Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.550
Peer-review started: October 1, 2023
First decision: December 5, 2023
Revised: December 11, 2023
Accepted: December 26, 2023
Article in press: December 26, 2023
Published online: February 15, 2024
Processing time: 123 Days and 19.4 Hours
Light chain (AL) amyloidosis is a plasma cell dyscrasia characterized by the pathologic production and extracellular tissue deposition of fibrillar proteins derived from immunoglobulin AL fragments secreted by a clone of plasma cells, which leads to progressive dysfunction of the affected organs. The two most commonly affected organs are the heart and kidneys, and liver is rarely the dominant affected organ with only 3.9% of cases, making them prone to misdiagnosis and missed diagnosis.
A 65-year-old woman was admitted with a 3-mo history of progressive jaundice and marked hepatomegaly. Initially, based on enhanced computed tomography scan and angiography, Budd-Chiari syndrome was considered and balloon dilatation of significant hepatic vein stenoses was performed. However, addi
AL amyloidosis with isolated liver involvement is very rare, and can be easily misdiagnosed as a vascular disease.
Core Tip: Light chain (AL) amyloidosis is a systemic disease, with heart, kidneys, and peripheral nerves being the most commonly affected organs. The proportion of patients with only liver involvement alone is quite low, and these patients are highly prone to misdiagnosis and missed diagnosis. We present a case of AL amyloidosis with isolated liver involvement and severe cholestasis as the predominant manifestations.
- Citation: Zhang X, Tang F, Gao YY, Song DZ, Liang J. Hepatomegaly and jaundice as the presenting symptoms of systemic light-chain amyloidosis: A case report. World J Gastrointest Oncol 2024; 16(2): 550-556
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/550.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.550
Systemic light chain (AL) amyloidosis can affect all organs except the central nervous system[1]. The most frequently affected organs are the kidneys and the heart, either separately or combined[2]. Although hepatic deposition of AL amyloid is prevalent, it is usually clinically silent, and the liver is rarely the most severely affected organ[3]. 33%-92% of patients experience liver enlargement due to the accumulation of hepatic amyloid proteins and moderate-to-severe cholestasis[4]. Cases with jaundice as the primary manifestation are rare, accounting for less than 5% of documented cases[5]. AL amyloidosis, characterized by hepatomegaly and jaundice, is easily confused with diseases such as lymphoma, drug-induced liver injury (DILI), sinusoidal obstruction syndrome (SOS), and Budd-Chiari syndrome. Here, we report the case of a 64-year-old woman diagnosed with liver enlargement and progressive jaundice without obvious involvement of other organs. Diagnosis could not be ascertained by medical history, physical examination, or laboratory examination. Finally, the diagnosis of hepatic amyloidosis was confirmed by transjugular liver biopsy. Immunofluorescence, flow cytometry, and bone marrow smear further confirmed the diagnosis of AL amyloidosis.
A 64-year-old female patient was transferred to our hospital with progressive jaundice that had persisted for 3 mo.
The patient had developed jaundice without obvious causes over the previous 3 mo, accompanied by decreased appetite. For 4 wk, diammonium glycyrrhizinate and ursodeoxycholic acid were given in conjunction with Chinese herbal medicines. However, the jaundice gradually worsened.
The patient had undergone hysterectomy for uterine fibroids 18 years previously and cholecystectomy for cholecystitis 6 years ago. Preoperative examination revealed abnormal liver function manifested as elevated alkaline phosphatase (ALP) and glutamyl transpeptidase (GGT) levels. Liver tests were negative for hepatitis viruses, autoimmune liver disease, and metabolic liver disease. The cause of liver damage was not identified. After more than 2 mo of treatment with ursodeoxycholic acid, it was discontinued without further treatment or follow-up.
The patient had no history of habitual alcohol consumption and no significant history of liver injury-inducing drug exposure. The patient had no chronic or familial inherited diseases.
Physical examination revealed marked jaundice, cutaneous spider naevi and an enlarged, hard, non-tender liver.
The patient’s liver function profile showed the following: Total bilirubin, 197.2 μmol/L (normal range: 3.4-20.5 μmol/L); direct bilirubin, 149.0 μmol/L (normal range: 0-3.4 μmol/L); aspartate aminotransferase, 88 IU/L (normal range: 15-40 IU/L); alanine aminotransferase, 11 IU/L (normal range: 9-50 IU/L); gamma-glutamyl transferase, 245 U/L (normal range: 10-60 U/L); ALP, 457 U/L (normal range: 45-125 U/L). The patient’s white blood cell count was 11.47 × 109/L [normal range: (3.5-9.5) × 109/L]; her neutrophils were 73.2%. In addition, there were no abnormalities in routine coagulation, renal function, electrocardiogram, myocardial enzymes, or type B natriuretic peptide levels. Liver tests were negative for hepatitis viruses, autoimmune liver diseases, and metabolic liver diseases such as hepatolenticular degeneration and hemochromatosis. Carbohydrate antigen 199 levels were slightly increased at 138.0 U/mL (normal range: < 60 U/mL), and alpha fetoprotein levels were normal. Monoclonal immunoglobulins were not detected in the serum or urine using immunofixation electrophoresis.
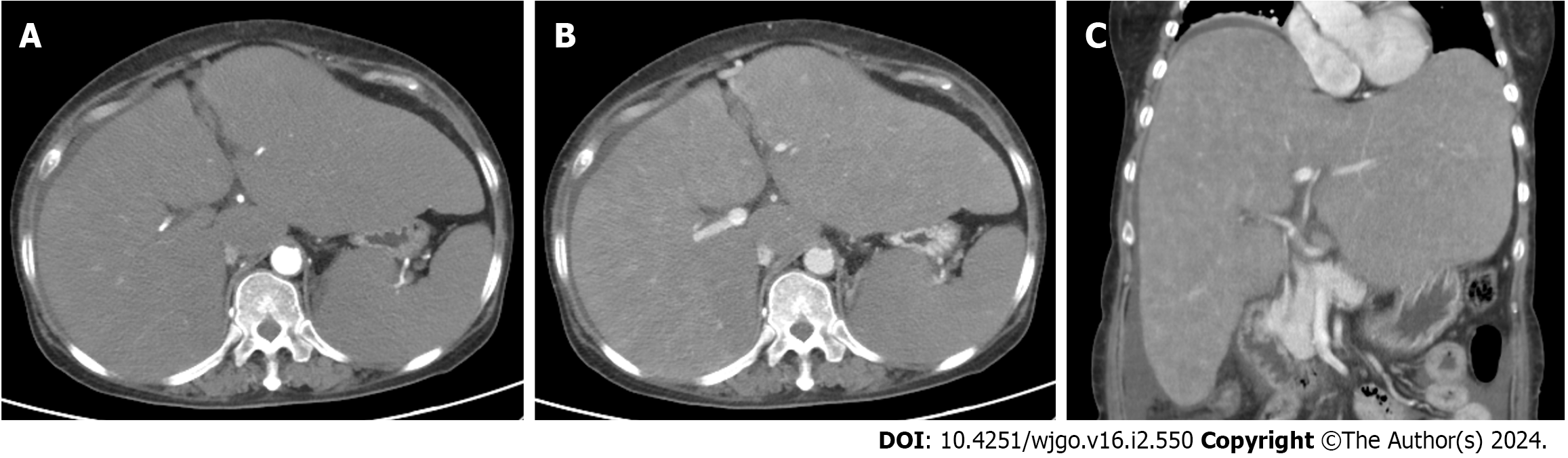
Echocardiography indicated reduced left ventricular diastolic function and mitral regurgitation, while electrocardiography showed no abnormalities. Gastroscopy revealed small esophageal varices. Abdominal enhanced computed tomography revealed an enlarged liver and spleen, irregular liver contour, uneven density of the liver parenchyma, and nodular enhancement in the arterial phase (Figure 1). Angiography suggested staged stenosis of the right and middle hepatic veins with local occlusion at the entrance to the inferior vena cava. The inferior vena cava was slightly narrow, which may have been related to liver enlargement and compression. Budd-Chiari syndrome with occlusion of the hepatic vein was considered, and balloon dilatation was performed to dilate the narrow segment of the hepatic vein and resume the blood stream. Radionuclide bone imaging did not reveal any abnormalities.
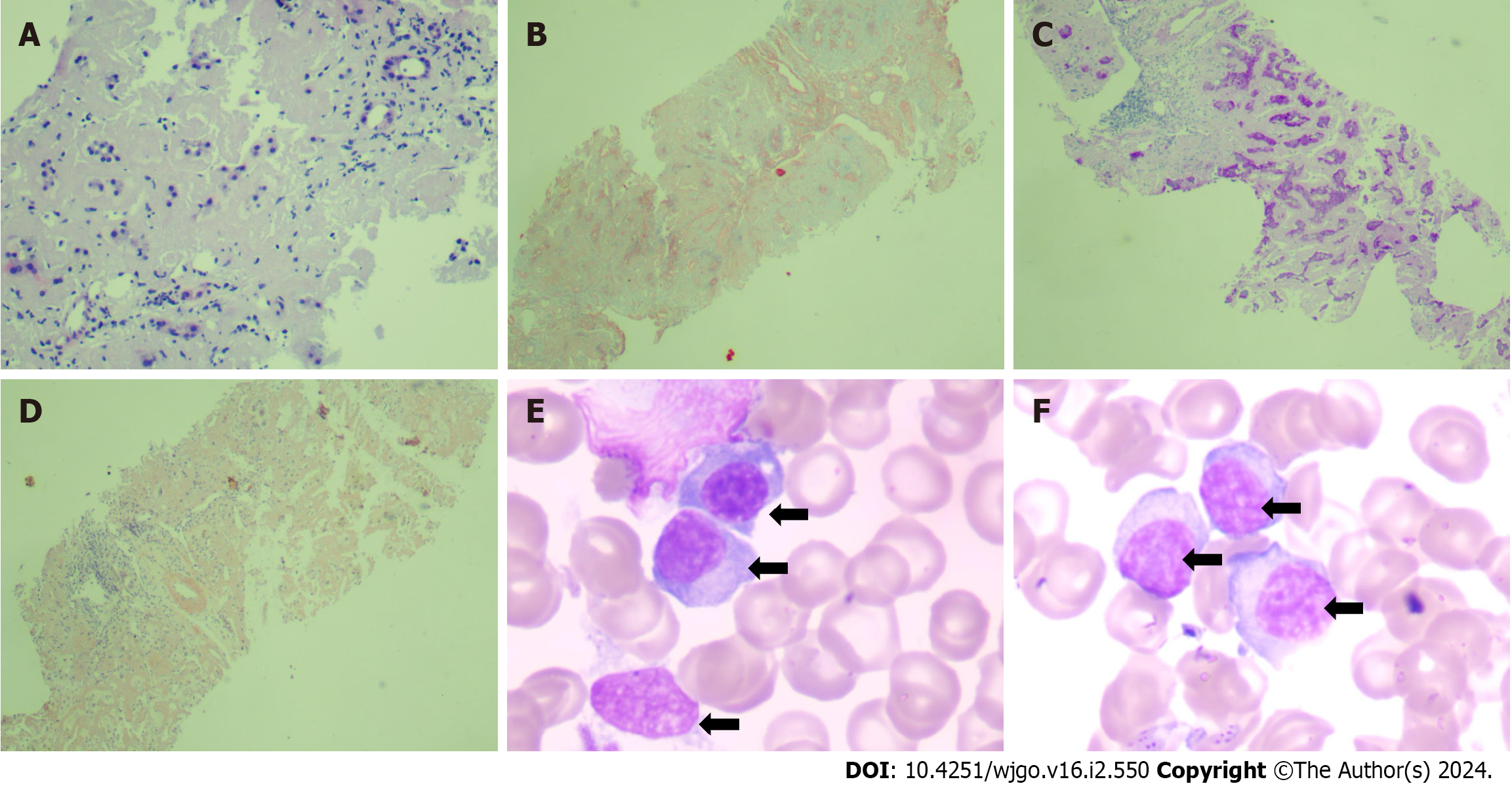
Liver biopsy was performed, and the hepatic venous pressure gradient (wedged hepatic venous pressure minus free hepatic venous pressure) was found to be 25 mmHg. Liver pathology suggested disruption of the structure of the hepatic lobules, hepatocellular atrophy, and deposition of pink amorphous material in the portal area, vascular wall, and sinusoids between the liver plates. Congo red staining was positive, indicating hepatic amyloidosis (Figure 2A-D). Bone marrow aspiration smear showed abnormal proliferation of plasma cell system (Figure 2E and F). Flow cytometry immunofluorescence revealed that 3.88% of monoclonal plasma cells were visible, with CD38+ and CD138+ immunophenotypes and intracellular immunoglobulin kappa AL restriction expression, supporting AL amyloidosis.
The final diagnosis of the presented case was liver involvement of AL amyloidosis.
After the dilatation of the hepatic vein, the patient's liver function did not improve, but instead the jaundice deepened progressively. In addition to liver-protective drug therapy, a double-plasma molecular adsorption liver support system was added.
However, the patient's bilirubin levels gradually increased, reaching up to 540 µmol/L. The patient developed bloody ascites and was considered to have experienced spontaneous rupture and bleeding of the liver capsule. After antibiotic and diuretic treatment ascites resolved completely. Considering the risks and costs of related treatment drugs, such as daretozumab and bortezomib, the family decided not to follow the corresponding treatment. The further course of the disease was progressive and the patient eventually died 5 mo after the onset of jaundice.
AL amyloidosis is the most common form of systemic amyloidosis, accounting for 70% of all cases[6-8]. It is characterized by deposition of misfolded monoclonal ALs secreted from malignant plasma cell clones. The misfolded proteins form highly ordered beta pleated sheet configuration, with the contiguous beta pleated sheets winding together into an insoluble fibrillar configuration instead of the typical alpha helical pattern of most proteins[9], which deposit in tissues interfering with the normal function of organs[10]. The clinical manifestations depend on the organ preference for ALs and the degree of organ dysfunction. The most commonly affected organs include the kidneys, heart, liver, peripheral nervous system, soft tissue, gastrointestinal tract, and less commonly, the lungs[11]. The findings of a study examining the correlation between immunoglobulin free ALs and clinical characteristics in a cohort of 730 recently diagnosed individuals with AL amyloidosis indicate that the type of AL has an impact on the range of organ involvement; κ-AL has more involvement in gastrointestinal and liver, while renal involvement is more common in λ-AL patients[12]. λ-AL is more common than κ-AL; as reported the ratio of λ/κ is about 3.5[13], and the overall survival of the two is similar. The overall survival of patients is primarily influenced by the numerical disparity between the clonal light-chain and the other light-chain, patients exhibiting a higher numerical difference experience significantly diminished survival duration[12]. The main manifestations in this patient were liver involvement, such as hepatomegaly and cholestasis, and no dysfunction of other organs such as the heart and kidneys. Flow cytometry immunofluorescence revealed monoclonal kappa chain restriction expression, which is consistent with previous reports[12].
Owing to the lack of specific symptoms, the time from the onset to diagnosis of AL amyloidosis is usually longer (6–10 mo) as reported previously[14-16]. A previous study showed that only 7.6% of patients were diagnosed with amyloidosis after consulting only one physician, whereas 31.8% of patients had consulted more than five physicians before being correctly diagnosed[1]. However, early diagnosis is crucial because early intervention can prevent irreversible organ damage and improve the prognosis. This patient had been diagnosed with abnormal liver function 6 years previously, mainly manifested by elevated ALP and GGT levels. Though liver disease-related investigations were performed; no clear cause was identified. Furthermore, the patient refrained from seeking subsequent medical intervention, resulting in a delayed diagnosis of the disease. Finally, when severe cholestasis occurred, the patient sought medical attention again, and a clear diagnosis was made based on liver pathology. Unfortunately, treatment opportunities were missed. The main symptoms of this case were progressive jaundice and liver enlargement. Therefore, we suggest that patients with unexplained elevated ALP and GGT levels accompanied by a significant increase in liver volume should be aware of the possibility of invasive diseases, such as liver amyloidosis.
Liver biopsy is not recommended as the first choice of evaluation for patients with suspected liver amyloidosis. Coagulation dysfunction is a recognized manifestation of AL amyloidosis. Multiple mechanisms have been proposed to explain this phenomenon, including vascular fragility caused by amyloid infiltration, presence of thrombin inhibitors, abnormal fibrinogen, potential disseminated intravascular coagulation and fibrinolysis, and acquired coagulation factor deficiency[17]. At the same time, due to excessive deposition of amyloid proteins, the liver enlarges and the tension of the liver capsule increases, posing a risk of spontaneous liver rupture. Therefore, percutaneous liver biopsy is associated with a high risk of inducing liver capsule rupture and abdominal bleeding. Subcutaneous fat biopsy or bone marrow biopsy can be considered when a liver biopsy is difficult to complete. The risks of both these tests is very low, with a comprehensive diagnostic sensitivity of 85%[18]. There are also reports suggesting that hepatocyte growth factor can serve as a useful non-invasive auxiliary marker for diagnosing primary systemic amyloidosis and predicting prognosis[19]. However, for patients with AL amyloidosis with only liver involvement, we can rely only on liver pathology to establish the diagnosis. Considering the risks of percutaneous liver biopsy, transjugular liver biopsy is a safe alternative method[20]. The patient was diagnosed based on the pathology obtained through a transjugular liver biopsy.
Hepatic AL amyloidosis is caused by excessive deposition of monoclonal globulin ALs or fragments produced by ALs in the liver tissue, mainly in the liver parenchyma, along the space of Disse, or in the vascular wall. When a large amount of amyloid protein is deposited in the liver, the liver cells are severely compressed and are likely to atrophy or disappear[21,22]. The pathological manifestations in this patient were consistent with the significant deposition of amyloid proteins, atrophy of liver cells, and destruction of lobular structures. Patients often exhibit liver enlargement and elevated cholestasis enzymes. Total bilirubin levels may also increase in some patients with advanced systemic hepatic amyloidosis. Because of the main manifestations of liver enlargement, jaundice, and even ascites, imaging findings indicating an uneven density of the liver parenchyma, as well as narrowing of the hepatic veins due to compression; these are similar to the manifestations of liver vascular diseases, such as acute Budd-Chiari syndrome and SOS, and are prone to be misdiagnosed. Our patient had a history of use of traditional Chinese herbal medicines, and we initially considered a diagnosis of SOS. However, after a detailed inquiry into the history of the present illness, it was found that the patient took non-standard drugs after the onset of jaundice, which did not meet the causal temporal relationship of the DILI diagnosis. Thus, the diagnosis of SOS remained unclear. In addition, during the transjugular liver biopsy, the patient underwent hepatic venography, which revealed stenosis of the right and middle hepatic veins. The interventional radiologists considered it to be consistent with the manifestation of Budd–Chiari syndrome and performed balloon dilation. However, in the end, the examination of liver pathology provided a definitive response, corroborating the notion that liver amyloidosis imitated Budd-Chiari syndrome rather than Budd-Chiari syndrome itself. We believe that the hepatic vein stenosis in this case was related to vascular compression caused by excessive deposition of amyloid in the liver. It is noteworthy that hepatic vascular diseases, such as Budd Chiari syndrome or SOS, commonly present with ascites in the early stages of the disease. However, in the early stages of hepatic amyloidosis, ascites rarely manifests, which could serve as a significant diagnostic differentiator.
Bortezomib is widely used for the treatment of AL amyloidosis. A chemotherapy regimen based on bortezomib can be used as a first-line treatment for newly diagnosed and relapsed patients[23]. However, patients with end-stage heart disease and liver failure are usually unsuitable for chemoimmunotherapy and cannot tolerate anti-clonal therapy that suppresses abnormal plasma cell clones[24,25]. Liver transplantation (LT) may be a practical alternative for patients with major liver involvement. In the literature on solid organ transplantation in AL amyloidosis, nine patients received orthotopic LT, and the 1-year and 5-year survival rates were 33% and 22%, respectively[26]. Among them, six patients received post-transplant chemotherapy, and interestingly, four patients with basal renal amyloidosis developed rapidly progressive proteinuria. Overall, the LT data of patients with AL amyloidosis are limited, and further exploration is needed. The patient lost effective treatment opportunities owing to severe liver damage and ultimately died of liver failure.
The liver, is often affected by AL amyloidosis in conjunction multiple organ involvement such as the heart and kidneys. Isolated liver involvement is rare. The number of organs affected by AL amyloidosis mainly depends on the light-chain phenotype. Hepatic amyloidosis is characterized by a mild elevation of cholestasis enzymes in the early stages without obvious clinical symptoms and signs, making timely diagnosis difficult. As the disease progresses, symptoms of liver enlargement and jaundice may occur, and it can be easily misdiagnosed as a vascular disease, such as SOS or Budd-Chiari syndrome. Severe cholestasis is associated with severe damage to the liver structure and function, indicating poor prognosis, poor tolerance to effective treatment drugs, and loss of effective treatment opportunities. Therefore, in populations with unexplained elevated ALP and GGT levels, we need to be vigilant about the possibility of liver amyloidosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oh SH, South Korea; van Buuren H, Netherlands S-Editor: Zhang H L-Editor: A P-Editor: Zhang XD
| 1. | Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1065] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 2. | Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Gertz MA, Kyle RA. Hepatic amyloidosis (primary [AL], immunoglobulin light chain): the natural history in 80 patients. Am J Med. 1988;85:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 4. | Tam M, Seldin DC, Forbes BM, Connors LH, Skinner M, Oran B, Quillen K, Sanchorawala V. Spontaneous rupture of the liver in a patient with systemic AL amyloidosis undergoing treatment with high-dose melphalan and autologous stem cell transplantation: a case report with literature review. Amyloid. 2009;16:103-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Peters RA, Koukoulis G, Gimson A, Portmann B, Westaby D, Williams R. Primary amyloidosis and severe intrahepatic cholestatic jaundice. Gut. 1994;35:1322-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Rajkumar SV, Gertz MA. Advances in the treatment of amyloidosis. N Engl J Med. 2007;356:2413-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 821] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 8. | Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Bhat A, Selmi C, Naguwa SM, Cheema GS, Gershwin ME. Currents concepts on the immunopathology of amyloidosis. Clin Rev Allergy Immunol. 2010;38:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Merlini G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am Soc Hematol Educ Program. 2017;2017:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45-59. [PubMed] |
| 12. | Kumar S, Dispenzieri A, Katzmann JA, Larson DR, Colby CL, Lacy MQ, Hayman SR, Buadi FK, Leung N, Zeldenrust SR, Ramirez-Alvarado M, Clark RJ, Kyle RA, Rajkumar SV, Gertz MA. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116:5126-5129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Shimazaki C, Hata H, Iida S, Ueda M, Katoh N, Sekijima Y, Ikeda S, Yazaki M, Fukushima W, Ando Y. Nationwide Survey of 741 Patients with Systemic Amyloid Light-chain Amyloidosis in Japan. Intern Med. 2018;57:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Palladini G, Kyle RA, Larson DR, Therneau TM, Merlini G, Gertz MA. Multicentre vs single centre approach to rare diseases: the model of systemic light chain amyloidosis. Amyloid. 2005;12:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Huang XH, Liu ZH. The Clinical Presentation and Management of Systemic Light-Chain Amyloidosis in China. Kidney Dis (Basel). 2016;2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Dispenzieri A. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129:2111-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 17. | Abdallah N, Muchtar E, Dispenzieri A, Gonsalves W, Buadi F, Lacy MQ, Hayman SR, Kourelis T, Kapoor P, Go RS, Warsame R, Leung N, Rajkumar SV, Kyle RA, Pruthi RK, Gertz MA, Kumar SK. Coagulation Abnormalities in Light Chain Amyloidosis. Mayo Clin Proc. 2021;96:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Miyazaki K, Kawai S, Suzuki K. Abdominal subcutaneous fat pad aspiration and bone marrow examination for the diagnosis of AL amyloidosis: the reliability of immunohistochemistry. Int J Hematol. 2015;102:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Karasuyama T, Honma Y, Kumamoto K, Shibata M, Watanabe T, Shimajiri S, Abe S, Yamashita T, Harada M. Hepatocyte Growth Factor and Primary Systemic Amyloidosis. J UOEH. 2021;43:227-233. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M, Patch D, Burroughs AK. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 21. | Shin YM. Hepatic amyloidosis. Korean J Hepatol. 2011;17:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Wang YD, Zhao CY, Yin HZ. Primary hepatic amyloidosis: a mini literature review and five cases report. Ann Hepatol. 2012;11:721-727. [PubMed] |
| 23. | Ryšavá R. AL amyloidosis: advances in diagnostics and treatment. Nephrol Dial Transplant. 2019;34:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, Gertz MA. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 25. | Kastritis E, Dimopoulos MA. Recent advances in the management of AL Amyloidosis. Br J Haematol. 2016;172:170-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Sattianayagam PT, Gibbs SD, Pinney JH, Wechalekar AD, Lachmann HJ, Whelan CJ, Gilbertson JA, Hawkins PN, Gillmore JD. Solid organ transplantation in AL amyloidosis. Am J Transplant. 2010;10:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |