Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.945
Peer-review started: November 25, 2023
First decision: December 7, 2023
Revised: December 25, 2023
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: March 15, 2024
Processing time: 108 Days and 2.9 Hours
Gastric cancer (GC) is a highly aggressive malignancy with a heterogeneous nature, which makes prognosis prediction and treatment determination difficult. Inflammation is now recognized as one of the hallmarks of cancer and plays an important role in the aetiology and continued growth of tumours. Inflammation also affects the prognosis of GC patients. Recent reports suggest that a number of inflammatory-related biomarkers are useful for predicting tumour prognosis. However, the importance of inflammatory-related biomarkers in predicting the prognosis of GC patients is still unclear.
To investigate inflammatory-related biomarkers in predicting the prognosis of GC patients.
In this study, the mRNA expression profiles and corresponding clinical infor
A prognostic model consisting of three inflammatory-related genes (MRPS17, GUF1, and PDK4) was constructed. Independent prognostic analysis revealed that the risk score was a separate prognostic factor in GC patients. According to the risk score, GC patients were stratified into high- and low-risk groups, and patients in the high-risk group had significantly worse prognoses according to age, sex, TNM stage and Lauren type. Consensus clustering identified three subtypes of inflammation that could predict GC prognosis more accurately than traditional grading and staging. Finally, the study revealed that patients in the low-risk group were more sensitive to certain drugs than were those in the high-risk group, indicating a link between inflammation-related genes and drug sensitivity.
In conclusion, we established a novel three-gene prognostic signature that may be useful for predicting the prognosis and personalizing treatment decisions of GC patients.
Core Tip: Our study identified a novel signature consisting of three inflammatory response-related genes that could precisely predict the prognosis of patients with gastric cancer (GC). The specific underlying mechanism of inflammatory response related genes and tumor immunity in GC is still unclear, which deserves further study. Taken together, our work will help shed light on their role in tumorgenesis, particularly in the areas of immune response, tumor microenvironment and drug resistance, which are critical for the development of personalized cancer therapies.
- Citation: Hu JL, Huang MJ, Halina H, Qiao K, Wang ZY, Lu JJ, Yin CL, Gao F. Identification of a novel inflammatory-related gene signature to evaluate the prognosis of gastric cancer patients. World J Gastrointest Oncol 2024; 16(3): 945-967
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/945.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.945
Gastric cancer (GC) is one of the most common malignancies worldwide and is the fourth leading cause of cancer mortality, with a worldwide incidence of one million new cases and more than 700 thousand fatalities each year[1,2]. Multiple factors, such as family history, diet, alcohol consumption, smoking, Helicobacter pylori (H. pylori) and Epstein–Barr virus (EBV) infections, have been noted to have a significant impact on the increased risk of developing GC. According to the Lauren division, two histological subtypes of GC can be distinguished: intestinal and diffuse. The indeterminate type was also included to characterize infrequent histology. Additionally, patients were considered to have diffuse-subtype signature ring cell carcinoma. Different classifications exhibit different characteristics, including clinical features, genetics, morphology, epidemiology and expansion properties[3]. With regard to the range of gastrectomy, this division also has an impact on surgical decision-making. The tumour, lymph node, and metastasis (TNM) staging system and histological differentiation degree have all been frequently employed for cancer categorization, prognosis prediction and therapeutic decision-making[4]. However, the TNM staging system has not been sufficient in practice to predict the prognosis of patients with GC or to formulate treatment plans[5]. Currently, surgery, chemotherapy, and molecular targeted therapy are used for GC treatment[6]. Although the therapeutic modalities available have improved, the clinical prognosis has not improved significantly. Due to the high incidence of local recurrence and distant metastases, the 5-year survival rate of GC patients is less than 30%, and identifiable early GC symptoms are lacking[7]. Therefore, identifying novel prognostic biomarkers for GC patients is critical, as these biomarkers could be used as practical therapeutic targets.
The tumour microenvironment (TME) is a specialized ecosystem of host components designed by tumour cells for the successful development and metastasis of tumours[8]. It contains various cell types, such as infiltrating immune cells, cancer-associated fibroblasts, vascular cells, and inflammatory cells. In addition to the cellular components within the tumour, various soluble substances, including cytokines, chemokines, inflammatory factors and cellular metabolic products, are present. Different components of the TME provide favourable environments for tumour growth and survival. Over the past few decades, there has been great renewed interest in the contribution of the immune system and inflammation to cancer development, progression and treatment. The link between inflammation and cancer is well recognized. Currently, tumour-associated inflammation is considered the seventh biological feature of cancer[9]. In the early stages of tumorigenesis, inflammatory cells are powerful tumour promoters that produce a favourable environment for tumour growth, facilitating genomic instability and promoting angiogenesis. In the middle, the inflammatory cells, chemokines and cytokines that they produce influence the whole tumour organ, regulating the growth, migration and differentiation of all cell types in the TME, including tumour cells, fibroblasts and endothelial cells. Subsequently, tumour cells can also alter inflammatory mechanisms, such as selectin-ligand interactions, MMP production, and chemokine function, to promote tumour spread and metastasis. However, inflammatory responses can also be counterproductive to tumour development and may represent an attempt by the host to suppress tumour growth. Therefore, inflammation can both promote and inhibit cancer[10,11]. In recent years, more has been learned about the relationship between inflammation and cancer[12,13]. Approximately 20% of cancer cases are accompanied by persistent infection caused by chronic inflammation[14], such as H. pylori-induced gastritis and hepatitis B/C virus-induced hepatitis, which increase the risk of GC and hepatocellular carcinoma (HCC), respectively[15,16]. Patients with inflammatory bowel disease are at a higher risk of colorectal cancer (CRC) due to the pro-neoplastic effects of chronic intestinal inflammation[17]. In GC, chronic inflammation of the gastric mucosa caused by H. pylori infection is an established risk factor for tumour development and causes immune cell migration to the stomach and the production of chemokines and cytokines, which eventually leads to the transformation from chronic atrophic gastritis to metaplasia, epithelial dysplasia, and eventually adenocarcinoma[11]. Therefore, inflammation plays an important role in the occurrence and development of GC. By analysing routinely available blood parameters, people can explore the relationship between cancer incidence and inflammatory marker levels. Many studies have shown that the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) reflect patients’ inflammation and immune status and that they are prognostic factors for multiple tumours (including rectal, prostate, lung, and breast cancer and head and neck squamous cell carcinoma)[18-20]. Furthermore, Mcmillan[20] reported that the Glasgow prognostic score, which consists of peripheral blood inflammatory marker data, was an independent prognostic factor in cancer patients. In addition to blood parameters, several inflammatory response-related genes have also been used to assess cancer recurrence and metastasis. These studies suggest that the IRGS can be used to predict the prognosis and immune status of breast cancer patients, HCC patients, bladder ancer patients, and pancreatic cancer patients[21-24]. Polymorphisms in inflammatory response-related genes are involved in the modulation of the inflammatory response in the pathogenesis of GC[25]. However, there is no reliable model according to inflammation-related genes for predicting the prognosis of GC patients.
Genomic profiling provides prognostic and predictive information about tumour biology and can aid in clinical decision-making and improve treatment options for cancer patients[26,27]. In our study, we downloaded the mRNA expression profiles and corresponding clinical data of patients with GC from a public database. Then, we constructed a prognostic signature comprising three differentially expressed genes (DEGs) related to the inflammatory response. We employed univariate and multivariate Cox regression analyses to evaluate the prognosis of patients with GC from the Gene Expression Omnibus (GEO) cohort independently and validated the stability and reliability of the findings. Furthermore, we analysed the associations between prognostic gene expression and immune infiltrate types and between prognostic gene expression and chemotherapeutic sensitivity. We believe that this powerful prognostic signature could help improve the risk stratification of GC patients, provide a more effective assessment for clinical management and provide new therapeutic targets for the treatment of these patients.
The RNA sequencing (RNA-seq) gene expression data and relevant clinical data of GC patients were acquired from the GEO website (http://www.ncbi.nlm.nih.gov/geo/). The normalization of gene expression profiles was performed by the “limma” and “SVA” R packages. A total of 300 GC patients from the GSE66229 cohort (from the GEO database) were used as the training set, and another 432 GC patients from the GSE26253 cohort were used as the verification set. A total of 200 inflammatory-related genes were selected and downloaded from the Molecular Signatures Database (MSigDB) (http://www.gseamsigdb.org/gsea/msigdb/cards/HALLMARK_INFLAMMATORY_RESPONSE.html) and are shown in Supplementary Table 1. The clinicopathological data, including survival information, age, sex, TNM stage, and Lauren type, were collected. Because all the data were from public databases, ethical review was not needed.
Univariate Cox analysis of overall survival (OS) was carried out to identify the inflammatory-related genes with prognostic significance. We utilized the log-rank test to adjust the P value. To reduce the number of prognostically significant candidate inflammatory-related genes, we employed least absolute shrinkage and selection operator (LASSO) Cox regression analysis for OS, which utilized the above mentioned screened genes as input. The most vital value in the LASSO Cox regression analysis was λ. With different λ values, LASSO Cox regression analysis can be used to screen different genes. We used cross-validation to determine the optimal λ for disease free survival (DFS) and OS. With these λ values, the genes associated with OS were identified via LASSO Cox regression analysis. Using these λ values, the genes associated with OS were identified by LASSO Cox regression analysis, which were showed in Supplementary Table 2. We then performed multivariate Cox regression analysis to determine the coefficients for the prognostic models of OS. The prognostic models were represented as risk scores, which are expressed as follows:
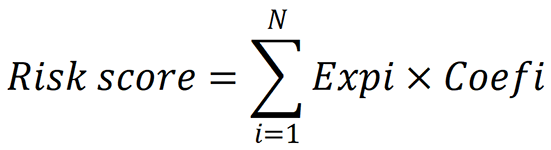
Patients with OS survival status and survival time were divided into low- and high-risk groups by the optimal cut-off point determined by the surv_cutpoint function in the “Survminer” package in R. Through the R packages “survival” and “survminer,” Kaplan-Meier analysis was employed to compare OS between the low- and high-risk groups. We also performed the same procedures for patients in all stages (stages I, II, and III), patients in stage I, patients in stages I and II, and patients in stage III. To assess the predictive power of the risk score, we plotted receiver operating characteristic (ROC) curves and displayed the 1-, 3- and 5-year projections as functions of survival using the “survivalROC” R package.
Gene Ontology (GO) analysis is a major bioinformatics tool for annotating genes and analysing the biological processes associated with these genes[28]. GO enrichment analysis was conducted with R software based on the genes identified via univariate Cox analysis of OS. A protein-protein interaction (PPI) network of the genes was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) database (STRING; http://string-db.org) (version 10.0)[29], and an interaction with a combined score > 0.4 was considered to be statistically significant. To visualize the PPI network, we used Cytoscape software (version 3.4.0) (https://cytoscape.org/), an open bioinformatics platform for visualizing the molecular interaction network[30]. Hub genes in the PPI network were screened by CytoHubba in Cytoscape, and the top hub genes were selected for further analysis.
Univariate and multivariate Cox analyses were used to determine whether the signature was an independent risk factor. To explore the relevance of the established prognostic model to clinical information, univariate Cox regression analyses were performed on patients with OS to generate risk scores, taking into account other clinical characteristics, including stage, sex, and age. Risk scores and other clinical factors were considered to be significantly related when the P value was < 0.05. We used a multivariate Cox proportional hazard model to examine the association between the risk score and other clinical factors and OS. The results of the multivariate Cox regression model were visualized in a combined forest plot. We also applied the same procedure to OS patients and the validation dataset from the GEO (GSE26253).
To facilitate the clinical application of our model, we created a nomogram that predicts 1-, 3-, and 5-year survival probabilities. ROC curve analysis was subsequently performed to determine the area under the curve (AUC) and evaluate the ability of the model to predict prognostic risk. The same procedure was used for the OS patients and the validation cohort. A calibration curve was produced to validate the nomogram’s predictive value by using the calibration function in the “rms” package in R. Furthermore, we conducted a decision curve analysis (DCA) to quantify and evaluate the clinical value of the nomogram[31]. DCA was performed to determine the clinical net benefit of the nomogram compared with that of any of the other strategies[32].
The inflammatory-related genes identified in the univariate Cox analysis of OS were clustered on the basis of their expression profiles, and a consistency matrix was constructed to identify the corresponding immune subtypes and gene modules. The partition around medoids algorithm using the “1-Pearson correlation” distance metric was applied, and 500 bootstraps were performed, each involving 80% of the patients in the discovery cohort. The cluster sets varied from 2 to 7, and the optimal partition was determined by evaluating the consensus matrix and the consensus cumulative distribution function. The inflammatory subtypes were subsequently validated in the validation cohort with the same settings. The consistency of inflammatory subtypes between the discovery and validation cohorts was quantified by calculating the intragroup proportions and Pearson correlations in the centroids of the gene module scores.
The prognostic values of the inflammatory subtypes were assessed through the log-rank test and univariate Cox regression with signature, stage and clinically distinct subtypes as covariates and OS as the endpoint. ANOVA was used to evaluate the associations of inflammatory subtypes with different inflammatory-related molecular and cellular characteristics.
The present study investigated hub genes via LASSO Cox regression using several online databases: (1) Oncomine database analysis. The Oncomine database (http://www.oncomine.org) is a tumour microarray database and online data analysis tool that contains information from many “multiple arrays”[33]. This tool was used to identify gene expression signatures in various types of cancers. The database was accessed at a P value threshold of 0.001 and a fold change threshold of 2, and gene expression levels were obtained by comparing mRNA expression in tumour tissue to that in normal tissue. Genes with a P value < 1E-4, a fold change > 2, or a top gene rank of 10% were considered[34]; (2) Gene correlation analysis was performed via Gene Expression Profiling Interactive Analysis (GEPIA). The online database GEPIA[35] (http://gepia.cancer-pku.cn/index.html) was used to confirm significantly correlated genes via Tumour Immune Estimation Resource (TIMER); (3) The Human Protein Atlas (HPA) database contains immunohistochemistry (IHC)-based expression data for approximately the 20 most common types of cancer and 12 individual tumours of each cancer type; and (4) cBioPortal analysis The cBioPortal website (https://www.cbioportal.org/) developed by the Memorial Sloan Kettering Cancer Center is a comprehensive open network platform based on The Cancer Genome Atlas (TCGA) database that integrates data mining, data integration, and visualization. The genetic alterations of four inflammatory-related genes were obtained from the TCGA cBioPortal. The cBioPortal for Cancer Genomics (http://cbioportal.org) was constructed specifically to decrease the difficulty of obtaining complex datasets and promote the translation of genomic data into novel biological knowledge, treatments, and clinical trials[36]. This platform allows researchers to visualize gene alteration patterns across samples, compare alteration frequencies across multiple tumour studies, and aggregate alterations of all related genomes in a single cancer sample. Various genomic data types, such as somatic mutations, DNA copy number alterations, mRNA and microRNA expression, DNA methylation, protein abundance, and phosphoprotein abundance, can be analysed via the cBioPortal[37].
The TIMER (https://cistrome.shinyapps.io/timer/) is a web interaction platform that is used to analyse tumour-infiltrating immunocytes systematically. We utilized TIMER to investigate the effect of inflammatory response-related genes on the status of the TME. The relationship between gene expression in the risk score model and tumour-infiltrating immune cells was assessed by the purity-correlated partial Spearman correlation and statistical significance[38]. CIBERSORT is a tool in which expression data are used to represent the cell composition of complex tissues based on preprocessed gene expression profiles. LM22 of CIBERSORT defines 22 immune cell subsets obtained from the CIBERSORT web portal (http://CIBERSORT.stanford.edu/). The RNA-Seq data of the GSE66229 cohort were analysed via CIBERSORT to obtain the abundance ratio matrix of 22 immune cells[34]. The differential abundance of immune infiltrates was obtained by comparing the distribution of immune cells in the low- and high-gene groups using R software. Correlation analysis was subsequently conducted among the levels of immune cells.
To evaluate the role of the signature in predicting the sensitivity of GC to chemical drugs, we calculated the half-maximal inhibitory concentration (IC50) of common chemotherapy drugs applied for the clinical treatment of GC. The Wilcoxon signed-rank test was used to compare the IC50 values between the high- and low-risk groups.
Student's t test was used to compare gene expression between tumour tissues and adjacent nontumorous tissues. Differences in proportions were compared by the chi-square test. We used Kaplan-Meier analysis with the log-rank test to compare OS between different groups. Univariate and multivariate Cox regression analyses were implemented to identify independent predictors of OS. The prognostic performance of the risk score for survival prediction was evaluated by ROC curve analysis, and the AUC was calculated. The decision curve analysis (DCA) results were plotted to quantify and assess the clinical value of the nomogram. Spearman correlation analysis was used to test the associations between the risk scores of the prognostic models and immune cell counts. The IC50 values were compared between the two groups by the Wilcoxon test. All the statistical analyses were performed with R software (version 3.5.3). If not specified above, a P value less than 0.05 was considered to indicate statistical significance.
The workflow of our research is illustrated in Supplementary Figure 1. A total of 300 GC patients were selected as the training set from the GEO database (GSE66229), and 432 GC patients were selected as the validation set from the GEO database (GSE26253). We collected clinicopathologic data from both the training and validation cohorts, the results of which are summarized in Table 1. The clinical characteristics of the GC patients included age, sex, TNM stage, Lauren type, survival time, and survival status. Patients with incomplete information were excluded from our analysis.
| Characteristics | GSE66229 (n = 300) | GSE26253 (n = 432) |
| Age group | ||
| Young | 106 (35.3) | 309 (71.5) |
| Old | 194 (64.7) | 123 (28.5) |
| Gender | ||
| Male | 199 (33.7) | 280 (64.8) |
| Female | 101 (66.3) | 152 (35.2) |
| TNM Stage | ||
| I | 30 (10.0) | 68 (15.7) |
| II | 97 (32.3) | 167 (38.7) |
| III | 96 (32.0) | 130 (30.1) |
| IV | 77 (25.7) | 67 (15.5) |
| Lauren type | ||
| Intestinal | 150 (50.0) | 139 (32.1) |
| Diffuse | 142 (47.3) | 280 (64.8) |
| Mixed | 8 (2.7) | 13 (3.0) |
The differentially expressed genes (DEGs) obtained from the GEO datasets were intersected with the inflammatory-related gene set to obtain “differentially expressed inflammatory-related genes”. A total of 173 genes were identified by matching the GSE66229 cohort with inflammatory-related genes, for which a total of 200 inflammatory response-related genes were obtained through the MSigDB. We then performed a univariate Cox regression analysis to investigate the association of these inflammatory-related genes with the prognosis of GC patients. Ultimately, 23 inflammatory-related genes were found to be associated with OS (OS-related DEGs) (P < 0.05) in the training cohort.
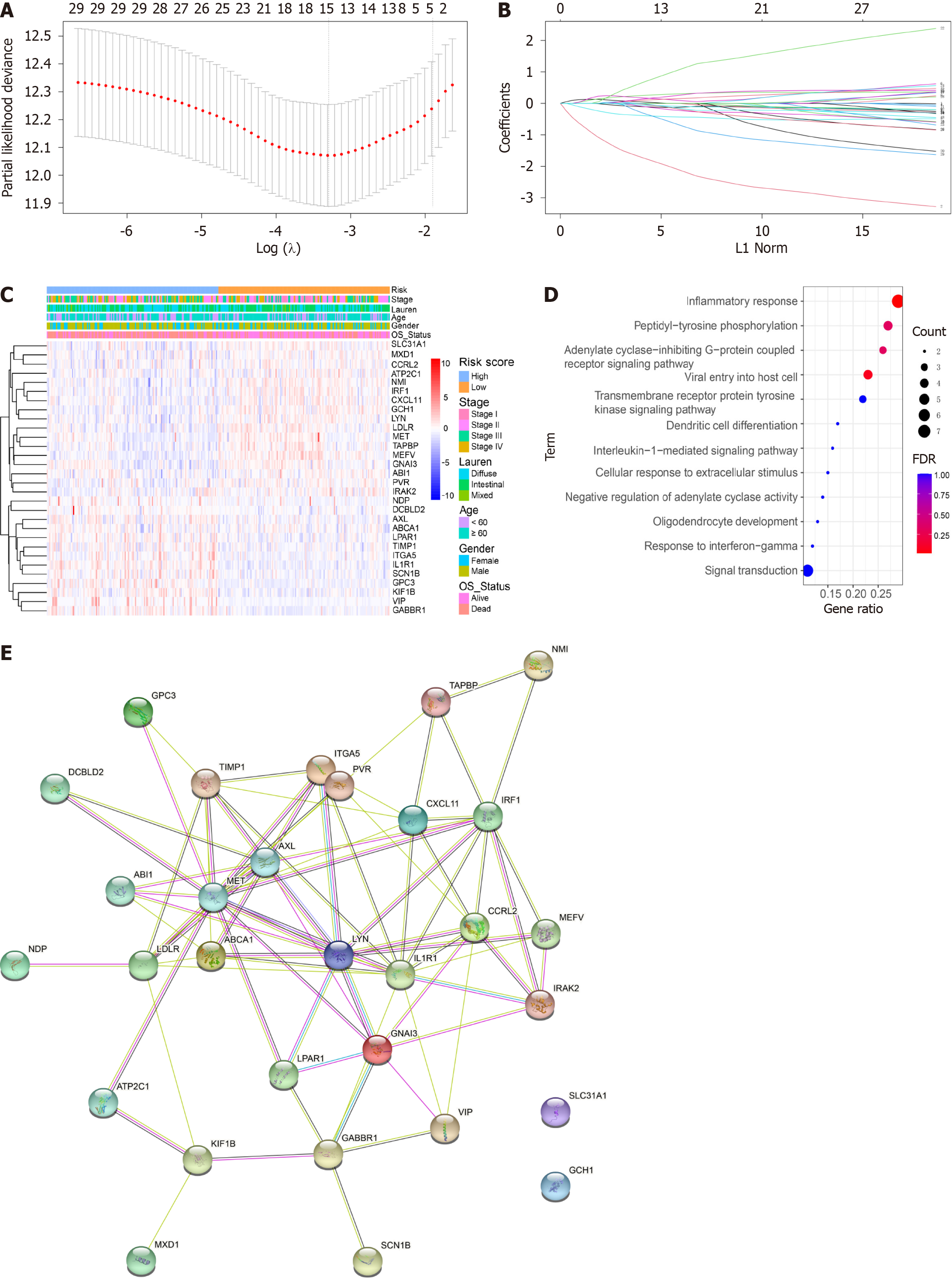
In our study, we utilized LASSO Cox regression analysis and multivariate Cox regression analyses to identify DEGs associated with OS to establish an inflammation-related prognostic model. Through our analysis, we identified five OS-related DEGs (L1R1, GCH1, VIP, GPC3, and MEFV) (Figure 1A and B). These DEGs were further subjected to LASSO Cox regression and multivariate Cox regression analysis (Figure 1C) to obtain the coefficients for the prognostic models of OS.
To further explore the correlation between risk scores and clinical characteristics, we analysed the differences in risk scores among the various subgroups stratified by clinical characteristics. As depicted in Figure 1C. GO analysis was carried out to investigate the potential biological importance of the three DFS-related DEGs and eight OS-related DEGs. GO enrichment revealed the top pathways that were positively correlated with inflammation in OS, such as the inflammatory response, viral entry into host cells, and the interleukin-1-mediated signalling pathway (Figure 1D). These findings suggest that GO enrichment is critically important in GC patients and is strongly associated with inflammation, especially in the inflammatory response. In addition, a PPI network of prognostic inflammatory-related genes was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) database (version 10.0) and visualized using Cytoscape (Figure 1E) to better understand the interactions of the genes with one another.
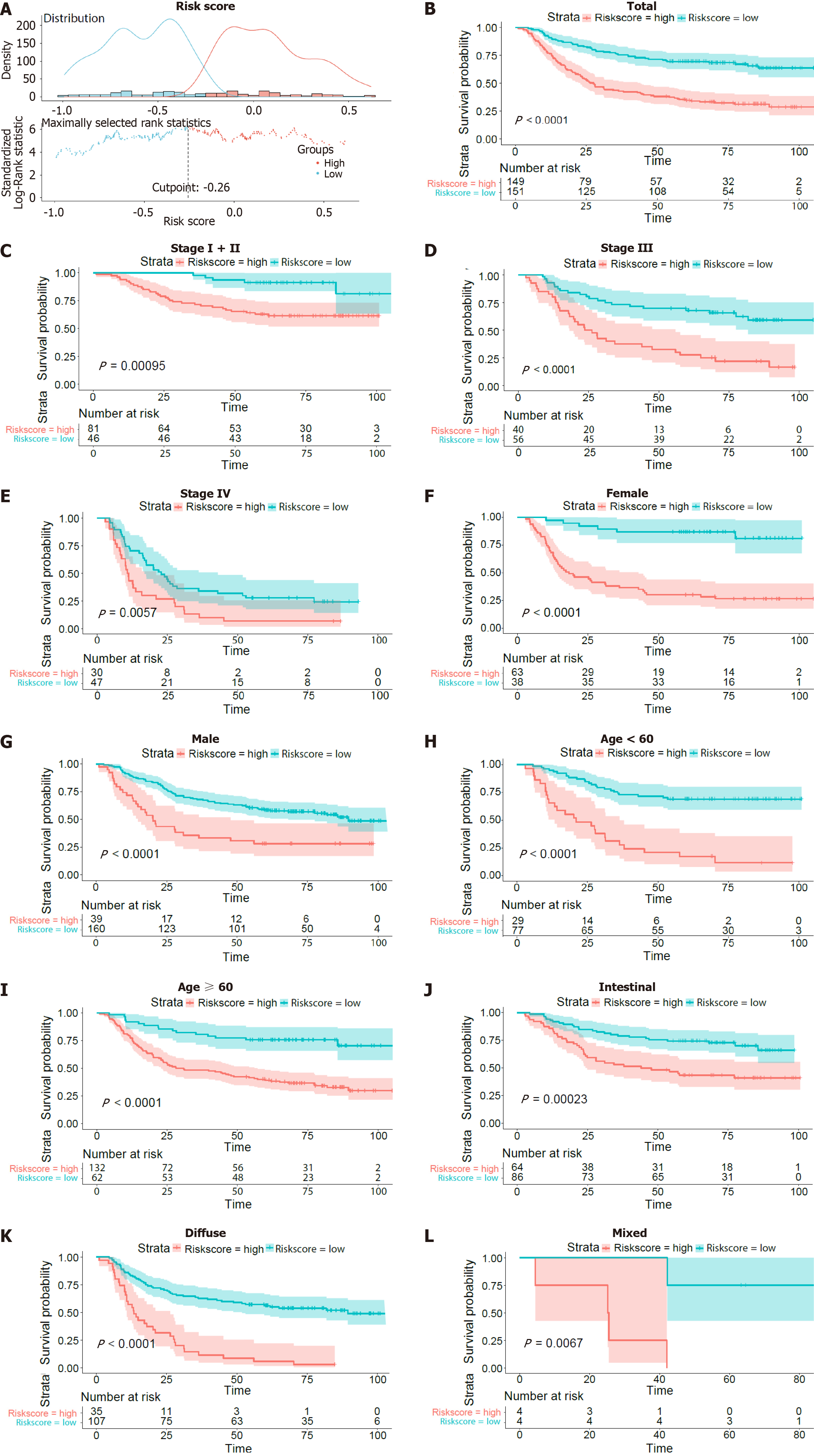
We further investigated the prognostic value of the inflammatory-related gene signature stratified by TNM stage (I + II, III or IV), sex (female or male), age (> 60 years or ≤ 60 years), and Lauren type (intestinal, diffuse, mixed). The optimal risk score cut-off point for OS status and time was -0.26, as illustrated in Figure 2A. Based on this cut-off point, we divided the OS patients into high- and low-risk groups, as shown in Figure 2A. K-M survival analysis was also conducted to evaluate the significance of differences in survival outcomes between the high-risk and low-risk patients. The Kaplan-Meier (KM) survival curve of the OS patients under the optimal cut-off point is depicted in Figure 2B (P value < 0.0001). The survival curves of the patients with stage I + II, III and IV disease are depicted in Figure 2C-E, with P < 0.05. Moreover, the survival curves according to sex (Figure 2F-G), age (Figure 2H-I) and Lauren type (Figure 2J-L) are shown. K-M analysis suggested that patients with high risk scores had worse outcomes than did those with low risk scores in all the subgroups (all P < 0.05). These results suggested that the risk score might serve as an effective indicator for predicting the OS of patients with GC.
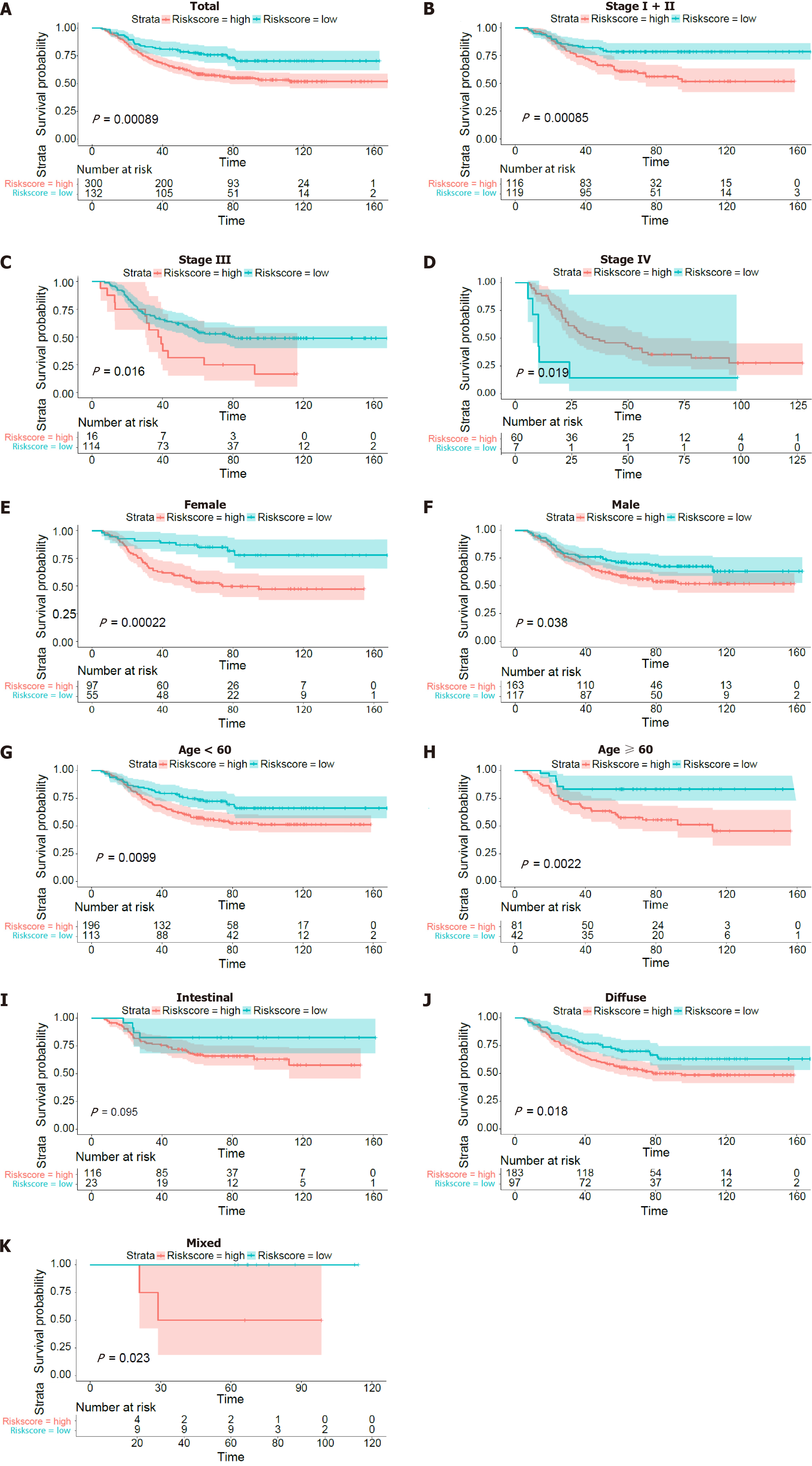
The Kaplan-Meier survival curves of OS for the validation dataset also showed that patients had different TNM stages (Figure 3B-D), sexes (Figure 3E and F), ages (Figure 3G and H) and Lauren types (Figure 3I-K).
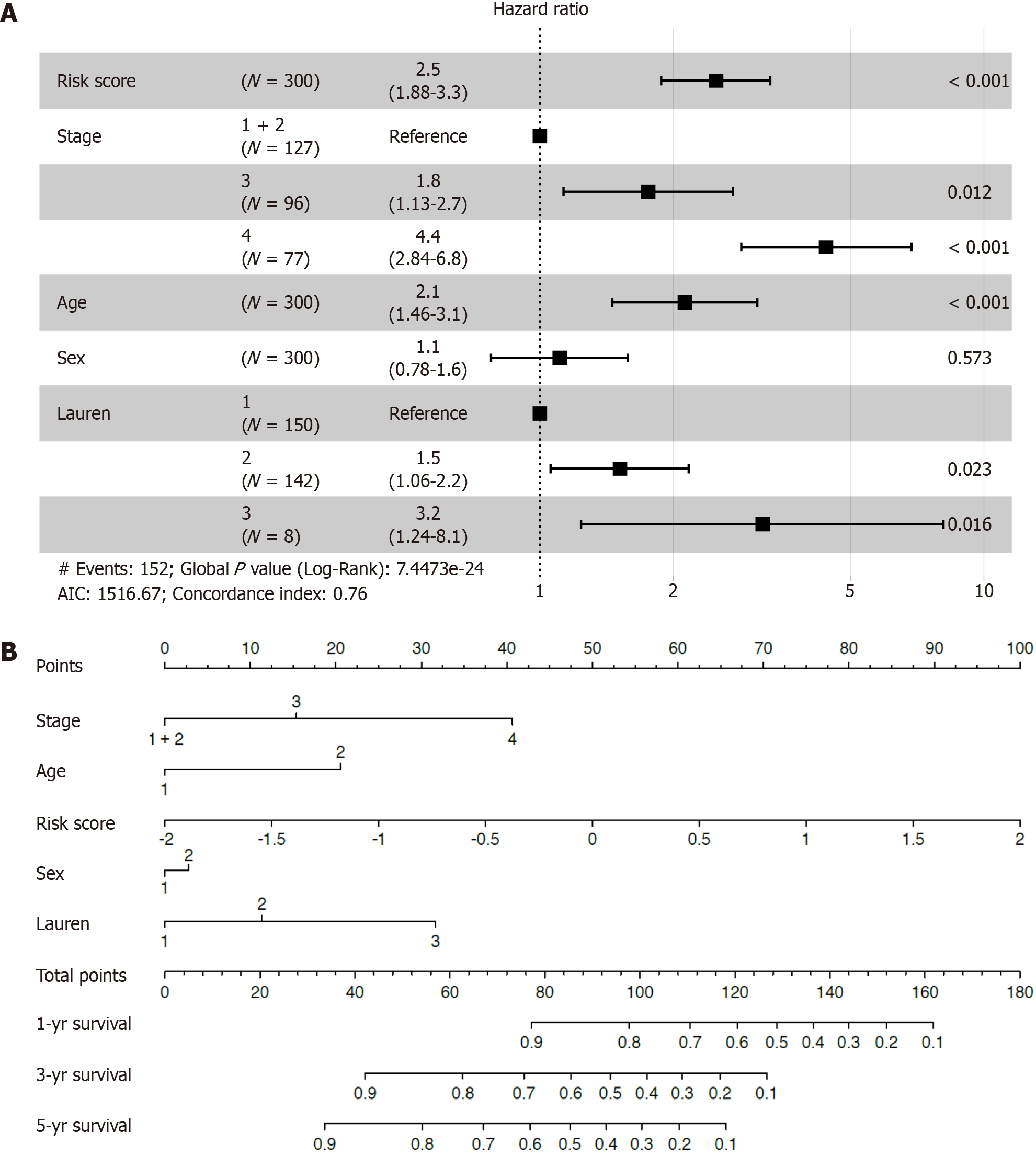
Univariate and multivariate Cox regression analyses were performed to explore the independence of the inflammatory-related genes as a signature by comparing clinical features, including TNM stage, age, sex and Lauren type. Cox multivariate regression analysis revealed that this prognostic model was an independent prognostic factor for OS (Figure 4A). To improve the practicality of the proposed approach for clinicians, a nomogram was established to predict 1-, 3-, and 5-year OS rates in the training cohort by incorporating risk scores and clinicopathological parameters, such as stage, age, sex, and Lauren type (Figure 4B). To construct the nomogram, a vertical line up to the top point row was drawn to assign points for each variable. Then, the total points for a patient can be summed, and the probabilities of 1-, 3- and 5-year OS can be obtained by drawing a vertical line from the total points row. The C-index of OS was 0.748. The AUCs were 0.774, 0.724, and 0.709 at the 1-, 3-, and 5-year follow-ups, respectively.
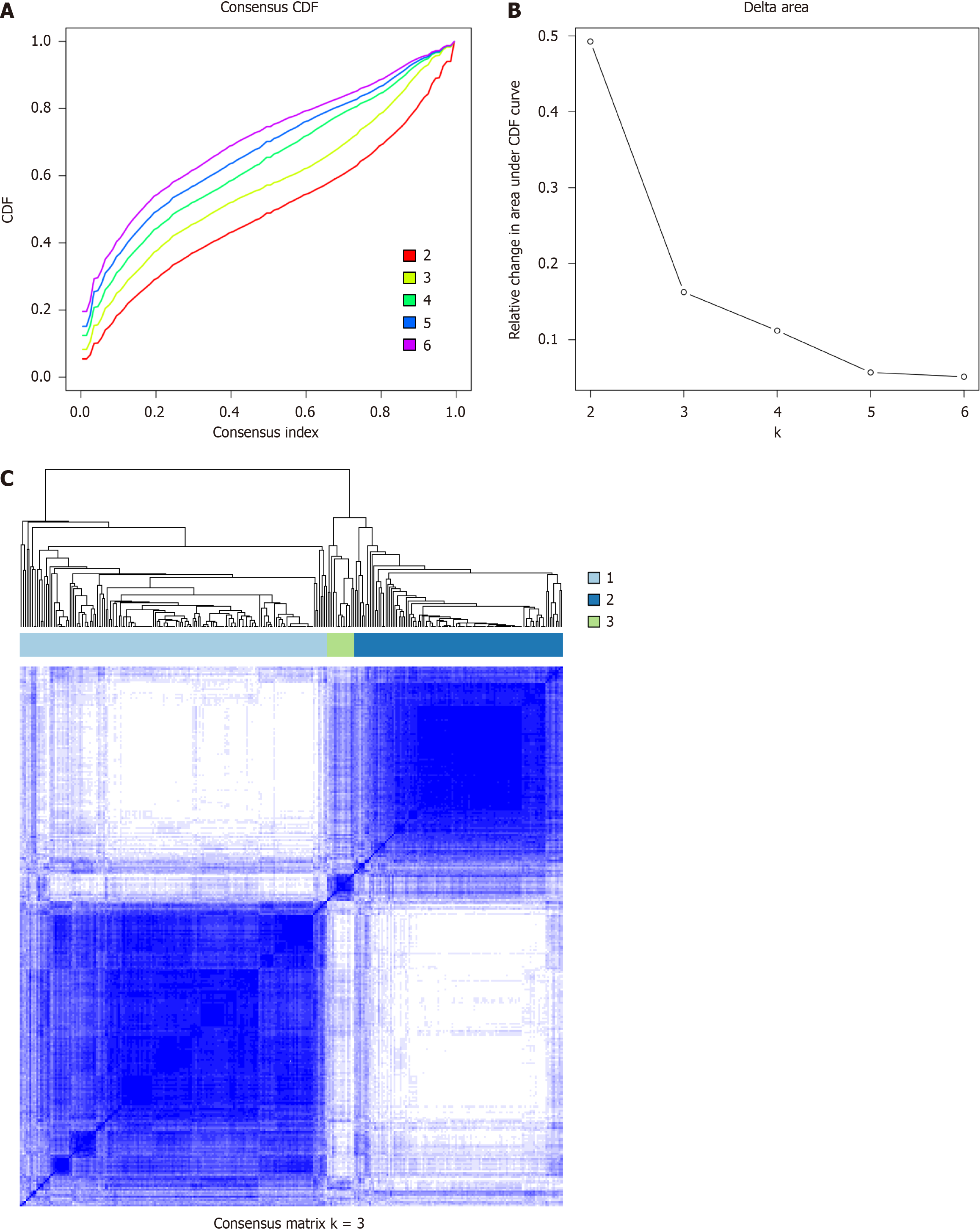
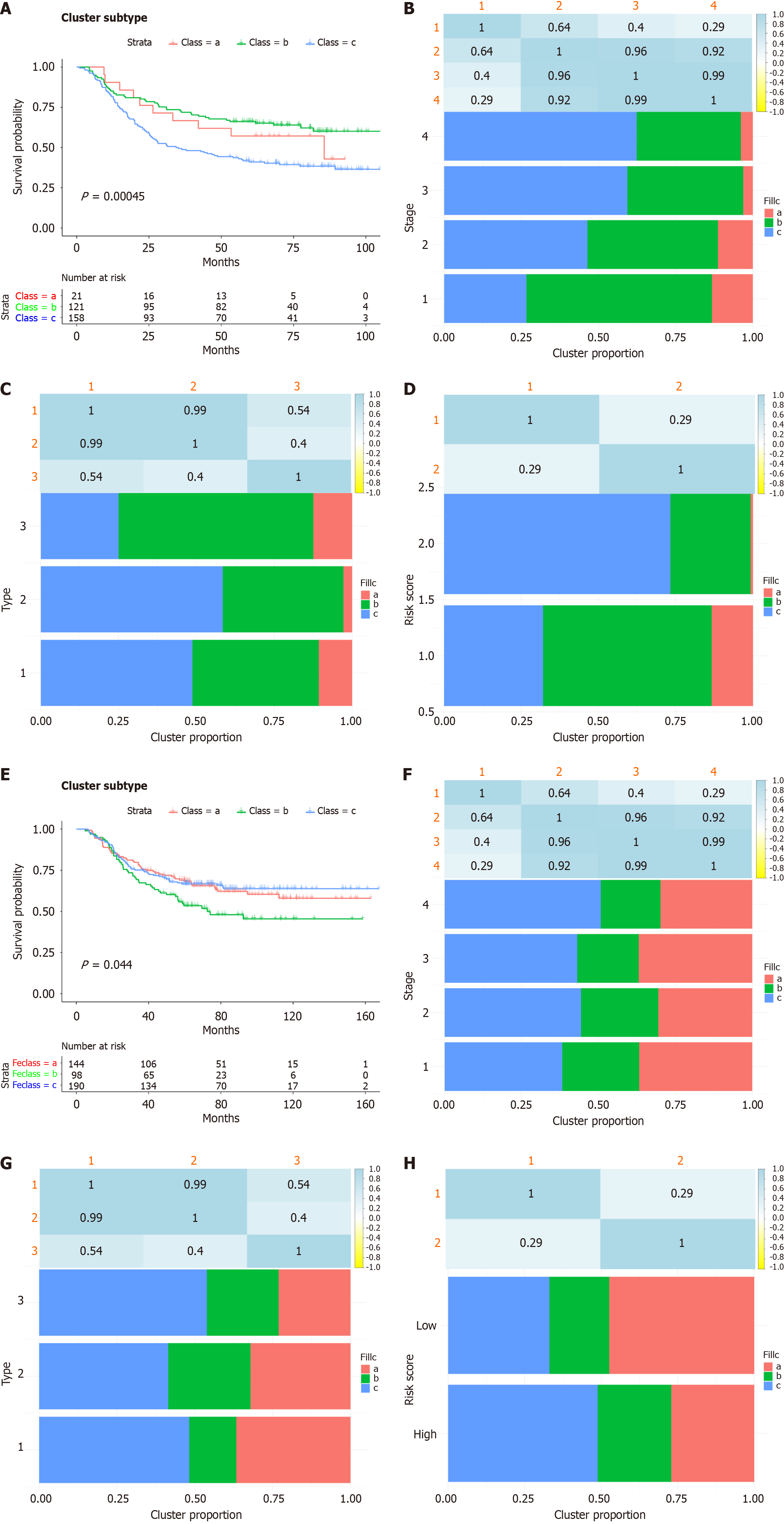
Inflammatory subtypes can be used to reflect the immune status of the tumour and its microenvironment, thereby helping to identify patients suitable for vaccination. Subsequently, we identified inflammation-based clusters utilizing consensus clustering. We analysed the expression profiles of 200 inflammation-related genes in 229 samples from the derivation cohort to construct a consensus clustering cohort. Based on their cumulative distribution function and functional delta area, we chose k = 3, where inflammatory-related genes appeared to be stably clustered (Figure 5A and B). After K-means clustering, we identified 3 inflammatory subtypes, designated FS1-FS3 (Figure 5C). The expression levels of inflammatory-related genes varied among the different clusters. Survival analysis revealed that patients in Cluster FS1 and FS2 had a better prognosis than patients in Cluster FS3 did (Figure 6A). The distribution of tumour stage and grade among the subtypes indicated that an irregular cluster of patients was diagnosed at different stages (Figure 6B), while both Grade 1 and Grade 4 were significantly associated with FS1 (Figure 6C). Consistent with the results of the GSE66229 cohort (Figure 6E), the inflammatory subtype was also a prognostic factor in the GSE26253 cohort (Figure 6E) and significantly varied at different stages (Figure 6F), and both Grade 1 and 4 were strongly correlated with FS1 (Figure 6H). Overall, the inflammatory subtype can be utilized to predict the prognosis of patients with GC, and its accuracy is better than that of traditional grading and staging methods, which are consistent across different cohorts.
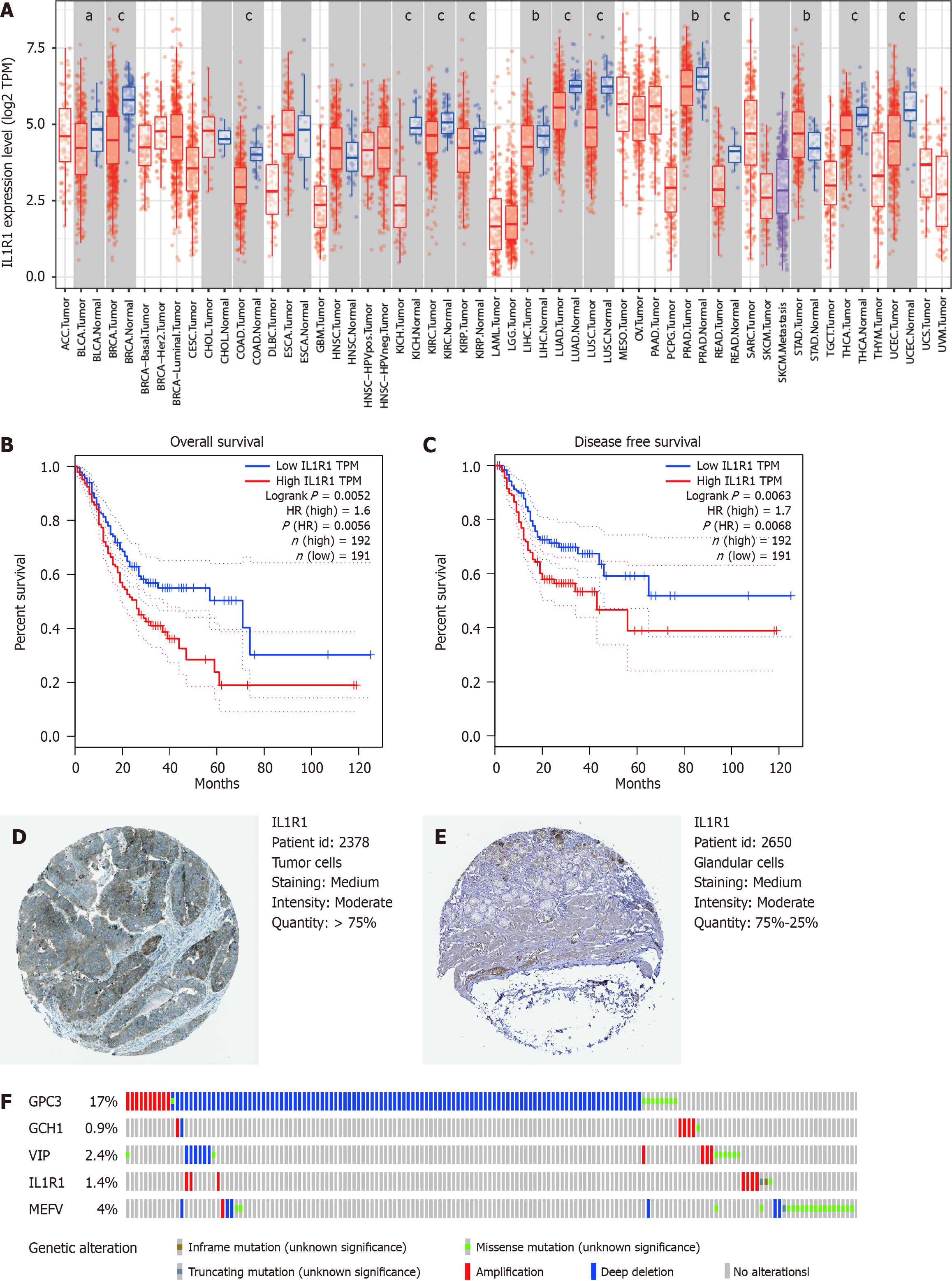
The expression levels of the prognostic genes were verified using an online database. Consistent with our findings, the level of IL1R1 mRNA expression in the Oncomine microarray database (https://www.oncomine.org) was significantly greater in GC tumour tissues than in normal tissues. (Figure 7A). Survival analysis of patients in the GEPIA database revealed that patients with high IL1R1 expression had significantly shorter OS (Figure 7B and C). However, the expression of HAMP, NOX4 and SLC1A5 had no significant effect on OS. By using an online database, we further reviewed the proteomic data and found that the protein level of IL1R1 was significantly downregulated in GC and that this protein could form an inflammatory circuit during the development of H. pylori-associated GC (https://www.oncotarget.com/article/7239/text/). The representative protein expression levels of IL1R1 in human normal and GC tissues were determined using the HPA, as shown in Figure 7D and E. The frequencies of genetic alterations in the five predictive genes in GC were evaluated using the cBioPortal database. The results from the cBioPortal database revealed that among the four genes included in the risk score model, IL1R1 was associated with genetic alterations (1.4%), and amplification was the most common variant (Figure 7F). Taken together, these findings indicate that IL1R1 is aberrantly expressed and that low IL1R1 expression predicts adverse outcomes and is a potential prognosticator.
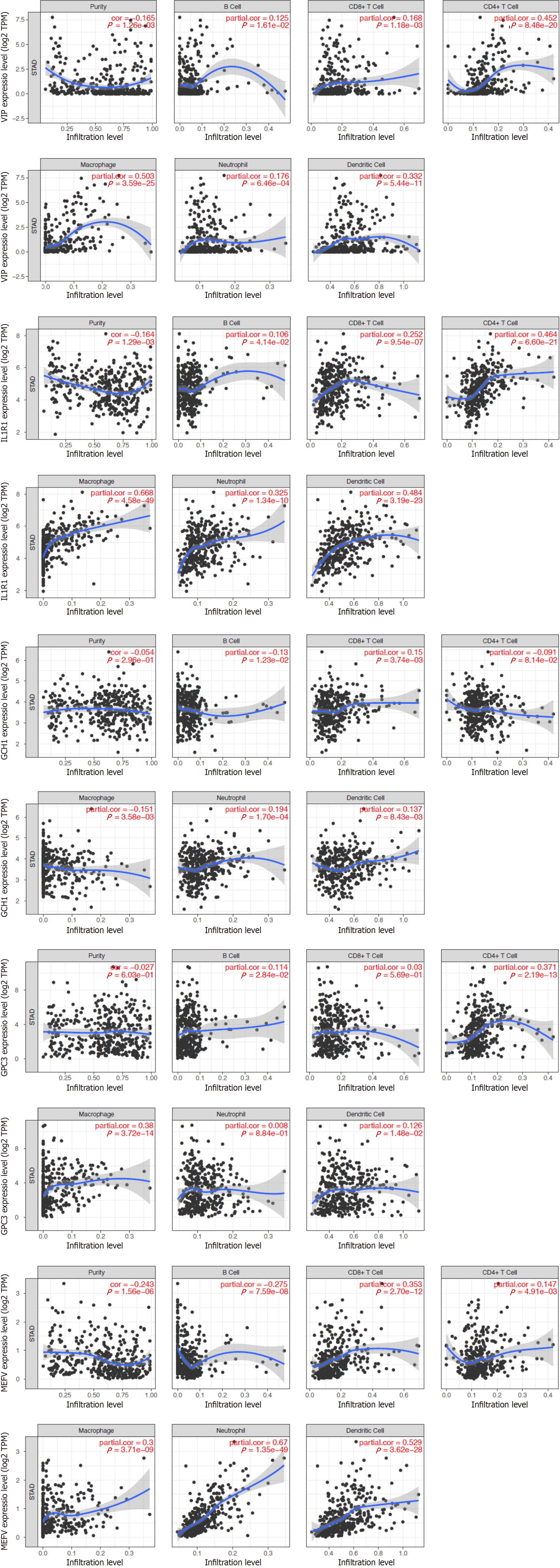
Given the important role of inflammatory responses in the tumour immune microenvironment, we performed correlation analysis between inflammatory-related genes and immune infiltration levels in GC patients by using the online tool TIMER, as shown in Figure 8. The scatterplots showed that the expression level of IL1R1 was positively associated with all six types of immune infiltration in GC patients (Figure 8). However, the expression levels of the other genes were irregular and not clearly associated with immune infiltration. These results suggest that IL1R1 may play a crucial role in the immune response.
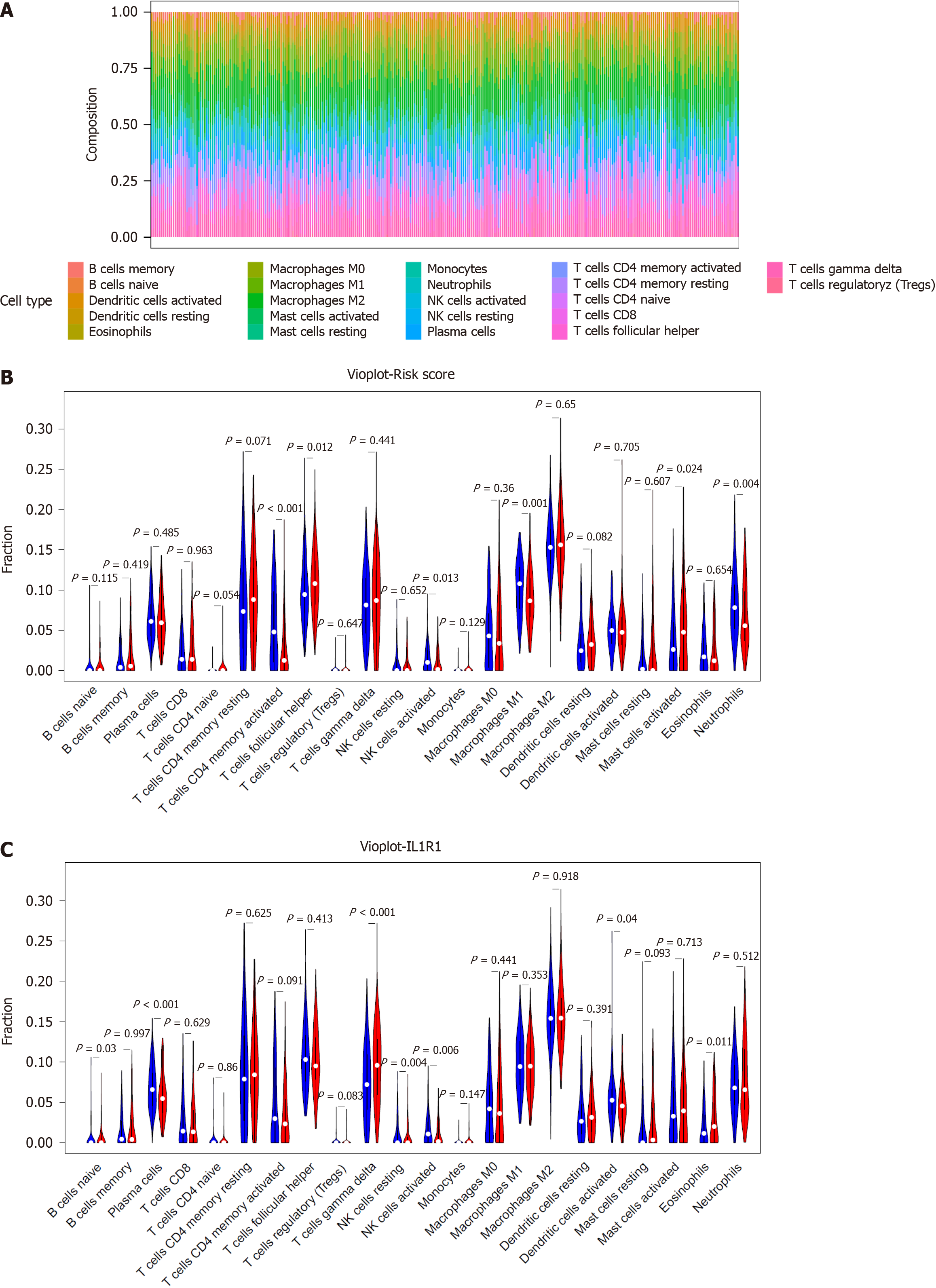
To explore the relationship between the risk signature and the tumour immune landscape, the CIBERSORT algorithm was used to evaluate immune infiltration between the low- and high-risk groups. The correlation matrix showed the relationships between different infiltrating immune cells in GC samples according to the results of the CIBERSORT algorithm (Supplementary Figure 2). According to the correlation heatmap, the proportions of 22 infiltrating immune cells were gradually correlated. Among the GC samples in the GSE66229 cohort, 22 immune cell infiltration landscapes with a marked difference in the percentage of immunocytes among the samples were observed (Figure 9A). In addition, the violin plot revealed that the fractions of T follicular helper cells and activated mast cells were greater in the high-risk group. However, the numbers of activated memory CD4 T cells, activated NK cells, M1 macrophages, and neutrophils were greater in the low-risk group (Figure 9B). Moreover, compared with those in the low-risk group, the fractions of naive B cells, gamma delta T cells, and eosinophils were greater in the high-risk group. Moreover, the numbers of plasma cells, activated NK cells, and activated dendritic cells were greater in the low-risk group (Figure 8C). Thus, we concluded that activated NK cells might play an important role in GC patients.
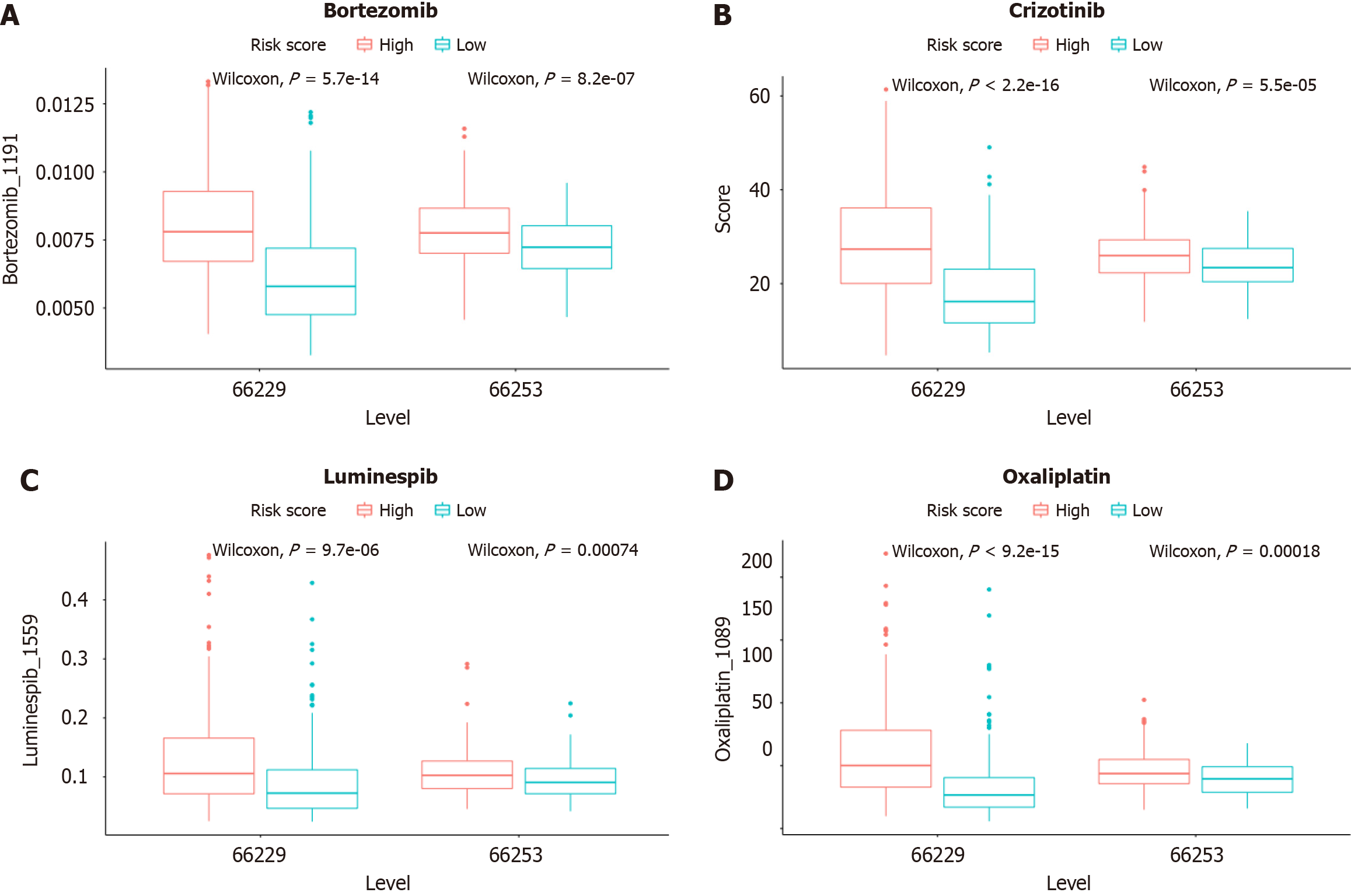
To explore the relationship between the signature and response to therapeutic drugs currently used for GC, we selected common chemotherapeutic drugs and targeted agents to evaluate the sensitivities of patients in the low- and high-risk groups. The results showed that drugs such as bortezomib, crizotinib, luminespib, and oxaliplatin had lower IC50s in the low-risk group than in the high-risk group, which suggested that patients in the low-risk group were more sensitive to these drugs than were those in the high-risk group (Figure 10). As a result, the model shows great potential for predicting chemotherapy sensitivity and may help clinicians choose optimal chemotherapy regimens.
Genomic analysis is a promising approach for providing prognostic and predictive information to guide clinical diagnosis and treatment decisions in cancer patients. Due to the scarcity of useful biomarkers, early diagnosis of GC and reliable prediction of patient response to chemotherapy and immune checkpoint inhibitors are often unavailable. To our knowledge, inflammation is an important hallmark of cancer and plays an integral role in the development and progression of cancer. Previous studies have indicated that inflammatory-related gene signatures can predict the prognosis and impact the immune status of HCC[22], lung adenocarcinoma[39], head and neck squamous cell carcinoma[40], and pancreatic adenocarcinoma[41]. These findings suggest that inflammatory-related genes could be used as tumour prognostic markers and are closely related to tumorigenesis. In addition, some reports have suggested that serum biomarkers related to the inflammatory response, such as the preoperative NLR, derived NLR, PLR, and lymphocyte-to-monocyte ratio, can serve as good predictors of GC prognosis[42]. However, the potential of an inflammatory-related gene signature as a prognostic marker for GC has not been investigated.
In our study, we systematically explored the expression of inflammatory response-related genes in GC tissues and their relationship with DFS and OS. A total of 173 genes were identified by matching the GSE66229 cohort with inflammatory-related genes. Univariate Cox analysis revealed that 23 DEGs were associated with OS (OS-related DEGs). To establish a prognostic model for OS, we used LASSO regression analysis to identify three inflammatory-related genes based on the GSE66229 dataset and validated the results using the GSE39582 dataset from the GEO. The validity of the novel signature was shown in the training, validation, and stage subgroups. The signature exhibited robust prognostic capacity, especially for the short-term survival of patients with GC. In addition, the OS of patients at high risk was shorter than that of patients at low risk according to the TNM stage. In addition, multivariate Cox regression analysis indicated that our prognostic model was an independent prognostic parameter for OS (hazard ratio > 1, P < 0.010). The AUC was used to verify the predictive power of the features. Additionally, the forest plot suggested that the risk score was an independent parameter according to the multivariate Cox regression analyses. Moreover, a nomogram integrating the inflammatory-related gene signature and clinical features was built to predict 1-, 3-, and 5-year DFS, and its predictive ability was validated in the GSE26253 dataset. Therefore, our nomogram may provide simple and accurate prognostic predictions for GC.
Subsequently, we used consensus clustering to identify inflammatory-based subtypes. After K-means clustering, we identified three subtypes of inflammation, namely, FS1, FS2 and FS3. Survival analysis revealed that patients in the FS1 and FS2 groups had better prognoses than patients in the FS3 group. In conclusion, inflammatory subtypes can be used to predict the prognosis of GC patients with better accuracy than traditional grading and staging methods.
Moreover, the expression levels of the three prognostic genes were verified using an online database. Among the three genes included in the prognostic model, IL1R1 was found to be obviously overexpressed at the mRNA level in GC tumours compared to normal tissues in the GEPIA database (Figure 7A, S10C). Survival analysis revealed that patients with high IL1R1 expression had significantly shorter OS and DFS (Figure 7B and C). Patients with high IL1R1 expression in the GEPIA cohort had markedly shorter DFS and OS than patients with low IL1R1 expression. Moreover, the IL1R1 results were consistent with the DFS and OS results in the GSE17536 cohort in PrognoScan. Taken together, our findings indicate that IL1R1 is aberrantly expressed and that high IL1R1 expression is a potential prognosticator of adverse outcomes.
IL1R1 (interleukin 1 receptor, type 1) is a cytokine receptor that belongs to the interleukin-1 receptor family and is an important mediator involved in many cytokine-induced immune and inflammatory responses. IL1R1, an essential participant in the IL-1R signalling pathway, is the only receptor that can bind to both agonistic ligands, IL-1α and IL-1β, subsequently mediating positive signal transduction via the NF-κB and MAP kinase pathways and participating in the pathogenesis of cancer[43,44]. The literature has illustrated the potential value of IL1R1 antagonists and anti-IL-1 monoclonal antibodies in inhibiting primary tumour growth and reversing acquired chemotherapy and ICB resistance in a variety of models[45-47]. Several clinical trials have evaluated the therapeutic value of targeting IL1R1 and the synergistic effect of IL1R1 antagonists, such as anakinra, with existing therapeutic strategies[48]. In GC, Zhang et al[49] reported that the IL-1R1 concentration was an adverse independent prognosticator and was associated with inferior responsiveness to both ACT and ICB. Therefore, they suggested that IL1R1 might be associated with tumour progression in GC. Furthermore, they found that IL1R1 promoted an immunosuppressive TME and was associated with particular genotypes, especially a loss of MSI status and increased genomic stability. Since multiple clinical trials have been carried out to evaluate the therapeutic value of IL1R1 antagonists alone or in combination with existing chemotherapeutic agents in a large variety of cancers, IL1R1 blockade might be a novel approach for GC treatment in the near future[49].
The TME is a pivotal component of cancer[50], and inflammation is a crucial component of the TME[51]. Immunocytes are essential ingredients of the TME. These immunocytes differentiate into subsets with distinct effects, and inflammatory reprogramming occurs during this process[52]. These immunocytes in the TME have inflammatory characteristics that differ from those in nontumorous tissues[53]. OS was dramatically worse in patients with low CD8+ T-cell infiltration than in those with high CD8+ T-cell infiltration. The survival rate of patients with high CD8+ T-cell infiltration was 100%. Moreover, peritumoural CD8+ T-cell infiltration has an antitumour effect on patients with CRC[54]. CD8+ T-cell expansion and function rely on glycolysis. However, the mechanisms underlying CD8+ T-cell metabolism remain unclear[55]. Our previous study demonstrated that increasing B-cell infiltration, clonal expansion, and mutational frequency from the caecum to the sigmoid colon were linked to an increasing number of reactive bacterial species[56]. Numerous B-cell clones are distributed into two broad networks: one includes the blood, bone marrow, spleen, and lung, whereas the other is distributed to the digestive tract, including colorectal tissues. B-cell clonal lineages are the basis for investigations of tissue-based immunity, including infection, vaccine response, autoimmunity, and tumours[57]. Inflammatory reprogramming of tumour cells and the TME is emerging as a critical characteristic affecting tumour development, metastasis, and response to treatment[58]. A better understanding of inflammatory communication among tumour cells, the intestinal flora, and immunocyte populations will open new avenues for identifying strategies to boost antitumoral immune responses in GC patients. Therefore, we also explored the relationship between the inflammatory-related gene signature and the tumour immunity landscape. The CIBERSORT algorithm results showed that the 22 immune cell infiltration landscapes exhibited a marked difference in the percentage of immunocytes among the samples. In patients in the low-risk group, greater proportions of infiltrating CD4+ memory T cells, activated NK cells, M1 macrophages, neutrophils, plasma cells, activated NK cells, and activated dendritic cells were found. In addition, the fractions of T follicular helper cells, activated mast cells, naive B cells, gamma delta T cells, and eosinophils were greater in the high-risk group. Thus, we concluded that activated NK cells might play an important role in GC patients.
Chemical treatment is one of the most basic and traditional methods for treating tumours and is widely used in clinical practice. However, there is a major problem of resistance to chemotherapeutic drugs, which results in a much less effective therapeutic effect. Therefore, we analysed the role of the risk model in differentiating chemosensitivity. The IC50s of several common chemotherapeutic agents were significantly different among the different risk groups. We observed that patients in the low-risk group were more sensitive to bortezomib, crizotinib, Luminex, and oxaliplatin than were those in the high-risk group, suggesting that inflammation-related genes are associated with drug sensitivity. Therefore, this prognostic model can be used to predict the prognosis of patients with GC, helping to elucidate the molecular mechanism of GC and providing new ideas for targeted therapy. Inflammation-related genes can be used as predictors of GC clinical outcomes and therapeutic response and even as therapeutic targets for overcoming drug resistance or adjuvant drug sensitivity.
Despite the use of various methods to optimize our model, there are still several limitations and shortcomings that need to be addressed. First, the analysis was based on data from public databases, and all the samples were obtained retrospectively, indicating that prospective multicentre trials and in vivo/in vitro experimental studies are needed to explore the specific mechanism of these genes in relation to the prognosis of GC. Furthermore, although our prognostic nomogram integrates the IRG signature and clinical features, we cannot determine the contribution of each IRG to this signature. In addition, important risk factors for GC, such as family history, diet, alcohol consumption, smoking status, and H. pylori and EBV infection, were not included in the analysis. We will continue to study this area in the future. Finally, this study preliminarily explored the potential relationship between the IRG risk signature and immune cell infiltration, and further studies are needed to reveal the potential underlying mechanism involved.
In summary, we identified a novel signature consisting of three inflammatory response-related genes that could precisely predict the prognosis of patients with GC. However, the specific underlying mechanism of inflammatory response-related genes and tumour immunity in GC is still unclear and deserves further study. Taken together, our work will help shed light on the role of these genes in tumorigenesis, particularly in the areas of immune response, TME and drug resistance, which are critical for the development of personalized cancer therapies.
Gastric cancer (GC) is a highly aggressive malignancy with a heterogeneous nature, which makes prognosis prediction and treatment determination difficult. Inflammation is now recognized as one of the hallmarks of cancer and plays an important role in the aetiology and continued growth of tumours. Inflammation also affects the prognosis of GC patients. Recent reports suggest that a number of inflammatory-related biomarkers are useful for predicting tumour prognosis.
The importance of inflammatory-related biomarkers in predicting the prognosis of GC patients is still unclear.
We established a novel three-gene prognostic signature that may be useful for predicting the prognosis and personalizing treatment decisions of GC patients.
We downloaded the mRNA expression profiles and corresponding clinical data of patients with GC from a public database. Then, we constructed a prognostic signature comprising three differentially expressed genes (DEGs) related to the inflammatory response. We employed univariate and multivariate Cox regression analyses to evaluate the prognosis of patients with GC from the GEO cohort independently and validated the stability and reliability of the findings. Furthermore, we analysed the associations between prognostic gene expression and immune infiltrate types and between prognostic gene expression and chemotherapeutic sensitivity.
A prognostic model consisting of three inflammatory-related genes (MRPS17, GUF1, and PDK4) was constructed. Independent prognostic analysis revealed that the risk score was a separate prognostic factor in GC patients. According to the risk score, GC patients were stratified into high- and low-risk groups, and patients in the high-risk group had significantly worse prognoses according to age, sex, TNM stage and Lauren type. Consensus clustering identified three subtypes of inflammation that could predict GC prognosis more accurately than traditional grading and staging. Finally, the study revealed that patients in the low-risk group were more sensitive to certain drugs than were those in the high-risk group, indicating a link between inflammation-related genes and drug sensitivity.
We identified a novel signature consisting of three inflammatory response-related genes that could precisely predict the prognosis of patients with GC. However, the specific underlying mechanism of inflammatory response-related genes and tumour immunity in GC is still unclear and deserves further study. Taken together, our work will help shed light on the role of these genes in tumorigenesis, particularly in the areas of immune response, tumour microenvironment and drug resistance, which are critical for the development of personalized cancer therapies.
We believe that this powerful prognostic signature could help improve the risk stratification of GC patients, provide a more effective assessment for clinical management and provide new therapeutic targets for the treatment of these patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM
| 1. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47137] [Article Influence: 3366.9] [Reference Citation Analysis (5)] |
| 2. | La Vecchia S, Sebastián C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 3. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 857] [Article Influence: 171.4] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1337] [Article Influence: 334.3] [Reference Citation Analysis (2)] |
| 5. | Jiang Y, Li T, Liang X, Hu Y, Huang L, Liao Z, Zhao L, Han Z, Zhu S, Wang M, Xu Y, Qi X, Liu H, Yang Y, Yu J, Liu W, Cai S, Li G. Association of Adjuvant Chemotherapy With Survival in Patients With Stage II or III Gastric Cancer. JAMA Surg. 2017;152:e171087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13166] [Article Influence: 1880.9] [Reference Citation Analysis (4)] |
| 7. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64634] [Article Influence: 16158.5] [Reference Citation Analysis (176)] |
| 8. | Tiwari A, Trivedi R, Lin SY. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J Biomed Sci. 2022;29:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 194] [Reference Citation Analysis (0)] |
| 9. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2085] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 10. | Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 2431] [Article Influence: 405.2] [Reference Citation Analysis (0)] |
| 11. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11281] [Article Influence: 490.5] [Reference Citation Analysis (2)] |
| 12. | Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13:5094-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 14. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8181] [Article Influence: 545.4] [Reference Citation Analysis (0)] |
| 15. | Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract. 2013;2013:393015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Rositch AF. Global burden of cancer attributable to infections: the critical role of implementation science. Lancet Glob Health. 2020;8:e153-e154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 18. | Takahashi Y, Kawamura M, Hato T, Harada M, Matsutani N, Horio H. Neutrophil-Lymphocyte Ratio as a Prognostic Marker for Lung Adenocarcinoma After Complete Resection. World J Surg. 2016;40:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Cai H, Zhang ZH, Zhou YJ, Liu J, Chen HQ, Lin RY. The Prognostic Value of Preoperative Plasma Fibrinogen and Neutrophil-to-Lymphocyte Ratio in Patients With Laryngeal Squamous Cell Carcinoma. Ear Nose Throat J. 2021;100:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 1011] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 21. | Zhao R, Xie C, Gong Y, Wei S, Yuan M, Gan J, Chen W. A Novel Inflammatory Response-Related Gene Signature Predicts Immune Status and Prognosis of Breast Cancer. J Oncol. 2022;2022:5468858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Lin Z, Xu Q, Miao D, Yu F. An Inflammatory Response-Related Gene Signature Can Impact the Immune Status and Predict the Prognosis of Hepatocellular Carcinoma. Front Oncol. 2021;11:644416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 23. | Zheng H, Luo W, Li Y, Peng G, Zhou D, Tang D, Cheng J, Wu S. Identification and Development of Inflammatory Response-Related Genes Signature Associated With Prognosis Evaluation and Immune Status of Bladder Cancer. Front Cell Dev Biol. 2022;10:837849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Xie F, Huang X, He C, Wang R, Li S. An Inflammatory Response-Related Gene Signature Reveals Distinct Survival Outcome and Tumor Microenvironment Characterization in Pancreatic Cancer. Front Mol Biosci. 2022;9:876607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Furuya TK, Jacob CE, Tomitão MTP, Camacho LCC, Ramos MFKP, Eluf-Neto J, Alves VAF, Zilberstein B, Cecconello I, Ribeiro U Jr, Chammas R. Association between Polymorphisms in Inflammatory Response-Related Genes and the Susceptibility, Progression and Prognosis of the Diffuse Histological Subtype of Gastric Cancer. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 27. | Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4524] [Cited by in RCA: 4435] [Article Influence: 211.2] [Reference Citation Analysis (0)] |
| 28. | Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29963] [Cited by in RCA: 28966] [Article Influence: 1158.6] [Reference Citation Analysis (1)] |
| 29. | Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808-D815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3099] [Cited by in RCA: 3361] [Article Influence: 280.1] [Reference Citation Analysis (0)] |
| 30. | Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3224] [Cited by in RCA: 3542] [Article Influence: 236.1] [Reference Citation Analysis (0)] |
| 31. | Asplund A, Edqvist PH, Schwenk JM, Pontén F. Antibodies for profiling the human proteome-The Human Protein Atlas as a resource for cancer research. Proteomics. 2012;12:2067-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 32. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3480] [Article Influence: 183.2] [Reference Citation Analysis (1)] |
| 33. | Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2463] [Cited by in RCA: 2831] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 34. | Wilson BJ, Giguère V. Identification of novel pathway partners of p68 and p72 RNA helicases through Oncomine meta-analysis. BMC Genomics. 2007;8:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX, Liu J, Luo XJ, Meng Q, Pu HY, Wang YN, Hu PS, Liu ZX, Zeng ZL, Zhao Q, Deng R, Zhu XF, Ju HQ, Xu RH. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 36. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12803] [Article Influence: 984.8] [Reference Citation Analysis (0)] |
| 37. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11255] [Article Influence: 937.9] [Reference Citation Analysis (0)] |
| 38. | Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017;77:e108-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 4092] [Article Influence: 511.5] [Reference Citation Analysis (0)] |
| 39. | Zhai WY, Duan FF, Chen S, Wang JY, Lin YB, Wang YZ, Rao BY, Zhao ZR, Long H. A Novel Inflammatory-Related Gene Signature Based Model for Risk Stratification and Prognosis Prediction in Lung Adenocarcinoma. Front Genet. 2021;12:798131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Han Y, Ding Z, Chen B, Liu Y. A Novel Inflammatory Response-Related Gene Signature Improves High-Risk Survival Prediction in Patients With Head and Neck Squamous Cell Carcinoma. Front Genet. 2022;13:767166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Deng ZL, Zhou DZ, Cao SJ, Li Q, Zhang JF, Xie H. Development and Validation of an Inflammatory Response-Related Gene Signature for Predicting the Prognosis of Pancreatic Adenocarcinoma. Inflammation. 2022;45:1732-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 43. | Voronov E, Dinarello CA, Apte RN. Interleukin-1α as an intracellular alarmin in cancer biology. Semin Immunol. 2018;38:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50:778-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 728] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 45. | Garlanda C, Mantovani A. Interleukin-1 in tumor progression, therapy, and prevention. Cancer Cell. 2021;39:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 46. | Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 47. | Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, Dinarello CA, Voronov E, Apte RN. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 321] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 48. | Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, Geyer SM, Campbell ME, Kyle RA, Rajkumar SV, Greipp PR, Kline MP, Xiong Y, Moon-Tasson LL, Donovan KA. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Zhang P, Gu Y, Fang H, Cao Y, Wang J, Liu H, Zhang H, Li H, He H, Li R, Lin C, Xu J. Intratumoral IL-1R1 expression delineates a distinctive molecular subset with therapeutic resistance in patients with gastric cancer. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 50. | Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1534] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 51. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 52. | Biswas SK. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity. 2015;43:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 513] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 53. | Xiao Z, Dai Z, Locasale JW. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun. 2019;10:3763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 54. | Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309-313. [PubMed] |
| 55. | Hu Z, Qu G, Yu X, Jiang H, Teng XL, Ding L, Hu Q, Guo X, Zhou Y, Wang F, Li HB, Chen L, Jiang J, Su B, Liu J, Zou Q. Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8(+) T Cells. Cell Metab. 2019;30:290-302.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 56. | James KR, Gomes T, Elmentaite R, Kumar N, Gulliver EL, King HW, Stares MD, Bareham BR, Ferdinand JR, Petrova VN, Polański K, Forster SC, Jarvis LB, Suchanek O, Howlett S, James LK, Jones JL, Meyer KB, Clatworthy MR, Saeb-Parsy K, Lawley TD, Teichmann SA. Distinct microbial and immune niches of the human colon. Nat Immunol. 2020;21:343-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 57. | Meng W, Zhang B, Schwartz GW, Rosenfeld AM, Ren D, Thome JJC, Carpenter DJ, Matsuoka N, Lerner H, Friedman AL, Granot T, Farber DL, Shlomchik MJ, Hershberg U, Luning Prak ET. An atlas of B-cell clonal distribution in the human body. Nat Biotechnol. 2017;35:879-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 58. | Ngwa VM, Edwards DN, Philip M, Chen J. Microenvironmental Metabolism Regulates Antitumor Immunity. Cancer Res. 2019;79:4003-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |