Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.761
Peer-review started: October 19, 2023
First decision: December 4, 2023
Revised: December 19, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: March 15, 2024
Processing time: 144 Days and 21.7 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the second leading cause of cancer deaths worldwide. It is often diagnosed at an advanced stage and therefore its prognosis remains poor with a low 5-year survival rate. HCC patients have increasingly complex and constantly changing characteristics, thus up-to-date and comprehensive data are fundamental.
To analyze the epidemiology and main clinical characteristics of HCC patients in a referral center hospital in the northwest of Italy between 2010 and 2019.
In this retrospective study, we analyzed the clinical data of all consecutive patients with a new diagnosis of HCC recorded at "Santa Croce e Carle" Hospital in Cuneo (Italy) between 1 January 2010 and 31 December 2019. To highlight possible changes in HCC patterns over the 10-year period, we split the population into two 5-year groups, according to the diagnosis period (2010-2014 and 2015-2019).
Of the 328 HCC patients who were included (M/F 255/73; mean age 68.9 ± 11.3 years), 154 in the first period, and 174 in the second. Hepatitis C virus infection was the most common HCC risk factor (41%, 135 patients). The alcoholic etiology rate was 18%, the hepatitis B virus infection etiology was 5%, and the non-viral/non-alcoholic etiology rate was 22%. The Child-Pugh score distribution of the patients was: class A 75%, class B 21% and class C 4%. The average Mayo end-stage liver disease score was 10.6 ± 3.7. A total of 55 patients (17%) were affected by portal vein thrombosis and 158 (48%) by portal hypertension. The average nodule size of the HCC was 4.6 ± 3.1 cm. A total of 204 patients (63%) had more than one nodule < 3, and 92% (305 patients) had a non-metastatic stage of the disease. The Barcelona Clinic Liver Cancer (BCLC) staging distribution of all patients was: 4% very early, 32% early, 23% intermediate, 34% advanced, and 7% terminal. Average survival rate was 1.6 ± 0.3 years. Only 20% of the patients underwent treatment. Age, presence of ascites, BCLC stage and therapy were predictors of a better prognosis (P < 0.01). A comparison of the two 5-year groups revealed a statistically significant difference only in global etiology (P < 0.05) and alpha-fetoprotein (AFP) levels (P < 0.01).
In this study analyzing patients with a new diagnosis of HCC between 2010-2019, hepatitis C virus infection was the most common etiology. Most patients presented with an advanced stage disease and a poor prognosis. When comparing the two 5-year groups, we observed a statistically significant difference only in global etiology (P < 0.05) and AFP levels (P < 0.01).
Core Tip: Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the second leading cause of cancer deaths worldwide. HCC patients have increasingly complex and constantly changing characteristics, thus up-to-date and comprehensive data are fundamental. In this study, we analyzed the epidemiology and main clinical characteristics of 328 HCC patients in a referral hospital in the northwest of Italy between 2010 and 2019. We found that hepatitis C virus infection was the most common etiology and most patients presented with advanced stage disease and a poor prognosis.
- Citation: Bracco C, Gallarate M, Badinella Martini M, Magnino C, D'Agnano S, Canta R, Racca G, Melchio R, Serraino C, Polla Mattiot V, Gollè G, Fenoglio L. Epidemiology, therapy and outcome of hepatocellular carcinoma between 2010 and 2019 in Piedmont, Italy. World J Gastrointest Oncol 2024; 16(3): 761-772
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/761.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.761
There are more than 850000 new cases of primary liver cancer per year worldwide[1]. With a 5-year survival of 18%, after pancreatic cancer, this neoplasm is currently the second leading oncological cause of death globally and this figure is constantly increasing[2,3]. Hepatocellular carcinoma (HCC) is the most common primary liver cancer, accounting for 90% of cases[1,4]. The World Health Organization estimates that more than one million patients will die of HCC in 2030.
In Italy, there were an estimated 12800 new cases of HCC in 2018, about 3% of all new cases of cancer, with a 5-year survival rate of 20% and a 10-year survival rate of 10%. Various risk factors for the development of HCC are well defined, such as liver cirrhosis (regardless of its etiology), chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV), alcohol abuse and metabolic syndrome[5]. Universal HBV vaccination and the extensive implementation of HCV-action antiviral agents are likely to change the etiological landscape of HCC. However, the increase in non-alcoholic fatty liver disease (NAFLD), along with metabolic syndrome and obesity, will soon become one of the most important HCC causes in Western countries[6].
Significant discoveries have been made in understanding the epidemiology, risk factors and molecular profiles of HCC. Rational approaches to prevention, diagnosis and treatment have been applied to high incidence populations, and are effective in preventing HCC and reducing its overall mortality.
The fact that risk factors can be eliminated, however, does not always translate into global improvements, for example, due to the suboptimal implementation of treatments in underdeveloped areas. Similarly, although surveillance is cost-effective in HCC, the global implementation of such programs is still suboptimal and is estimated to involve < 50% of the target population in Western countries[7].
The incidence and mortality for HCC thus continues to increase in many countries and in most patients this neoplasm still occurs at an advanced stage[8]. New diagnostic approaches and recent therapeutic advances could achieve a reduction in HCC morbidity and mortality within the next few decades.
HCC patients have increasingly complex and constantly changing characteristics, which require an individualized and evidence-based approach. This implies the need for recent and exhaustive data on this type of patient. The impact of changing risk factors, demographics and new therapies remain unclear to date with few studies dedicated to these areas[9-11].
This study, therefore, analyzed the epidemiology, presentation and main clinical characteristics and therapeutic management of HCC patients in north-west Italy from 2010 to 2019.
In this retrospective analysis, the clinical data of 328 newly diagnosed HCC patients were evaluated and analyzed at the "Santa Croce e Carle" General Hospital of Cuneo (Piedmont, Italy) between 1 January 2010 and December 31, 2019. To highlight possible changes in HCC patterns in the 1st 5 years and the 2nd 5 years, we divided the population according to their diagnosis period (2010-2014 or 2015-2019).
We selected patients by searching the clinical information system and medical records database of the hospital with the codes for HCC (according to the International Classification of Disease, 9th revision - Clinical Modification classification system). HCC patients were etiologically classified according to the most likely anamnestic cause as follows: (1) HBV: If patients had a history of hepatitis B virus infection with a positive test for the hepatitis B surface antigen (HbSAg). Patients with negative HbSAg but with positive anti-HBc antibodies and a history of antiviral drug therapy were also considered in this group; (2) HCV: If patients had a history of hepatitis C virus infection with positive anti-HCV serum antibodies and HCV-RNA titers. Patients with positive serum anti-HCV antibodies but negative HCV-RNA and a history of eradicating drug therapy were also considered in this group; (3) Alcoholic: In cases of anamnestic history of daily intake of ethanol > 60 g for women, and > 80 g for men for more than 10 years; (4) Multi-etiology: If there was a combination of causal factors (viral and alcoholic); (5) NAFLD: In cases of steatosis on ultrasound and/or biopsy, and absence of significant alcohol consumption; (6) Hereditary hemochromatosis: In cases of alterations in transferrin saturation (> 45%) and positivity to the genetic test for one of the HFE/HJV and HAMP/TFR2/SLC40A1 genes; (7) Not known/idiopathic: In cases of absence of recognized causes and/or unspecified etiology.
Laboratory findings had been recorded at the time of initial HCC diagnosis. If they were not available on the exact date, results closest to the date of diagnosis within 90 d were considered. The severity of liver dysfunction was assessed according to the Child-Pugh classification. The model for end-stage liver disease (MELD) score, the chronic liver disease severity score system, was calculated to predict survival at 3 mo. It was specified where the diagnosis of HCC was confirmed by biopsy and histological examination. HCC was classified as unifocal, paucifocal (≤ 3 nodules), multifocal (> 3 nodules), infiltrative and/or massive (infiltrating growth pattern and/or a mass of > 10 cm diameter and an indefinite limit). The tumor size of the expanding nodules was also measured (in cases of multinodular tumors, the largest was measured). The HCC stage was assessed according to the Barcelona Clinic Liver Cancer (BCLC) staging system, updated to 2022[12]. Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) score.
Regarding treatment, patients were divided into those who had received curative therapy for HCC after diagnosis (liver transplant, surgical resection or percutaneous ablation) and patients who received non-curative therapy [trans-arterial chemo embolization (TACE), selective internal radiation therapy (SIRT), sorafenib chemotherapy or palliation].
Continuous variables were presented as mean and deviation standards, and categorical variables as absolute and relative frequencies. Group comparisons were performed using the ANOVA test for continuous variables, and the Pearson χ2 test for categorical variables. Survival was calculated from the time HCC was diagnosed to death. The data were censored after 5 years of follow-up. The Kaplan-Meier method was used for the survival analysis and the difference between the survival curves was assessed using the Log-Rank test. A Cox proportional risk model was adopted to test the role of prognostic factors associated with the probability of death. Statistical significance was assumed by accepting an alpha < 0.05 error. All statistical analyses were performed using Rstudio 2022.02.0 based on R version 4.1.2.
The study included 328 patients with HCC, 154 in the first period (2010-2014) and 174 in the second period (2015-2019). The mean age at diagnosis was 68.9 years, with no difference between the two groups, while the mean age at death was 69.7 years in the first period and 72.4 years in the second period (P < 0.05). The male/female ratio was 255/73 (Table 1).
The most common cause of liver disease was HCV infection (135 cases, 41%), followed by alcoholic etiology (58 cases, 18%). HBV infection was identified in only 15 cases (5%), while multiple etiology (viral and alcoholic) was found in 36 patients (11%). A total of 7 patients (2%) had NAFLD, and 4 patients (1%) had hereditary hemochromatosis. In 73 patients (22%), the etiology was unknown/idiopathic. Most cases were patients with viral and/or alcoholic etiology (244 patients, 74%), while cases with non-viral-non-alcoholic etiology accounted for 26% (84 patients). The overall difference between the etiological causes between the two periods was statistically significant (P < 0.05) (Table 1).
| Characteristics | 2010-2014, 154 patients | 2015-2019, 174 patients | All patients, 328 |
| Sex, n (%) | |||
| Male | 122 (79) | 133 (76) | 255 (78) |
| Female | 32 (21) | 41 (24) | 73 (22) |
| Age in yr, mean ± SD | |||
| At diagnosis | 67.6 ± 11.8 | 70 ± 10.7 | 68.9 ± 11.3 |
| At death | 69.7 ± 12.2a | 72.4 ± 10.5a | 71 ± 11.4 |
| Etiology, n (%) | |||
| HCV | 65 (42) | 70 (40) | 135 (41) |
| HBV | 10 (6) | 5 (3) | 15 (5) |
| Alcoholic | 18 (12) | 40 (23) | 58 (18) |
| Multiple etiology1 | 16 (10) | 20 (11) | 36 (11) |
| NAFLD | 5 (3) | 2 (1) | 7 (2) |
| Hereditary hemochromatosis | 0 (0) | 4 (2) | 4 (1) |
| Unknown/idiopathic | 40 (26) | 33 (19) | 73 (22) |
Overall, there were 90 (27%) patients with type 2 diabetes mellitus (T2DM). Diabetes was present in 1 out of 15 patients with HBV etiology (7%), 28 out of 135 patients with HCV etiology (21%), 18 of 58 patients with alcoholic etiology (31%), 7 of 7 patients with NAFLD (100%), 9 of 36 patients with multiple etiology (viral and alcoholic) (25%), 1 of 4 patients with hemochromatosis (25%), and 26 of 73 patients with idiopathic/unknown etiology (36%). We found a statistically significant difference in the presence of T2DM with respect to the various etiological classes (P < 0.01). In addition, cases with non-viral/non-alcoholic etiology presenting with T2DM, overall accounted for 40% compared to those with viral or alcoholic etiology, which accounted for 23% (P < 0.01). In relation to age, diabetes mellitus was more frequent in patients over 65-years-old (P < 0.01).
HCC was diagnosed by histology in 45 patients (14%). Overall, 55 patients (17%) had portal thrombosis at the time of diagnosis. The likelihood of portal thrombosis was statistically higher in patients with more advanced BCLC stage (100% of cases in BCLC C-D stage) (P < 0.01), with alpha-fetoprotein (AFP) > 200 ng/dL (83% of cases) (P < 0.01), with a non-unifocal HCC (78% of cases) (P < 0.01) and with portal hypertension (85% of cases) (P < 0.01).
According to Child-Pugh's classification, 247 patients (75%) were class A, 68 patients (21%) were class B and 13 patients (4%) were class C. The mean MELD score was 10.6 (± 3.7). Patients were grouped into five different stages according to the BCLC system: 12 patients (4%) belonged to BCLC stage 0, 105 (32%) to BCLC stage A, 74 (23%) to BCLC stage B, 113 (34%) to BCLC C stage and 24 (7%) to BCLC stage D (Table 2).
| Characteristics | 2010-2014, 154 patients | 2015-2019, 174 patients | All patients, 328 |
| BCLC stage, n (%) | |||
| BCLC 0 | 4 (3) | 8 (5) | 12 (4) |
| BCLC A | 47 (31) | 58 (33) | 105 (32) |
| BCLC B | 31 (20) | 43 (25) | 74 (23) |
| BCLC C | 56 (36) | 57 (33) | 113 (34) |
| BCLC D | 16 (10) | 8 (5) | 24 (7) |
| Child-Pugh stage, n (%) | |||
| Class A | 114 (74) | 133 (76) | 247 (75) |
| Class B | 30 (19) | 38 (22) | 68 (21) |
| Class C | 10 (6) | 3 (2) | 13 (4) |
| MELD score, mean ± SD | 10.7 ± 3.8 | 10.4 ± 3.6 | 10.4 ± 3.6 |
| Nodule characteristics, n (%) | |||
| Unifocal | 65 (42) | 75 (43) | 140 (43) |
| Paucifocal | 24 (16) | 40 (23) | 64 (20) |
| Multifocal | 52 (34) | 40 (23) | 92 (28) |
| Infiltrative/massive | 13 (8) | 19 (11) | 32 (10) |
| Dimension in cm, mean ± SD | 4.3 ± 2.9 | 4.9 ± 3.3 | 4.6 ± 3.1 |
At the time of diagnosis, 140 HCCs were unifocal (43%), 64 were paucifocal (20%), 92 were multifocal (28%) and 32 were massive (10%). The global average size of the nodules was 4.6 cm (Table 2). Extrahepatic metastases were found in 23 patients (8%): 12 patients had lymph node metastases (4%), 6 had pulmonary metastases (2%), and 5 had bone metastases (2%).
All patients were subdivided according to the ECOG score: 210 patients scored 0 (64%), 95 patients scored 1-2 5 (29%), and 23 patients scored 3-4 (7%).
A total of 64 patients (20%) underwent curative therapeutic procedures: surgical resection in 8 cases (2%) and percutaneous radiofrequency ablation in 56 cases (17%). A total of 264 patients (80%) underwent non-curative therapeutic procedures: TACE in 124 cases (38%), SIRT in 46 cases (14%) and systemic chemotherapy with sorafenib in 17 cases (5%). A total of 77 patients (23%) received palliation or no therapy at all (Table 3). No patients underwent liver transplantation. Only one patient underwent evaluation for transplantation.
| Therapy | 2010-2014, 154 patients | 2015-2019, 174 patients | All patients, 328 |
| Surgical resection | 3 (2) | 5 (3) | 8 (2) |
| Percutaneous radiofrequency ablation | 31 (20) | 25 (14) | 56 (17) |
| TACE | 58 (38) | 66 (38) | 124 (38) |
| SIRT | 13 (8) | 33 (19) | 46 (14) |
| Sorafenib | 11 (7) | 6 (3) | 17 (5) |
| Palliation | 38 (25) | 39 (22) | 77 (23) |
The likelihood of curative therapy was statistically higher in patients with unifocal HCC (73% of cases) (P < 0.01), early BCLC stage (62% of cases in BCLC stage 0-A) (P < 0.01), good performance status (81% of cases with ECOG score 0) (P < 0.01), AFP < 199 ng/dL (77% of cases) (P < 0.05), without ascites (91% of cases) (P < 0.05). A statistically significant difference in the likelihood of curative or non-curative therapy between the etiological groups was not evaluated.
Among patients with viral etiology, 21 out of 91 (23%) patients in the first period and 20 out of 95 patients (21%) in the second period underwent antiviral therapy. Overall, among patients with viral etiology, 41 patients (22%) underwent antiviral therapy.
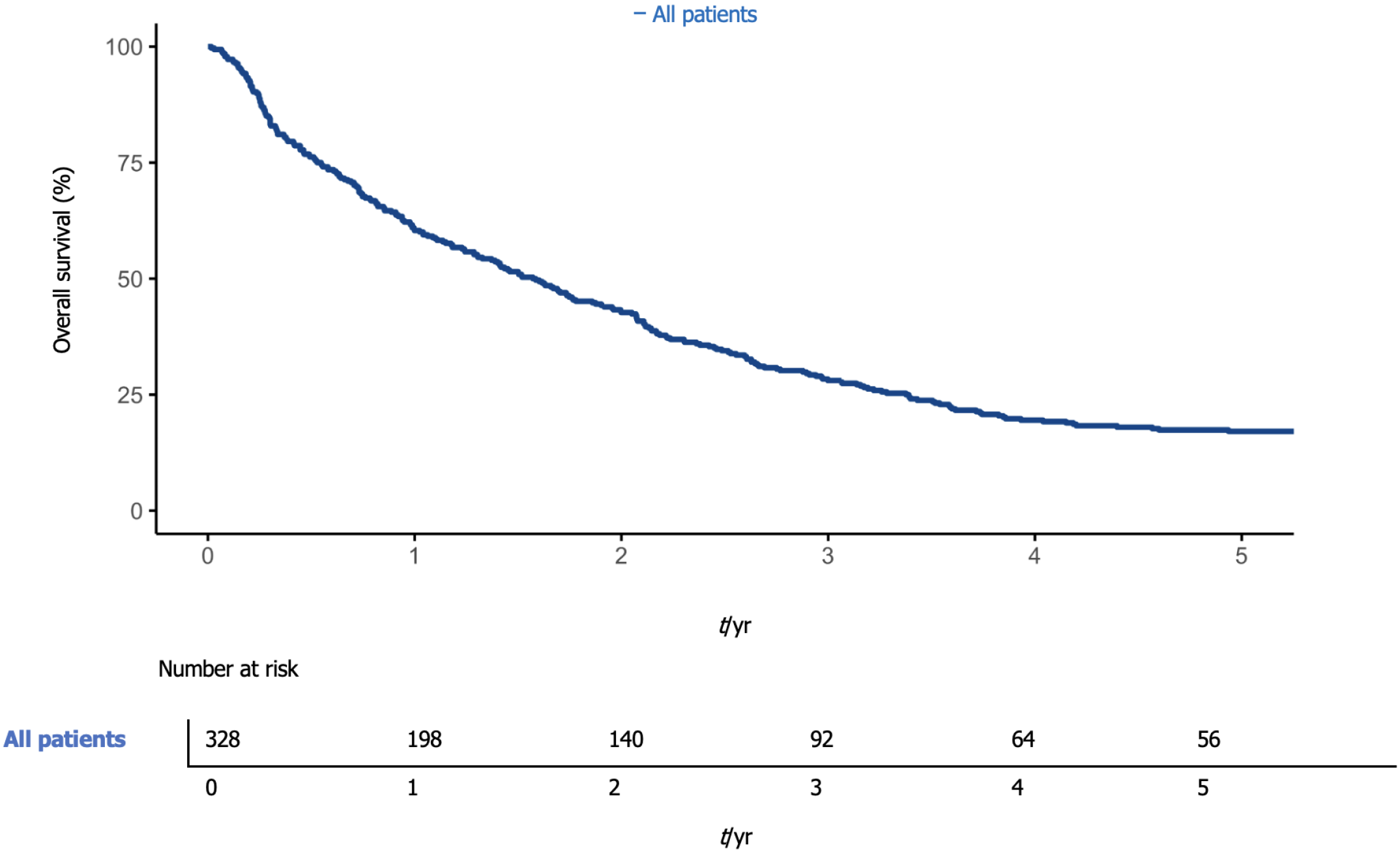
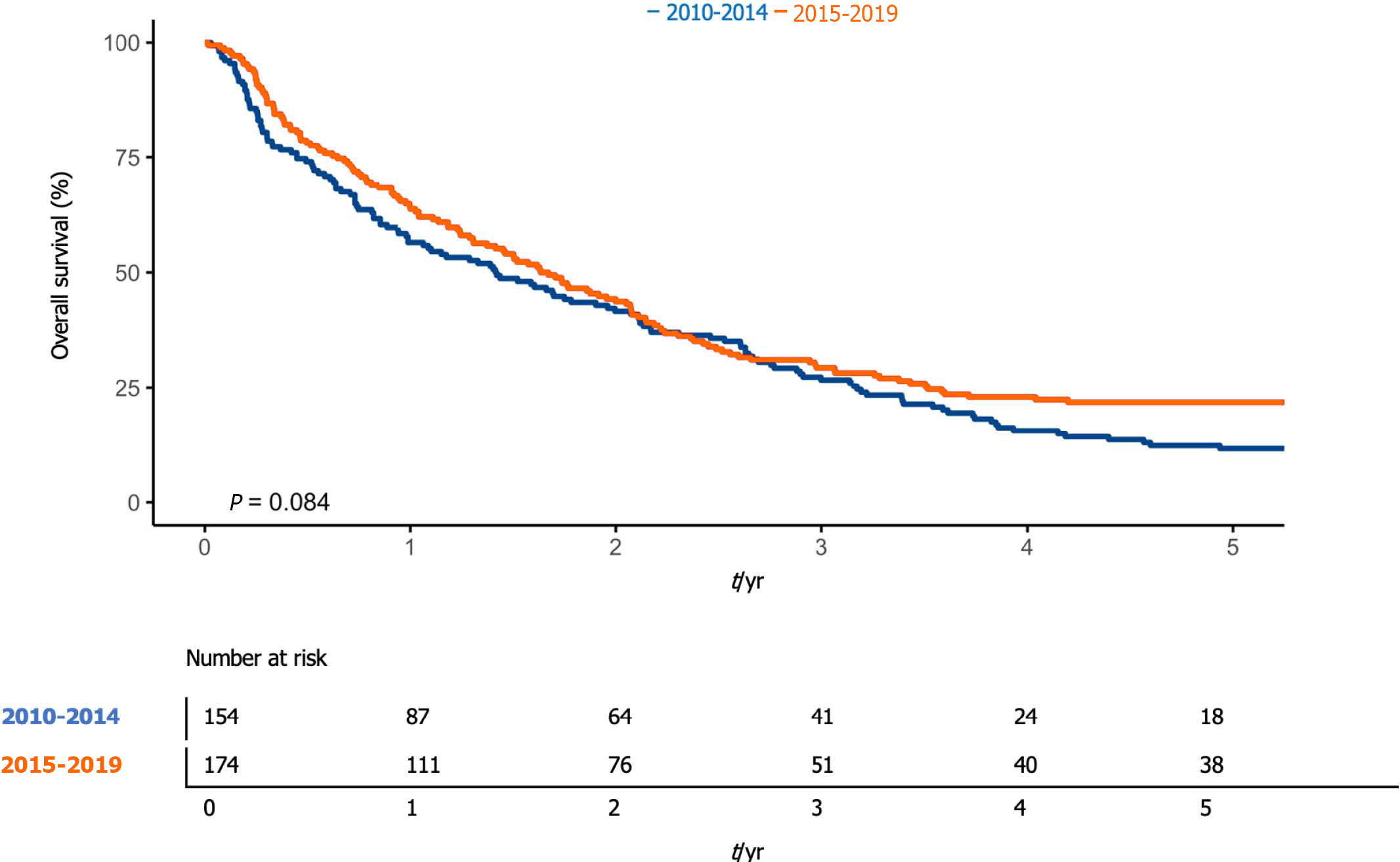
Overall, 1-year survival was 60.4% (95%CI: 55.3-65.9), while 5-year overall survival was 17.1% (95%CI: 13.5-21.7) (Figure 1). The median overall survival was 576 d (95%CI: 476-695). One-year survival was 56.5% (95%CI: 49.1-64.9%) and 63.8% (95%CI: 57.0-71.3%) in the first and second periods, respectively. The median survival was 517 d [95%CI 361-757], and 603 d (95%CI: 476-757) (P < 0.08) in the first and second periods, respectively (Figure 2).
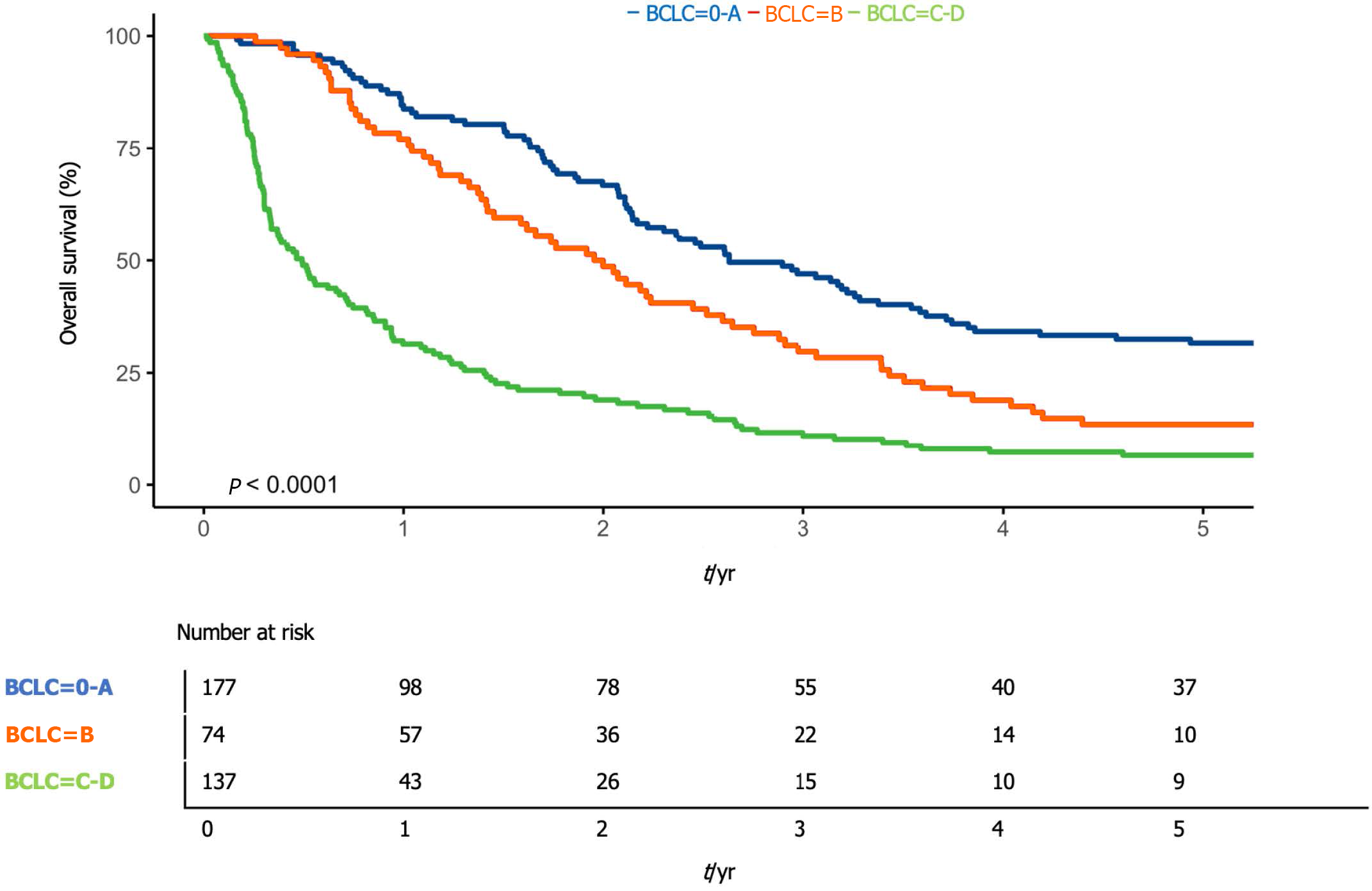
Compared to the BCLC stage, 1-year survival was 83.8% (95%CI: 77.3-90.7) in patients with BCLC stage 0 or A (early), 77.0% (95%CI: 68.0-87.2) in patients with BCLC B (intermediate) stage, and 31.4% (95%CI: 24.5-40.2) in patients with BCLC C or D (advanced) stage (P < 0.01) (Figure 3).
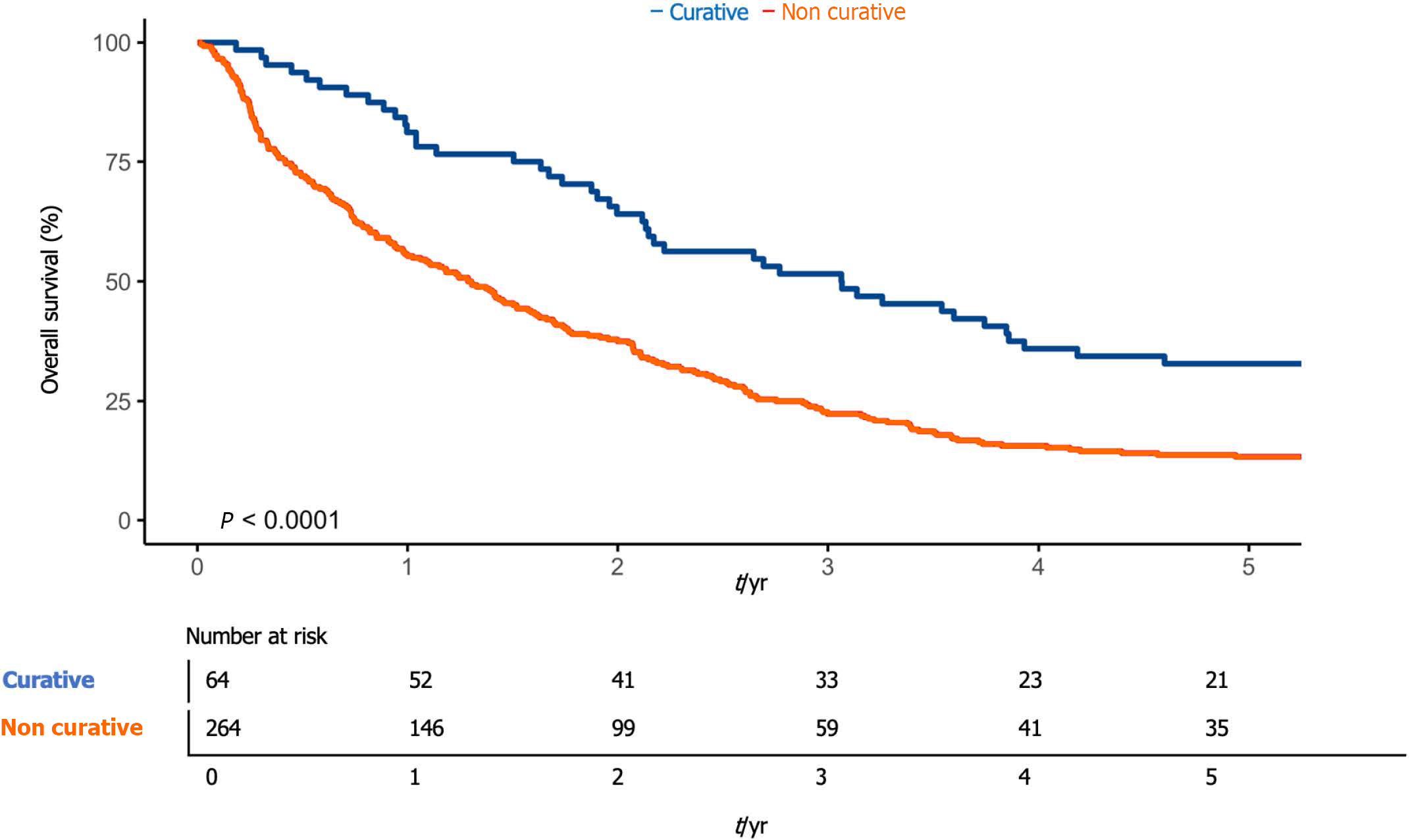
Compared to therapy, 1-year survival was 81.3% (95%CI: 72.2-91.4) in patients undergoing curative therapeutic treatment (resection or ablation) and 55.3% (95%CI: 49.6-61.6) in patients undergoing non-curative therapeutic treatment (TACE, SIRT, chemotherapy with sorafenib or palliation) (P < 0.01) (Figure 4).
At the multivariate analysis, age (≥ 65 vs < 65: HR = 1.41; 95%CI: 1.07-1.86) (P < 0.01), the presence of ascites (HR = 1.45; 95%CI: 1.06-2.00) (P < 0.01), the BCLC stage [B vs 0-A: HR = 1.42; 95%CI: 1.01-1.99 (P < 0.05); C-D vs 0-A: HR = 2.14; 95%CI: 1.26-3.64 (P < 0.01)] and the type of therapy performed (non-curative treatments vs curative treatments: HR = 1.92; 95%CI: 1.35-2.71) (P < 0.01) emerged as significant predictors of survival. There were no significant differences in outcome regarding periods, sex, or etiology.
This long-term retrospective study was conducted on a cohort of 328 HCC patients consecutively assessed between 2010 and 2019 at the "Santa Croce e Carle" General Hospital in Cuneo, a reference center for diagnosis and treatment of HCC in the southwest of Piedmont in Italy.
The aim of this study was to analyze the clinical characteristics of a large cohort of HCC patients treated at a second level hospital. Previous forecasts predicted that the incidence of HCC would gradually start to decrease as a consequence of the probable reduction of cirrhosis related to HCV infection[13]. In reality, however there has recently been a constant increase in HCC incidence rates[14]. Our study revealed an increase in newly diagnosed HCC cases in 2015-2019 (174) compared to 2010-2014 (154).
The causes of this trend are not clear. A key role may have been played by the still high prevalence of HCV infection (due to the general underdiagnosis and therefore lack of treatment of these patients) and the share of HCV-eradicated patients who nevertheless present with cirrhosis as an underlying disease, as well as the progressive increase in HCC due to nonalcoholic fatty liver disease over the past two decades[15].
However, at the same time, we found that the mean age of newly diagnosed HCC patients increased from 67.6 years to 70.0 years (P < 0.1). Progressive aging of HCC patients is a known trend and in line with other cohorts[16]. This could be explained by the impaired cohort effect of chronic HCV infection and by the delayed development of HCC in HCV and HBV infected patients due to the antiviral therapy. In addition, there has been a change in daily clinical practice with more and more elderly patients being referred to specialist centers for evaluation of treatment options.
The main risk factor for HCC in western Europe appears to be HCV infection, followed by alcohol abuse[14]. In our study there was also a significant proportion (22%) of HCC apparently of idiopathic etiology. This is however in line with other previous cases in Europe[9,10]. We also found a low rate of etiology related to a clear diagnosis of NAFLD (2%). However, note that 36% of the cases labeled as idiopathic in our sample were actually affected by T2DM.
Underdiagnosis of NAFLD in the general population is a widely recognized and debated problem. However, there is now incontrovertible evidence that NAFLD contributes to the development of HCC and is becoming an increasingly common cause of this disease worldwide[17]. In fact, about 10%-30% of NAFLD cases likely progress to cirrhosis[18] and in the main developed nations, the incidence of HCC due to NAFLD will probably increase by more than 120% between 2016 and 2030[19].
Currently, however, there are no unambiguous evidence-based recommendations that this disease should be screened for HCC, as in other high-risk groups of HCC[20]. We also found that only 22% of the HCV-positive patients had undergone antiviral therapy. Even stratifying these patients for the two different periods, the values did not change.
Our data thus confirm widespread underutilization of antiviral therapy in HCV positive patients[21]. In fact, depending on the stage of the tumor and life expectancy, patients with HCV positive viral hepatitis should always be considered for antiviral treatment for the prevention of HCC development as well as during treatment of HCC.
Regarding tumor stage, during the entire study period, the most common HCC presentation pattern was a single nodule with a diameter of between 3 cm and 5 cm. This is in line with the previous case series in Italy[9]. Regarding liver function, the majority of our population was in Child-Pugh A class, in line with other studies in Italy[22,23]. In the second period, we found a trend (within the limits of significance) regarding the decrease in the multifocal pattern at diagnosis, and a clear significance with regard to lower AFP values at diagnosis.
Regarding the staging of HCC, we highlighted two incidence peaks in BCLC stage A (early) and stage C (advanced) at the time of diagnosis. A total of 42% of the total sample was either in the advanced or terminal stage (BCLC stage C-D). By dividing this data into two 5-year periods, this percentage dropped from 47% in the first to 37% in the second (this trend was almost significant). All these variations could be due to possibly earlier diagnoses and improved surveillance in high-risk patients in the second period. However, there is still ample room for improvement.
We found that curative therapies (surgical resection/radiofrequency ablation) had been used in 20% of cases, despite the fact that 36% of cases had 0-A (early) BCLC staging. Similar results have been obtained in previous series carried out in Italy[24,25]. This could have two possible explanations. First, the BCLC staging system does not take into account patients’ age, leading to the possibility that non-curative treatments were preferred to curative therapies in elderly patients, given the slow growth of HCC and the survival benefit provided by non-curative therapies[26]. Second, the lack of real-word applications of the BCLC staging system leads to an incorrect choice of therapies for early HCC patients.
The prognosis in HCC patients is in fact determined by the stage of the tumor, by the underlying liver function reserve, and by the general state of health[20]. The BCLC staging system takes all of these factors into account, and is currently recommended as the reference staging system for HCC[12]. Given the complexity of HCC management, we strongly emphasize the need to adopt a common staging system in order to easily define patient groups for correct therapies and stratify their prognosis. The BCLC staging system meets all of these characteristics.
In our population the most frequently used curative therapy was percutaneous radiofrequency ablation and only 8 patients were treated with liver resection. Although this therapeutic option is considered first-line and therefore recommended in patients with 0-A (early) BCLC staging, percutaneous radiofrequency ablation is less invasive and less expensive than surgical resection. In addition it is supported by good survival results and considered as a plausible alternative in selected patients[27]. This preference may be correlated with the relatively high median age and frailty of our population. In addition, 87 of 117 patients with 0-A (early) BCLC staging had cirrhosis, and 24 patients had two or more nodules. In fact, ablation allows for a shorter hospital stay with less serious postoperative complications, which is important given the relatively high median age of our population.
Overall, a large percentage of our population (38%) received a TACE in the study period. The use of TACE has increased significantly in recent years, principally in intermediate stage HCC. Current evidence proves that TACE improves survival in patients who cannot benefit from curative treatment and who do not have severe hepatic impairment, vascular invasion and/or hepatic spread[28].
SIRT is another treatment approach frequently used in patients with BCLC B stage, and we found that its usage was greater, compared to similar series carried out in Italy.
Evidence of greater chances of survival as a result of radioembolization has not yet been demonstrated for randomized clinical trials compared to the standard of care, which is TACE in the intermediate stages and sorafenib in the advanced stages. SIRT is generally used with those patients who are not good candidates for TACE due to a large tumor burden, vascular invasion, or progression to previous TACE[29].
We found that the use of chemotherapy remained constant compared to the previous decade. This is despite the fact that several subsequent studies have revealed the stable benefit of sorafenib in all regions of the world and in all etiologies of HCC, with a median survival of about 10 mo[30]. This is probably linked to the fact that sorafenib is subject to some restrictions, such as poor liver function and the poor tolerability of the therapy[27].
Finally, 23% of our patients did not receive any specific treatment for HCC, but underwent palliation. These, as expected, most commonly had intermediate-to-advanced HCC. The likelihood of undergoing curative therapy was statistically higher in patients with unifocal HCC, in early BCLC stage, and with good performance status. As expected, our patients' prognosis was characterized by a 60% survival rate at 1 year, and only 17% at 5 years. This was in line with the numerous literature data[20]. Comparing the survivals of the two 5-year periods in the study showed a difference at the limits of significance (56% vs 64%, P = 0.08). This variation, in line with the previously reported data (decrease in the multifocal pattern and decrease in BCLC advanced or terminal stage patients), could also perhaps be due to the early diagnosis and better surveillance capacity in high-risk patients in the second period, in line with other case studies[31].
Age, BCLC stage and the type of therapy performed, as well as the presence of ascites, emerged as significant predictors of survival. However, the period of diagnosis, sex or etiology did not prove to be significant predictors. The survival benefit in patients treated with curative therapy underlines the need to choose the right treatment for patients with newly diagnosed HCC, also taking into account BCLC staging.
Unfortunately, the retrospective observational nature of the study prevented us from assessing some potentially interesting variables, e.g., body mass index. Furthermore, the exclusion of patients for whom it was not possible to derive the variables required for the study may have led to some selection bias. Finally, given that our population was from just one hospital our data may have been subject to reference bias.
We have presented detailed information on periodic changes in risk factors and liver function characteristics, tumor stage and treatment modalities performed at diagnosis in a large cohort of patients with HCC in a referral center in the north of Italy. Throughout the world, the current approach to HCC is far from adequate and HCC remains undertreated or inappropriately treated despite the positive advances in diagnosis and treatment in recent years. We hope these findings will be a stimulus for the improved surveillance of patients at risk according to the guidelines of scientific societies and with subsequent better use of the various therapies available.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the second leading cause of cancer deaths worldwide. It is often diagnosed at an advanced stage and therefore its prognosis remains poor, with a low 5-year survival rate.
HCC patients have increasingly complex and constantly changing characteristics, thus up-to-date and comprehensive data are fundamental.
To analyze the epidemiology and main clinical characteristics of HCC patients in a referral hospital in the northwest of Italy between 2010 and 2019.
In this retrospective study, we analyzed the clinical data of all consecutive patients with a new diagnosis of HCC recorded at the "Santa Croce e Carle" Hospital in Cuneo (Italy) between 1 January 2010 and 31 December 2019. To highlight possible changes in HCC patterns over the 10-year period, we split the population into two 5-year groups, according to the diagnosis period (2010-2014 and 2015-2019).
A total of 328 HCC patients were included (M/F 255/73; mean age 68.9 ± 11.3 years), 154 in the first period and 174 in the second. Hepatitis C virus infection was the most common HCC risk factor (41%, 135 patients). The alcoholic etiology rate was 18%, the hepatitis B virus infection etiology was 5%, and the non-viral/non-alcoholic etiology rate was 22%. The Child-Pugh score distribution of the patients was: class A 75%, class B 21% and class C 4%. The average model for end-stage liver disease score was 10.6 ± 3.7. A total of 55 patients (17%) were affected by portal vein thrombosis, and 158 (48%) by portal hypertension. The average nodule size of the HCC was 4.6 ± 3.1 cm. A total of 204 patients (63%) had more than one nodule < 3, and 92% (305 patients) had non-metastatic stage of disease. The Barcelona Clinic Liver Cancer (BCLC) staging distribution of all patients was: 4% very early, 32% early, 23% intermediate, 34% advanced, and 7% terminal. The average survival rate was 1.6 ± 0.3 years. Only 20% of the patients underwent treatment. Age, presence of ascites, BCLC stage and therapy were predictors of a better prognosis (P < 0.01). A comparison of the two 5-year groups revealed a statistically significant difference only in global etiology (P < 0.05) and alpha-fetoprotein (AFP) levels (P < 0.01).
In this study, analyzing patients with a new diagnosis of HCC between 2010-2019, hepatitis C virus infection was the most common etiology. Most patients presented with an advanced stage disease and poor prognosis. When comparing the two 5-year groups, we observed a statistically significant difference only in global etiology (P < 0.05) and AFP levels (P < 0.01).
HCC remains undertreated or inappropriately treated despite the positive advances in diagnosis and treatment in recent years. We hope these findings will be a stimulus for the improved surveillance of patients at risk according to the guidelines of scientific societies, with the subsequent better use of the various therapies available.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li M, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 2. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5238] [Article Influence: 523.8] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1110] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Projections of mortality and causes of death, 2016 to 2060. Available from: http://www.who.int/healthinfo/global_burden_disease/projections/en/. |
| 5. | European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4518] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 6. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1871] [Article Influence: 207.9] [Reference Citation Analysis (4)] |
| 8. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2960] [Article Influence: 370.0] [Reference Citation Analysis (0)] |
| 9. | Fenoglio L, Serraino C, Castagna E, Cardellicchio A, Pomero F, Grosso M, Senore C. Epidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma cases. World J Gastroenterol. 2013;19:3207-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Weinmann A, Koch S, Niederle IM, Schulze-Bergkamen H, König J, Hoppe-Lotichius M, Hansen T, Pitton MB, Düber C, Otto G, Schuchmann M, Galle PR, Wörns MA. Trends in epidemiology, treatment, and survival of hepatocellular carcinoma patients between 1998 and 2009: an analysis of 1066 cases of a German HCC Registry. J Clin Gastroenterol. 2014;48:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Reinders MTM, van Meer S, Burgmans MC, de Jong KP, Klümpen HJ, de Man RA, Ramsoekh DS, Sprengers D, Tjwa ETTL, de Vos-Geelen J, van Erpecum KJ, van der Geest LGM; Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG). Trends in incidence, diagnosis, treatment and survival of hepatocellular carcinoma in a low-incidence country: Data from the Netherlands in the period 2009-2016. Eur J Cancer. 2020;137:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2593] [Article Influence: 864.3] [Reference Citation Analysis (59)] |
| 13. | Bosetti C, Bianchi C, Negri E, Colombo M, La Vecchia C. Estimates of the incidence and prevalence of hepatocellular carcinoma in Italy in 2002 and projections for the years 2007 and 2012. Tumori. 2009;95:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 778] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 15. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 741] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 16. | Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, Del Poggio P, Rapaccini G, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Giannini EG, Caturelli E, Chiaramonte M, Bernardi M, Trevisani F. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2012;56:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 18. | World Gastroenterology Organisation Global Guidelines. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. 2012. Accessed June 5, 2022. Available from: http://www.worldgastroenterology.org/assets/export/userfiles/2012_NASH%20and%20NAFLD_Final_long.pdf. |
| 19. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1307] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 20. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 21. | Roderburg C, Tacke F, Trautwein C. Antiviral Therapy in Patients with Viral Hepatitis and Hepatocellular Carcinoma: Indications and Prognosis. Visc Med. 2016;32:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Borzio M, Colloredo G, Pioltelli P, Quagliuolo M; Gruppo Epatologico Lombardo (GEL). Epidemiology and outcome of hepatocellular carcinoma in Lombardy. Dig Liver Dis. 2007;39:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Trevisani F, Magini G, Santi V, Morselli-Labate AM, Cantarini MC, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F, Zoli M, Borzio F, Giannini EG, Caturelli E, Bernardi M; Italian Liver Cancer (ITA. LI.CA) Group. Impact of etiology of cirrhosis on the survival of patients diagnosed with hepatocellular carcinoma during surveillance. Am J Gastroenterol. 2007;102:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Farinati F, Sergio A, Baldan A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M, Borzio F, Giannini EG, Caturelli E, Trevisani F. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer. 2009;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 27. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3595] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 28. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 29. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 30. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, Porta C, Gerken G, Marrero JA, Nadel A, Shan M, Moscovici M, Voliotis D, Llovet JM. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 653] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 31. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |