Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1412
Peer-review started: March 28, 2023
First decision: May 17, 2023
Revised: May 30, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: August 15, 2023
Processing time: 135 Days and 4.8 Hours
Hepatocellular carcinoma (HCC) has very low overall survival. According to global cancer statistics, approximately 905677 new cases were reported in 2020, with at least 830180 of them being fatal. Cluster of differentiation 147 (CD147) is a novel, transmembrane glycoprotein that is expressed in a wide variety of tumor cells and plays an important role in various stages of tumor development. Based on the reports described previously, we theorize that CD147 may be used as a novel biological indicator to predict the prognosis of HCC. To study this possi
To explore the pattern of CD147 expression and its applicability in the prognosis of HCC. To establish HRs and probability points for predicting the prognosis of HCC by correlating CD147 expression with clinical characteristics. To determine if CD147 can be a reliable biomarker in HCC prognosis.
The CD147 expression profile in HCC and corresponding clinical data were obtained from TCGA database. The expression patterns of CD147 were then validated by analyzing data from the GEO database. In addition, CD147 immunohistochemistry in HCC was obtained from the Human Protein Atlas. CD147 expression patterns and clinical characteristics in the prognosis of HCC were analyzed by accessing the UALCAN web resource. Accuracy, sensitivity, and specificity of the CD147 expression profile in predictive prognosis were deter
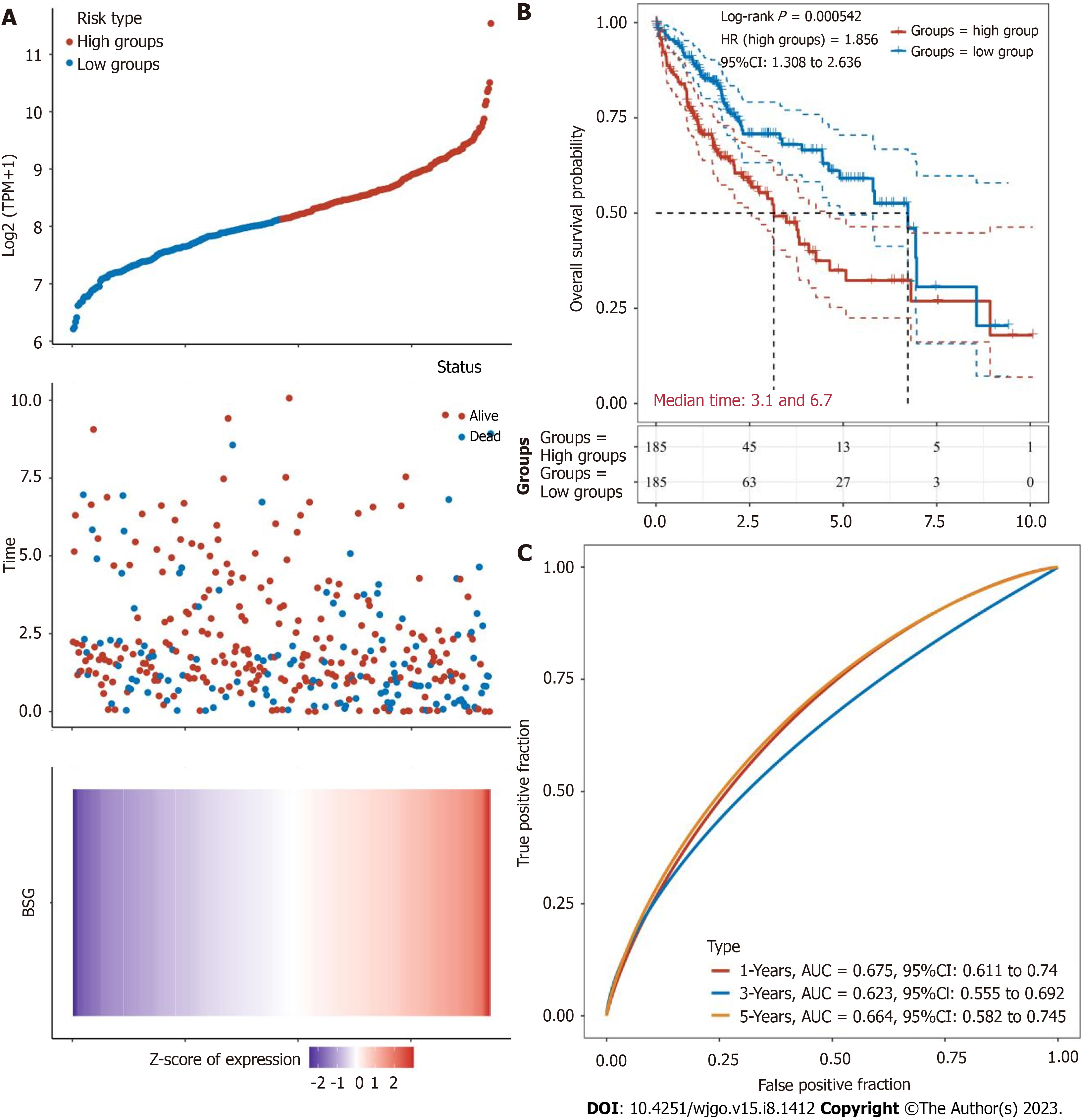
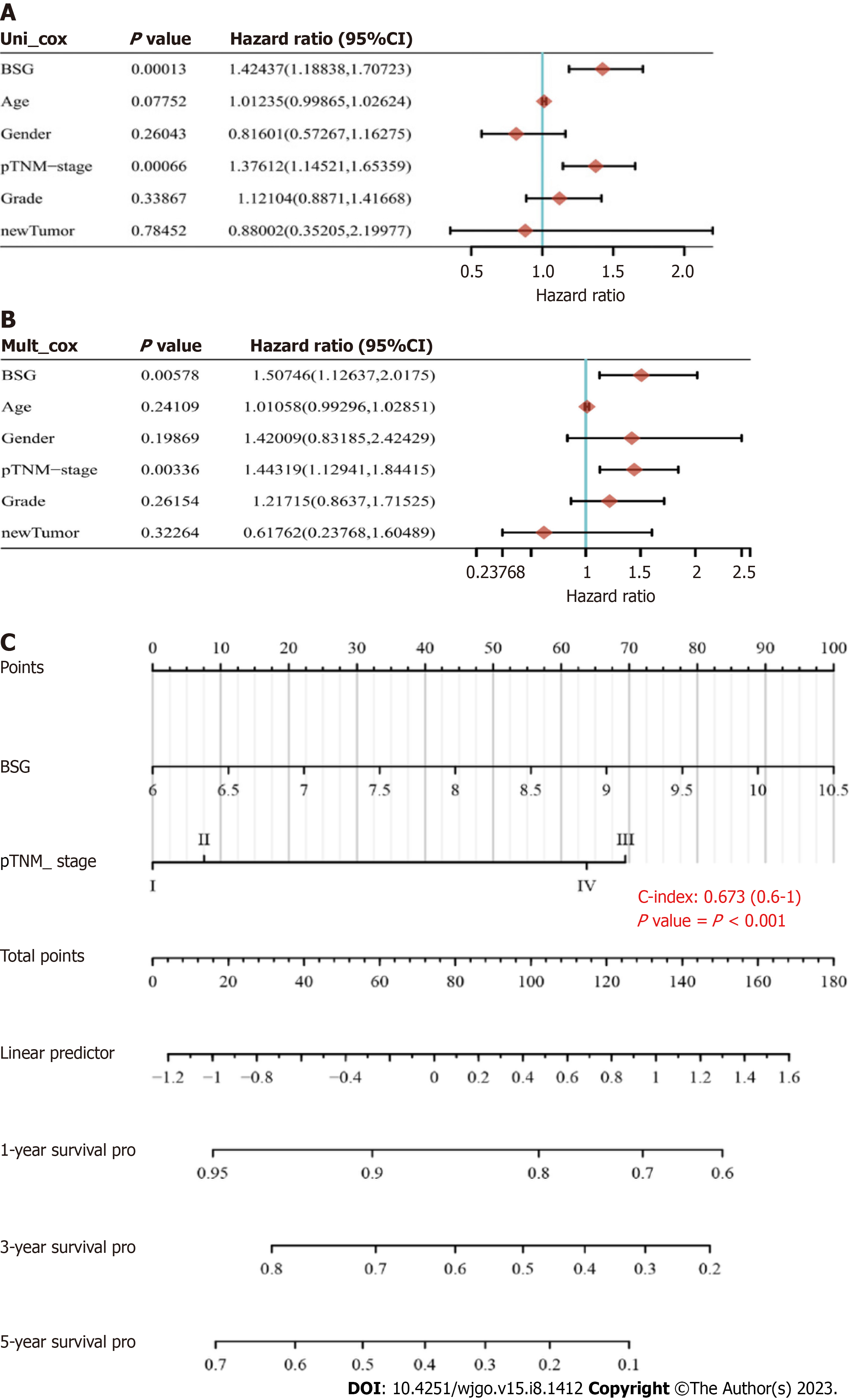
Data from TCGA and GEO databases revealed that CD147 was significantly overexpressed in HCC (P = 1.624 × 10-12 and P = 1.2 × 10-5, respectively). The expression of CD147 and prognosis of HCC were significantly correlated with the clinical characteristics of HCC as per the data from the UALCAN web resource (P < 0.05). Kaplan-Meier analysis of CD147 expression in HCC revealed that the high expression groups showed poor prognosis and an HR of survival > 1 [log-rank test, P = 0.000542, HR (in high expression group): 1.856, 95% confidence interval (CI): 1.308 to 2.636]. ROC curves were plotted to analyze the 1-year, 3-year, and 5-year survival rates. The area under the ROC curve values were 0.675 (95%CI: 0.611 to 0.740), 0.623 (95%CI: 0.555 to 0.692), and 0.664 (95%CI: 0.582 to 9.745), respectively. Univariate Cox analysis of CD147 expression and clinical characteristics of HCC and multivariate Cox analysis of CD147 patterns and pathological tumor-node-metastasis stage showed significant differences (univariate Cox, P = 0.00013, HR: 1.424, 95%CI: 1.884 to 1.707 and P = 0.00066, HR: 1.376, 95%CI: 1.145 to 1.654, respectively; multivariate Cox, P = 0.00578, HR: 1.507, 95%CI: 1.126 to 2.018 and P = 0.00336, HR: 1.443, 95%CI: 1.129 to 1.844, respectively). Nomograms were plotted to establish the probability points and predict prognosis. The total points ranged from 0 to 180, and the C-index value was 0.673 (95%CI: 0.600 to 1.000, P < 0.01).
Overexpression of CD147 was correlated with poor prognosis in HCC. The CD147 expression profile combined with clinical characteristics can reliably predict the prognosis of HCC. CD147 can serve as a biomarker to predict the prognosis of HCC.
Core Tip: Hepatocellular carcinoma (HCC) has very low overall survival. The discovery of a new biomarker related to prognosis would improve the prognosis of HCC. Cluster of differentiation 147 (CD147) is a novel transmembrane glycoprotein that is expressed in a wide variety of tumor cells and plays an important role in various stages of tumor development. In our study, we found that CD147 correlated with clinical characteristics and prognosis of HCC. Therefore, we suggest that CD147 can serve as a biomarker to predict the prognosis of HCC.
- Citation: Xu YJ, He HJ, Wu P, Li WB. Expression patterns of cluster of differentiation 147 impact the prognosis of hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(8): 1412-1423
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1412.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1412
Liver cancer is a highly malignant tumor, with a 5-year survival rate of only 10%[1]. Therefore, its treatment is challenging worldwide[1,2]. Hepatocellular carcinoma (HCC) and cholangiocarcinoma account for 80%-90% and 10%-15% of all primary liver cancers, respectively[3]. According to global cancer statistics, approximately 905677 new cases were reported in 2020, with at least 830180 of them being fatal[4]. It is estimated that by 2025, more than one million individuals will be affected by liver cancer annually[5]. Surveillance Epidemiology End Results reported HCC to be the fastest-growing cause of cancer-related deaths in the United States since the early 2000s. If this trend continues, it is predicted to become the third leading cause of cancer-related mortality by 2030[6].
The major risk factors for HCC include chronic alcohol consumption, diabetes-related or obesity-related nonalcoholic steatohepatitis, and infection by hepatitis B virus (HBV) or hepatitis C virus (HCV). The high-risk factors for HCC include chronic alcohol consumption, diabetes, and infection by HBV or HCV[7,8]. According to Barcelona Clinic Liver Cancer staging system, several approaches are available for the treatment of HCC, such as surgery (early stage), liver trans
Cluster of differentiation 147 (CD147) is a novel, transmembrane glycoprotein that is expressed in a wide variety of tumor cells and plays an important role in various stages of tumor development[10-12]. CD147 is encoded by the Basigin (BSG) gene located on chromosome 19 at the p13.3 locus[11,12]. Epithelial and fetal tissues have low expression levels of CD147[13]. CD147 promotes tumor proliferation, invasion, and metastasis[14,15], probably by triggering a matrix metalloproteinase (MMP) on the tumor surface[16,17]. The expression of CD147 is upregulated in various tumors including breast cancer, bladder cancer, colorectal cancer, ovarian cancer, melanoma, and osteosarcoma[18,19] as well as in HCC[20]. CD147 is highly expressed in ovarian cancer and in combination with human epididymis protein 4 may act as a novel indicator for the diagnosis and treatment of early ovarian cancer[21].
Based on the reports described previously, we theorize that CD147 may be used as a novel biological indicator to predict the prognosis of HCC. To study this possibility, expression profiles of CD147 and corresponding clinical data from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases were analyzed, and a hazard ratio (HR) was established.
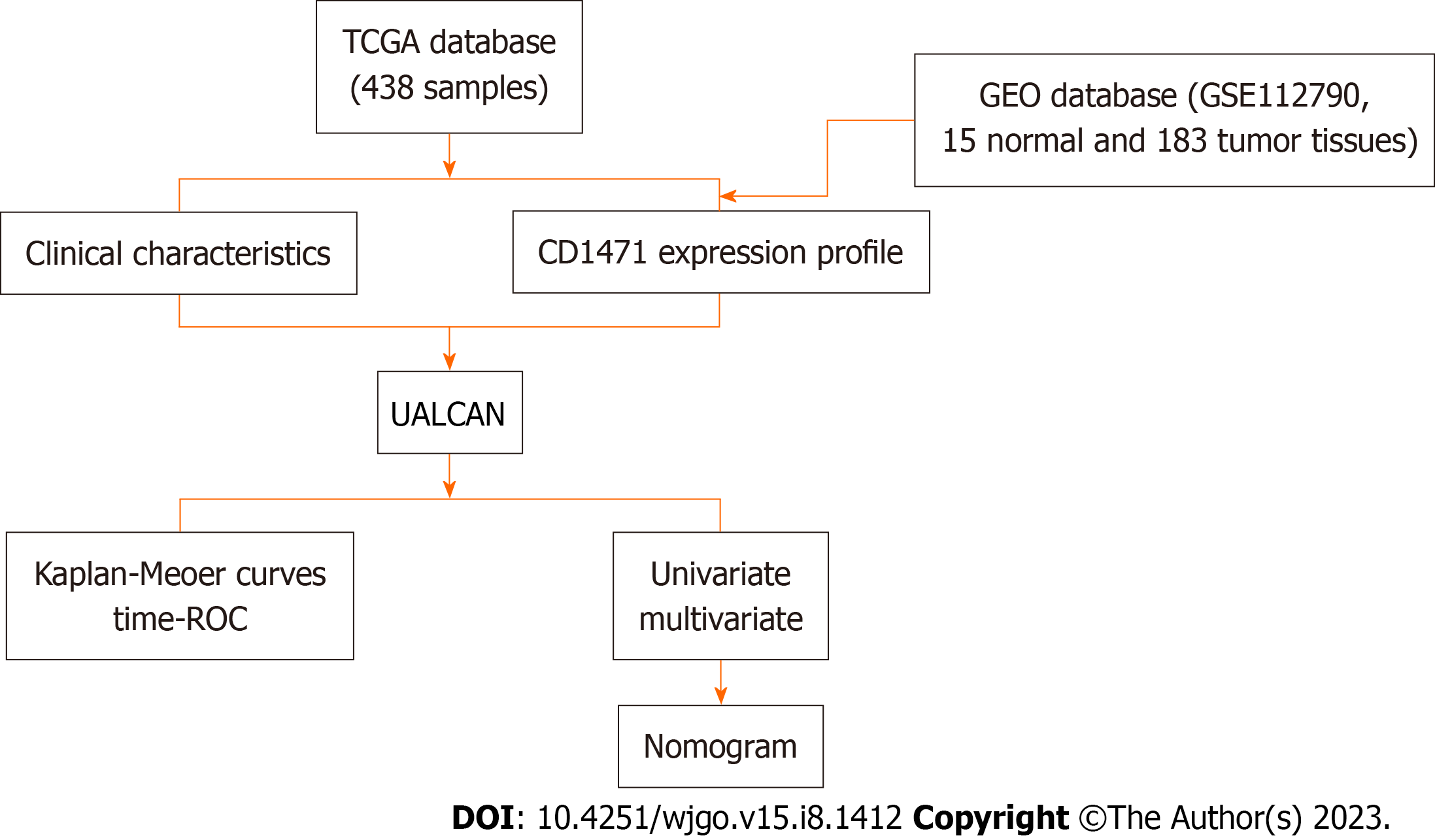
The transcriptome profile of HCC was obtained from TCGA using the TCGAbiolinks package. The dataset included 50 normal and 371 tumor samples [TCGA-Liver Hepatocellular Carcinoma (LIHC)]. The GSE112790 dataset (based on the GPL570 platform), which included 15 normal and 183 tumor tissues, was acquired from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) for external validation of the prognostic gene signature. The workflow is illustrated in Figure 1.
To determine the expression patterns of CD147, gene expression analysis was performed on the TCGA-LIHC dataset using the R package cluster profiler. The samples with no significant expression value and insufficient survival information were excluded. The clinical characteristics including age, sex, pathological tumor-node-metastasis (pTNM), tumor grade, metastatic status, overall survival (OS) time, and survival status were obtained from the patients’ data. The expression profile of CD147 was analyzed, and its prognostic value was validated in the GSE112790 dataset.
UALCAN (http://ualcan.path.uab.edu) is an interactive web resource designed to analyze the relative mRNA expression patterns of potential genes (TCGA and MET500 transcriptome sequencing) and their relationship with various tumor subtypes. UALCAN was utilized to obtain the mRNA expression profile of CD147 in HCC tissues and ascertain its association with clinical characteristics.
The correlation between the expression of CD147 and OS of patients with HCC was analyzed by univariate and multivariate Cox proportional hazards regression analyses. The timedependent receiver operating characteristic (ROC) curve and Kaplan-Meier curve were generated to assess the prognostic ability of CD147. A prognostic nomogram was also constructed based on the results obtained from the multivariate Cox regression analysis to predict the 1-year, 3-year, and 5-year survival rates and overall recurrence. The log-rank test was used for calculating HR with a 95% confidence interval (CI).
All analyses were performed using R software (version 4.0.3, foundation for statistical computing, 2020) and its related packages unless stated otherwise. The Student’s t-test was used to determine if the differences between the two groups were statistically significant. A value of P < 0.05 was considered to be statistically significant.
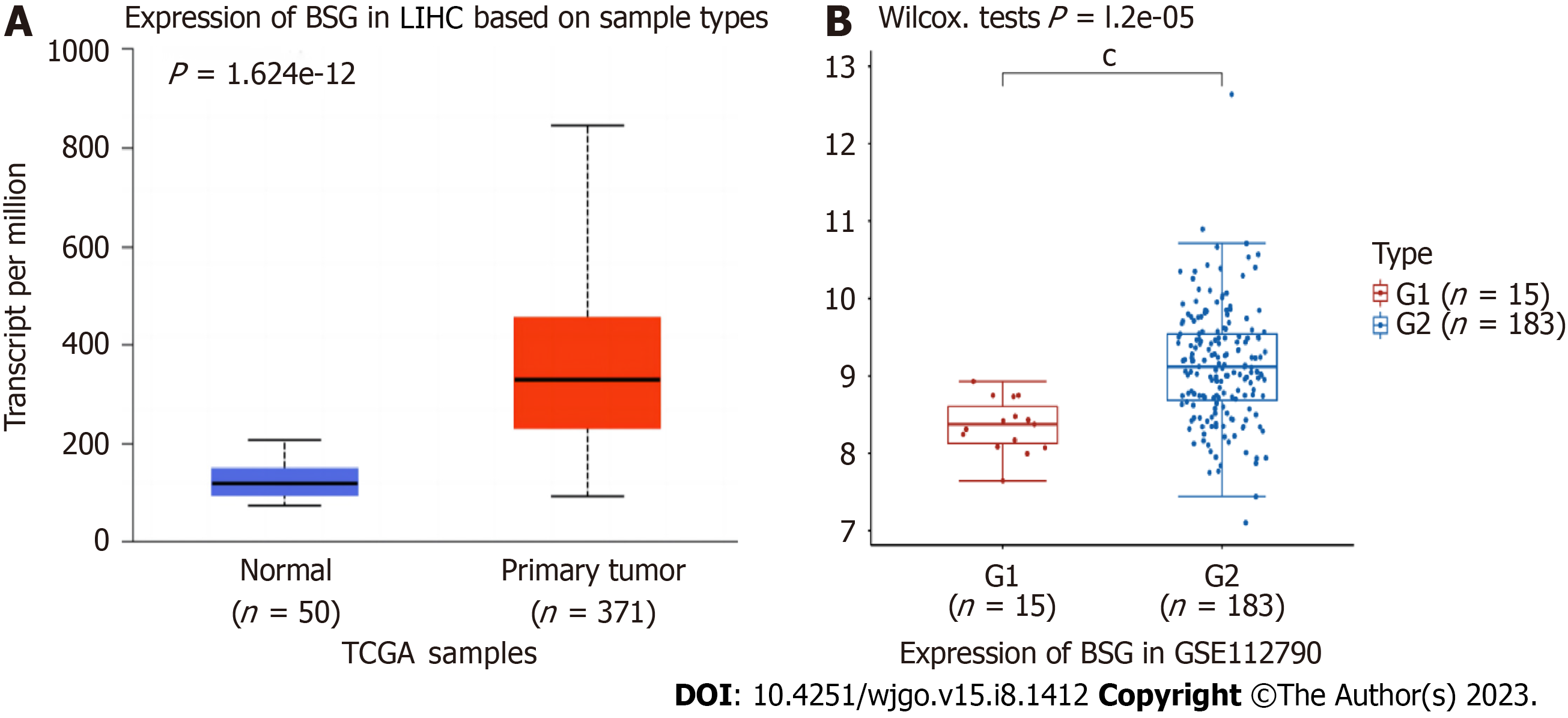
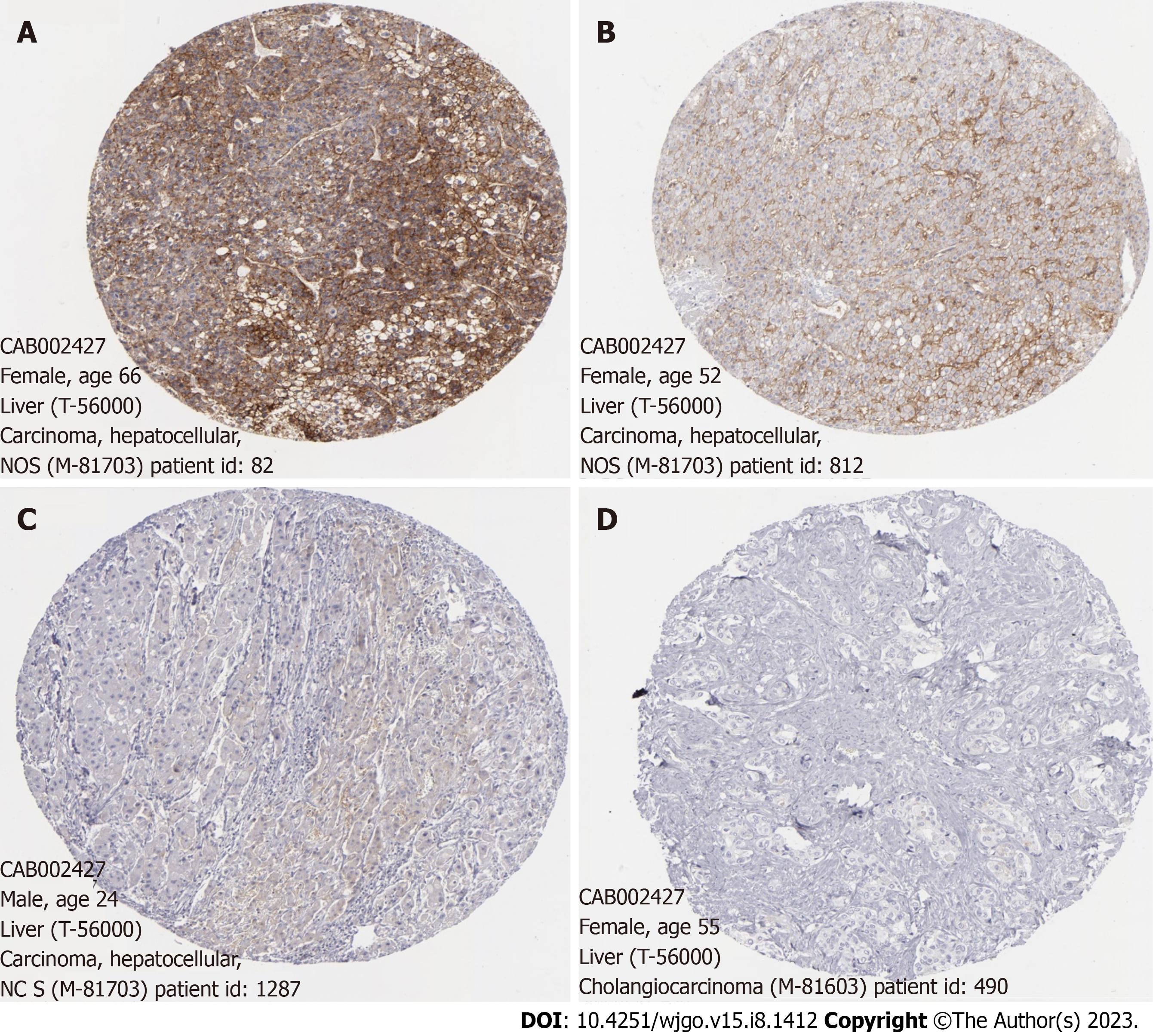
The CD147 expression profile was obtained from the TCGA database and GSE112790 dataset from the GEO database. The analysis revealed that CD147 was significantly overexpressed in tumors compared to normal tissues (P = 1.624 × 10-12 and P = 1.2 × 10-5, respectively, Figure 2). In addition, the immunohistochemistry profile of CD147 in HCC was obtained from the Human Protein Atlas (https://www.proteinatlas.org) (Figure 3).
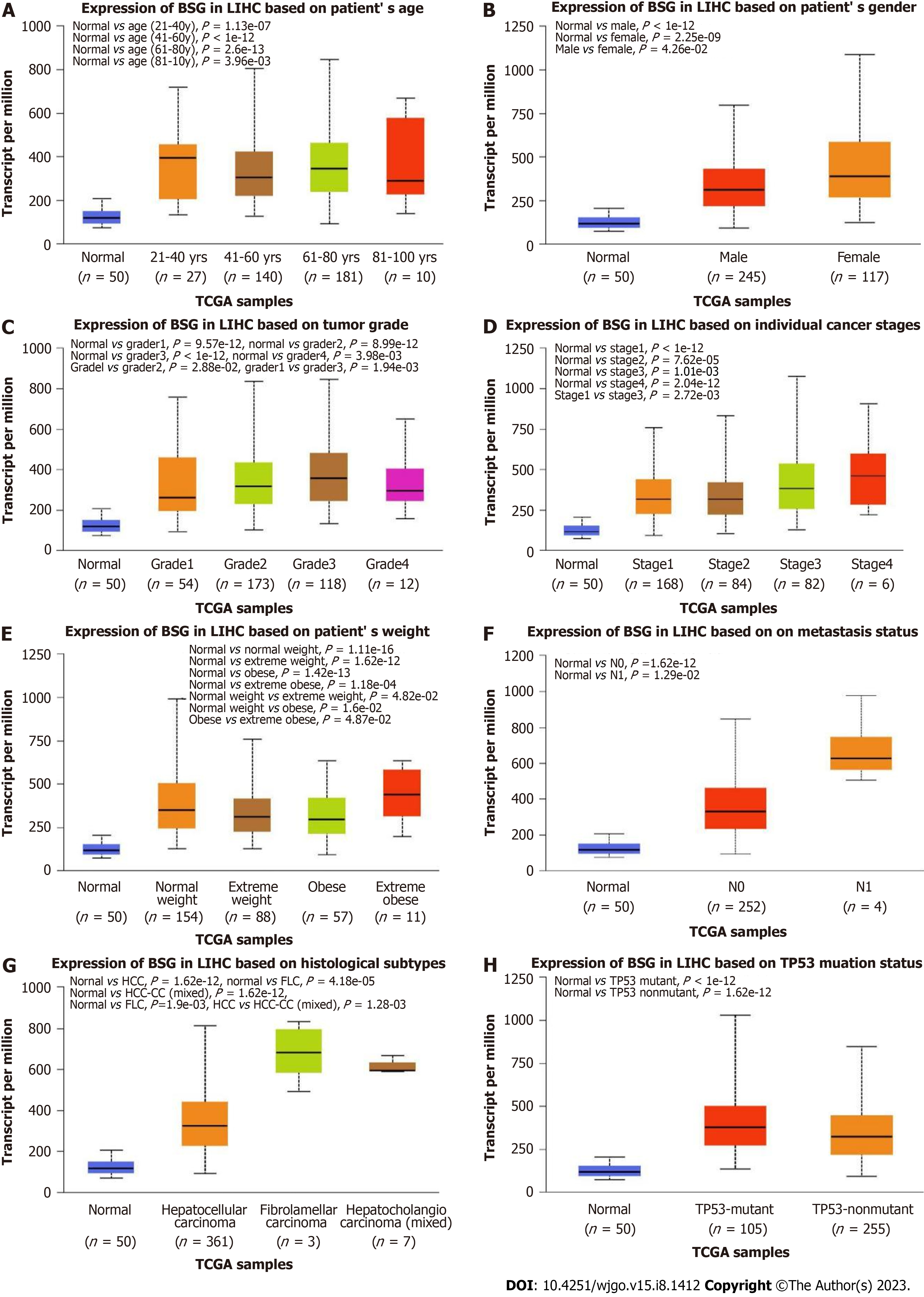
The expression of CD147 in comparison to clinical characteristics of HCC obtained from the TCGA database was analyzed using UALCAN. The expression of CD147 was compared to age, sex, tumor grade, pTNM, weight, histology, metastatic status, and mutation in the TP53 gene. The expression of CD147 showed significant differences in each criterion (P < 0.05, Figure 4).
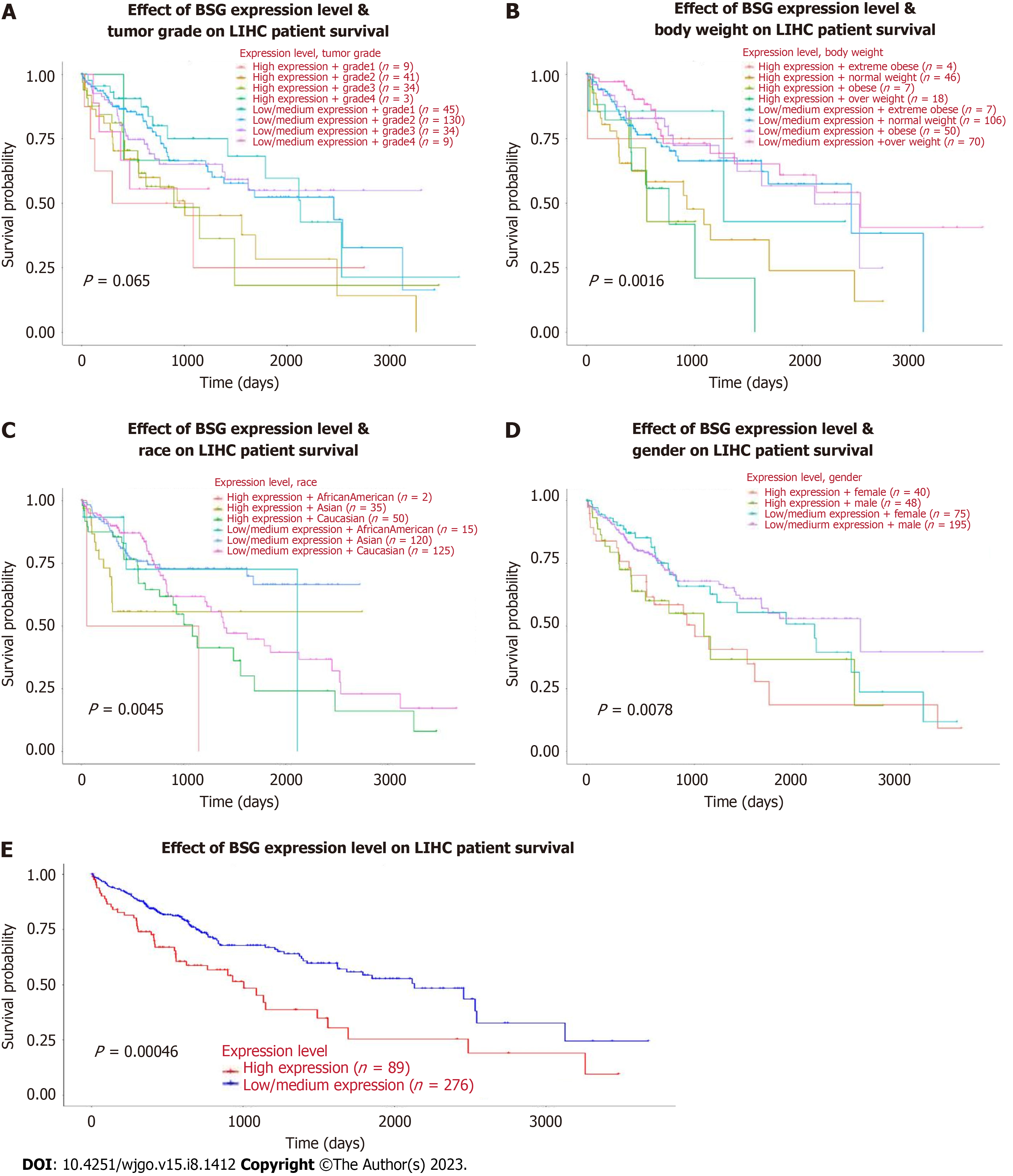
The correlation between CD147 expression and prognosis of HCC concerning tumor grade, weight, race, sex, and OS time data obtained from the TCGA database showed statistically significant differences (P = 0.065, P = 0.0016, P = 0.0045, P = 0.0078, P = 0.00046, respectively, Figure 5). Kaplan-Meier curve based survival analysis in low-expression and high-expression groups of CD147 was conducted by the log-rank test (Figure 6). The high-expression groups demonstrated poor prognosis and HR > 1 (log-rank P = 0.000542, HR: 1.856, 95%CI: 1.308 to 2.636, Figure 6B). Based on the area under the ROC curve values obtained from ROC curves, the 1-year, 3-year, and 5-year survival rates were 0.675 (95%CI: 0.611 to 0.740), 0.623 (95%CI: 0.555 to 0.692), and 0.664 (95%CI: 0.582 to 9.745) (Figure 6C).
CD147 expression and age, sex, pTNM-stage, tumor grade, and HR of a new HCC tumor was analyzed by univariate and multivariate Cox regression analysis. The CD147 expression and pTNM-stage showed significant differences (univariate, P = 0.00013, HR: 1.424, 95%CI: 1.884 to 1.707 and P = 0.00066, HR: 1.376, 95%CI: 1.145 to 1.654, respectively, Figure 7A; multivariate, P = 0.00578, HR: 1.507, 95%CI: 1.126 to 2.018 and P = 0.00336, HR: 1.443, 95%CI: 1.129 to 1.844, respectively, Figure 7B). Accordingly, the factors of CD147 expression and pTNM-stage (P < 0.05) were selected to establish a prognostic nomogram for HCC. The total points ranged from 0 to 180, and the C-index value was 0.673 (95%CI: 0.600 to 1.000, P < 0.01, Figure 7C).
The expression profile of CD147 in HCC was obtained from the TCGA database and was verified in the GSE112790 dataset obtained from the GEO database. The expression of CD147 was significantly higher in HCC than in normal tissue. In addition, analysis using UALCAN revealed that the expression of CD147 was closely associated with clinical characteristics of HCC, including age, sex, pTNM, tumor grade, metastatic status, weight, histology, and mutation in the TP53 gene.
Even though CD147 is closely associated with tumor proliferation, invasion, and metastasis[14,15], the underlying mechanisms are still unclear. CD147 overexpression may be associated with tumor cell migration and activation of the extracellular signal-regulated kinase signaling pathway[22]. In addition, CD147 regulates MMPs and vascular endothelial growth factor implicated in tumor and stromal cells[22,23]. CD147 induced MEK-mediated intracellular signaling pathway and MMP-9 activity, which promoted tumor proliferation, invasion, and metastasis in hypopharyngeal carcinoma[24]. CD147 was overexpressed in HCC cells, and the knockdown of CD147 significantly inhibited the proliferation, migration, and invasion of HCC cells[25]. Hypophosphorylation of CD147 promotes the invasion and metastasis of HCC, and CD147 may be utilized as a novel biomarker in the prognosis of HCC[26]. CD147 expression has been associated with lymph node metastasis in cervical cancer and laryngeal squamous cell carcinoma[27]. CD147 was overexpressed in oral cancer, and knockout of CD147 significantly reduced the proliferation and invasion of cal27 cells, indicating that CD147 may be a potential therapeutic target in oral cancer[28].
CD147 has been proposed as a novel, prognostic biomarker in HCC. We analyzed the role of the expression of CD147 in the prognosis of HCC. High expression of CD147 significantly shortened the prognosis of HCC and related clinical characteristics. In our study, we found CD147 had an HR > 1 implying that is a poorly reliable factor in prognosis. The CI of the univariate and multivariate Cox analyses of the expression of CD147 and clinical characteristics was 0.673, significantly greater than 0.5, indicating that it has a high predictive value. Since CD147 plays an important role in the prognosis of HCC, it may be considered a potential biomarker in the prediction of tumor prognosis.
Several studies have shown that CD147 has a good prognostic value in many tumors. The methylation levels of CD147 were ascertained using cfDNA in non-small cell lung cancer (NSCLC) tissues and were inversely related to tumor size, lymph node metastasis, and TNM stage[29]. Targeted methylation of CD147 could inhibit NSCLC invasion and metastasis. CD147 and MMP-9 were closely correlated with the pathological stage, metastasis, and differentiation of tumors in breast cancer cases (P < 0.05) but were poor independent risk factors for prognosis in triple-negative breast cancer (PCD147 = 0.023, PMMP-9 = 0.015)[30]. In cancer patients older than 50 years, CD147 was an independent prognostic indicator[30]. Expression patterns of CD147 in different stages of esophageal squamous cell carcinoma were able to reliably predict prognosis in patients[31].
CD147 also plays an important role in other diseases such as HIV type 1, HCV, HBV, Kaposi’s sarcoma-associated herpes virus, and severe acute respiratory syndrome coronavirus infections[32]. Interestingly, CD147 is involved in severe acute respiratory syndrome coronavirus 2 tropism and may be a potential therapeutic target for coronavirus disease 2019[32].
Matuzumab (anti-CD147) was confirmed to be a safe treatment method for NSCLC[33]. Recently, chimeric antigen receptor T-cell immunotherapy targeting CD147 demonstrated antitumor efficacy in patients with HCC[20]. Thus, CD147 expression in correlation with clinical characteristics may serve as a predictive biomarker for pathological type prognosis of tumor and as a target for tumor treatment.
Overexpression of CD147 was correlated with poor prognosis in HCC. CD147 expression profile combined with clinical characteristics can reliably predict the prognosis of HCC. CD147 can serve as a biomarker to predict the prognosis of HCC.
Hepatocellular carcinoma (HCC) has very low overall survival. Searching for a new biomarker related to prognosis will be helpful in improving the prognosis of HCC. Cluster of differentiation 147 (CD147) is a novel transmembrane glycoprotein that is expressed in a wide variety of tumor cells and plays an important role in various stages of tumor development.
CD147 is highly expressed in various tumors and is associated with prognosis.
To explore the pattern of CD147 expression and its applicability in the prognosis of HCC. To establish hazard ratios and probability points for predicting the prognosis of HCC by correlating CD147 expression with clinical characteristics. To determine if CD147 can be a reliable biomarker in HCC prognosis.
Using The Cancer Genome Atlas and Gene Expression Omnibus databases, R language was used to analyze the expression of CD147 in HCC. The online website UALCAN was used to analyze the correlation between clinical characteristics and survival time of The Cancer Genome Atlas-Liver Hepatocellular Carcinoma and CD147 expression. Subsequently, time-dependent receiver operating characteristic curves were used to analyze the accuracy, sensitivity, and specificity of CD147 in HCC. Finally, univariate and multivariate Cox regression proportional hazards analyses of CD147 expression levels and clinical characteristics as prognostic factors of HCC were performed. Nomograms were used to establish probability points and predict prognosis.
CD147 was overexpressed in HCC, and the prognosis of HCC was significantly correlated with the clinical characteristics of HCC. The overexpression of CD147 showed poor prognosis and a hazard ratio of survival > 1 in HCC. Multivariate Cox analysis of CD147 patterns and pathological tumor-node-metastasis-stage showed significant differences. Nomograms showed CD147 can predict prognosis.
CD147 is overexpressed and is associated with clinical characteristics in HCC. CD147 overexpressed in HCC has a poor prognosis. In addition, CD147 can predict the prognosis of HCC.
Based on our study and the reports described previously, we theorize that CD147 may be used as a novel biological indicator to predict the prognosis of HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ker CG, Taiwan; Perelli L, Italy S-Editor: Li L L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 2. | Yamashita T, Kaneko S. [Liver Cancer]. Rinsho Byori. 2016;64:787-796. [PubMed] |
| 3. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 736] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 5. | World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2018. IARC. 2020. [cited 13 June 2023]. Available from: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_groupearth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D. |
| 6. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5140] [Article Influence: 467.3] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236]. J Hepatol. 2019;70:817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3243] [Article Influence: 463.3] [Reference Citation Analysis (1)] |
| 9. | Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018;25:1073274817744621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 10. | Hahn JN, Kaushik DK, Yong VW. The role of EMMPRIN in T cell biology and immunological diseases. J Leukoc Biol. 2015;98:33-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Kaname T, Miyauchi T, Kuwano A, Matsuda Y, Muramatsu T, Kajii T. Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3. Cytogenet Cell Genet. 1993;64:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Landras A, Reger de Moura C, Jouenne F, Lebbe C, Menashi S, Mourah S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Liao CG, Kong LM, Song F, Xing JL, Wang LX, Sun ZJ, Tang H, Yao H, Zhang Y, Wang L, Wang Y, Yang XM, Li Y, Chen ZN. Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol Cell Biol. 2011;31:2591-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323-11330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Imai K. [Matrix metalloproteinases and cancer cell invasion and metastasis]. Tanpakushitsu Kakusan Koso. 1997;42:1694-1700. [PubMed] |
| 17. | Kelly T, Yan Y, Osborne RL, Athota AB, Rozypal TL, Colclasure JC, Chu WS. Proteolysis of extracellular matrix by invadopodia facilitates human breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin Exp Metastasis. 1998;16:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Xin X, Zeng X, Gu H, Li M, Tan H, Jin Z, Hua T, Shi R, Wang H. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci Rep. 2016;6:32804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Hu X, Su J, Zhou Y, Xie X, Peng C, Yuan Z, Chen X. Repressing CD147 is a novel therapeutic strategy for malignant melanoma. Oncotarget. 2017;8:25806-25813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Tseng HC, Xiong W, Badeti S, Yang Y, Ma M, Liu T, Ramos CA, Dotti G, Fritzky L, Jiang JG, Yi Q, Guarrera J, Zong WX, Liu C, Liu D. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020;11:4810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 21. | Gao L, Nie X, Gou R, Qi Y, Liu J, Lin B. Interaction of CD147 and human epididymis protein 4 promotes invasion and metastasis of ovarian cancer. J Cancer. 2021;12:7422-7435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé C, Menashi S, Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009;114:5547-5556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016;159:481-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 24. | Suzuki S, Toyoma S, Kawasaki Y, Nanjo H, Yamada T. CD147 promotes invasion and MMP-9 expression through MEK signaling and predicts poor prognosis in hypopharyngeal squamous cell carcinoma. Adv Clin Exp Med. 2021;30:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Pope ED 3rd, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA 3rd, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Jin J, Wang SJ, Cui J, Li L, Li JY, Liu FL, Sun XX, Jiang JL, Cui HY, Chen ZN. Hypo-phosphorylated CD147 promotes migration and invasion of hepatocellular carcinoma cells and predicts a poor prognosis. Cell Oncol (Dordr). 2019;42:537-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Guo W, Abudumijiti H, Xu L, Hasim A. CD147 promotes cervical cancer migration and invasion by up-regulating fatty acid synthase expression. Int J Clin Exp Pathol. 2019;12:4280-4288. [PubMed] |
| 28. | Pan S, Su Y, Sun B, Hao R, Gao X, Han B. Knockout of CD147 inhibits the proliferation, invasion, and drug resistance of human oral cancer CAL27 cells in Vitro and in Vivo. Int J Biol Macromol. 2021;181:378-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Liao CG, Liang XH, Ke Y, Yao L, Liu M, Liu ZK, He L, Guo YX, Bian H, Chen ZN, Kong LM. Active demethylation upregulates CD147 expression promoting non-small cell lung cancer invasion and metastasis. Oncogene. 2022;41:1780-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Zhang W, Liu H, Jiang J, Yang Y, Wang W, Jia Z. Expression of CD147 after neoadjuvant chemotherapy and its relationship with prognosis in patients with triple negative breast cancer. Am J Transl Res. 2022;14:2952-2961. [PubMed] |
| 31. | Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C, Daniele T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 32. | Zhang Z, Zhang Y, Sun Q, Feng F, Huhe M, Mi L, Chen Z. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol Cancer Ther. 2015;14:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Zhang RY, Wei D, Liu ZK, Yong YL, Wei W, Zhang ZY, Lv JJ, Zhang Z, Chen ZN, Bian H. Doxycycline Inducible Chimeric Antigen Receptor T Cells Targeting CD147 for Hepatocellular Carcinoma Therapy. Front Cell Dev Biol. 2019;7:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |