Published online Jul 15, 2023. doi: 10.4251/wjgo.v15.i7.1283
Peer-review started: March 27, 2023
First decision: May 4, 2023
Revised: May 17, 2023
Accepted: June 8, 2023
Article in press: June 8, 2023
Published online: July 15, 2023
Processing time: 107 Days and 3.3 Hours
Obstruction or fullness after feeding is common in gastric cancer (GC) patients, affecting their nutritional status and quality of life. Patients with digestive obstruction are generally in a more advanced stage. Existing methods, including palliative gastrectomy, gastrojejunostomy, endoluminal stent, jejunal nutrition tube and intravenous chemotherapy, have limitations in treating these symptoms.
To analyze the efficacy of continuous gastric artery infusion chemotherapy (cGAIC) in relieving digestive obstruction in patients with advanced GC.
This study was a retrospective study. Twenty-nine patients with digestive obstruction of advanced GC who underwent at least one cycle of treatment were reviewed at The Second Affiliated Hospital of Zhejiang University School of Medicine. The oxaliplatin-based intra-arterial infusion regimen was applied in all patients. Mild systemic chemotherapy was used in combination with local treatment. The clinical response was evaluated by contrast-enhanced computed tomography using Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Digestive tract symptoms and toxic effects were analyzed regularly. A comparison of the Karnofsky Performance Status (KPS) score and Stooler’s Dysphagia Score before and after therapy was made. Univariate survival analysis and multivariate survival analysis were also performed to explore the key factors affecting patient survival.
All patients finished cGAIC successfully without microcatheter displacement, as confirmed by arteriography. The median follow-up time was 24 mo (95%CI: 20.24-27.76 mo). The overall response rate was 89.7% after cGAIC according to the RECIST criteria. The postoperative Stooler’s Dysphagia Score was significantly improved. Twenty-two (75.9%) of the 29 patients experienced relief of digestive obstruction after the first two cycles, and 13 (44.8%) initially unresectable patients were then considered radically resectable. The median overall survival time (mOS) was 16 mo (95%CI: 9.32-22.68 mo). Patients who received radical surgery had a significantly longer mOS than other patients (P value < 0.001). Multivariate Cox regression analysis indicated that radical resection after cGAIC, intravenous chemotherapy after cGAIC, and immunotherapy after cGAIC were independent predictors of mOS. None of the patients stopped treatment because of adverse events.
cGAIC was effective and safe in relieving digestive obstruction in advanced GC, and it could improve surgical conversion possibility and survival time.
Core Tip: This was a retrospective study to evaluate the effectiveness of continuous gastric artery infusion chemotherapy (cGAIC) in relieving digestive obstruction in advanced gastric cancer patients. The overall response rate was 89.7% after cGAIC. A total of 75.9% of patients experienced relief of digestive obstruction after the first two cycles, and 44.8% of initially unresectable patients were then considered radically resectable. The median overall survival was 16 mo. C-arm computed tomography angiography helped to precisely confirm the tumor-feeding artery. Our new treatment can not only help relieve patients with digestive obstruction but also provide a good prognosis in treating tumors.
- Citation: Tang R, Chen GF, Jin K, Zhang GQ, Wu JJ, Han SG, Li B, Chao M. Efficacy of continuous gastric artery infusion chemotherapy in relieving digestive obstruction in advanced gastric cancer. World J Gastrointest Oncol 2023; 15(7): 1283-1294
- URL: https://www.wjgnet.com/1948-5204/full/v15/i7/1283.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i7.1283
Gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third most common cause of cancer-related death worldwide[1]. Due to the insidious onset, 80%-90% of patients in China are initially diagnosed at an advanced stage without the possibility of radical surgery. The poor prognosis objective response rate (ORR) varies from 29% to 47%, and the median overall survival time (mOS) ranges from 7.2 to 14.6 mo[2]. Obstruction or fullness after feeding is common, occurring in 31.6% of GC patients, affecting their nutritional status and quality of life[3]. Chen et al[3] found that GC with digestive obstruction was more aggressive and metastatic, indicating that patients with digestive obstruction are generally in a more advanced stage. Therefore, relieving these symptoms and resuming oral feeding as much as possible have become the main therapeutic aim of patients with advanced GC accompanied by digestive obstruction.
National Comprehensive Cancer Network (NCCN) gastric cancer guidelines and Japanese gastric cancer treatment guidelines recommend that intravenous chemotherapy should be considered first in patients with advanced GC, while palliative gastrectomy, gastrojejunostomy, endoluminal stent, jejunal nutrition tube and other treatments are available to relieve digestive obstruction[1,4]. However, surgical operations are not suitable for patients in poor general condition. Despite the efficacy of endoluminal stents and jejunal nutritional tubes, the incidence of postoperative complications is high, such as stent displacement (16%-36%) and restenosis (17%-36%)[5]. In addition, no significant oral feeding improvement was obtained in more than 50% of patients following radiotherapy[6]. Previous studies found the potential role of chemotherapy in relieving digestive obstruction[7,8]. Neoadjuvant chemotherapy can also decrease the stage (40.7%-73.3%) in patients with locally advanced GC[9-12]. However, systemic intravenous chemotherapy is hardly tolerated by patients in poor condition.
In recent years, the usage of intra-arterial chemotherapy has gradually increased as an alternative to intravenous chemotherapy, such as hepatic artery infusion chemotherapy[13]. Research has shown mild adverse effects and significant efficacy, with better tolerance than intravenous chemotherapy[9,11,14]. It has also achieved encouraging results (ORR: 59.5%-85.4%, mOS: 9-30 mo) in patients with advanced GC[15-17]. However, no studies on relieving digestive obstruction have been reported. Thus, this study retrospectively analyzed patients with advanced GC-induced cardia or pyloric obstruction treated by constant gastric artery infusion chemotherapy (cGAIC). This study aimed to assess the safety and efficacy of this new method in relieving digestive obstruction.
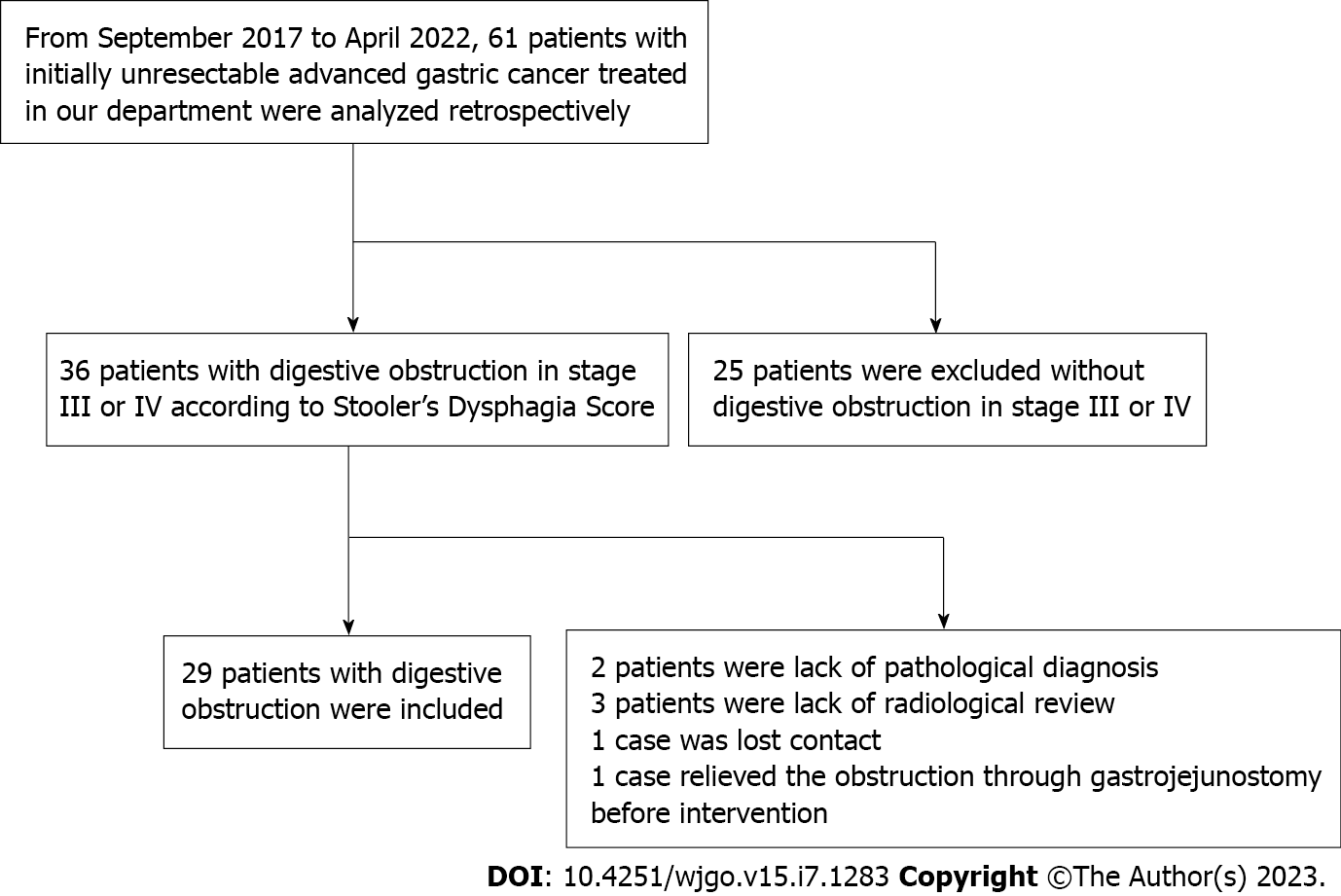
From September 2017 to April 2022, a total of 61 patients with advanced GC were analyzed retrospectively. Among them, 25 patients without obvious digestive obstruction symptoms were excluded. Of the remaining 36 patients, 6 were excluded because of inadequate follow-up, of which 2 lacked a pathological diagnosis, 3 did not undergo radiological review, and 1 lost contact. In addition, 1 case was removed due to having to relieve the obstruction through gastrojejunostomy before intervention. Finally, twenty-nine advanced GC-induced digestive obstruction patients (20 male, 9 female, aged 45-82 years, mean 64.28 ± 8.49 years) who were unable to undergo radical surgery or failed to respond to radiotherapy or intravenous chemotherapy within a sufficient observation time were eligible for this study. All patients passed the examination and approval of the Ethics Committee of The Second Affiliated Hospital of Zhejiang University School of Medicine (Approval No. I2020001737) and gave signed informed consent. All patients were diagnosed by endoscopy and confirmed by pathology. In this study, advanced GC included locally advanced unresectable GC or metastatic GC. Clinical tumor-node-metastasis (cTNM) stage distribution was confirmed by contrast-enhanced computed tomography (CT) according to the 8th edition American Joint Committee and Union International Center Cancer (AJCC/UICC)[18]. Details of the patient selection processes are shown in Figure 1.
Patients with advanced GC who met the following criteria were included: (1) Experienced obstruction or fullness after feeding in stage III or IV according to Stooler’s Dysphagia Score; (2) had a Karnofsky Performance Status (KPS) score > 50; (3) had initially unresectable advanced GC according to a gastrointestinal surgeon; (4) had no indication for further radical surgery; and (5) had no contraindications for interventional therapy and chemotherapy.
Patients with inadequate follow-up, acute infection, severe liver and kidney dysfunction or blood coagulation disturbance and pregnant and breastfeeding women were excluded.
The framework of the chemotherapy regimen is based on the SOX (S-1 and oxaliplatin) intravenous chemotherapy regimen according to The NCCN Clinical Practice Guidelines for Gastric Cancer. In this study, the treatment regimen consisted of intra-arterial infusion of oxaliplatin 100 mg in 5 h and oral S-1 40 mg/m2 twice a day for 14 d[14,19,20]. 5-Fluorouracil 2600 mg/m2 d1 was administered intravenously if the patients could not take S-1 orally. Symptomatic antiemetic treatment and rehydration were used postcGAIC. Laboratory examination and abdominal contrast-enhanced CT were performed after 2 wk[21]. The KPS score and Stooler’s Dysphagia Score were obtained from telephone follow-up after leaving the hospital. This cycle was repeated once every 3 wk.
Interventional treatments were performed by two interventional radiologists with more than 10 years of experience. After femoral artery puncture using Seldinger’s approach, a microcatheter was used for angiography of possible tumor-feeding arteries, including the left gastric artery, right gastric artery, right gastroepiploic artery or anomalous origin of the gastric artery. C-arm computed tomographic angiography (CACTA) during interventional operation was then used to confirm the tumor-feeding artery according to preoperative abdominal contrast-enhanced CT. When selection of the tumor-feeding artery was complete, a vascular sheath and catheters were fixed to the skin, and a mixture of oxaliplatin 100 mg and 250 mL 5% glucose solution was infused by a chemotherapy pump over 5 h (50 mL/h). If 2 tumor-feeding arteries existed, 2 microcatheters were placed at the same time through the bilateral femoral artery, and oxaliplatin 50 mg was equally infused into these arteries (Table 1). After infusion, angiography was performed again to confirm the location of the microcatheter.
| Infusion area | Gastroesophageal junction obstruction, n | Pyloric obstruction, n | Anastomosis obstruction, n |
| Left gastric artery | 13 | 5 | 1 |
| Right gastric artery | 1 | 3 | 0 |
| Right gastroepiploic artery | 0 | 13 | 2 |
| Left gastric artery and right gastroepiploic artery | 5 | 0 | 0 |
| Right gastric artery and right gastroepiploic artery | 0 | 23 | 0 |
| Left gastric artery and right gastric artery | 1 | 1 | 0 |
| Others | 71 | 62 | 12 |
The cycles of cGAIC depended on the following stop criteria: (1) The patient could not tolerate continuing chemotherapy; (2) the patient was evaluated as having progressive disease after 2 cycles of cGAICs; (3) the patient was evaluated as having a complete response after cGAIC; (4) the initially unresectable patient was considered resectable and received radical distal subtotal gastrectomy; and (5) the digestive obstruction was relieved, but the patient was still not resectable, and palliative treatment continued.
Radiology responses were assessed by two experienced radiologists independent from the clinical teams. Response Evaluation Criteria In Solid Tumors (RECIST) criteria were used to evaluate tumor response[22]. Complete disappearance of the tumor was considered to be complete response (CR); at least 30% decrease in tumor size was defined as partial response (PR); at least 20% increase in tumor size was defined as progressive disease (PD); and neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease was considered stable disease (SD). KPS scores from 0 to 100 were used to reflect general physical condition. Digestive obstruction was divided into five levels according to Stooler’s Dysphagia Score: 0, normal swallowing; I, semidry food; II, soft food; III, fluid; and IV, completely unable to feed orally. Adverse events in this study included postoperative nausea and vomiting, abdominal pain, gastrointestinal hemorrhage, bone marrow hypocellular, paresthesia and increased levels of liver enzyme. Adverse events were assessed according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Comparison of the KPS score and Stooler’s Dysphagia Score before and after therapy was performed using the Wilcoxon signed-rank test. Comparisons of parameter variables were performed using the paired samples test. Univariate survival analysis was performed using the Kaplan-Meier method, and multivariate survival analysis was performed using a Cox regression model. Factors with P < 0.05 in univariate survival analysis were included in the Cox regression model. Statistical analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, United States). Two-tailed P < 0.05 was considered significant.
Among all patients, the median follow-up time was 24 mo (95%CI: 20.24-27.76 mo). All patients were given a total of 82 cGAIC cycles, and the median number of cycles was 3 (1-6). Of these patients, 6 had gastroesophageal junction cancer (Siewert III), 2 had recurrent tumors on anastomosis, and the remaining 21 had GC in the pylorus. During the follow-up, 13 (44.8%) initially unresectable patients were still unresectable and received radical distal subtotal gastrectomy (Billroth II) combined with D2 lymph node dissection, including 1, 3, 4 sb, 5, 6, 7, 8a, 9, 11p, 12a, and 14 V if necessary. Further postoperative intravenous chemotherapy was applied in these 12 patients, excluding 1 patient with poor health status after gastrectomy. Metastatic disease is mainly treated by systemic therapy, including intravenous chemotherapy or immunotherapy. Only in a few patients were implantation metastases dissected for biopsy; details are shown in the Supplementary Table 1. Other patients continued to carry immune checkpoint inhibitor treatment only (2 patients), intravenous chemotherapy only (5 patients), immune checkpoint inhibitors combined with intravenous chemotherapy (2 patients), best supportive care (5 patients), or gave up further treatments for poor health status and death (2 patients). All patients finished cGAIC successfully without microcatheter displacement, as confirmed by arteriography.
None of the twenty-nine patients showed PD after the treatment evaluated by the RECIST criteria, while the overall response (OR) was 89.7%, and the objective response rate (ORR) was 86.2% after the first cGAIC, 88.9% after the second cGAIC, 84.2% after the third cGAIC, and 100% after the 4-6th cycles of cGAIC by the RECIST criteria (Table 2).
| After the first cGAIC, n (%) | After the second cGAIC, n (%) | After the third cGAIC, n (%) | After the fourth cGAIC, n (%) | After the fifth cGAIC, n (%) | After the sixth cGAIC, n (%) | |
| CR | 0 (0) | 1 (3.7) | 4 (21.1) | 0 (0) | 0 (0) | 0 (0) |
| PR | 25 (86.2) | 23 (85.2) | 12 (63.2) | 3 (100) | 2 (100) | 2 (100) |
| SD | 4 (13.8) | 3 (11.1) | 3 (15.8) | 0 (0) | 0 (0) | 0 (0) |
| PD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 29 | 27 | 19 | 3 | 2 | 2 |
Significant differences were found in Stooler’s Dysphagia Score before and after treatment (before: 3.24 ± 0.43, after: 2.13 ± 0.78, P value < 0.001). Nineteen patients experienced relief of digestive obstruction after the first cGAIC, 3 patients experienced relief of digestive obstruction after the second cGAIC, and 7 patients did not recover from digestive obstruction (Table 3). Two temporarily recovered patients developed obstruction again after the third cGAIC. The KPS (before: 61.79 ± 6.58, after: 71.43 ± 5.15, P value < 0.001) score increased after cGAIC.
| Before cGAIC, n | After cGAIC, n | P value | |
| Stooler’s Dysphagia Score | < 0.001 | ||
| I | 0 | 6 | |
| II | 0 | 14 | |
| III | 22 | 8 | |
| IV | 7 | 1 | |
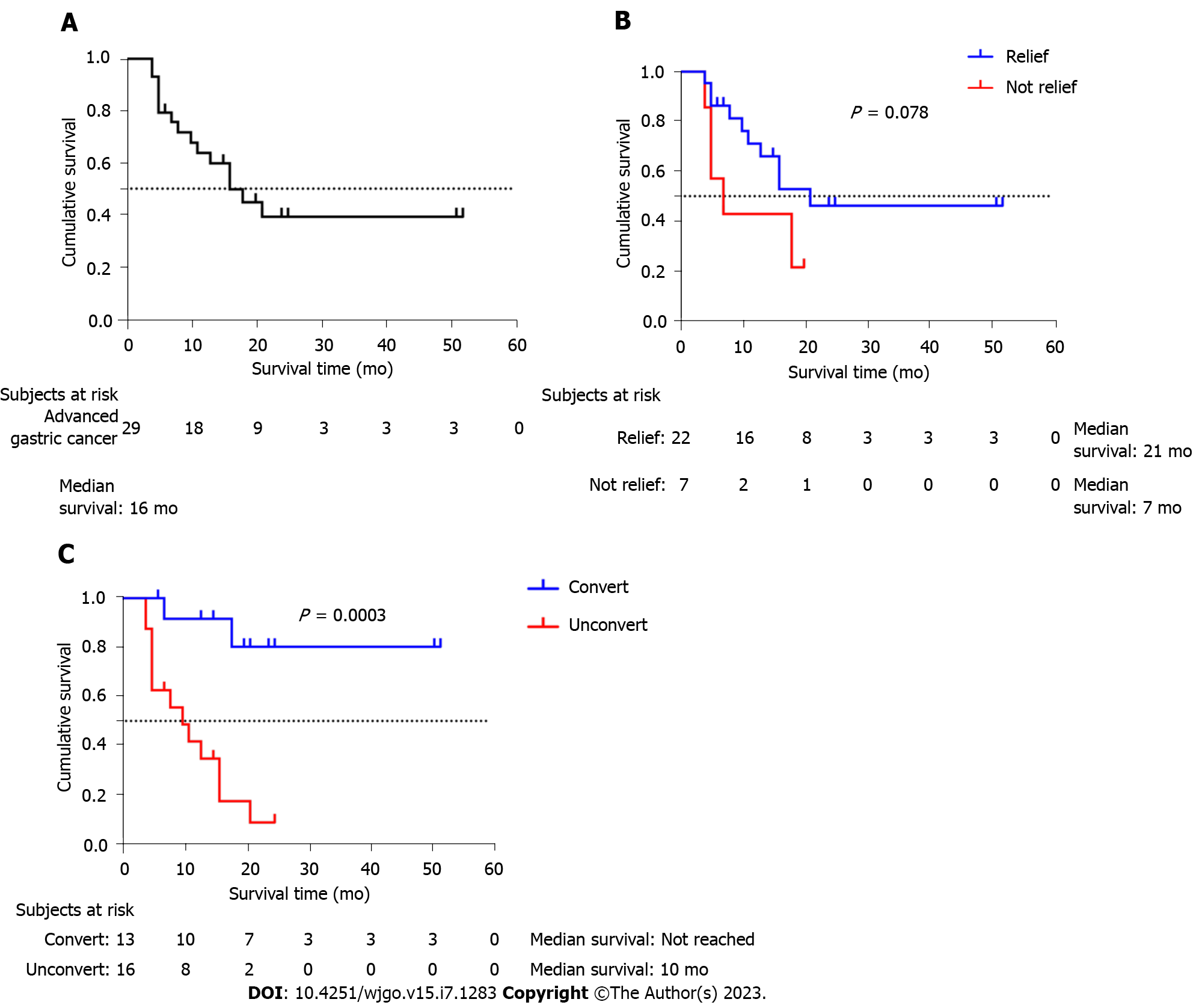
The median overall survival time (mOS) was 16 mo (95%CI: 9.32-22.68 mo, Figure 2A). Subgroup analysis showed that patients who were relieved of obstruction had a slightly longer mOS than patients who were not (21 vs 7 mo, P = 0.078, Figure 2B). Patients who received radical surgery had a significantly longer mOS than other patients (unobtained for low follow-up time vs 10 mo, P value < 0.001, Figure 2C).
In the exploratory univariate analysis shown in Table 4, there were significant associations between mOS and intravenous chemotherapy history, pathology, radical resection after cGAIC, intravenous chemotherapy after cGAIC, and immunotherapy after cGAIC (all P < 0.05). In addition, multivariate Cox regression analysis indicated that radical resection after cGAIC, intravenous chemotherapy after cGAIC, and immunotherapy after cGAIC were independent predictors of mOS (all P < 0.05). We also observed that relieving digestive obstruction was not significantly associated with mOS.
| Variables | n (%) | Univariate survival analysis | Multivariate survival analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Gender | |||||||
| Male | 20 (69) | 1.53 | 0.49-4.85 | 0.466 | |||
| Female | 9 (31) | 1 | |||||
| Intravenous chemotherapy history | |||||||
| No | 22 (76) | 0.30 | 0.11-0.83 | 0.020 | |||
| Yes | 7 (24) | 1 | |||||
| Gastric cancer surgery history | |||||||
| No | 27 (93) | 0.51 | 0.12-2.29 | 0.381 | |||
| Yes | 2 (7) | 1 | |||||
| Pathology | |||||||
| Squamous cell carcinoma | 2 (7) | 9.24 | 1.62-52.69 | 0.012 | |||
| Signet ring cell carcinoma | 8 (27) | 0.66 | 0.18-2.44 | 0.535 | |||
| Adenocarcinoma | 19 (66) | 1 | |||||
| Tumor invasion | |||||||
| ≤ T4a | 19 (66) | 0.48 | 0.17-1.32 | 0.153 | |||
| T4b | 10 (34) | 1 | |||||
| Metastasis | |||||||
| No | 9 (31) | 0.63 | 0.20-1.98 | 0.425 | |||
| Yes | 20 (69) | ||||||
| Obstructive location | |||||||
| Gastroesophageal junction | 6 (21) | 1.21 | 2.24-6.10 | 0.816 | |||
| Pylorus | 21 (72) | 0.35 | 0.07-1.68 | 0.188 | |||
| Anastomosis | 2 (7) | 1 | |||||
| Stooler’s Dysphagia Score | |||||||
| III | 22 (76) | 0.34 | 0.11-1.02 | 0.055 | |||
| IV | 7 (24) | 1 | |||||
| Relieve digestive obstruction | |||||||
| No | 7 (24) | 2.55 | 0.84-7.72 | 0.098 | |||
| Yes | 22 (76) | ||||||
| Radical resection after cGAIC | |||||||
| No | 16 (55) | 9.56 | 2.11-43.44 | 0.003 | 8.48 | 1.27-56.51 | 0.027 |
| Yes | 13 (45) | ||||||
| Intravenous chemotherapy after cGAIC | |||||||
| No | 10(34) | 6.20 | 2.14-18.00 | 0.001 | 8.61 | 1.42-52.17 | 0.019 |
| Yes | 19(66) | ||||||
| Immunotherapy after cGAIC | |||||||
| No | 18(62) | 4.12 | 0.93-18.28 | 0.063 | 13.09 | 1.64-104-12 | 0.015 |
| Yes | 11(38) | ||||||
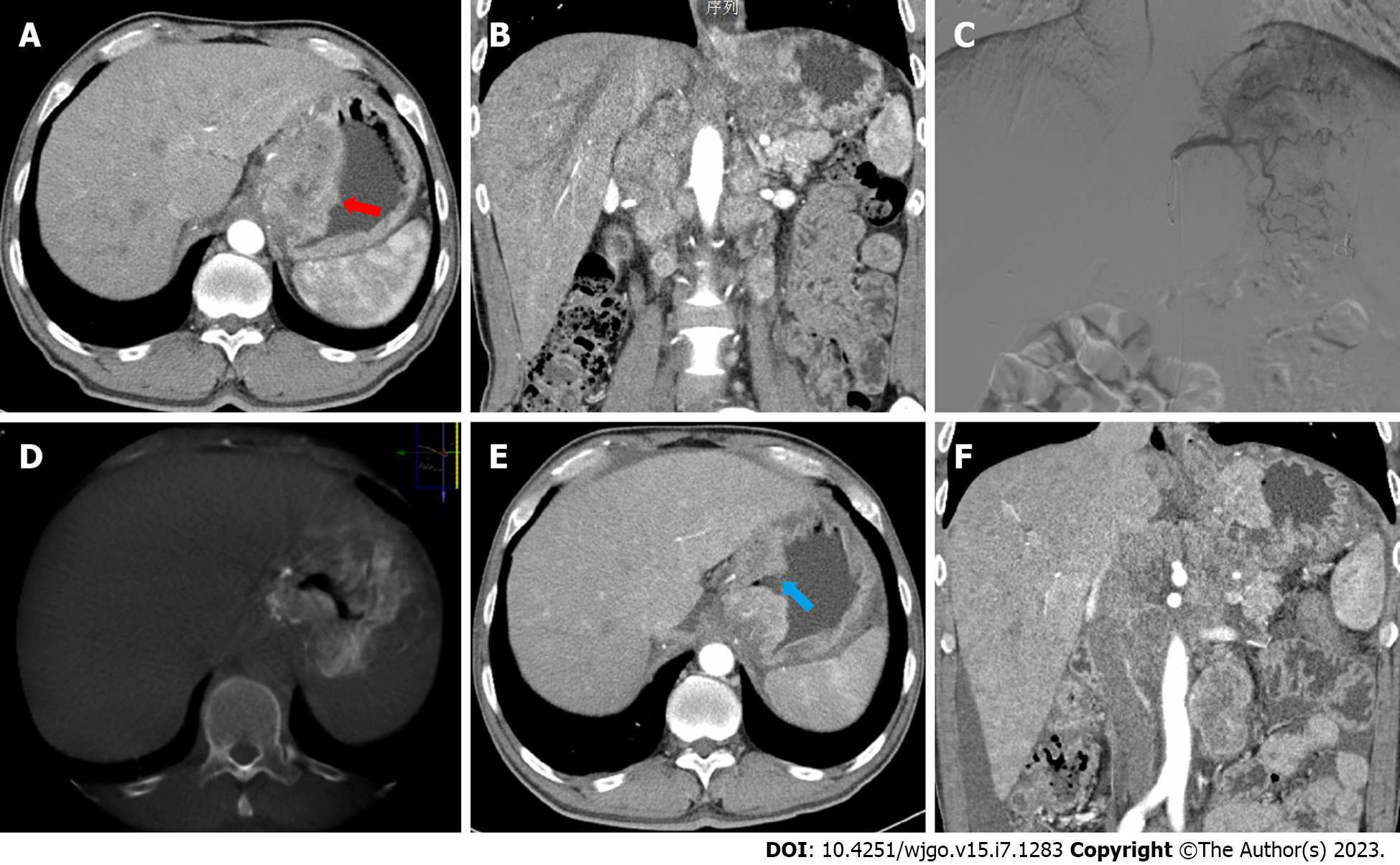
Obvious imaging changes were found in the review of abdominal contrast-enhanced CT compared with preoperative CT. Pyloric obstruction or gastroesophageal junction obstruction (Figure 3A and B) manifested as thickening of the gastric wall, blurring of the boundary, and narrowing or disappearance of the digestive tract. After the first cGAIC, the abnormally thick gastric wall became thin, the blurred boundary became clear, and the blocked digestive tract opened again (Figure 3E and F).
Adverse events after intra-arterial infusion chemotherapy show a similar representation but a lower degree compared with those in intravenous chemotherapy in past studies (Table 5). No serious complications, such as gastrointestinal perforation or hemorrhage, were found. All the chemotherapy-related adverse events during cGAIC were not greater than grade 2 according to CTCAE and disappeared rapidly in the following week. None of the patients stopped treatment because of severe adverse events.
| Adverse events | Grade 1, n (%) | Grade 2, n (%) |
| Nausea and vomiting | 4 (13.8) | 2 (6.9) |
| Abdominal pain | 3 (10.3) | 1 (3.4) |
| Gastrointestinal hemorrhage | 0 (0) | 0 (0) |
| Neutropenia | 2 (6.9) | 2 (6.9) |
| Anemia | 1 (3.4) | 0 (0) |
| Thrombocytopenia | 2 (6.9) | 2 (6.9) |
| Paresthesia | 1 (3.4) | 1 (4.3) |
| Liver enzyme increased | 1 (3.4) | 3 (10.3) |
The efficacy and safety of intra-arterial infusion chemotherapy have been confirmed in the treatment of advanced GC by inhibiting cell proliferation and inducing apoptosis. Zhang et al[16] found that preoperative intra-arterial infusion chemotherapy was an independent factor for the long-term survival of patients with advanced GC. Zhang et al[17] found an mOS of 25 mo in advanced GC while using intra-arterial chemotherapy, which was better than the mOS of 9 mo when using single intravenous chemotherapy. In addition, studies have shown that weight loss or malnutrition during neoadjuvant chemotherapy are independent risk factors for the effect, which indicates the importance of relieving digestive obstruction[23]. However, relief of digestive obstruction is not mentioned in the previous literature, and the intra-arterial infusion chemotherapy regimen lacks standardized guidance. Therefore, this study aimed to explore the efficacy and safety of cGAIC in advanced GC patients with digestive obstruction.
After the first treatment, patients immediately achieved an 86.2% ORR, which is better than the ORR (29%-47%) in a previous study in advanced GC with digestive obstruction, and no patient exhibited disease progression in the following treatment[1]. In addition, the clinical symptoms of digestive obstruction were relieved quickly in the follow-up visits, indicated by most patients beginning to resume oral feeding, which was an encouraging result for advanced GC patients with poor oral feeding. The KPS score was also improved compared with that before treatment, with better quality of life. No patients stopped treatment because of severe adverse events, which represented good clinical compliance. There may be some key factors in our regimen that are important for the satisfactory clinical effect.
The first key factor is to infuse the tumor-feeding artery accurately using CACTA. In recent studies, the infusion area of intra-arterial chemotherapy was chosen empirically according to the tumor location instead of being guided by CACTA. This may lead to choosing the wrong infusion area, resulting in weak responses compared with intravenous chemotherapy and unnecessary adverse reactions such as ischemia and ulcers. Tao et al[24] and Ji et al[25] found that the clear tumor blood supply in GC is an independent risk factor for OS. Wang et al[26] also confirmed that distinct tumor staining intraoperatively could affect prognosis. Therefore, it is important to place the microcatheter in the right location. In this study, the combination of intraoperative CACTA with preoperative contrast-enhanced CT may help with accurate treatment (Figure 3C and D) and avoid injuring important organs. For example, we should place the microcatheter in the right gastroepiploic artery when possible and avoid the pancreaticoduodenal artery and duodenal artery to prevent pancreatitis or duodenal ulcers. One case in this study failed to relieve digestive obstruction because of an error in evaluating the dual feeding artery. In the following treatment, both arteries were infused, and the obstruction was relieved.
Another reason contributing to the better treatment may be the infusion time. Compared to 2-6 h continuous infusion in standard intravenous chemotherapy regimens, we infused concentration-dependent drugs through the arterial pathway persistently by a chemotherapy pump in 5 h. In previous studies, drugs were injected into the target area within a few minutes to produce a higher local drug concentration, which is simple and convenient but is not conducive to maintaining blood concentration for a long period[15]. In the postoperative biopsy in Zhang et al’s study, they found gastric mucosal necrosis and scarring in the area of intra-arterial infusion treatment[17]. A high concentration of drugs was confirmed in blood samples from the portal vein by the left gastric intra-arterial route, which was 4-40-fold of the group by intravenous administration[26]. This higher local drug concentration may lead to arterial inflammation, promoting tumor cell ischemia and necrosis. A constant higher drug concentration may induce tumor cell apoptosis more effectively, which may be the pathophysiological mechanism of this treatment.
Therefore, in our accurate and constant intra-arterial infusion chemotherapy, the mOS was 16 mo, which was greater than expected. Because cGAIC has a high efficiency in shrinking the local lesion in the cardia or pylorus, the obstruction symptoms can be quickly relieved in the first two cycles of treatment in most patients, which results in a better nutritional status. In addition, 13 unresectable patients were then considered resectable and had an obviously longer median OS time than the others (P < 0.001, Figure 2C). Furthermore, based on the effectiveness of local treatment, a single oral or intravenous chemotherapy drug was used in combination. This combination obtained better regional benefits and systemic control in distant metastasis and reduced the dosage of chemotherapy drugs and adverse events. Thus, locally advanced GC patients could acquire higher surgical conversion possibilities and better prognoses.
In another aspect from multivariate Cox regression analysis, appropriate treatments after cGAIC were more relevant with a higher mOS, including radical resection, intravenous chemotherapy and immunotherapy after cGAIC. Although digestive obstruction symptoms of 76% of patients were relieved and life quality and nutritional status improved, relieving digestive obstruction did not become an independent predictor for long-term survival because cGAIC only made up a part of the whole comprehensive treatment. We believe that this treatment indirectly improved survival by improving the radical surgery rate. Two patients with squamous cell carcinoma were also treated with this treatment. In the following univariate survival analysis, this pathological type had a higher hazard ratio than signet ring cell carcinoma and adenocarcinoma, which demonstrated that this treatment was more suitable for advanced gastric or gastroesophageal junction cancer (Siewert type III). In addition, we found that patients with no chemotherapy history were more likely to benefit from cGAIC.
Although intra-arterial chemotherapy has been suggested to be safe and effective, there is still controversy surrounding chemotherapeutic drug selection and the speed of administration[27]. In this study, fluorouracil plus oxaliplatin was used as the preferred chemotherapy regimen for GC recommended by the NCCN, while the dosage of oxaliplatin was reduced by half for patients in poor general condition. A few patients with mild gastrointestinal reactions and leukopenia quickly returned to normal after symptomatic treatment.
This study was limited by the small sample size and short observation period. As a new effective method in theory, we did not set a control group at the same period, so we can only compare the effectiveness with the external control group. Moreover, this was a retrospective analysis but not prospective randomized, which may cause selection and recall biases. In addition, we did not embolize the tumor-feeding artery. In addition, the optimal cycles of cGAIC need to be explored in the future. The safety of embolization needs to be verified in randomized trials to avoid the occurrence of serious adverse effects, such as ischemia, ulcer, perforation and bleeding.
In conclusion, this study preliminarily demonstrated the efficacy and safety of cGAIC in relieving digestive obstruction in advanced GC, which improved radical resection possibility after cGAIC and survival time. Intraoperative CACTA can help with precise definition of the perfusion area.
Patients with digestive obstruction generally have advanced gastric cancer, affecting their quality of life and survival.
Existing methods cannot relieve digestive obstruction very well.
Continuous gastric artery infusion chemotherapy (cGAIC) was effective and safe in relieving digestive obstruction.
Twenty-nine patients with digestive obstruction of advanced gastric cancer treated by cGAIC were reviewed retrospectively. Interventional treatments combined with C-arm computed tomographic angiography were performed to accurately infuse oxaliplatin into the tumor-feeding artery. Radiology responses, Stooler’s Dysphagia Score and toxic effects were evaluated.
The overall response rate was 89.7% after cGAIC. The postoperative Stooler’s Dysphagia Score was significantly reduced. Twenty-two (75.9%) of the 29 patients experienced relief of digestive obstruction after the first two cycles, and 13 (44.8%) initially unresectable patients were then considered radically resectable. The median overall survival time was 16 mo.
The efficacy and safety of cGAIC in relieving digestive obstruction were demonstrated, and cGAIC could improve radical resection after cGAIC and survival time. Intraoperative CACTA can help with precise definition of the perfusion area.
To improve the efficacy of chemotherapy using interventional methods.
We gratefully acknowledge the assistance of all the patients and their families volunteer to participate in the clinical study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Augustin G, Croatia; Kawabata H, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 678] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 2. | Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer. 2016;35:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Chen JH, Wu CW, Lo SS, Li AF, Hsieh MC, Shen KH, Lui WY. Outcome of distal gastric cancer with pyloric stenosis after curative resection. Eur J Surg Oncol. 2007;33:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 5. | Jain P. Self-expanding metallic esophageal stents: a long way to go before a particular stent can be recommended. World J Gastroenterol. 2011;17:5327-5328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tey J, Zheng H, Soon YY, Leong CN, Koh WY, Lim K, So JBY, Shabbir A, Tham IWK, Lu J. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med. 2019;8:1447-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Jiao X, Zhou Y. Investigation of the potential role of preoperative chemotherapy in treatment for gastric cancer with outlet obstruction. Mol Clin Oncol. 2015;3:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Long B, Yu ZY, Li Q, Du HR, Wang ZJ, Zhan H, Jiao ZY. A systematic review and network meta-analysis protocol of neoadjuvant treatments for patients with gastric cancer. Medicine (Baltimore). 2018;97:e0392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1648] [Article Influence: 274.7] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J; RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 11. | Guo H, Ding P, Sun C, Yang P, Tian Y, Liu Y, Lowe S, Bentley R, Li Y, Zhang Z, Wang D, Zhao Q. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: A single-arm, open-label, phase II trial. Front Oncol. 2022;12:927781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 12. | Newman E, Marcus SG, Potmesil M, Sewak S, Yee H, Sorich J, Hayek M, Muggia F, Hochster H. Neoadjuvant chemotherapy with CPT-11 and cisplatin downstages locally advanced gastric cancer. J Gastrointest Surg. 2002;6:212-23; discussion 223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Ouyang G, Pan G, Xu H, Wu Y, Liu Z, Lu W, Yi B, Chen X. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy in Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2020;54:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Satake H, Miki A, Kondo M, Kotake T, Okita Y, Hatachi Y, Yasui H, Imai Y, Ichikawa C, Murotani K, Hashida H, Kobayashi H, Kotaka M, Kato T, Kaihara S, Tsuji A. Phase I study of neoadjuvant chemotherapy with S-1 and oxaliplatin for locally advanced gastric cancer (Neo G-SOX PI). ESMO Open. 2017;2:e000130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Li Y, Chen J, He Q, Ji X, Wang X, Fan C, Li G. Clinical efficacy of neoadjuvant chemotherapy regimens FLEEOX vs. XELOX in patients with initially unresectable advanced gastric cancer: a propensity score analysis. Oncotarget. 2017;8:86886-86896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zhang CW, Zou SC, Shi D, Zhao DJ. Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer. World J Gastroenterol. 2004;10:3070-3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Zhang C, Li G, Fan C, Xu J, Cao J, Liu S, Li N. Comparison of efficacy of different route of administration of chemotherapy on unresectable, advanced gastric cancer. World J Surg Oncol. 2012;10:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 19. | Inoue K, Nakane Y, Kogire M, Fujitani K, Kimura Y, Imamura H, Tamura S, Okano S, Kwon AH, Kurokawa Y, Shimokawa T, Takiuchi H, Tsujinaka T, Furukawa H. Phase II trial of preoperative S-1 plus cisplatin followed by surgery for initially unresectable locally advanced gastric cancer. Eur J Surg Oncol. 2012;38:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Wang M, Wu M, Wang W, Wang Q, Wang Y. Docetexal plus S-1 versus oxaliplatin plus S-1 for first-line treatment of patients with advanced gastric cancer: a retrospective study. Oncol Res Treat. 2014;37:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kruk-Bachonko J, Krupski W, Czechowski M, Kurys-Denis E, Mądro P, Sierocińska-Sawa J, Dąbrowski A, Wallner G, Skoczylas T. Perfusion CT - A novel quantitative and qualitative imaging biomarker in gastric cancer. Eur J Radiol. 2017;95:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13078] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 23. | Jiang L, Ma Z, Ye X, Kang W, Yu J. Clinicopathological factors affecting the effect of neoadjuvant chemotherapy in patients with gastric cancer. World J Surg Oncol. 2021;19:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ji X, Yang Q, Qin H, Zhou J, Liu W. Tumor blood supply may predict neoadjuvant chemotherapy response and survival in patients with gastric cancer. J Int Med Res. 2019;47:2524-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Wang J, Shi H, Yang G, Han G, Zhao M, Duan X, Mi L, Han X, Li N, Shi J, Yin X, Yin F. Combined intra-arterial and intravenous chemotherapy for unresectable, advanced gastric cancer has an improved curative effect compared with intravenous chemotherapy only. Oncol Lett. 2018;15:5662-5670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Li M, Zhang J, Wang D, Zhong B, Tucker S, Lu C, Cheng J, Cao C, Xu J, Pan H. A phase II study of intra-arterial chemotherapy of 5-fluorouracil, cisplatin, and mitomycin C for advanced nonresectable gastric cancer. Anticancer Drugs. 2009;20:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |