Zhang Y, Zhao J, Tan R. The role of the symbiotic microecosystem in cancer: gut microbiota, metabolome, and host immunome. Front Immunol. 2023;14:1235827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 33. | Popa AD, Niță O, Gherasim A, Enache AI, Caba L, Mihalache L, Arhire LI. A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Chen C, Chai D, Kuang T, Deng W, Wang W. Alterations of gut mycobiota profiles in intrahepatic cholangiocarcinoma. Front Microbiol. 2022;13:1090392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919848184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Li JQ, Li JL, Xie YH, Wang Y, Shen XN, Qian Y, Han JX, Chen YX, Fang JY. Saccharomyces cerevisiae may serve as a probiotic in colorectal cancer by promoting cancer cell apoptosis. J Dig Dis. 2020;21:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Shock T, Badang L, Ferguson B, Martinez-Guryn K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J Nutr Biochem. 2021;95:108631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1253] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 40. | Lili Z, Junyan W, Hongfei Z, Baoqing Z, Bolin Z. Detoxification of cancerogenic compounds by lactic acid bacteria strains. Crit Rev Food Sci Nutr. 2018;58:2727-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Huang R, He K, Duan X, Xiao J, Wang H, Xiang G. Changes of Intestinal Microflora in Colorectal Cancer Patients after Surgical Resection and Chemotherapy. Comput Math Methods Med. 2022;2022:1940846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Mingdong W, Xiang G, Yongjun Q, Mingshuai W, Hao P. Causal associations between gut microbiota and urological tumors: a two-sample mendelian randomization study. BMC Cancer. 2023;23:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 43. | Huang X, Chen C, Xie W, Zhou C, Tian X, Zhang Z, Wang Q, Chang H, Xiao W, Zhang R, Gao Y. Metagenomic Analysis of Intratumoral Microbiome Linking to Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Int J Radiat Oncol Biol Phys. 2023;117:1255-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 44. | Yang G, Liu R, Rezaei S, Liu X, Wan YY. Uncovering the Gut-Liver Axis Biomarkers for Predicting Metabolic Burden in Mice. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |