Published online Nov 15, 2024. doi: 10.4251/wjgo.v16.i11.4436
Revised: August 13, 2024
Accepted: September 10, 2024
Published online: November 15, 2024
Processing time: 185 Days and 9.1 Hours
Trastuzumab-targeted therapy is currently the standard of care for advanced human epidermal growth factor receptor 2 (HER2)-positive gastric cancer. How
To identify the key genes associated with trastuzumab resistance. These results provide a basis for the development of interventions to address drug resistance and improve patient outcomes.
High-throughput sequencing and bioinformatics were used to identify the differentially expressed pivotal gene BIRC3 and delineate its potential function and pathway regulation. Tumor samples were collected from patients with HER2-positive gastric cancer to evaluate the correlation between BIRC3 expression and trastuzumab resistance. We established gastric cancer cell lines with both highly expressed and suppressed levels of BIRC3, followed by comprehensive in vitro and in vivo experiments to confirm the involvement of BIRC3 in trastuzumab resistance and to elucidate its underlying mechanisms.
In patients with HER2-positive gastric cancer, there is a significant correlation between elevated BIRC3 expression in tumor tissues and higher T stage, tumor node metastasis stage, as well as poor overall survival and progression-free survival. BIRC3 is highly expressed in trastuzumab-resistant gastric cancer cell lines, where it inhibits tumor cell apoptosis and enhances trastuzumab resistance by promoting the phosphorylation and activation of the phosphoinositide 3-kinase-Akt (PI3K-AKT) pathway in HER2-positive gastric cancer cells, both in vivo and in vitro.
This study revealed a robust association between high BIRC3 expression and an unfavorable prognosis in patients with HER2-positive gastric cancer. Thus, the high expression of BIRC3 stimulated PI3K-AKT phosphorylation and activation, stimulating the proliferation of HER2-positive tumor cells and suppressing apoptosis, ultimately leading to trastuzumab resistance.
Core Tip: Our study discovered that the overexpression of BIRC3 leads to the stimulation of phosphoinositide 3-kinase-Akt phosphorylation and activation. Consequently, this enhances the proliferation of human epidermal growth factor receptor 2-positive tumor cells and inhibits apoptosis, resulting in resistance to trastuzumab.
- Citation: Li SL, Wang PY, Jia YP, Zhang ZX, He HY, Chen PY, Liu X, Liu B, Lu L, Fu WH. BIRC3 induces the phosphoinositide 3-kinase-Akt pathway activation to promote trastuzumab resistance in human epidermal growth factor receptor 2-positive gastric cancer. World J Gastrointest Oncol 2024; 16(11): 4436-4455
- URL: https://www.wjgnet.com/1948-5204/full/v16/i11/4436.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i11.4436
Gastric cancer is a highly prevalent and lethal form of cancer worldwide[1,2]. As our understanding of the molecular landscape of gastric cancer continues to improve, new therapeutic targets and drugs have been discovered and developed, leading to improved treatment outcomes. In the context of cancer, human epidermal growth factor receptor 2 (HER2) expression is considered to be significantly associated with cancer development and the escalation of resistance to chemotherapy drugs[3]. Trastuzumab is considered the standard and optimal treatment for patients with HER2-positive metastatic gastric cancer[4].
Trastuzumab inhibits the growth of HER2-positive tumor cells through immune-related mechanisms, such as antibody-dependent or complement-dependent cytotoxicity[5,6]. However, with the continued use of HER2-targeted therapies such as trastuzumab, most patients experience reduced therapeutic sensitivity resulting in tumors more tolerant to HER2-targeted therapies, leading to disease relapse and progression, which significantly affects patient prognosis. Several potential mechanisms of trastuzumab resistance have been proposed. These factors include HER2 heterogeneity, formation of HER2 heterodimers, and alterations in intracellular signaling, among others. Notably, the abnormal activation of the phosphoinositide 3-kinase (PI3K) pathway is recognized as a significant mechanism underlying resistance to HER2-targeted therapy[7].
Multiple studies have identified a new PI3K signaling pathway-related protein: BIRC3, also known as baculoviral inhibitor of apoptosis (IAP) repeat containing 3. BIRC3 encodes cIAP2 (cellular IAP 2) and belongs to the IAP family[8,9]. These apoptosis-inhibitory proteins are highly conserved and inhibit apoptosis by suppressing caspase activity and regulating immune-related signaling pathways. Studies have shown that IAPs are frequently overexpressed in cancer, and that their expression levels are associated with tumorigenesis, chemotherapy resistance, disease progression, and survival differences[10].
Previous studies have demonstrated that high BIRC3 expression is linked to clinicopathological characteristics and poor prognosis in colorectal cancer, bladder cancer, and glioblastoma[11,12]. Furthermore, BIRC3 overexpression is associated with chemotherapy resistance in breast cancer and oral squamous cell carcinoma. However, its role in gastric cancer, particularly in relation to resistance to trastuzumab-targeted therapy in HER2-positive gastric cancer, remains unexplored. Hence, our study aimed to investigate the involvement of BIRC3 in the development of trastuzumab-targeted therapy resistance and to unravel the underlying mechanism with the goal of overcoming trastuzumab resistance in HER2-positive gastric cancer.
We examined 28 HER2-positive tumor tissue specimens from patients with gastric cancer who underwent curative gastrectomy in the Department of General Surgery at Tianjin Medical University General Hospital (Tianjin, China) between March 2018 and May 2021. The expression intensity of HER2 was assessed using immunohistochemistry (IHC), employing a four-grade scoring system (ranging from 0 to 3 +) to determine the proportion of stained tumor cells. HER2 overexpression was characterized by an IHC score of 3 +. In instances where HER2 staining was ambiguous (IHC 2 +), Fluorescence in situ hybridization was employed to validate the amplification status of the HER2. None of the enrolled patients underwent neoadjuvant therapy before gastrectomy. Follow-up evaluations were conducted every three months during the initial two years post-radical surgery and every six months thereafter.
Two-terminal sequencing was performed using an Illumina NovaSeq 6000 (LC Bio Technology Co., Ltd. Hangzhou, Zhejiang Province, China) according to standard protocols in the PE150 sequencing mode. Subsequent bioinformatics analyses were performed on the sequencing data, and R language was used to conduct correlation analyses of gene expression within the samples.
To identify the “BIRC3” gene in the gene bank of the national center for biotechnology information website, we selected the transcript-id “NM_001165.5”. This enabled us to obtain the coding sequence. The BamHI and NotI restriction sites were selected for plasmid generation. The expression plasmid pLVX-IRES-puro-BIRC3 was synthesized by Hongxun Biotechnology Co., Ltd (Suzhou, Jiangsu Province, China). For the siRNA, featuring the sequence hs-BIRC3-si: Forward 5’-CAGUUCGUACAUUUCUUUCAUdTdT-3,’ design and synthesis was carried out by Hongxun Biotechnology Co., Ltd (Suzhou, Jiangsu Province, China). Verification of the sequencing results ensured alignment with the original sequence sequenced by Hongxun Biotechnology Co., Ltd (Suzhou, Jiangsu Province, China).
In this study, the NCI-N87 cell line, originating from human HER2-positive gastric carcinoma, was used. The cell line was procured from the Institute of General Surgery (Tianjin, China). To induce resistance to trastuzumab, the concentration of trastuzumab was systematically increased from 10 μg/mL to 1000 μg/mL, and viability was examined using the cell counting kit-8 (CCK8) assay. The resultant trastuzumab-resistant cell line was denoted as NCI-N87R and was sustained for approximately half a year. The cells were grown at 37 °C under a 5% carbon dioxide atmosphere in a humid setting. Transfection trials were performed in a the trastuzumab-resistant NCI-N87R cell line using BIRC3 siRNA and a non-coding siRNA (siRNA-NC). Additionally, the trastuzumab-sensitive NCI-N87 cell line was transfected with a BIRC3 overexpression plasmid pLVX-IRES-puro-BIRC3 or the empty plasmid pLVX-IRES-puro-NC. The efficacy of transfection was confirmed through quantitative reverse transcription polymerase chain reaction (RT-qPCR) and western blot analyses, and alterations in the cellular proliferation capability were examined using CCK8 and 5-ethynyl-2’-deoxyuridine (EdU) assays.
To evaluate cell viability, a manual lauryl sodium sulfate cell counting kit-8 (CCK8) assay was performed. We plated 2500 cells per well in 96-well plates. Various concentrations of trastuzumab were introduced into the culture medium, and after four days, a CCK8 assay was carried out to quantify cell viability at optical density 450 nm. A total of nine trastuzumab concentration gradients in five replicate wells were established in the medium, ranging from 0 μg/mL to 1000 μg/mL: 0 μg/mL, 1 μg/mL, 10 μg/mL, 20 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, 500 μg/mL, and 1000 μg/mL.
BIRC3 levels were analyzed using RT-qPCR. The ChamQ universal synergetic binding reagent qPCR master mix was used for RT-qPCR assessments, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as the reference gene. The primers utilized for BIRC3 in the RT-qPCR analysis were as follows: Forward 5’-TTTCCGTGGCTCTTATTCAAACT-3’ and reverse 5’-GCACAGTGGTAGGAACTTCTCAT-3c’; for GAPDH, forward 5’-GCACCGTCAAGGCTGAGAAC-3’ and reverse 5’-TGGTGAAGACGCCAGTGGA-3’. The RT-qPCR process was repeated three times, and the data were analyzed using GraphPad Prism 9.0 to determine the 2-Ct values. Statistical significance was set at P ≤ 0.05.
IHC analyses were performed to assess BIRC3 protein expression in paraffin-embedded sections of tissues obtained from patients with gastric cancer. Additionally, the expression levels of BIRC3, phosphorylated AKT (pAKT), and caspase 3 in paraffin sections of tumors formed from different groups of mice were analyzed. The IHC scoring formula was as follows: IHC score = staining intensity score (a) × percentage score of positive cells (b), where (a) represents staining intensity, with a negative score of 0, a weak positive score of 1, a positive score of 2, and a strong positive score of 3, and (b) represents the proportion of positive cells, with a score of 0 for 0% positive cells, a score of 1 for 1%-25% positive cells, a score of 2 for 26%-50% positive cells, a score of 3 for 51%-75% positive cells, and a score of 4 for 76%-100% positive cells. Low expression was indicated by an IHC score of less than 4 points, whereas high expression was indicated by a score of 4 points or more.
In the dynamic tumor model, the transgenic strain N87-LV-BIRC3 with stable overexpression was developed by lentiviral transfection of the BIRC3 gene. Subsequently, the transgenic strain N87R-LV-BIRC3-si with a stable knockdown of BIRC3 was created by lentiviral transfection with BIRC3 siRNA.
For the in vivo tumorigenesis experiment, specific pathogen free (SPF) BALB/c nude mice were divided into five groups. All appropriate measures were taken to minimize pain or discomfort, and comply with ARRIVE guidelines. The group that received NCI-N87 cell inoculation was designated with the N87-NC group. The group that received NCI-N87 cells transfected with the overexpressed LV-GFP-puro-BIRC3 virus was labeled the N87-LV-BIRC3 group. The group that received NCI-N87 cells transfected with the overexpressed LV-GFP-puro-BIRC3 virus and treated with an allosteric AKT inhibitor MK-2206 was denoted as the N87-BIRC3-AKTi group. The NCI-N87R group, inoculated with drug-resistant cells, was designated as the N87R group. The group that received NCI-N87R cells transfected with the knockout LV-GFP-puro-BIRC3-si virus was named N87R-LV-BIRC3-si.
All experimental groups received a trastuzumab injection (200 µg/kg) into the abdominal cavity every two days. Furthermore, the N87-LV-BIRC3-AKTi group received 10 mg/kg AKT inhibitor (MK-2206) at the same intervals for seven administrations.
Each experiment was replicated at least three times, and the data presented reflect the average outcomes. Statistical analysis was performed using χ2 test for IHC scores and patient pathological features. the statistical review of the study was performed by a biomedical statistician. Differences were considered statistically significant at P < 0.05.
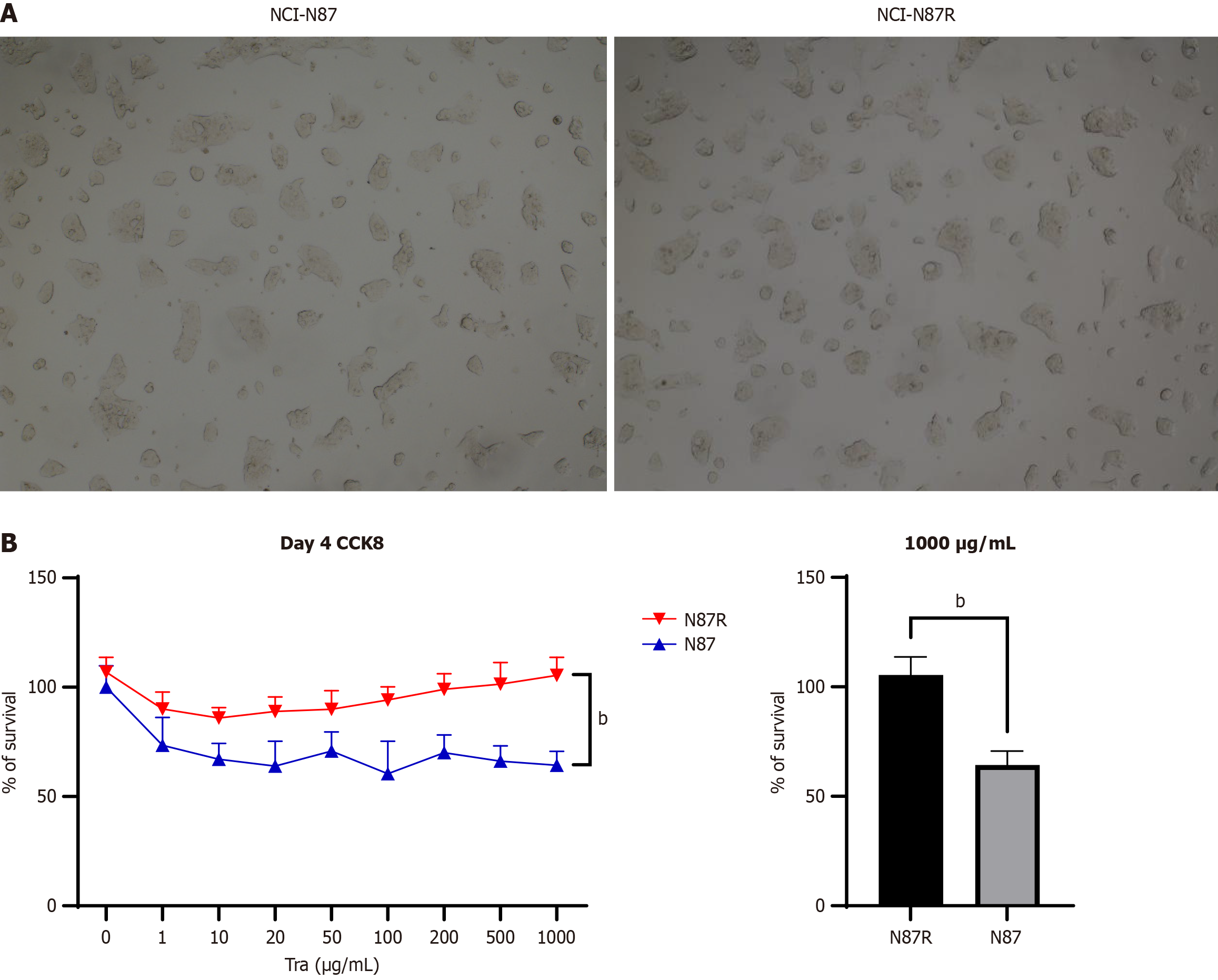
We created a trastuzumab-resistant variant HER2-positive gastric cancer cell line NCI-N87, designated NCI-N87R. The morphology of NCI-N87R cells closely mirrored that of its parental cell line, NCI-N87 (Figure 1A). As shown in Figure 1B, NCI-N87R cells exhibited remarkable resistance to trastuzumab when subjected to a concentration of 1000 μg/mL, as determined by a CCK-8 assay. The proliferative activity of NCI-N87R was (105.4 ± 8.240), whereas that of NCI-N87 cells was (64.34 ± 6.371, P < 0.0001). Consequently, we successfully generated a trastuzumab-resistant cell line, NCI-N87R, by using a low-concentration stepwise addition method.
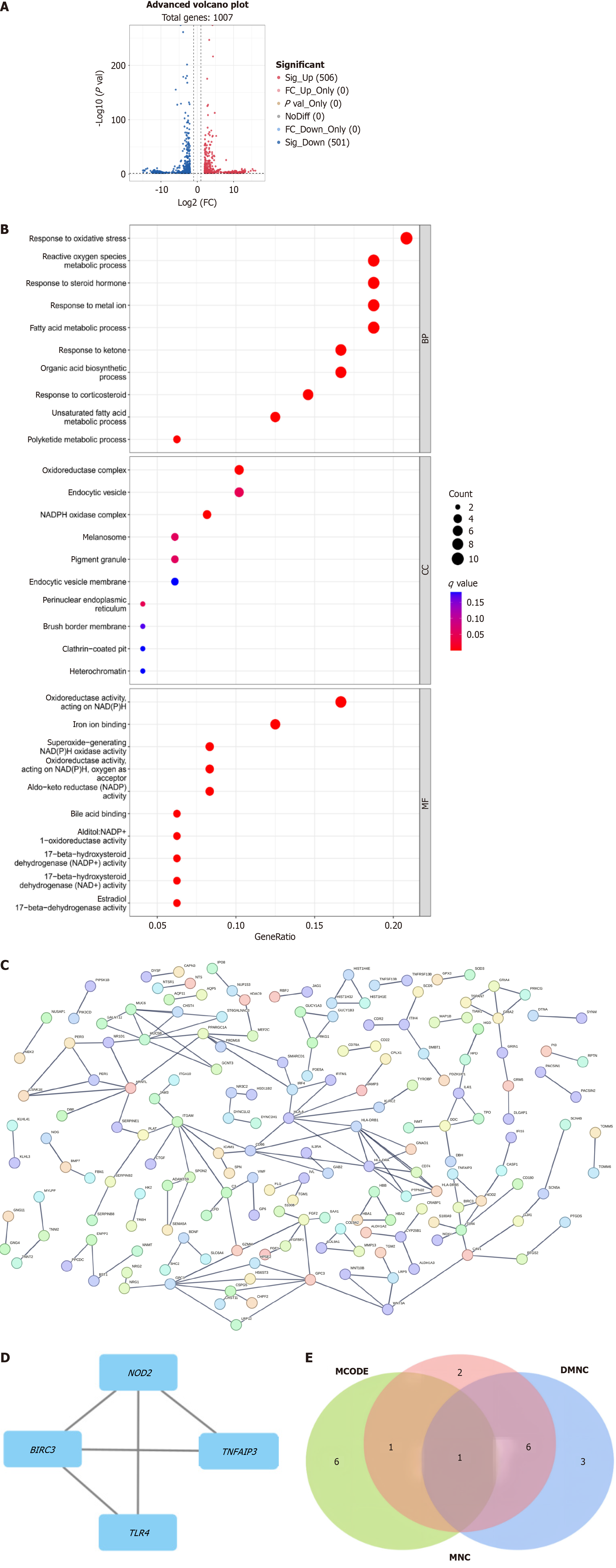
Following the successful generation of a trastuzumab-resistant cell line, we conducted high-throughput sequencing and bioinformatics analyses. By comparing the NCI-N87R cells with normal NCI-N87 cells and applying a filter with |log2 (fold change)| ≥ 2 and P ≤ 0.05, we identified a total of 506 up-regulated genes and 501 down-regulated genes. A volcano map depicting these differentially expressed genes is shown in Figure 2A. Subsequently, we performed gene ontology and protein interaction network analyses of these differentially expressed genes, as shown in Figure 2B and C, respectively. We then utilized the maximum neighborhood component centrality (DMNC) and MNC algorithms in Cytoscape 3.9.1 software to identify the top ten differentially expressed genes. The molecular complex detection algorithm was employed to screen genes, resulting in the identification of the two modules with the highest scores (score = 3.33), as illustrated in Figure 2D, Table 1 and Table 2. After consolidating the genes, we visualized the top ten genes selected using different algorithms from Cyto Hubba using a Venn plot (Figure 2E). We identified BIRC3 as a pivotal gene that may contribute to trastuzumab resistance in patients with HER2-positive gastric cancer.
| DMNC | MNC |
| HLA-DRB5 | HLA-DRB1 |
| PTPN22 | HLA-DRA |
| CD74 | MUC6 |
| CD86 | MUC5B |
| HLA-A | HLA-DRB5 |
| HLA-DRB1 | PER1 |
| HLA-DRA | CSNK1E |
| PER1 | BIRC3 |
| CSNK1E | NOD2 |
| BIRC3 | PTPN22 |
| Cluster | Score | Nodes | Edges | Node IDs |
| 1 | 3.333 | 4 | 5 | GRIA2, GRIA4, TSPAN7, PRKCG |
| 2 | 3.333 | 4 | 5 | NOD2, TNFAIP3, BIRC3, TLR4 |
| 3 | 3 | 3 | 3 | WNT10B, LRP5, WNT3A |
| 4 | 3 | 3 | 3 | HIST1H1E, HIST1H4E, HIST1H3J |
| 5 | 3 | 3 | 3 | HBB, HBA2, HBA1 |
| 6 | 3 | 3 | 3 | GUCY1B3, GUCY1A3, PRKG1 |
| 7 | 3 | 3 | 3 | HLA-DRA, GNAO1, HLA-DRB1 |
| 8 | 3 | 3 | 3 | TPO, DDC, IL4I1 |
| 9 | 3 | 3 | 3 | PER3, CSNK1E, PER1 |
| 10 | 3 | 3 | 3 | MMP13, COL9A1, COL9A2 |
| 11 | 3 | 3 | 3 | SPON2, ADAMTS9, SEMA5A |
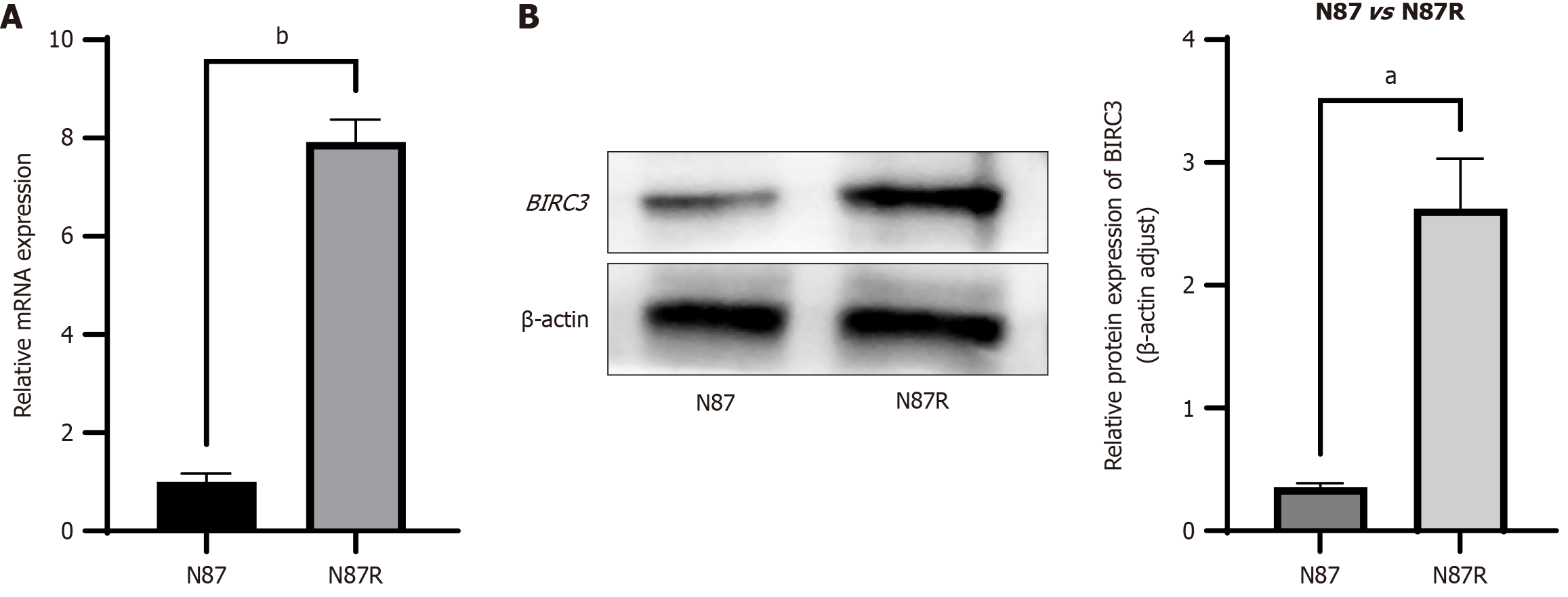
RT-qPCR analysis revealed a significant upregulation in the mRNA levels of BIRC3 in NCI-N87R cells compared to that in control parental NCI-N87 cells (P < 0.0001) (Figure 3A). Western blotting confirmed that the protein levels of BIRC3 were significantly higher in NCI-N87R cells than in NCI-N87 cells (P < 0.001) (Figure 3B). These findings suggest that trastuzumab-resistant gastric cancer cells exhibit increased expression of BIRC3 compared to trastuzumab-sensitive cells.
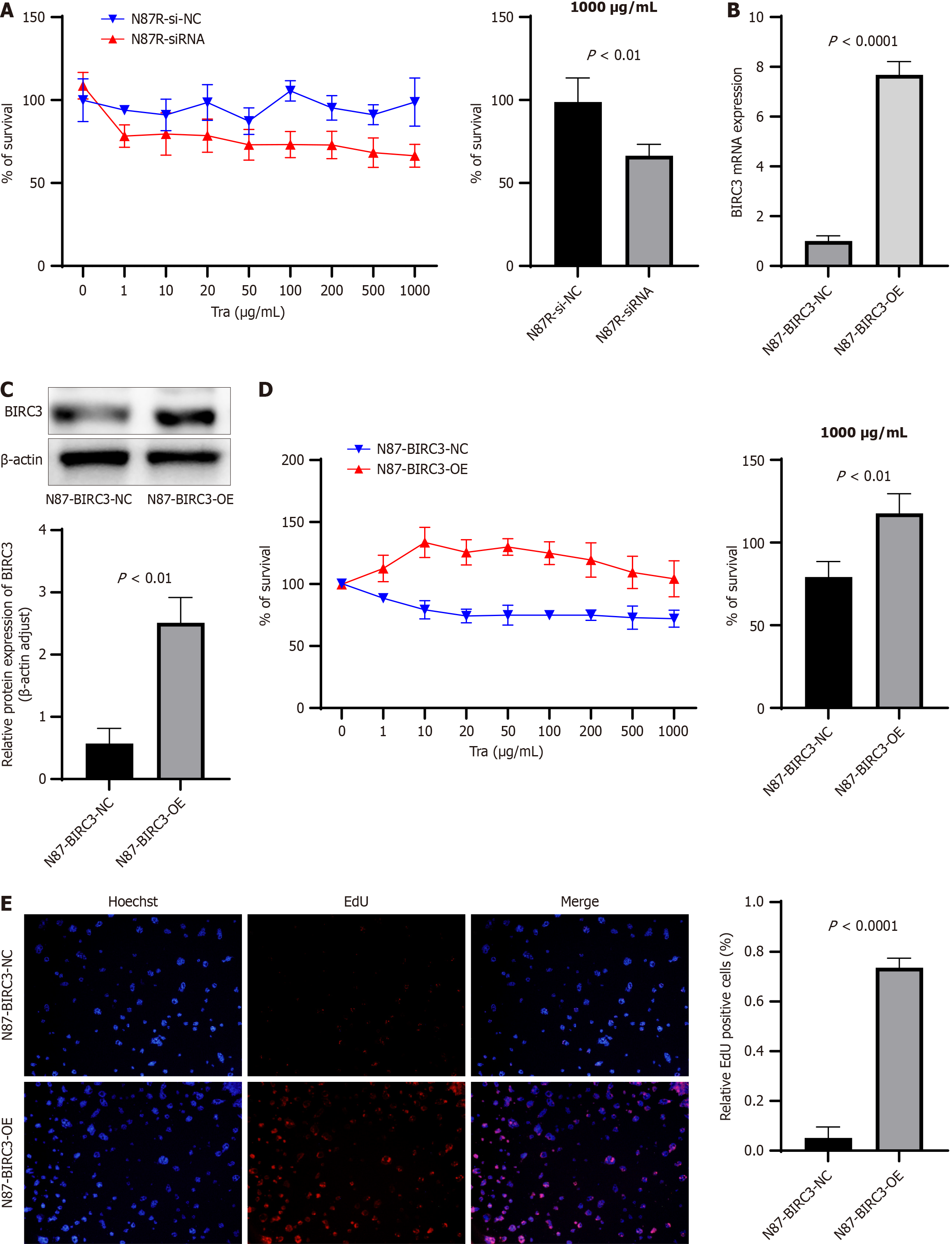
To investigate the role of BIRC3 in the response of human HER2-positive gastric cancer cells to trastuzumab, we used BIRC3-siRNA to downregulate the levels of BIRC3 in NCI-N87R cells. Cell viability was evaluated using CCK8 assays, revealing significant reductions in cell survival (66.38 ± 6.93 vs 98.78 ± 14.54, P = 0.0047) (P < 0.0001). Notably, the degradation of BIRC3 markedly decreased cell growth in NCI-N87R cells when exposed to 1000 μg/mL of trastuzumab compared to the control group (Figure 4A). Consequently, the decreased expression of BIRC3 results in diminished trastuzumab resistance in trastuzumab-resistant gastric cancer cells.
Subsequently, we introduced the BIRC3 overexpressing plasmid pLVX-IRES-puro-BIRC3 or control empty plasmid pLVX-IRES-puro-NC into the HER2-positive gastric cancer cell line NCI-N87, designated N87-BIRC3-OE and N87-BIRC3-NC, respectively. The mRNA and protein levels of BIRC3 were significantly higher in N87-BIRC3-OE cells than in N87-BIRC3-NC cells, as shown in Figure 4B and C. Upon exposure to trastuzumab, both cell types exhibited decreased cell viability. However, the decline in cell viability was less pronounced in N87-BIRC3-OE cells compared to N87-BIRC3-NC cells, as demonstrated by the CCK8 assay (104.2 ± 14.59 vs 72.06 ± 6.859, P = 0.0021) and EdU assay (P < 0.0001) (Figure 4D and E). Therefore, the overexpression of BIRC3 promoted trastuzumab resistance in the HER2-positive gastric cancer cell line NCI-N87.
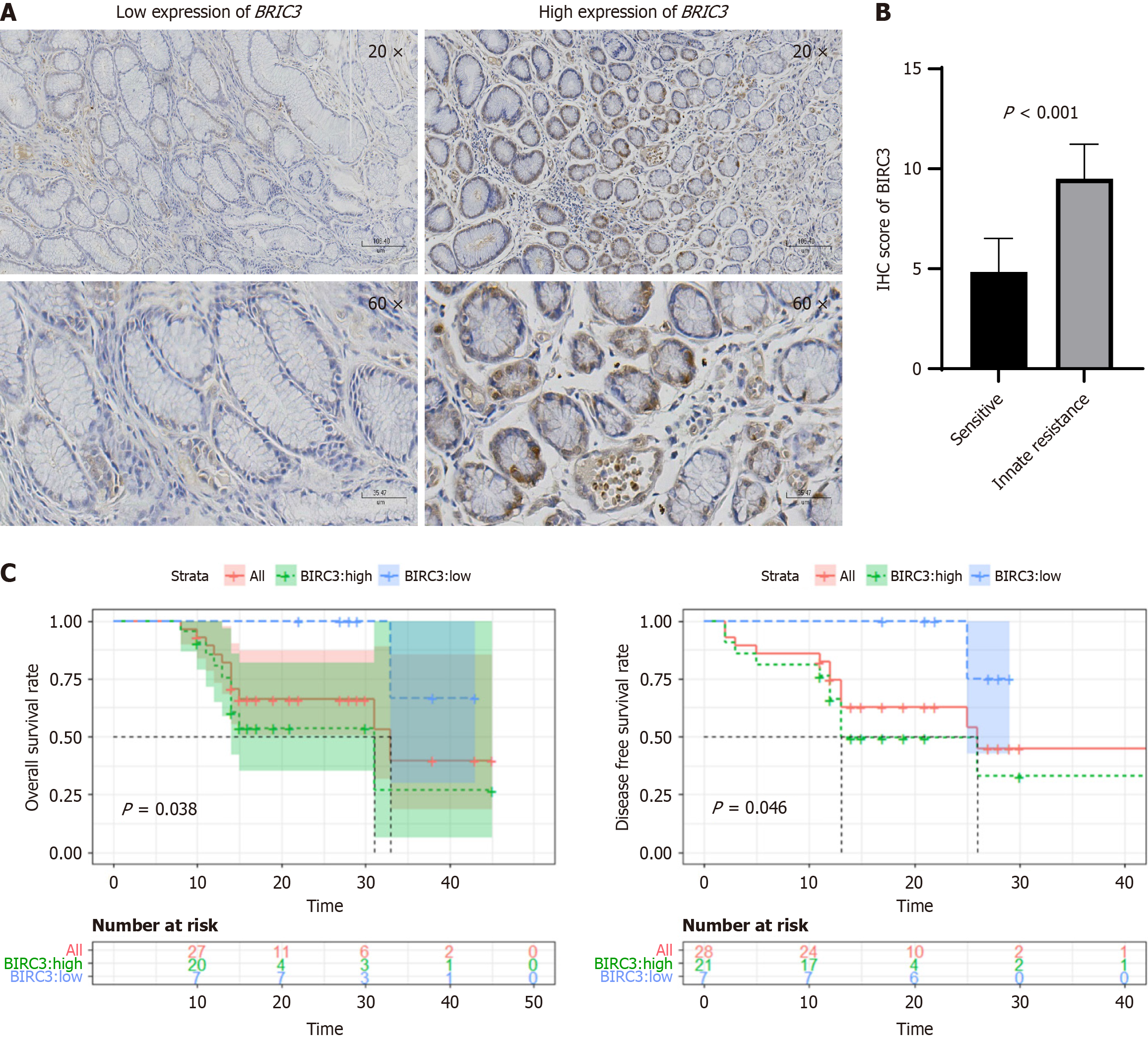
To further investigate this correlation, we procured 28 paraffin-embedded tissue samples from individuals diagnosed with HER2-positive gastric cancer for IHC analysis of BIRC3 protein levels. Notably, BIRC3 was predominantly localized to the cytoplasm, characterized by discernible brown particles (Figure 5A). Subsequently, these gastric cancer specimens were stratified into two distinct cohorts based on their BIRC3 expression profiles: The BIRC3 high and BIRC3 Low groups. To further elucidate the relationship between BIRC3 expression and prognosis among patients with HER2-positive gastric cancer, sophisticated analytical tools, such as the gene expression omnibus and Kaplan-Meier plotter, were employed. The results are presented in Table 3 and underscore a significant association, revealing that elevated BIRC3 levels were statistically associated with advanced T stage (P = 0.030), elevated tumor node metastasis stage (P = 0.016), increased risk of overall survival (P = 0.038), and decreased disease-free survival rate (P = 0.046) within the cohort of 28 HER2-positive gastric cancer patients (Figure 5B). To verify the robustness of our findings, a parallel analysis was conducted on 32 HER2-negative patients, yielding results that were not statistically different (Table 4).
| BIRClow group (n = 7) | BIRChigh group (n = 21) | P value | |
| Sex (male) | 6 (85.7) | 19 (90.5) | 1.000 |
| Age (year) | 63.71 ± 8.45 | 63.95 ± 8.52 | 0.9492 |
| BMI (kg/m2) | 22.94 ± 2.81 | 24.56 ± 3.40 | 0.2702 |
| Tumor size (cm) | 3.64 ± 2.34 | 4.90 ± 1.78 | 0.1462 |
| Differentiation | 0.4291 | ||
| Poor | 3 (42.9) | 14 (66.7) | |
| Moderate | 4 (50.0) | 6 (28.6) | |
| High | 0 (0.0) | 1 (4.8) | |
| T stage | 0.030 | ||
| 1 | 1 (14.3) | 0 (0) | |
| 2 | 2 (28.6) | 2 (9.5) | |
| 3 | 4 (57.1) | 2 (9.5) | |
| 4 | 0 (0.0) | 17 (81.0) | |
| N stage | 0.245 | ||
| 0 | 3 (42.9) | 5 (23.8) | |
| 1 | 2 (28.6) | 9 (42.9) | |
| 2 | 2 (28.6) | 3 (14.3) | |
| 3 | 0 (0.0) | 4 (19.0) | |
| M stage | - | ||
| 0 | 7 (100.0) | 19 (90.5) | |
| 1 | 0 (0.0) | 2 (9.5) | |
| TNM stage | 0.016 | ||
| I | 1 (4.8) | 0 (0.0) | |
| II | 2 (9.5) | 0 (0.0) | |
| III | 4 (19) | 19 (90.5) | |
| IV | 0 (0.0) | 2 (9.5) | |
| Survival | 0.5711 | ||
| Live | 6 (85.7) | 11 (52.4) | |
| Dead | 1 (14.3) | 10 (47.6) | |
| OS (month) | |||
| mean ± SD | 31.43 ± 7.138 | 17.0 ± 8.63 | 0.0012 |
| Median (range) | 29 (22-43) | 14 (8-45) | - |
| Recurrence | 0.5711 | ||
| No | 6 (85.7) | 10 (47.6) | |
| Recurrence | 1 (14.3) | 11 (52.4) | |
| DFS (month) | |||
| mean ± SD | 24.14 ± 4.34 | 14.76 ± 9.89 | 0.0022 |
| Median (range) | 25 (17-29) | 13 (2-45) | - |
| BIRClow group (n = 23) | BIRChigh group (n = 37) | P value | |
| Sex (male) | 19 (82.6) | 24 (81.1) | 0.2701 |
| Age (year) | 65.43 ± 10.77 | 64.97 ± 7.58 | 0.1102 |
| BMI (kg/m2) | 23.46 ± 3.43 | 24.84 ± 3.77 | 0.8922 |
| HER2 | 0.047 | ||
| HER2 (-) | 16 (69.6) | 16 (43.2) | |
| HER2 (+) | 7 (30.4) | 21 (56.8) | |
| Tumor size (cm) | 3.43 ± 2.02 | 4.59 ± 1.84 | 0.5002 |
| Differentiation | 0.489 | ||
| Poor | 9 (39.1) | 24 (64.9) | |
| Moderate | 14 (60.9) | 10 (27.0) | |
| High | 0 (0.0) | 3 (8.1) | |
| T stage | 0.001 | ||
| 1 | 5 (13.5) | 1 (2.7) | |
| 2 | 7 (18.9) | 6 (16.2) | |
| 3 | 10 (27.0) | 11 (29.7) | |
| 4 | 1 (2.7) | 19 (51.4) | |
| N stage | 0.208 | ||
| 0 | 11 (29.7) | 8 (21.6) | |
| 1 | 10 (27.0) | 20 (54.1) | |
| 2 | 2 (5.4) | 5 (13.5) | |
| 3 | 0 (0.0) | 4 (10.8) | |
| M stage | 0.691 | ||
| 0 | 23 (100.0) | 35 (94.6) | |
| 1 | 0 (0.0) | 2 (5.4) | |
| TNM stage | 0.007 | ||
| I | 4 (10.8) | 1 (2.7) | |
| II | 5 (13.5) | 1 (2.7) | |
| III | 12 (32.4) | 24 (64.9) | |
| IV | 2 (5.4) | 11 (29.7) | |
| OS (month) | |||
| mean ± SD | 32.2 ± 16.33 | 21.81 ± 15.17 | 0.0152 |
| Median (range) | 33 (3-53) | 15 (5-53) | - |
| DFS (month) | |||
| mean ± SD | 26.74 ± 14.80 | 18.57 ± 14.21 | 0.0402 |
| Median (range) | 26 (3-52) | 15 (2-53) | - |
Within the scope of this study, 20 of 28 patients received a treatment regimen that combined trastuzumab with chemotherapy (Figure 5C). Among these, 10 of 20 patients received capecitabine and oxaliplatin chemotherapy, 5 of 20 received tegafur gimeracil oteracil potassium capsule and oxaliplatin chemotherapy, and 5 of 20 received treatment with Tegio or capecitabine chemotherapy. Following trastuzumab therapy, four patients displayed disease progression within a 3-month timeframe, while the remaining 13 patients exhibited sensitivity to trastuzumab during the same period. Additionally, at the time of analysis, three patients had not yet completed the full 3-month treatment protocol. IHC results indicated markedly elevated expression levels of BIRC3 in the trastuzumab-resistant group compared to the trastuzumab-sensitive group.
These findings underscore the correlation between BIRC3 expression in tumor cells, resistance to trastuzumab treatment, and adverse prognostic implications in patients diagnosed with gastric cancer.
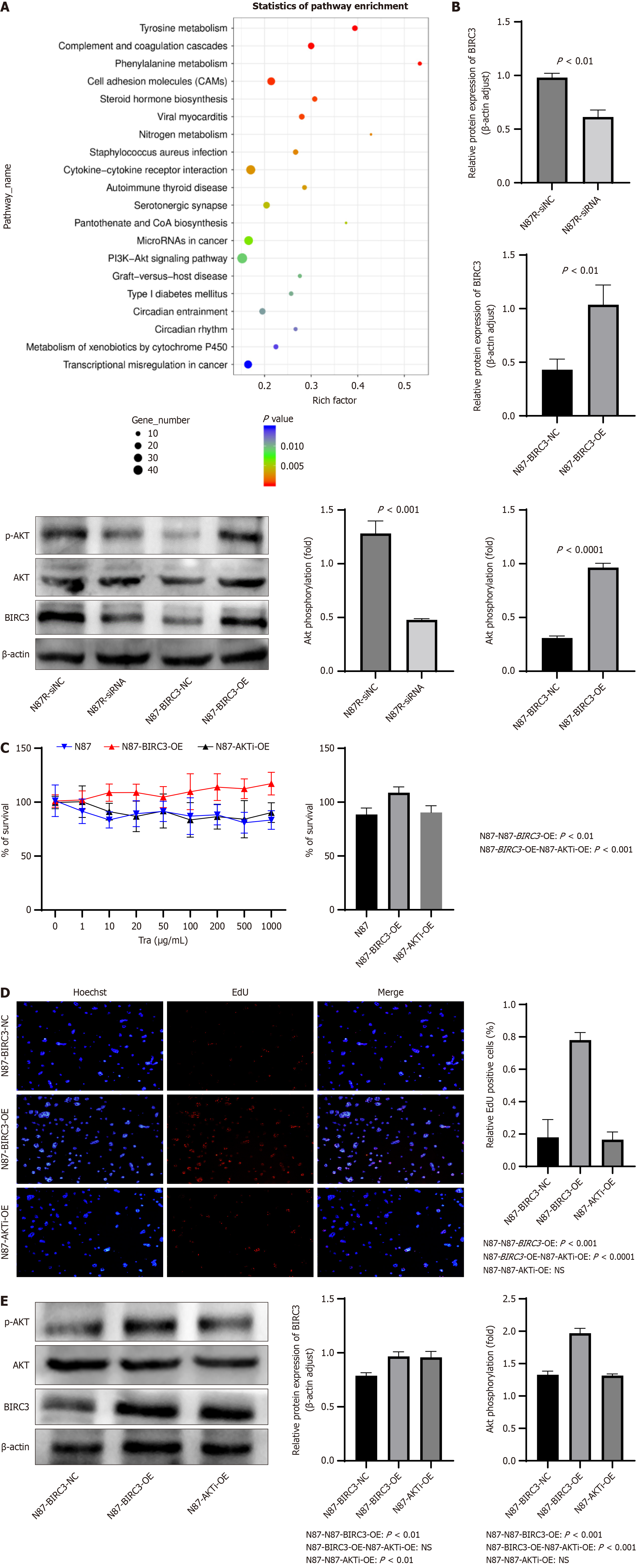
In this study, we explored the mechanism underlying BIRC3 action in HER2-positive gastric cancer cells. Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis revealed that the differentially expressed genes were predominantly enriched in the PI3K-AKT and Ras pathways (Figure 6A). Existing literature has documented that aberrant activation of the PI3K-AKT pathway in HER2-positive tumor cells can trigger resistance to targeted trastuzumab therapy. Moreover, it has been observed that anomalous expression of BIRC3 can stimulate the activation of the AKT signaling pathway.
BIRC3 siRNA or siRNA-NC was transfected into NCI-N87R cells, resulting in two distinct cohorts: N87-siRNA and N87-si-NC. We conducted qPCR to assess the expression of BIRC3. As illustrated in Figure 6B, the N87-siRNA group displayed a notable decrease in BIRC3 protein expression compared to the N87-si-NC group (0.6134 ± 0.06462 vs 0.9811 ± 0.03973, P = 0.0011). Furthermore, western blotting indicated that the downregulation of BIRC3 significantly diminished the expression of AKT and pAKT in NCI-N87R cells (0.4776 ± 0.01184 vs 1.282 ± 0.1165, P = 0.0003). Additionally, Figure 6B shows that the expression levels of BIRC3 (1.037 ± 0.1829 vs 0.4307 ± 0.09833, P = 0.0072), AKT, and pAKT proteins (0.9639 ± 0.04096 vs 0.3095 ± 0.01921, P < 0.0001) were markedly elevated in N87-BIRC3-OE cells compared to those in N87-BIRC3-NC cells.
To investigate the activation of the AKT pathway by BIRC3 and its role in trastuzumab resistance, we conducted an AKT pathway blockade experiment. NCI-N87 cells were pretreated with MK-2206, an AKT inhibitor, and transfected with the overexpression plasmid pLVX-IRES-puro-BIRC3, to generate N87-AKTi-OE cells. As illustrated in Figure 6C and D, the CCK8 and EdU detection assays demonstrated a significantly higher cell proliferation activity in the N87-BIRC3-OE group compared to the N87-NC and N87-AKTi-OE groups, with statistically significant differences (P < 0.001). However, there was no discernible difference in cell proliferation between the N87-NC and N87-AKTi-OE groups. These results indicated that transfection with BIRC3 significantly enhanced the proliferative capacity of N87-NC cells, which was attenuated by AKT inhibitors (P < 0.001). Furthermore, western blot analysis revealed a substantial increase in AKT phosphorylation in the sensitive cell line NCI-N87 upon BIRC3 overexpression, whereas AKT inhibitors reduced AKT phosphorylation (Figure 6E). This suggested that BIRC3 augmented the proliferative ability of NCI-N87 cells by activating the AKT pathway.
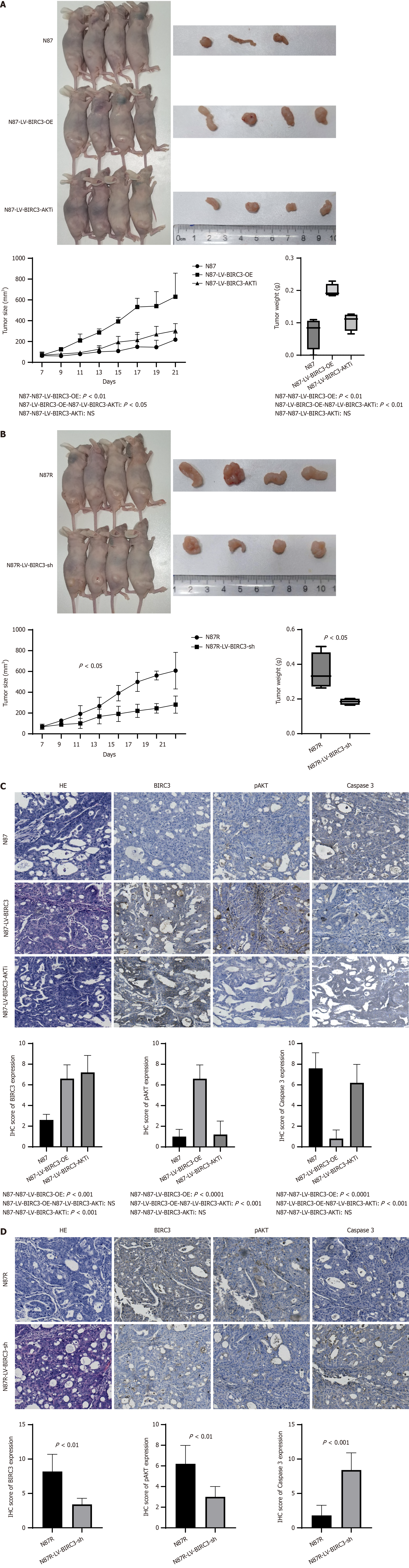
To examine tumorigenesis, SPF BALB/c nude mice were inoculated with the corresponding cells according to the described grouping plan. Tumor growth was monitored in nude mouse on the 7th day. Following the experimental design, the treatments were intraperitoneally administered every two days for two weeks.
As shown in Figure 7A, one nude mouse in the N87-NC group was administered trastuzumab for two weeks after tumor formation, resulting in complete tumor regression. The N87-LV-BIRC3 group displayed a significantly accelerated tumor growth rate and increased weight compared with both the N87-NC (P = 0.0084) and N87-LV-BIRC3-AKTi groups
According to the IHC results, the expression of BIRC3 was substantially enhanced in the N87R-LV-BIRC3-si (2.600 ± 0.5477 vs 6.7 ± 1.342, P = 0.0003) and N87-LV-BIRC3-AKTi (2.600 ± 0.5477 vs 7.20 ± 1.643, P = 0.0003) groups compared to the N87-NC group. Conversely, caspase 3 staining exhibited the opposite trend (P < 0.001). Additionally, the expression of pAKT in the N87-LV-BIRC3 group was notably higher than that in the N87-NC (6.600 ± 1.342 vs 1.000 ± 0.7071, P < 0.0001) and N87-LV-BIRC3-AKTi groups (6.600 ± 1.342 vs 1.200 ± 1.304, P = 0.0002) (Figure 7C). Furthermore, when compared to the N87R group, the expression of BIRC3 (8.200 ± 2.490 vs 3.400 ± 0.8944, P = 0.0036) and pAKT (6.200 ± 1.789 vs 3.000 ± 1.000, P = 0.0082) in the N87R-LV-BIRC3-si group significantly decreased, whereas the caspase 3 staining exhibited an opposite trend (1.800 ± 1.483 vs 8.400 ± 2.510, P = 0.0010) (Figure 7D).
These findings suggest that BIRC3 potentiates the inhibition of apoptosis and stimulates tumor cell proliferation through activation of the pAKT pathway, ultimately leading to the resistance of HER2-positive gastric cancer cells to trastuzumab.
In this study, we established a trastuzumab-resistant cell line, NCI-N87R, using advanced sequencing and bioinformatics analyses. Our screening process identified the key gene, BIRC3, which is responsible for resistance to targeted therapy with trastuzumab.
We investigated the functional role of BIRC3 in human gastric cancer, by focusing on its relationship with HER2. HER2 plays a crucial role in the development of gastric cancer, with approximately 5%-25% of gastric cancer cases exhibiting overexpression of the HER2. Studies have indicated that HER2 overexpression is an independent risk factor for advanced gastric cancer, leading to poor outcomes and shorter survival times[13,14]. The specific ligand of HER2 is unknown, but it is known to form hetero- or homodimers with itself or with other members of its family (EGFR, HER3, and HER4). This dimerization leads to the phosphorylation of the tyrosine kinase domain, activating downstream signaling pathways such as the PI3K-AKT and MAPK pathways. These pathways play a crucial role in promoting cell proliferation, preventing cell apoptosis, and influencing cell growth, survival, and differentiation[15-17].
BIRC3 has been shown to have an anti-apoptotic role, as it directly binds and inhibits caspases 3, 7, and 9. Additionally, BIRC3 can bind to polyubiquitin linked with lysine through its ubiquitin-related domain and activate the NF-κB signal pathway. This activation helps protect cells from tumor necrosis factor (TNF)-α induced cell apoptosis and maintain the survival of tumor cells[18]. Several studies have reported that BIRC3 is overexpressed in more than 70% of human gastric cancers[19]. BIRC3 knockout in gastric cancer cell lines has been shown to increase apoptosis, decrease proliferation, and delay cell migration[20]. Yoon et al[21] discovered that high BIRC3 expression plays a crucial role in gastric cancer development after Helicobacter pylori infection. Furthermore, various studies have demonstrated that BIRC3 exhibits similar effects in different tumor types. For example, high BIRC3 expression is associated with clinicopathological characteristics and poor prognosis in colon cancer, pancreatic cancer, bladder cancer, and glioblastoma[9,22-25]. BIRC3 overexpression plays a significant role in chemotherapy resistance in breast cancer, temozolomide resistance in glioblastoma multiforme, and cisplatin resistance induced by COL11A1 in ovarian cancer[26-29]. These findings strongly suggest that BIRC3 acts as a tumor promoter and has the potential to be a prognostic indicator in patients with tumors. Moreover, it may serve as a novel target for tumor treatment.
According to current literature, resistance to trastuzumab treatment in HER2-positive tumors can be attributed to various mechanisms. One important mechanism is abnormal activation of the downstream PI3K-AKT pathway[30,31]. Once AKT is phosphorylated and activated, it regulates several cellular processes including cell proliferation, differentiation, apoptosis, and angiogenesis through the involvement of Iκ-B kinase, procaspase 9, and mammalian target of rapamycin phosphorylation at the serine/threonine site[27-29]. Previous studies have demonstrated that AKT activation is associated with progressive disease and poor prognosis in certain tumor types[32]. In triple-negative breast cancer, overexpression of BIRC3 activates the AKT signaling pathway, leading to tumor cell proliferation, metastasis, and poor prognosis[33]. Furthermore, research on resistance to HER2-positive tumor-targeted drugs has revealed that various molecular abnormalities, such as changes in receptor structure, co-expression with other transmembrane receptors, and abnormal activation of downstream pathways, can contribute to trastuzumab resistance[34-37].
The experimental results convincingly demonstrated that overexpression of BIRC3 and AKT in N87 cells elicited a notable increase in cell proliferation and concurrent resistance to trastuzumab. Conversely, upon silencing BIRC3 expression, the inverse effect was observed. Furthermore, inhibition of the AKT pathway resulted in a discernible decline in N87 cell proliferation, accompanied by increased susceptibility to trastuzumab. These findings suggest that BIRC3 amplifies the proliferative potential of HER2-positive gastric cancer cells by activating the AKT pathway, thereby inducing resistance to targeted therapy with trastuzumab. Moreover, in vitro cytological experiments substantiated these observations, affirming that increased BIRC3 expression activates the AKT pathway, resulting in enhanced proliferation of previously responsive cells after trastuzumab treatment, consequently fostering drug resistance. To confirm these conclusions, tumorigenesis experiments were conducted in nude mice. Additionally, a responsive experimental group was designed to provide further compelling evidence supporting the assertion that BIRC3 induces trastuzumab-targeted drug resistance through the activation of the AKT pathway.
In the clinical data analysis section, several limitations are evident. This study is a single-center investigation and lacks data from larger multicenter samples. Consequently, a greater number of gastric cancer patients must be evaluated to enhance the robustness of the results. Although we conducted multiple repeated experiments to ensure the accuracy of our findings, additional results from diverse experimental methods are necessary for further improvement. Ultimately, more work is required to provide tangible benefits to clinical patients.
In conclusion, our findings illustrate that the knockdown of BIRC3 diminishes the resistance of the trastuzumab-resistant cell line NCI-N87R. Conversely, high BIRC3 expression induced resistance to trastuzumab. Moreover, upon transfection with the BIRC3 overexpression vector, N87 cells exhibited resistance to trastuzumab. In addition, resistance to trastuzumab could be counteracted by AKT inhibitors, suggesting that BIRC3 may trigger resistance to trastuzumab therapy by activating the AKT pathway.
The authors would like to thank Tianjin Medical University General Hospital for support.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64590] [Article Influence: 16147.5] [Reference Citation Analysis (176)] |
| 2. | Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18:473-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 3. | Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 452] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Tarazona N, Gambardella V, Huerta M, Roselló S, Cervantes A. Personalised Treatment in Gastric Cancer: Myth or Reality? Curr Oncol Rep. 2016;18:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47115] [Article Influence: 3365.4] [Reference Citation Analysis (5)] |
| 6. | Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 785] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 7. | Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Yabal M, Müller N, Adler H, Knies N, Groß CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwälder M, Strasser A, Groß O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Wang D, Berglund A, Kenchappa RS, Forsyth PA, Mulé JJ, Etame AB. BIRC3 is a novel driver of therapeutic resistance in Glioblastoma. Sci Rep. 2016;6:21710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 418] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Bhosale PG, Pandey M, Cristea S, Shah M, Patil A, Beerenwinkel N, Schäffer AA, Mahimkar MB. Recurring Amplification at 11q22.1-q22.2 Locus Plays an Important Role in Lymph Node Metastasis and Radioresistance in OSCC. Sci Rep. 2017;7:16051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mendoza-Rodríguez M, Arévalo Romero H, Fuentes-Pananá EM, Ayala-Sumuano JT, Meza I. IL-1β induces up-regulation of BIRC3, a gene involved in chemoresistance to doxorubicin in breast cancer cells. Cancer Lett. 2017;390:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Kim JW, Im SA, Kim M, Cha Y, Lee KH, Keam B, Kim MA, Han SW, Oh DY, Kim TY, Kim WH, Bang YJ. The prognostic significance of HER2 positivity for advanced gastric cancer patients undergoing first-line modified FOLFOX-6 regimen. Anticancer Res. 2012;32:1547-1553. [PubMed] |
| 14. | Bayrak M, Olmez OF, Kurt E, Cubukcu E, Evrensel T, Kanat O, Manavoglu O. Prognostic significance of c-erbB2 overexpression in patients with metastatic gastric cancer. Clin Transl Oncol. 2013;15:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 16. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 872] [Article Influence: 51.3] [Reference Citation Analysis (2)] |
| 17. | Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 18. | Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, Xue W, Tenev T, da Fonseca PC, Zvelebil M, Bujnicki JM, Lowe S, Silke J, Meier P. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Li Z, Chen J, Chan KW, Qiao L, Wong BC. A possible role of cIAP2 in Helicobacter pylori-associated gastric cancer. Cancer Lett. 2011;313:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Chang CS, Chen WN, Lin HH, Wu CC, Wang CJ. Increased oxidative DNA damage, inducible nitric oxide synthase, nuclear factor kappaB expression and enhanced antiapoptosis-related proteins in Helicobacter pylori-infected non-cardiac gastric adenocarcinoma. World J Gastroenterol. 2004;10:2232-2240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Yoon H, Kim SG, Kim BK, Shin E, Kim N, Lee HJ, Kang GH, Jung HC. Helicobacter pylori Eradication Downregulates Cellular Inhibitor of Apoptosis Protein 2 in Gastric Carcinogenesis. Gut Liver. 2017;11:79-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Miura K, Karasawa H, Sasaki I. cIAP2 as a therapeutic target in colorectal cancer and other malignancies. Expert Opin Ther Targets. 2009;13:1333-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Esposito I, Kleeff J, Abiatari I, Shi X, Giese N, Bergmann F, Roth W, Friess H, Schirmacher P. Overexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancer. J Clin Pathol. 2007;60:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Ponnelle T, Chapusot C, Martin L, Bonithon-Kopp C, Bouvier AM, Plenchette S, Rageot D, Faivre J, Solary E, Piard F. Subcellular expression of c-IAP1 and c-IAP2 in colorectal cancers: relationships with clinicopathological features and prognosis. Pathol Res Pract. 2003;199:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Che X, Yang D, Zong H, Wang J, Li X, Chen F, Chen X, Song X. Nuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patients. Urol Oncol. 2012;30:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Rada M, Nallanthighal S, Cha J, Ryan K, Sage J, Eldred C, Ullo M, Orsulic S, Cheon DJ. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene. 2018;37:4809-4820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2760] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 28. | Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034-5037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 559] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636-3641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 579] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 30. | Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, Riches JC, Tuvy Y, Kundra R, Bouvier N, Vakiani E, Gao J, Heins ZJ, Gross BE, Kelsen DP, Zhang L, Strong VE, Schattner M, Gerdes H, Coit DG, Bains M, Stadler ZK, Rusch VW, Jones DR, Molena D, Shia J, Robson ME, Capanu M, Middha S, Zehir A, Hyman DM, Scaltriti M, Ladanyi M, Rosen N, Ilson DH, Berger MF, Tang L, Taylor BS, Solit DB, Schultz N. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018;8:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 31. | Gambardella V, Gimeno-Valiente F, Tarazona N, Martinez-Ciarpaglini C, Roda D, Fleitas T, Tolosa P, Cejalvo JM, Huerta M, Roselló S, Castillo J, Cervantes A. NRF2 through RPS6 Activation Is Related to Anti-HER2 Drug Resistance in HER2-Amplified Gastric Cancer. Clin Cancer Res. 2019;25:1639-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 1787] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 33. | Jo SJ, Park PG, Cha HR, Ahn SG, Kim MJ, Kim H, Koo JS, Jeong J, Park JH, Dong SM, Lee JM. Cellular inhibitor of apoptosis protein 2 promotes the epithelial-mesenchymal transition in triple-negative breast cancer cells through activation of the AKT signaling pathway. Oncotarget. 2017;8:78781-78795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Ross JS, Wang K, Sheehan CE, Boguniewicz AB, Otto G, Downing SR, Sun J, He J, Curran JA, Ali S, Yelensky R, Lipson D, Palmer G, Miller VA, Stephens PJ. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin Cancer Res. 2013;19:2668-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 641] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 36. | Pietrantonio F, Fucà G, Morano F, Gloghini A, Corso S, Aprile G, Perrone F, De Vita F, Tamborini E, Tomasello G, Gualeni AV, Ongaro E, Busico A, Giommoni E, Volpi CC, Laterza MM, Corallo S, Prisciandaro M, Antista M, Pellegrinelli A, Castagnoli L, Pupa SM, Pruneri G, de Braud F, Giordano S, Cremolini C, Di Bartolomeo M. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin Cancer Res. 2018;24:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | He F, Ru X, Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 1064] [Article Influence: 212.8] [Reference Citation Analysis (0)] |