Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.979
Peer-review started: October 11, 2023
First decision: December 8, 2023
Revised: December 16, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 15, 2024
Processing time: 152 Days and 17.8 Hours
Helicobacter pylori (H. pylori) is the primary risk factor for gastric cancer (GC), the Wnt/β-Catenin signaling pathway is closely linked to tumourigenesis. GC has a high mortality rate and treatment cost, and there are no drugs to prevent the progression of gastric precancerous lesions to GC. Therefore, it is necessary to find a novel drug that is inexpensive and preventive to against GC.
To explore the effects of H. pylori and Moluodan on the Wnt/β-Catenin signaling pathway and precancerous lesions of GC (PLGC).
Mice were divided into the control, N-methyl-N-nitrosourea (MNU), H. pylori + MNU, and Moluodan groups. We first created an H. pylori infection model in the H. pylori + MNU and Moluodan groups. A PLGC model was created in the remaining three groups except for the control group. Moluodan was fed to mice in the Moloudan group ad libitum. The general condition of mice were observed during the whole experiment period. Gastric tissues of mice were grossly and microscopically examined. Through quantitative real-time PCR (qRT-PCR) and Western blotting analysis, the expression of relevant genes were detected.
Mice in the H. pylori + MNU group showed the worst performance in general condition, gastric tissue visual and microscopic observation, followed by the MNU group, Moluodan group and the control group. QRT-PCR and Western blotting analysis were used to detect the expression of relevant genes, the results showed that the H. pylori + MNU group had the highest expression, followed by the MNU group, Moluodan group and the control group.
H. pylori can activate the Wnt/β-catenin signaling pathway, thereby facilitating the development and progression of PLGC. Moluodan suppressed the activation of the Wnt/β-catenin signaling pathway, thereby decreasing the progression of PLGC.
Core Tip: Helicobacter pylori (H. pylori) is a pathological bacteria. We explored the effects of H. pylori and the traditional Chinese medicine Moluodan on the Wnt/β-Catenin signaling pathway and precancerous lesions of GC (PLGC). Our experiments successfully established a mouse model of H. pylori infection and PLGC, which serves as a reference for others. Through the gene expression assay, we concluded that H. pylori accelerates the progression of PLGC by promoting the expression of Wnt/β-Catenin signaling pathway, epidermal growth factor (EGF) and c-Myc. Meanwhile, Moluodan can repair the gastric mucosa and delay the progression of PLGC by inhibiting the Wnt/β-Catenin signaling pathway and the expression of EGF and c-Myc.
- Citation: Wang YM, Luo ZW, Shu YL, Zhou X, Wang LQ, Liang CH, Wu CQ, Li CP. Effects of Helicobacter pylori and Moluodan on the Wnt/β-catenin signaling pathway in mice with precancerous gastric cancer lesions. World J Gastrointest Oncol 2024; 16(3): 979-990
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/979.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.979
Gastric cancer (GC) is a significant health issue in China that is characterized by its complex etiology, high prevalence and mortality rates, and challenges in treatment[1]. GC ranks among the top three cancers in terms of mortality worldwide[2]. Unfortunately, early symptoms of GC are often inconspicuous, leading to delayed detection and missed opportunities for timely treatment. Notably, the development of GC involves stages such as chronic atrophic gastritis, heterogeneous hyperplasia, and intestinal epithelial hyperplasia, which are all considered as precancerous lesions[3,4]. Thus, imple
Helicobacter pylori (H. pylori) is a type of bacteria that thrives in the stomach and damages the gastric mucosa primarily through the production of urease and induction of immune responses[5]. Numerous reports, including the Kyoto Global Consensus, have identified H. pylori as a leading risk factor for GC[6]. Consequently, the timely eradication of H. pylori has become crucial in delaying the progression to GC. The Wnt/β-catenin signaling pathway is a ubiquitous intracellular signaling pathway that plays a role in early embryonic development by promoting cell proliferation and epithelial mesenchymal transition (EMT); this pathway has also been implicated in the development of various tumors[7,8]. Notably, the Wnt/β-catenin signaling pathway has a strong association with GC. Studies have demonstrated that this pathway can facilitate the development of GC through mechanisms involving microRNA, ligands, receptors, and other factors[9-11]. The relationship between H. pylori and signaling pathways has garnered increasing attention, which this study aimed to investigate. Traditional Chinese medicines have gained popularity among medical practitioners and have been extensively used in clinical practice, particularly in infection, tumors, and digestive, nervous, and cardiovascular systems[12,13]. One such medicine is Moluodan, which is commonly used in the treatment of digestive system disorders such as chronic gastritis and indigestion[14]. However, its role in PGLC and GC prevention as well as its relationship with the Wnt/β-catenin signaling pathway have not been explored. Therefore, the second objective of this study was to examine the association between Moluodan and the Wnt/β-catenin signaling pathway and its potential preventive effects against the development and progression of PLGC. This research aimed to provide a novel approach for the prevention and treatment of GC.
H. pylori Sydney strain SS1 (donated by the Third Military Medical University) was retrieved from a −80°C ultra-low temperature refrigerator (Thermo). The strain was then resuscitated at room temperature and mixed using a pipette gun. Subsequently, 100 µL of the bacterial solution was inoculated onto a solid culture medium. The bacterial solution was allowed to dry on the surface of the medium before being inverted and placed in a CO2 incubator for incubation at 37°C with 5% O2, 10% CO2, and 85% N2, creating a microaerobic environment. After 48–72 h, pinpoint-sized transparent colonies were observed. The presence of H. pylori was confirmed through HE staining, rapid urease testing, and catalase testing. A sterile inoculation loop was used to scrape a small quantity of cultured H. pylori colonies, which were evenly mixed with a PBS buffer. Subsequently, 100 µL of the mixture was aspirated and inoculated onto the next solid medium; the growth of colonies was observed within 48–72 h. Prior to administration in mice via gavage, H. pylori colonies were selected and dissolved in a PBS buffer. The concentration of the resulting liquid was controlled using turbidimetric methods to achieve a density of 1 × 109 colony forming units per milliliter of H. pylori mixture.
The powdered form of N-methyl-N-nitrosourea (MNU) was stored in a refrigerator at 4°C, ensuring protection from light. For the experiments, a solution with a concentration of 240 ppm (0.024%) was prepared by diluting the MNU in distilled water. The pH of the solution was maintained at 4.5 using a citric acid configuration solution. Fresh solution was prepared twice a week, and the bottle containing the solution was stored away from light to ensure the well-being of the free-range mice.
In this study, 14 SPF-grade Balb/c male mice, aged 6–8 wk, were obtained from Changzhou Cavins Experiment Co. They were allocated into four groups: A control group consisting of 3 mice, the MNU group consisting of 4 mice, the H. pylori + MNU group consisting of 4 mice, and the Moluodan group consisting of 3 mice. The mice were housed in a sterile laboratory with laminar air flow for 1 wk. The mice were subjected to a 12-h light–dark cycle, and the room temperature was maintained at 22–24°C with a humidity of 40%–50%. The mice were provided with standard mouse maintenance chow and had unrestricted access to water. Then the subsequent treatments were administered as follows: (1) Control group: On the second week, mice were fasted for 24 h, gavaged with 0.5 mL of saline, and resumed their normal diet after 4 h. The procedure was repeated every 2 d for five consecutive times. On the third week, the mice were fed with normal maintenance chow and sterile distilled water; (2) MNU group: The first 2 wk were similar to that of the control group. From the third week, the mice were given 240 ppm of MNU solution to drink freely. They were also protected from light and fed with 0.03% ranitidine (Beijing Jingming Biotechnology Co.) with their diet for 2 d followed by fasting for 1 d. On the fasting day, 10% of NaCl solution was heated to 56°C, and mice were gavaged at a dose of 10 mL/kg. On week 23, one mouse was randomly sacrificed, and stomach tissues were taken, stained with HE, and microscopically observed to confirm the development of PLGC. The rest of the mice were continuously fed with normal chow and sterile distilled water until week 29; (3) H. pylori + MNU group: Mice were fasted for 12 h at the beginning of the second week. Each mouse received 0.2 mL of 2% HaHCO3 solution followed by 0.4 mL of H. pylori solution for 1 h; normal diet was resumed after 4 h. This procedure was repeated once every 2 d for five consecutive times. From the third week until the end of the 29th week, the feeding regimen of the MNU group was adopted. On week 10, one mouse was sacrificed; stomach tissues were taken, stained with HE and Giemsa, and tested with the rapid urease assay to confirm H. pylori infection; and (4) Moluodan group: Mice in this group were fed the same way as those in the H. pylori group for the first 23 wk. After successful PLGC model creation, distilled water was substituted for a solution of 10 g/L Moluodan small honey pills (Handan Pharmaceutical Co.) in combination with normal chow until the end of the 29th week.
At the end of the 29th week, mice were fasted for 24 h. The mice were then intraperitoneally injected with 10% trichloroacetaldehyde hydrate at a dosage of 3 mL/kg. Gastric tissues were then exposed, isolated, and incised along the greater curvature of the stomach and thoroughly rinsed with 0.9% NaCl solution. Subsequently, tissues were grossly observed for histopathological and morphological changes. To confirm H. pylori infection, gastric sinus tissues from both the H. pylori + MNU and Moluodan groups underwent HE and Giemsa staining as well as rapid urease assay. Additionally, gastric tissues in all mice underwent HE staining after fixation with 10% formaldehyde, allowing for the microscopic observation of pathological changes. A portion of the samples were immediately frozen in liquid nitrogen and stored in a refrigerator at −80°C for subsequent molecular testing.
Rapid urease method: The procedure was performed according to the manufacturer’s instructions (Guangzhou Shunaimi Biotechnology Co.). The transparent film covering the urease test paper was carefully removed up to the dotted line. Fresh gastric sinus tissue was obtained, cut into small pieces, and placed in the center of the rapid urease test paper. Subsequently, the film was closed to seal the test paper. A color change from yellow to red in the central area of the paper within 3 min indicated a positive result for H. pylori.
HE stain: Fresh gastric sinus tissues were processed for histological analysis. Tissues were embedded in paraffin, sectioned, and subsequently deparaffinized. Hematoxylin staining was performed to visualize the nuclei, followed by ammonia rebluing and eosin re-staining. Dehydration and transparency of the tissues were achieved by immersing them in anhydrous ethanol and xylene. Tissue sections were then sealed with neutral tree glue. The presence of short bluish-purple rod-shaped or curved bacteria indicated the presence of H. pylori.
Giemsa stain: Fresh gastric sinus tissues were fixed using a 10% paraformaldehyde solution and embedded in paraffin. The paraffin-embedded tissues were then sectioned into 4-µm thick slices. To enhance hydrophilicity, sections were sequentially dewaxed using xylene and alcohol. Giemsa stain was applied dropwise onto the sections and allowed to stand for 30 min at 37°C. Following staining, the slides were washed with distilled water and soaked in xylene for 5 min until they became clear. Finally, the slides were sealed using neutral tree glue.
Following sacrifice, a segment of stomach tissues of mice was preserved in a 10% formaldehyde solution. Subsequently, the tissues were embedded in paraffin and cut into 4-µm thick sections. After removing the paraffin, the sections were stained in a sequential manner using HE. Following dehydration, the slices were sealed with neutral gum. The gastric mucosa of the mice was then examined under a light microscope to observe any pathological and histological alterations.
Mouse stomach tissue specimens were removed from the −80°C ultra-low temperature refrigerator and thawed at room temperature. They were then thoroughly ground in TRIpure Total RNA Extraction Reagent (EP013 ELK Biotechnology) and centrifuged at 10000 rpm for 10 min at 4°C. The supernatant was collected and centrifuged again for 10 min with isopropanol, and the supernatant was discarded. The RNA precipitate was washed with 75% ethanol, and 100 µL of RNase-Free Water was added to completely solubilize the RNA. cDNA was synthesized by reverse transcription using the EntiLink™ 1st Strand cDNA Synthesis Super Mix kit (EQ031 ELK Biotechnology) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed using the EnTurbo™ SYBR Green PCR Supermix Kit (EQ001 ELK Biotechnology) according to the manufacturer’s instructions. Briefly, pre-denaturation was performed at 95°C for 30 s followed by denaturation at 95°C for 10 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. The total volume was 10 µL, and 40 cycles were performed. Finally, the relative amount of mRNA for each gene was determined using the 2-ΔΔCt method. The reverse transcription primers employed are listed in Table 1.
| Primer name | Primer information | Base sequences (5’-3’) | Tm value | Product length | |
| M-GAPDH | NM_008084.3 | Sense | TGAAGGGTGGAGCCAAAAG | 58.3 | 227 |
| Anti-sense | AGTCTTCTGGGTGGCAGTGAT | 58.4 | |||
| M-wnt1 | NM_021279 | Sense | GCAGCCTCTTCTCACTGCAG | 61.0 | 148 |
| Anti-sense | CCCAGGCTGGCTCTAATAAGT | 59.2 | |||
| M-β-catenin | NM_007614 | Sense | TCCGAGGACTCAATACCATTCC | 60.6 | 250 |
| Anti-sense | CGCTTCTTGTAATCCTGTGGC | 60.0 | |||
| M-cyclinD1 | NM_007631.2 | Sense | GGATGAGAACAAGCAGACCATC | 58.6 | 186 |
| Anti-sense | AGAAAGTGCGTTGTGCGGTA | 59.2 | |||
| M-EGF | NM_010113 | Sense | TTATGACCCTGTGGAAAGCAAG | 58.6 | 119 |
| Anti-sense | CAAGCGTATCTACTCCTTCTGTGAT | 60.2 | |||
| M-c-myc | NM_001177352.1 | Sense | GGACTGTATGTGGAGCGGTTT | 59.1 | 208 |
| Anti-sense | GTTGAGCGGGTAGGGAAAGA | 59.3 | |||
Gastric tissues were washed several times with PBS buffer, cut into pieces, and added to a tissue protein extraction reagent. The supernatant was collected after lysis in an ice bath. The BCA Protein Concentration Assay Kit (AS1086 ASPEN) was used to determine the protein concentration of the sample. After sample processing, SDS-PAGE electrophoresis and membrane transfer were conducted. The transferred membranes were incubated with the sealing solution for 1 h at room temperature, and the primary antibody (AS1061 ASPEN) was added and incubated overnight at 4°C. The membranes were again incubated for 1 h at room temperature with the sealing solution. After buffer rinsing, the secondary antibody (AS1058 ASPEN) was added, and the solution was re-incubated. Finally, the membranes were exposed, developed, and fixed, and the optical density values of the target bands were analyzed using AlphaEaseFC software.
Statistical analyses were performed using SPSS 27.0 software, while image depiction was performed using GraphPad Prism 9.0 software. Experimental data are presented as mean ± SD. One-way analysis of variance was conducted for between-group comparisons. P < 0.05 was considered statistically significant.
The control group exhibited an optimal mental condition as characterized by high activity levels, responsive reflexes, normal food consumption, glossy and thick fur, and regular and well-formed stools. Meanwhile, mice in the MNU group displayed inferior conditions than that of the control group. The H. pylori + MNU group exhibited the most severe deterioration, including a significantly reduced mental status, dull eyes, decreased activity levels, slow reflexes, decreased food intake, thinning and lackluster fur, and loose and irregular stools. Initially, mice in the Moluodan group showed a similar condition to those in the H. pylori + MNU group. However, upon the addition of Moluodan to the animals’ diet, the mental state, activity levels, feeding behavior, fur quality, and overall condition of mice improved significantly, and their stools became regular and well-formed.
Following rinsing of gastric tissues with physiological saline, the gastric mucosa appeared smooth, gastric tissues were elastic, and no abnormalities were detected along the gastric wall. In the MNU group, there was a decrease in mucus secretion in the stomach along with a slightly rough and swollen gastric mucosa, scattered hemorrhages, and thinning and reduced elasticity of the gastric wall. Gastric tissues in the H. pylori + MNU group exhibited only a small amount of mucus adhering to the stomach along with a more swollen and rough stomach wall, increased inflammatory manifestations, and decreased elasticity. In comparison, gastric tissues in the Moluodan group showed an improvement in the gastric mucosal condition compared to that of the H. pylori + MNU and MNU groups, with increased mucus secretion, mild swelling and scattered inflammatory changes and moderate elasticity of the gastric wall.
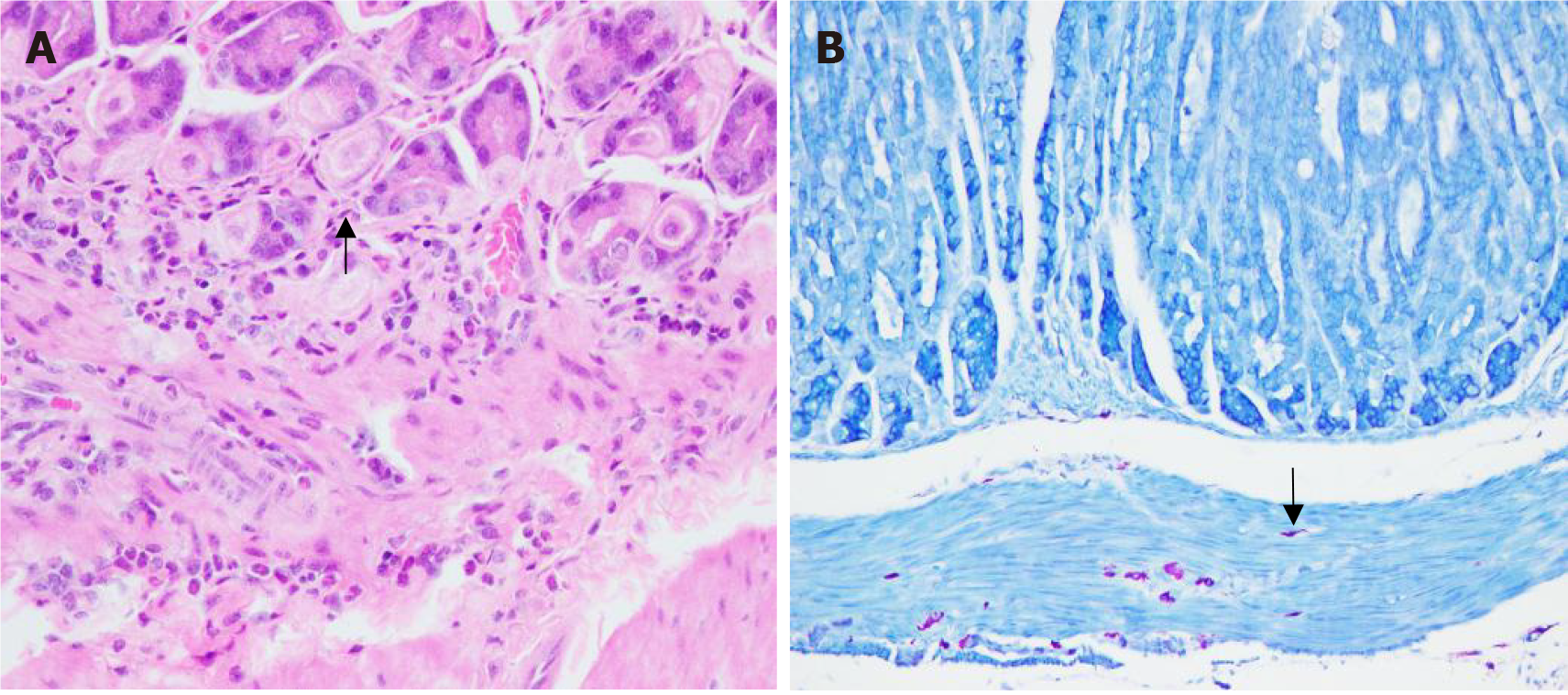
The findings indicated a positive urease test. HE staining revealed the presence of blue-purple rod-shaped H. pylori bacteria (Figure 1A). Additionally, Giemsa staining revealed purple short clostridial H. pylori bacteria (Figure 1B). Notably, all mice in both groups exhibited a 100% H. pylori infection rate.
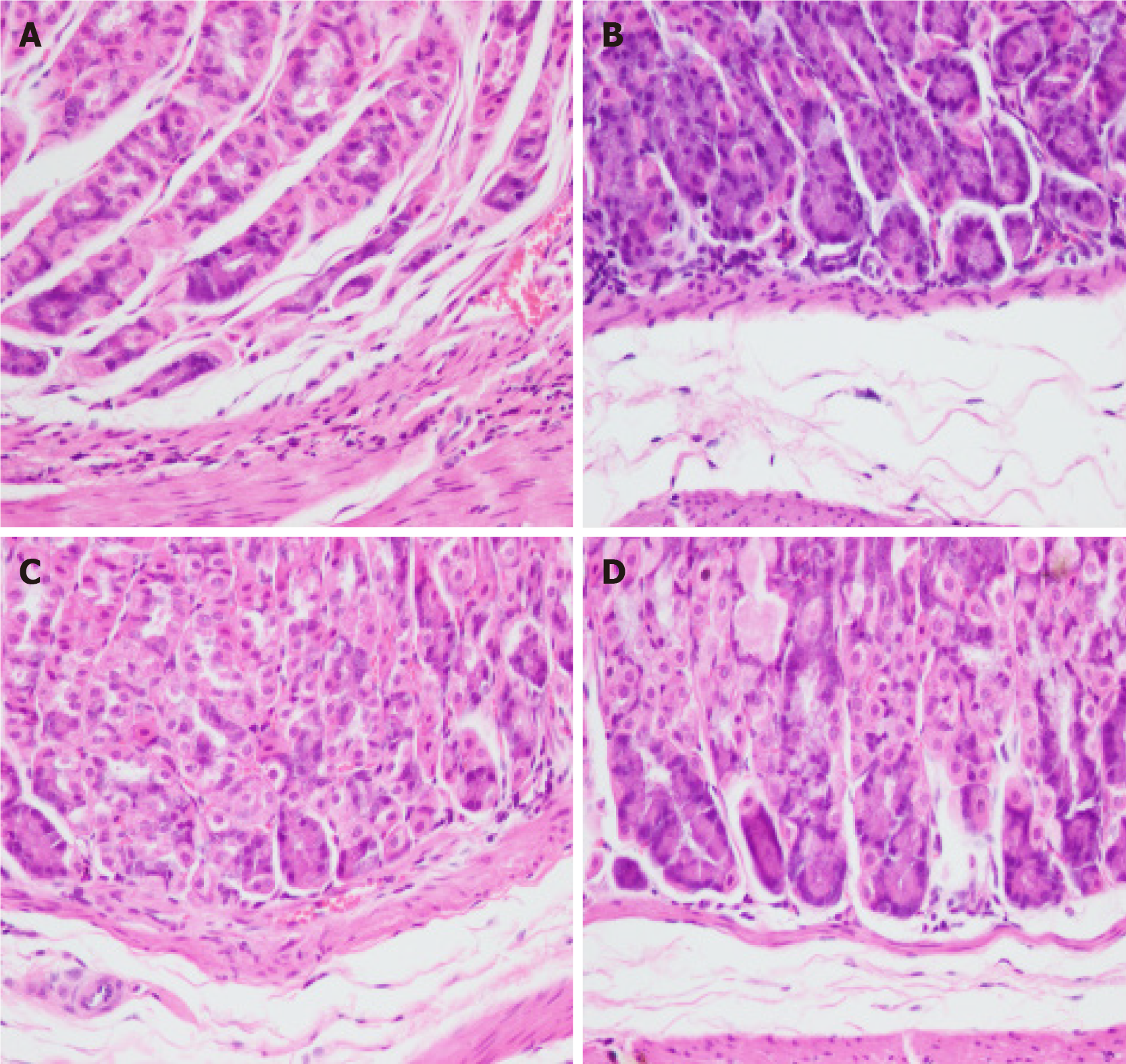
Under light microscopy, gastric tissues of mice in the control group exhibited intact gastric mucosal glandular architecture without any infiltration of inflammatory cells, abnormal cell proliferation, or pathological nuclear division (Figure 2A). In contrast, those of mice in the MNU group displayed a small number of heterogeneous cells with enlarged and deeply stained nuclei, moderate infiltration of inflammatory cells, and a few cells with pathological nuclear division (Figure 2B). The gastric glands of mice in the H. pylori + MNU group exhibited significant disorganization, evident cellular heterogeneity, an increased nucleoplasmic ratio, pronounced staining of the nucleus, fusion of some cells, extensive inflammatory cell infiltration, and an increase in pathological divisions (Figure 2C). Comparatively, the gastric mucosal condition of mice in the Moluodan group showed improvement compared to that of the H. pylori + MNU and MNU groups as characterized by reduced disorganization of the glandular structure and inflammatory cell infiltration as well as the absence of significant cellular anisotropy or pathological nuclear division (Figure 2D).
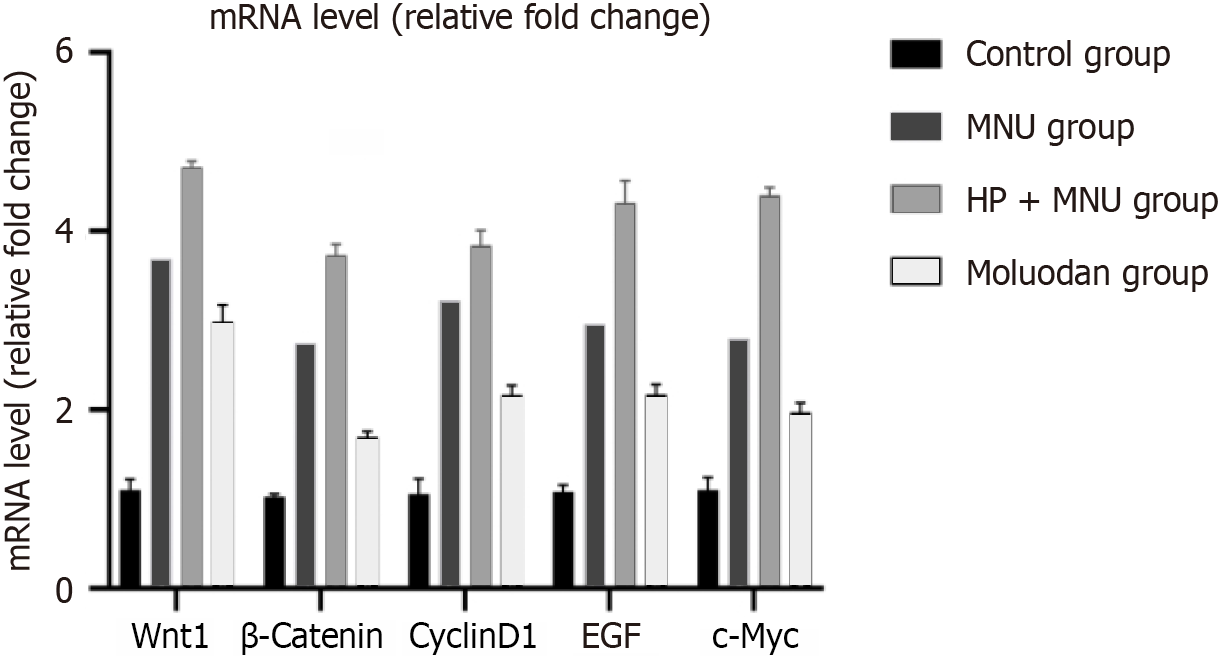
There was significantly increased Wnt1, β-catenin, cyclinD1, epidermal growth factor (EGF), and c-Myc m-RNA expression in the MNU group compared to the Moluodan and control groups (P < 0.05; Table 2). Wnt1, β-catenin, cyclinD1, EGF, and c-Myc m-RNA expression in the H. pylori + MNU group was significantly greater than that in the MNU, Moluodan, and control groups and was the highest among the four groups (P < 0.05). Meanwhile, the Moluodan group showed higher expression levels of Wnt1, β-catenin, cyclinD1, EGF, and c-Myc compared to the control group, but lower levels compared to the MNU and H. pylori + MNU groups; all differences were significant (P < 0.05; Figure 3).
| Norm | Control group | MNU group | H. pylori + MNU group | Moluodan group | F value | P value |
| Wnt1 | 1.10 ± 0.12 | 3.69 ± 0.16a | 4.72 ± 0.06a,b | 2.99 ± 0.19a,b,c | 347.51 | < 0.001 |
| β-catenin | 1.03 ± 0.03 | 2.74 ± 0.11a | 3.73 ± 0.12a,b | 1.70 ± 0.06a,b,c | 523.67 | < 0.001 |
| CyclinD1 | 1.06 ± 0.17 | 3.22 ± 0.07a | 3.84 ± 0.16a,b | 2.17 ± 0.11a,b,c | 248.70 | < 0.001 |
| EGF | 1.08 ± 0.07 | 2.96 ± 0.14a | 4.32 ± 0.24a,b | 2.17 ± 0.11a,b,c | 230.00 | < 0.001 |
| c-Myc | 1.10 ± 0.14 | 2.80 ± 0.14a | 4.40 ± 0.08a,b | 1.97 ± 0.11a,b,c | 411.91 | < 0.001 |
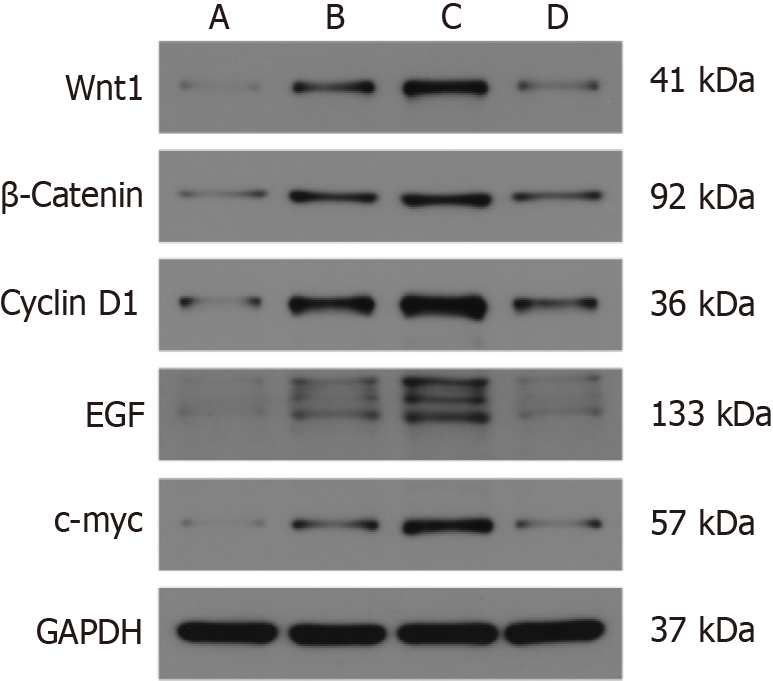
There was a significant increase in the expression of Wnt1, β-catenin, cyclinD1, EGF, and c-Myc in the MNU group compared to the Moluodan and control groups (P < 0.05); Table 3). Furthermore, Wnt1, β-catenin, cyclinD1, EGF, and c-Myc expression in the H. pylori + MNU group was significantly greater than that in the MNU, Moluodan, and control groups and was the highest among all groups (P < 0.05). In the Moluodan group, expression levels of Wnt1, β-catenin, cyclinD1, EGF, and c-Myc were higher than those in the control group but lower than those in the MNU and H. pylori + MNU groups; all differences were significant (P < 0.05; Figure 4).
| Norm | Control group | MNU group | H. pylori + MNU group | Moluodan group | F value | P value |
| Wnt1 | 0.05 ± 0.01 | 0.29 ± 0.02a | 0.58 ± 0.02a,b | 0.12 ± 0.02a,b,c | 479.39 | < 0.001 |
| β-catenin | 0.06 ± 0.04 | 0.26 ± 0.08a | 0.43 ± 0.06a,b | 0.16 ± 0.06a,b,c | 18.80 | < 0.001 |
| cyclinD1 | 0.09 ± 0.04 | 0.31 ± 0.12a | 0.60 ± 0.10a,b | 0.15 ± 0.07a,b,c | 11.59 | 0.003 |
| EGF | 0.06 ± 0.03 | 0.17 ± 0.02a | 0.39 ± 0.08a,b | 0.09 ± 0.02a,b,c | 35.26 | < 0.001 |
| c-Myc | 0.05 ± 0.01 | 0.23 ± 0.06a | 0.54 ± 0.06a,b | 0.13 ± 0.02a,b,c | 64.19 | < 0.001 |
H. pylori is highly prevalent and is distributed worldwide[15]. Over 4 billion individuals worldwide are infected with H. pylori, with a particularly high prevalence in Asian countries[16,17]. H. pylori infection can lead to various diseases, such as indigestion, gastrointestinal ulcers, MALT lymphoma, and even GC. Failure to promptly eradicate H. pylori infection can result in significant detrimental effects on human health[18]. Factors, such as the irrational selection of antibiotics, non-standard treatment, and the development of drug resistance, have contributed to an increasing number of cases wherein H. pylori is refractory to treatment. A study involving 180000 individuals revealed that gender, irregular medication use, smoking, alcohol consumption, previous stomach diseases, and obesity are risk factors for the failure of H. pylori eradication, with a higher failure rate observed in men compared to women[19]. Therefore, it is crucial to further investigate the mechanisms of interaction between H. pylori and the human body as well as to explore new methods for eradicating H. pylori and treating stomach diseases caused by H. pylori. In this study, a mouse model of H. pylori infection was established. Ultimately, both the H. pylori and Moluodan groups were successfully infected with H. pylori. The infection mode in mice was similar to that in humans, and we confirmed that after 8 wk of gavage, H. pylori could stably colonize the surface of the gastric mucosa and cause damage.
MNU, which is a chemical preparation commonly utilized in conjunction with H. pylori, is frequently employed to establish animal models of PLGC and GC. MNU has also been employed as a tumor inducing agent for modeling colorectal and prostate cancers, demonstrating its efficacy in this regard[20-22]. Throughout our experiment, when only H. pylori infection was induced, mice in the H. pylori + MNU and Moluodan groups had poorer diets than those in the other groups accompanied by reduced activity, poor mental status, and irregular and unformed stools. After introducing MNU into the PLGC model, a noticeable decline in the mental state of the mice was observed in all groups except for those in the control group. This decline was characterized by significant weight loss, reduced appetite, hair loss, and decreased activity; these symptoms closely resemble those seen during the chronic progression of human tumors. Additionally, the administration of Moluodan, which is a therapeutic agent that alleviates gastrointestinal bloating and belching, improved symptoms. Specifically, mice in the Moluodan group exhibited increased consumption of the agent when Moluodan was provided in their drinking water, leading to improvements in mental state, bowel movements, and activity levels. These findings further support the notion that Moluodan possesses stomach-protective and gastrointestinal symptom-improving properties.
After the administration of H. pylori and MNU to PLGC mice, gastric tissues were obtained and examined, showing that gastric tissues of mice in the H. pylori + MNU group exhibited the most pronounced tissue inflammation. Additionally, the gastric wall of was noticeably swollen with red and white spots indicating granular bleeding, consistent with chronic atrophic gastritis. Comparatively, gastric tissues in mice in the MNU group showed slightly less severe inflammation and atrophic gastritis. However, upon the addition of Moluodan, there was a significant improvement in the condition of the gastric mucosa accompanied by a reduction in symptoms. Furthermore, the eating and bowel movements of mice also showed significant improvement. These findings provide evidence that H. pylori infection can exacerbate damage to the gastric mucosa and accelerate the development and progression of malignant PLGC such as atrophic gastritis. Moluodan may have potential benefits in improving the inflammatory condition of the gastric mucosa by treating gastric mucosal lesions caused by H. pylori and potentially preventing or reversing the progression of precancerous lesions. Lee et al[23] reported the effects of H. pylori and MNU alone and in combination on the induction rate of GC in mice. Their results revealed that the group treated with both H. pylori and MNU had the highest induction rate of GC, reaching 37.5%; this was significantly higher compared to that in the other groups. It was suggested that this combination may decrease levels of the Rev-Erb protein and increase levels of IL-1β, thereby promoting the development of GC[23,24].
From a microscopic perspective, the glandular structure of gastric tissues in mice in the H. pylori + MNU group exhibited significant disorder as characterized by pronounced nucleolar staining and enlargement. Inflammatory cells were more prevalent with mitotic figures being the most common, and signs of precancerous lesions were highly evident. Conversely, in the MNU group, changes in the microscopic glandular structure, inflammatory infiltration, and pathological mitotic images were less frequent. However, following the administration of Moluodan, inflammatory cell infiltration in the Moluodan group significantly decreased, and the glandular structure reverted to its normal state. Pathological mitotic figures became imperceptible, and nuclei reverted to their normal size. These findings suggest that Moluodan exerts a robust tissue recovering capacity that is capable of visually and microscopically repairing gastric tissue. This mechanism enables the reversal of PLGC, thereby preventing the progression and development of GC. Amieva and Peek[25] conducted a simulation study on the effects of H. pylori infection on the gastric mucosal epithelium and discovered that external factors, such as diet, micronutrients, and gastrointestinal microbiota, contribute to changes in the epithelium. Additionally, virulence factors of H. pylori, including CagA, are significant pathogenic factors[26]. These findings provide valuable insights for future research and exploration in this field.
The Wnt/β-Catenin signaling pathway plays a crucial role in human growth and development, cell homeostasis, and tissue signal transmission[27,28]. It is also considered the most fundamental signaling pathway in the Wnt family signaling cascade and is involved in various biological processes such as cell proliferation, tissue self-renewal, and EMT, particularly during embryonic development[29,30]. Recently, research on the association between this pathway and the occurrence and progression of tumors, including gastric, endometrial, liver, and adrenal cortical, cancer have gained attention[31-34]. The Wnt/β-Catenin signaling pathway is comprised of the Wnt ligand, Wnt receptor (Frizzled and LRP5/6), intermediate β-Catenin protein, and downstream signaling molecules such as c-Myc and cyclinD1[7,35,36]. Activation of the pathway occurs when Wnt1 or other ligands stimulate the receptor, preventing the phosphorylation degradation of β-Catenin[37,38]. Subsequently, β-Catenin translocates to the nucleus and binds with cytokines TCF/LEF, promoting the transcription and expression of downstream genes[39]. Deletion or inactivation of the adenomatous polyposis coli gene can activate this pathway and is believed to be an initiating factor in the development of colorectal cancer[40]. Wu et al[10] confirmed that the long chain non-coding RNA SNHG11 facilitates the activation of the Wnt/β-Catenin signaling pathway by inducing the ubiquitination of the intermediate signal GSK-3β, thereby promoting the progression of gastric tumor cells. In our study, we employed qRT-PCR to detect the expression of mRNA for Wnt1, β-Catenin, and downstream Cyclin D1 signaling molecules on the Wnt/β-Catenin pathway. Additionally, we utilized Western blotting to assess the protein expression of these aforementioned genes. Our results revealed that the transcription of m-RNA and protein expression of these genes were highest in the H. pylori + MNU group followed by the MNU group and the Moluodan group, demonstrating that H. pylori can enhance the expression of the Wnt/β-Catenin signaling pathway, which decreased after Moluodan administration. However, the specific mechanisms or smaller signaling molecules through which they exert these effects require further investigation.
EGF is a cytokine that plays a crucial role in cellular growth, development, and tumorigenesis. The receptor for EGF, known as EGF receptor (EGFR), has been implicated in the progression and treatment of various types of tumors[41,42]. Sheng et al[43] demonstrated that calreticulin facilitates EGF-induced changes in the transformation of pancreatic cancer cells through the integrin/EGFR-ERK/MAPK signaling pathway. In our study, we assessed the transcription of m-RNA and protein expression levels in relation to EGF expression. Our findings revealed that the H. pylori + MNU group exhibited the highest expression of both m-RNA and corresponding proteins followed by the MNU group, while the Moluodan group displayed the lowest expression. This indicates that H. pylori enhances the expression of the tumor-associated factor EGF, whereas Moluodan reduces its expression. Additionally, C-Myc, which is an important proto-oncogene, can contribute to tumorigenesis when mutated or overexpressed. c-MYC plays a significant role in various cancer-related processes, such as cell reprogramming, immune evasion, and resistance to chemotherapy, primarily through epigenetic modifications[44,45]. In this study, m-RNA transcription and protein expression levels of c-MYC were assessed as part of the molecular assay. Results showed that the H. pylori + MNU group exhibited the highest levels of c-MYC expression followed by the MNU and Moluodan groups. Furthermore, H. pylori promoted the expression of c-MYC, while Moluodan inhibited its expression. However, the specific mechanisms or molecules through which these effects occur require further investigation. Moluodan, which is a commonly used Chinese proprietary medicine, is currently being explored for its potential therapeutic applications. Traditional Chinese medicine is gaining recognition on the international stage.
In conclusions, H. pylori infection promotes the activation of the Wnt/β-catenin signaling pathway and accelerates the progression of PLGC in mice. Furthermore, Moluodan inhibits the activation of the Wnt/β-catenin signaling pathway, protects the gastric mucosa, treats H. pylori -infected gastric mucosal lesions, and halts and reverses the development and progression of PLGC.
The early diagnosis of gastric cancer (GC) is difficult. It has the characteristics of high incidence rate and high mortality. Precancerous lesion is an important stage in the development of GC. Helicobacter pylori (H. pylori) is the primary risk factor of GC, Wnt/β-catenin signaling pathway is closely related to tumor development. So their relationships with precancerous lesion should be further explored in order to explore the specific mechanisms of GC occurrence and find new drugs to prevent the development of GC.
Constructing a dual model of H. pylori infection and precancerous lesions of GC (PLGC) in mice is rare, and we are inspired by some recent researches. Moluodan is a traditional Chinese patent medicine and commonly used to treat digestive tract diseases. It has not yet been used for the prevention and treatment of GC. We can explore its role in the prevention of GC so that to increase its broader clinical pharmacological effects.
Our aim is to establish a double mouse model of H. pylori infection and PLGC, and find a new traditional Chinese patent medicine that can prevent the development of GC.
We established a dual model of H. pylori infection and PLGC in mice. After successful modeling, the mice were freely fed with Moluodan aqueous solution to achieve the goal of drug treatment. We observed the general condition of mice throughout the entire experimental period. Subsequently, the mice were killed to detect the infection rate of H. pylori, and the pathological changes of the gastric tissue were detected by gross observation and light microscopy. The expression of Wnt/β-Catenin signaling pathway, EGF and c-Myc was detected by quantitative real-time PCR (qRT-PCR) and Western blot analyses.
Mice in the H. pylori + N-methyl-N-nitrosourea (MNU) group showed the worst performance in general condition, gastric tissue visual and microscopic observation, followed by the MNU group, Moluodan group and the control group. qRT-PCR and Western blotting analysis used to detect the expression of Wnt/β-Catenin signaling pathway, EGF and c-Myc showed that the H. pylori + MNU group had the highest expression, followed by the MNU group, Moluodan group and the control group.
H. pylori can promot the expression of Wnt/β-Catenin signaling pathway, EGF and c-Myc and accelerate the malignant progression of gastric tissues; Moluodan can inhibit the expression of Wnt/β-Catenin signaling pathway, EGF and c-Myc, protect the gastric mucosa, treat H. pylori -infected gastric mucous lesions, and prevent the malignant development of gastric tissues.
Moluodan has the effect of preventing the progression of PLGC, further in-depth researches can be conducted in the future to explore its deeper mechanisms of action.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2175] [Article Influence: 725.0] [Reference Citation Analysis (1)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2814] [Article Influence: 562.8] [Reference Citation Analysis (5)] |
| 3. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Liu ZC, Wu WH, Huang S, Li ZW, Li X, Shui GH, Lam SM, Li BW, Li ZX, Zhang Y, Zhou T, You WC, Pan KF, Li WQ. Plasma lipids signify the progression of precancerous gastric lesions to gastric cancer: a prospective targeted lipidomics study. Theranostics. 2022;12:4671-4683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 5. | Park AM, Tsunoda I. Helicobacter pylori infection in the stomach induces neuroinflammation: the potential roles of bacterial outer membrane vesicles in an animal model of Alzheimer's disease. Inflamm Regen. 2022;42:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1173] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 7. | Basu S, Cheriyamundath S, Ben-Ze'ev A. Cell-cell adhesion: linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 762] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 9. | Fan D, Ren B, Yang X, Liu J, Zhang Z. Upregulation of miR-501-5p activates the wnt/β-catenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J Exp Clin Cancer Res. 2016;35:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Wu Q, Ma J, Wei J, Meng W, Wang Y, Shi M. lncRNA SNHG11 Promotes Gastric Cancer Progression by Activating the Wnt/β-Catenin Pathway and Oncogenic Autophagy. Mol Ther. 2021;29:1258-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Giebel N, de Jaime-Soguero A, García Del Arco A, Landry JJM, Tietje M, Villacorta L, Benes V, Fernández-Sáiz V, Acebrón SP. USP42 protects ZNRF3/RNF43 from R-spondin-dependent clearance and inhibits Wnt signalling. EMBO Rep. 2021;22:e51415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, Cai H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 319] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 13. | Li S, Wu Z, Le W. Traditional Chinese medicine for dementia. Alzheimers Dement. 2021;17:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Yu Y, Yang X, Hu G, Yin S, Zhang F, Wen Y, Zhu Y, Liu Z. Clinical efficacy of moluodan in the treatment of chronic atrophic gastritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e32303. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | FitzGerald R, Smith SM. An Overview of Helicobacter pylori Infection. Methods Mol Biol. 2021;2283:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 198] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (15)] |
| 17. | Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 18. | Fischbach W, Malfertheiner P. Helicobacter Pylori Infection. Dtsch Arztebl Int. 2018;115:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 19. | Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, Wang JX, Zhang Y, Bajbouj M, Zhang LF, Li M, Vieth M, Liu RY, Quante M, Wang LH, Suchanek S, Zhou T, Guan WX, Schmid R, Classen M, You WC. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Bosland MC, Schlicht MJ, Acevedo N, Soto AM, Prins G. Effects of perinatal exposure to bisphenol A on induction of prostate cancer in Sprague Dawley rats by MNU and testosterone. Toxicology. 2023;484:153394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Liu CA, Cai PZ, Xu FP, Zhu WJ, Wang WW, Jiang HP. Omega-3PUFA Attenuates MNU-Induced Colorectal Cancer in Rats by Blocking PI3K/AKT/Bcl-2 Signaling. Onco Targets Ther. 2020;13:1953-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, Drake CG, Wang TC. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology. 2021;160:781-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 23. | Lee JY, Kim N, Choi YJ, Nam RH, Lee S, Choi D, Lee HS, Kim JW, Lee DH. Effect of N-Methyl-N-Nitrosourea on Helicobacter-induced Gastric Carcinogenesis in C57BL/6 Mice. J Cancer Prev. 2016;21:182-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Ke C, Xiaowen C, Yufeng W, Dongchang L, Huaqing Z, Zhengguang W. Posttranslational Modifications of Rev-Erbα Protein and Abnormal Inflammatory Response in Gastric Cancer. J Oncol. 2022;2022:6291656. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 637] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 26. | Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 27. | Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 1182] [Article Influence: 394.0] [Reference Citation Analysis (0)] |
| 28. | Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: A comprehensive review. Cell Biol Int. 2022;46:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 179] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 29. | Chu A, Yu X, Guo Q, Li Q, Sun M, Yuan Y, Gong Y. H. pylori slyD, a novel virulence factor, is associated with Wnt pathway protein expression during gastric disease progression. Microb Pathog. 2020;148:104428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Duan P, Bonewald LF. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 31. | Zhang X, Dong N, Hu X. Wnt/β-catenin Signaling Inhibitors. Curr Top Med Chem. 2023;23:880-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Patel L, Mills GB, Lu KH, Sood AK, Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, Broaddus RR, Zhang W. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Abitbol S, Dahmani R, Coulouarn C, Ragazzon B, Mlecnik B, Senni N, Savall M, Bossard P, Sohier P, Drouet V, Tournier E, Dumont F, Sanson R, Calderaro J, Zucman-Rossi J, Vasseur-Cognet M, Just PA, Terris B, Perret C, Gilgenkrantz H. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J Hepatol. 2018;68:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libé R, de Reyniès A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 501] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 35. | Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med. 2015;5:84-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 36. | Wang J, Cai H, Liu Q, Xia Y, Xing L, Zuo Q, Zhang Y, Chen C, Xu K, Yin P, Chen T. Cinobufacini Inhibits Colon Cancer Invasion and Metastasis via Suppressing Wnt/β-Catenin Signaling Pathway and EMT. Am J Chin Med. 2020;48:703-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 37. | Yu P, Wang Y, Yu Y, Wang A, Huang L, Zhang Y, Liu W, Wu H, Yao M, Du YA, Cheng X. Deep Targeted Sequencing and Its Potential Implication for Cancer Therapy in Chinese Patients with Gastric Adenocarcinoma. Oncologist. 2021;26:e756-e768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | A Najafi SMA. The Canonical Wnt Signaling (Wnt/β-Catenin Pathway): A Potential Target for Cancer Prevention and Therapy. Iran Biomed J. 2020;24:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Koelman EMR, Yeste-Vázquez A, Grossmann TN. Targeting the interaction of β-catenin and TCF/LEF transcription factors to inhibit oncogenic Wnt signaling. Bioorg Med Chem. 2022;70:116920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Bian J, Dannappel M, Wan C, Firestein R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 41. | Wang Z. ErbB Receptors and Cancer. Methods Mol Biol. 2017;1652:3-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 42. | Yang CC, Chang KW. Eicosanoids and HB-EGF/EGFR in cancer. Cancer Metastasis Rev. 2018;37:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Sheng W, Chen C, Dong M, Wang G, Zhou J, Song H, Li Y, Zhang J, Ding S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017;8:e3147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 44. | Fatma H, Maurya SK, Siddique HR. Epigenetic modifications of c-MYC: Role in cancer cell reprogramming, progression and chemoresistance. Semin Cancer Biol. 2022;83:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 45. | Dhanasekaran R, Deutzmann A, Mahauad-Fernandez WD, Hansen AS, Gouw AM, Felsher DW. The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat Rev Clin Oncol. 2022;19:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 512] [Article Influence: 170.7] [Reference Citation Analysis (0)] |