Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.925
Peer-review started: February 20, 2023
First decision: April 2, 2023
Revised: April 21, 2023
Accepted: April 28, 2023
Article in press: April 28, 2023
Published online: June 15, 2023
Processing time: 114 Days and 10 Hours
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies and is developing into the 2nd leading cause of cancer-related death. Often, the clinical and radiological presentation of PDAC may be mirrored by other inflammatory pancreatic masses, such as autoimmune pancreatitis (AIP) and mass-forming chronic pancreatitis (MFCP), making its diagnosis challenging. Differentiating AIP and MFCP from PDAC is vital due to significant therapeutic and prognostic implications. Current diagnostic criteria and tools allow the precise differentiation of benign from malignant masses; however, the diagnostic accuracy is imperfect. Major pancreatic resections have been performed in AIP cases under initial suspicion of PDAC after a diagnostic approach failed to provide an accurate diagnosis. It is not unusual that after a thorough diagnostic evaluation, the clinician is confronted with a pancreatic mass with uncertain diagnosis. In those cases, a re-evaluation must be entertained, preferably by an experienced multispecialty team including radiologists, pathologists, gastroenterologists, and surgeons, looking for disease-specific clinical, imaging, and histological hallmarks or collateral evidence that could favor a specific diagnosis. Our aim is to describe current diagnostic limitations that hinder our ability to reach an accurate diagnosis among AIP, PDAC, and MFCP and to highlight those disease-specific clinical, radiological, serological, and histological characteristics that could support the presence of any of these three disorders when facing a pancreatic mass with uncertain diagnosis after an initial diagnostic approach has been unsuccessful.
Core Tip: This article describes the flaws and hurdles of current diagnostic tools as well as disease specific imaging, serological and histological characteristics that play a significant role in the differentiation of focal autoimmune pancreatitis, mass-forming chronic pancreatitis and pancreatic ductal adenocarcinoma.
- Citation: Tornel-Avelar AI, Velarde Ruiz-Velasco JA, Pelaez-Luna M. Pancreatic cancer, autoimmune or chronic pancreatitis, beyond tissue diagnosis: Collateral imaging and clinical characteristics may differentiate them. World J Gastrointest Oncol 2023; 15(6): 925-942
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/925.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.925
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies; its incidence almost matches its fatality rate and continues to increase. PDAC is on track to becoming the 2nd leading cause of cancer-related death. It affects both sexes equally, and its 5-year survival rate is less than 10%[1,2]. Although novel chemotherapy schemes increase its prognosis, surgery seems to be the only treatment that offers better survival rates[3-5]. Such a dismal picture prompts an accurate and early diagnosis of PDAC that may offer better outcomes.
In most cases, the diagnosis of PDAC offers no difficulty; however, different benign conditions, such as inflammatory masses [chronic pancreatitis (CP) and autoimmune pancreatitis (AIP)] as well as other malignant conditions with different treatments and prognoses (e.g., metastases, islet cell tumors, complex cystic lesions, pancreatoblastoma, pancreatic lymphoma, etc.) often resemble its clinical, biochemical, and imaging appearance, posing a diagnostic challenge[6].
The most common inflammatory conditions that present as a pancreatic mass include mass-forming CP (MFCP), which accounts for 10%-30% of all cases of CP, and focal AIP, which is a variant of the classic diffusely enlarged pancreas that has been widely described. Focal AIP accounts for 28%-41% of all AIP cases[7,8].
The ability to differentiate among these masses (PDAC, MFCP, and AIP) remains a challenge, as most of the time the clinical picture is confusing, and gross imaging features seem to be similar, which hinders accurate diagnosis and may result in unnecessary surgery regardless of their benign or malignant nature.
Differential diagnosis requires considerable expertise and the use of a myriad of diagnostic tools that include computed tomography (CT), which remains the most available and cost-effective technique to evaluate the pancreas. However, considering that up to 5% to 10% of all pancreatic neoplasms present a contrast enhancement pattern similar to the rest of the pancreatic parenchyma and that its diagnostic accuracy for small lesions decreases significantly, the use of other imaging techniques is often necessary.
Magnetic resonance imaging (MRI) offers a more accurate evaluation of the pancreatic parenchyma; it is able to visualize up to 5% to 10% of the issoatenuated pancreatic masses seen in CT scans, and is capable of revealing lesions smaller than 2 cm in size[9].
Endoscopic ultrasound (EUS) is another valuable tool; it provides high-resolution images of the pancreas, nearby organs, and vascularity and is capable of detecting small lesions and providing information on potential vascular and adjacent organ involvement. A significant drawback is that it remains operator-dependent, which accounts for its variable diagnostic accuracy.
The advent of new techniques such as dynamic MRI, which calculates and evaluates perfusion parameters, may increase the diagnostic yield and provide relevant information regarding chemo
In addition to the former imaging techniques, serologic markers, histologic examination, and in some cases, therapeutic trials (e.g., steroid trial for presumed AIP) can and have been entertained.
Although international consensuses and criteria on the definition, diagnosis, and treatment of AIP and CP have been developed and disease-specific characteristics and available diagnostic tools usually allow precise differentiation of AIP, CP, and PDAC or at least differentiation of a benign lesion from a malignant lesion, their diagnostic accuracy is imperfect. In a significant number of cases, regardless of a multidisciplinary approach involving access to high notch technology and clinical expertise, an accurate diagnosis of a pancreatic mass remains elusive, and major pancreatic resections in benign conditions such as AIP are still being performed. Some surgical series have reported that focal AIP has been diagnosed in up to 2% of surgical specimens from patients who underwent surgery for presumed PDAC.
Some groups advise that any resectable pancreatic mass should undergo surgery even if a thorough diagnostic evaluation has failed to prove the presence of malignancy. It should not be overlooked that although pancreatic surgery mortality rates in expert hands range from 1%-2%, comorbidity rates, especially those related to a Whipple procedure, are considerable (40%-50%).
Every effort leading to a prompt and accurate diagnosis to avoid surgical delays of a resectable PDAC that can rapidly become nonresectable as well as efforts to avoid unnecessary surgery and its related complications should be considered[1,3].
PDAC’s clinical picture consisting of abdominal pain, jaundice, weight loss, etc., is usually shared by AIP and MFCP among other conditions, and although diagnostic suspicion could increase based on imaging and serum characteristics, these are not exclusive to PDAC, and pathology confirmation remains mandatory.
Serum carbohydrate antigen 19-9 (Ca 19-9) sensitivity is low. Increased levels (> 37 U/mL) are not exclusive to pancreatic cancer, and they may occur in biliary and other gastrointestinal carcinomas (i.e., neuroendocrine tumors of the pancreas, cholangiocarcinoma, or hepatocarcinoma). Expert guidelines do not recommend it as a screening tool for pancreatic cancer, but it is helpful in patient’s follow-up and, in some cases, in the selection of potential surgical candidates with an otherwise resectable appearing mass[10].
Ca 19-9 serum levels may increase in benign conditions such as acute and CP, liver cirrhosis, cholangitis, and cholelithiasis[11]. Up to 27% of focal AIP cases mimicking PDAC present with increased levels of Ca 19-9, but usually in numbers < 100 U/mL. Ca 19-9 is not a sensitive or a specific method that could help to distinguish malignant from benign processes[12].
The role of conventional surface US seems to be limited to the initial evaluation of jaundice. It may identify features suggestive of pancreatic cancer, such as the presence of a hypoechogenic mass in the pancreatic head and dilation of the pancreatic and biliary ducts. However, an accurate and complete assessment of the pancreas, specifically the body and tail, is frequently limited due to the interposition of intestinal loops and gas. The sensitivity and accuracy of abdominal US to assess the pancreas depends on the operator's experience, the patient’s body constitution and the location and burden of the disease. Its diagnostic accuracy ranges between 50% and 90% for detecting pancreatic cancer[13].
Contrast-enhanced CT (CECT) has 90% sensitivity, 87% specificity, and 89% accuracy in diagnosing pancreatic cancer and is considered the best method to evaluate the pancreas and pancreatic masses. Its main limitation is its low sensitivity for early lesions and tumors smaller than 2 cm[14] since they usually appear isoattenuating in relation to the surrounding pancreatic parenchyma[15,16].
PDAC usually presents as a poorly defined and spiculated hypoattenuating mass with distal atrophy of the gland. After contrast media administration, it persists as a hypoattenuating mass with respect to the rest of the parenchyma, but in late stages, it shows heterogeneous peripheral enhancement, although atypical forms of AIP and MFCP may share the same characteristics[17].
As will be discussed later, specific and some subtle changes in structure and contrast dynamics may help in the differential.
Some studies consider MRI a superior test compared to CT in characterizing pancreatic masses[18] and delineating the pancreatic ductal system. MRI has become the diagnostic alternative to endoscopic pancreatic cholangiopancreatography. The sensitivity, specificity, and accuracy of MRI for diagnosing pancreatic cancer have been reported to be 93%, 89%, and 90%, respectively[19,20].
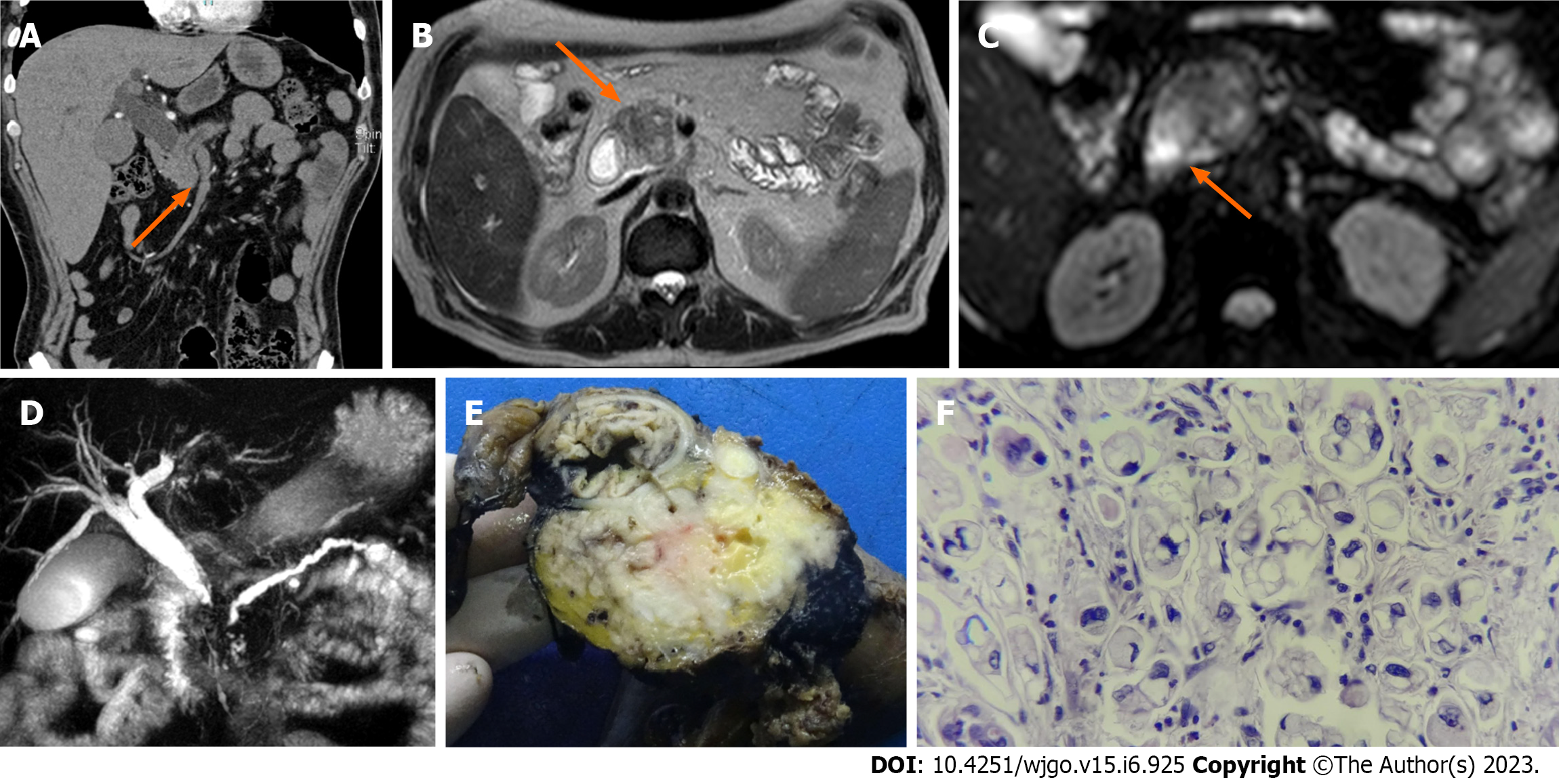
PDAC and MFCP appear hypointense on fat-suppressed T1 sequences[21], whereas they have a variable appearance on T2-weighted images (Figure 1)[22], as well as on diffusion-weighted images (DWI)[23]. For example, on the diffusion sequence, pancreatic masses show increased signal intensity relative to the normal pancreatic parenchyma and appear hypointense on the apparent diffusion coefficient (ADC)[24]. However, DWI may not be able to conclusively discriminate an inflammatory mass from a neoplastic solid lesion, as both present similar values in this modality[24,25].
When PDAC appears isointense, MRI can show indirect signs that suggest malignancy, such as gland atrophy and dilatation of the main pancreatic duct (usually > 4 mm)[26,27]. In contrast, in MFCP, the pancreatic duct is dilated to a lesser degree and usually coexists with lengthy and irregular ductal stenosis. In PDAC, duct dilation is uniform, comprising the total length of the upstream duct, and the ductal stenoses are associated or next to the mass (this can also be seen on CECT)[28,29]. Widening of the space between the pancreas, common bile duct (CBD), and duodenal lumen is another sign that is frequently observed in MFCP and not in PDAC[29].
All these characteristics can be observed in atypical forms of AIP and MFCP, making the distinction among them based on noncontrast MRI difficult. Reports on the differentiating capacity of dynamic contrast-enhanced MRI have reported high sensitivity, specificity, and diagnostic accuracy rates[30].
Since the diagnostic gold standard of PDAC, MFCP, and AIP is histology, EUS has become a valuable tool in the evaluation of pancreatic masses. EUS provides high-resolution images and allows us to obtain a tissue sample. Compared to abdominal US, CECT, and positron emission tomography (PET/CT) in the recognition of early pancreatic tumors, early-stage CP (Figure 2), loco-regional staging of PDAC, and deciding the best site for biopsy, EUS has a better performance[31,32].
For the detection of lesions smaller than 3 cm, EUS has a diagnostic sensitivity of 99% compared to 55% for CECT. It also has a high negative predictive value for ruling out pancreatic cancer[32].
EUS elastography and contrast-enhanced endoscopic US, although still not widely available, are rapidly growing techniques that have demonstrated to be able to characterize and differentiate pancreatic masses based on their stiffness and sonographic contrast dynamics, with promising results and ongoing research and development[33].
Considering all these caveats of imaging techniques, histology still stands as the diagnostic gold standard of pancreatic masses. EUS-guided biopsy has become the method of choice for obtaining pancreatic tissue, with 90% sensitivity and 96% specificity, a complication rate less than 0.8% and a reduced risk of seeding and dissemination[34].
However, its diagnostic yield depends on the quality of the sample, the selected site of puncture, and the interpretation of the results[31]. A 15% to 59% rate of inconclusive diagnoses resulting from the initial EUS-FNA has been reported[35-38].
Repeated EUS biopsy is an accepted strategy in patients with a suspicious pancreatic mass and inconclusive diagnosis after a first diagnostic approach that included a biopsy. The cumulative yield after repeat EUS-FNA for definite PDAC has been reported to be approximately 16%[39].
The atypical neoplastic glands of PDAC are usually embedded in a dense stroma (desmoplastic tumors). Biopsy’s limited diagnostic yield can be explained by PDAC’s associated desmoplastic reaction, which increases the chance of obtaining fibrotic tissue instead of cancer cells; since AIP and MFCP are rich in fibrotic tissue and inflammatory infiltrate, their presence does not rule out malignancy or confirm a benign inflammatory mass[40,41]. Other factors that may affect the diagnostic accuracy of EUS-guided biopsies include operator- and technique-related factors and extent of tumor necrosis.
Since a negative biopsy does not confidently rule out malignancy, if clinical suspicion is high enough, a biopsy should be repeated or if the mass is potentially resectable, surgery must be entertained.
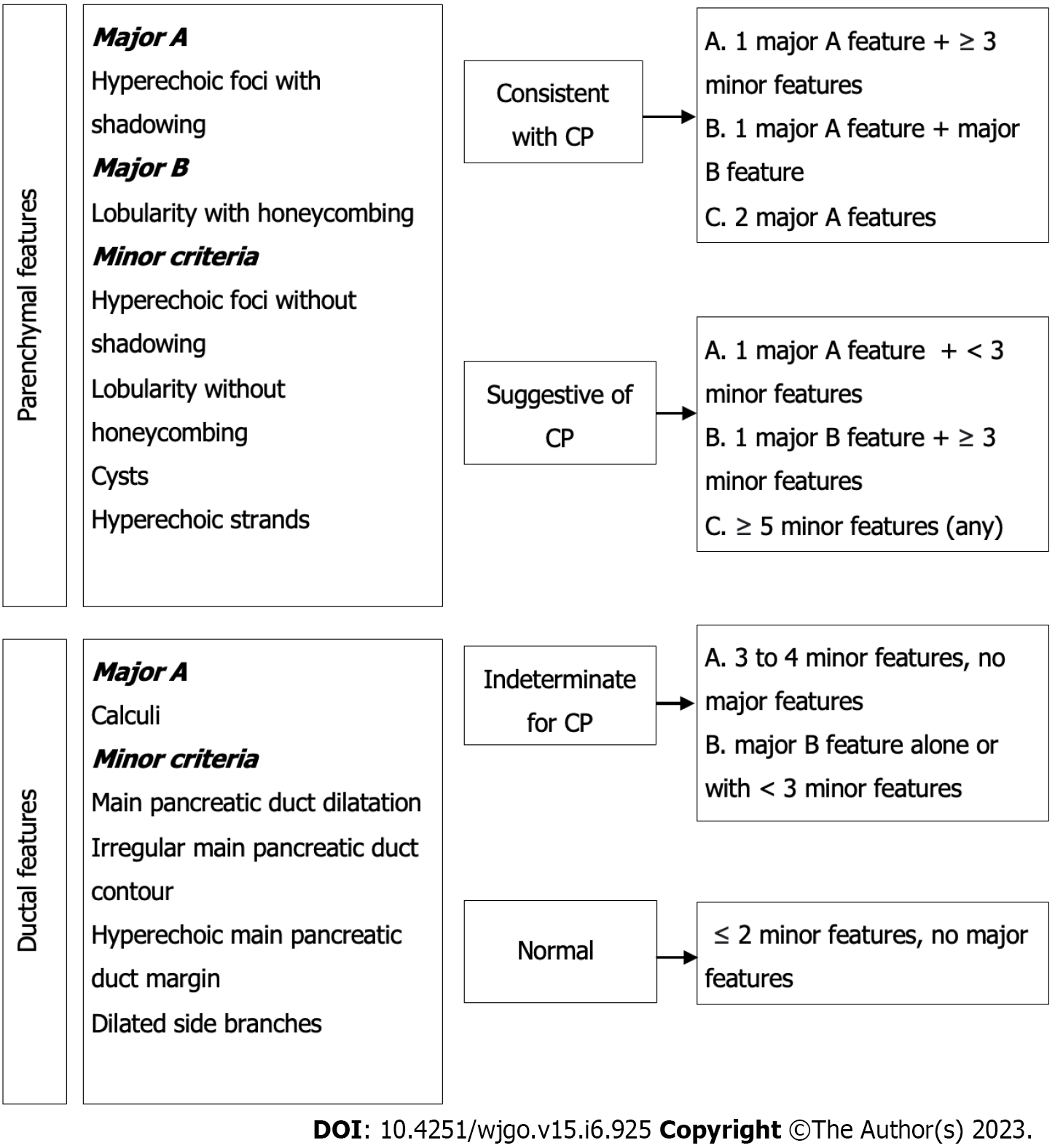
CP is a pathologic fibro-inflammatory syndrome of the pancreas in individuals with genetic, environmental, and/or other risk factors who develop persistent pathologic responses to parenchymal injury or stress[42].
Common features of established and advanced CP include pancreatic atrophy, fibrosis, pain syndromes, duct distortion, calcifications, pancreatic exocrine dysfunction, pancreatic endocrine dysfunction, and dysplasia[8,43]; up to 10% to 30% of cases may present as MFCP[44].
The incidence of PDAC is much higher than that in the general population (several genetic risk factors have been described as well as modifiable and nonmodifiable host factors), with almost 2% to 4% of patients with CP developing PDAC within ten years or more of diagnosis. The presence of a pancreatic mass in patients with CP represents a diagnostic challenge, with significant therapeutic and prognostic implications[4,5,8,45].
Discerning between PDCA and MFCP solely on clinical grounds is challenging; both may present as a pancreatic mass with recurrent abdominal pain, jaundice, weight loss, and pancreatic insufficiencies (exocrine and/or endocrine)[8].
Abdominal US does not discern among pancreatic cancer, AIP, and MFCP since all 3 may display similar imaging characteristics. Despite AIP having specific features such as diffuse enlargement of the pancreas, decreased echogenicity, and narrowing of the pancreatic duct due to compression of the affected parenchyma, the aforementioned technical limitations of abdominal US play a significant role in decreasing its diagnostic yield, especially in focal and atypical forms of AIP[46].
On the other hand, if intraparenchymal calcifications, heterogenicity with hyperechogenicity of the parenchyma, dilatation of the main pancreatic duct, and irregularity of the pancreatic contour can be identified, MFCP is to be suspected. However, some studies have reported that parenchymal calcifications can occur in other pancreatic disorders, such as intraductal papillary mucinous neoplasms (IPMN), with a 20% reported incidence. The calcification pattern in IPMN is indistinguishable from that in CP; calcifications can be found in the pancreatic duct, parenchyma, or diffusely scattered throughout the gland. Punctate calcification was the most common pattern (87%), followed by coarse calcification (33%).
Abdominal US has 67% sensitivity and 90% specificity for the diagnosis of established CP[47].
Contrast-enhanced imaging may discriminate among the different etiologies of a pancreatic mass[37]. A mass related to MFCP and AIP exhibits homogeneous enhancement similar to the rest of the pancreatic parenchyma, with the opposite occurring in PDAC, which shows poor enhancement in all phases along with poorly delimited margins[33]. These features can be secondary to a marked desmoplastic reaction, low vascularity, and the presence of necrosis and mucin in PDAC[48]. Unfortunately, in advanced stages of CP, MFCP pancreatic parenchyma presents significant fibrosis that modifies the enhancement pattern, especially during the arterial phase, although in some cases, the venous phase is preserved and may help in distinguishing MFCP from PDAC. For the diagnosis of CP, CECT has 75% sensitivity and 91% specificity[49].
Once considered the gold standard for assessing the pancreatic duct, EUS has been replaced by magnetic resonance cholangiopancreatography (MRCP) and EUS[50], and its current role is almost exclusively therapeutic.
Histologic examination is paramount when facing pancreatic masses. CP exhibits a fibrotic pattern that may resemble the one observed in PDAC-related desmoplasia; thus, the presence of chronic inflammatory infiltrate in pancreatic tissue does not rule out PDAC.
Biopsies may not always add to the differential between AIP and CP since the latter might also show lymphocytic and plasma cell infiltrate, as well as macrophage infiltrate and areas of interlobular fibrosis extending into the ductal structures that are also observed in type 1 and 2 AIP.
In CP, acinar and islet cells are usually spliced by fibrous tissue, and the ducts may contain protein plugs that can become calcified. Nevertheless, these features are often seen in elderly patients without CP, especially in those with diabetes mellitus[51].
Repeated biopsy should always be entertained as it improves the diagnostic yield. Mitchell et al[39] demonstrated that repeat FNA yields an altered diagnosis in 71% of patients. This is similar to other studies that showed that a second EUS-FNA alters the initial diagnosis in up to 63% to 82% of cases[36-38,52].
AIP is classified into type 1 and 2 AIP that may present as an acute or chronic form. Type 1 AIP is a manifestation of systemic IgG4-related disease (IgG4-RD), a systemic condition that can affect almost every organ but has a predilection for the pancreas and biliary tract[53,54].
Both types of AIP can mimic PDAC both in imaging appearance and clinical presentation. AIP can present with painless obstructive jaundice in up to 70% of cases as well as with abdominal pain and weight loss in up to 30%[55].
In late stages, if left untreated or if treatment is delayed, AIP can result in CP and present with exocrine pancreatic insufficiency and diabetes mellitus. Thus, MFCP can either be a primary disorder or a complication of AIP[53,56,57].
Criteria for diagnosis are based on imaging, serological, histological, and therapeutic response parameters[1,58].
International Consensus Diagnostic Criteria (ICDC) for type 1 and 2 AIP consider both typical and atypical presentations. The ICDC diagnostic yield for type 1 is high, with a reported sensitivity of 89%-95%, specificity of 100%, and accuracy of 94%. Biopsy is seldom needed since serum and imaging characteristics along with other organ involvement frequently provide enough diagnostic evidence.
Atypical forms of IgG4-related pancreatitis as well as type 2 AIP might require tissue acquisition. In both, but especially in type 2 AIP, collateral information from serum and other organ involvement is missing in most cases (only 25% of type 2 AIP may have concurrent inflammatory bowel disease), making biopsy almost mandatory since pancreatic cancer cannot confidently be ruled out[59-61].
IgG4 remains a good marker for the diagnosis of IgG4-RD[62], but increased levels can also occur in CP and other benign conditions as well as in up to 10% to 15% of PDAC, but levels are usually less than twice the upper limit of normal[63], which is considered the ideal cut off point to diagnose or differentiate IgG4-RD AIP from other non-IgG4-related diseases with a specificity of 92.6%. IgG4 levels are of no utility in ruling in or out the possibility of type 2 AIP[63,64].
Autoantibodies (Abs), including anti-nuclear Ab, anti-Ro/SSA, anti-La/SSB, anti-neutrophil cytoplasmic Ab, anti-lactoferrin Ab, anti-carbonic anhydrase II, anti-plasminogen-binding protein Ab, anti-inhibitor of pancreatic trypsin secretion Ab, anti-DNA, anti-Sm, anti-RNP, and cryoglobulins, are neither sensitive nor specific[65] and lack diagnostic value since they do not differentiate AIP from MFCP or PDAC. In some cases, they may increase the possibility of type 2 AIP[65-67].
On imaging, AIP may present as either diffuse, focal, or multifocal pancreas enlargement resembling MFCP and PDAC[68,69].
As discussed before, abdominal US does not have any diagnostic value other than being the initial tool to assess jaundice. Although significantly limited with regard to the extension of the pancreas that it can visualize, it can show a hypoechoic mass associated with hyperechoic foci and stranding as well as parenchymal heterogeneity but is unable to differentiate a benign from a malignant mass.
Contrast-enhanced US might provide more information in this regard, but again, the extension of the gland that can be examined as well as technical, operator-, and patient-related factors limit its diagnostic yield.
Abdominal CT allows visualization of the full extension of the pancreas and better characterization of any abnormality.
The classic form or level 1 evidence of AIP according to the ICDC is a diffusely enlarged pancreas with an increased anteroposterior diameter and smooth edges (“sausage pancreas”) and is usually accompanied by a hypodense halo or pseudocapsule surrounding the pancreas[1].
Ductwise, the level 1 evidence consists of an irregular and stenotic pancreatic main duct[70-72]. When contrast media is applied, in the early phase, a hypodense mass is usually observed, and in the late phase, it will become isodense. Although a similar behavior is observed in PDAC, AIP usually presents late enhancement in the venous phase.
Up to 30% of the time, AIP presents as a focal mass that is indistinguishable from MFCP and PDAC[6,73]. In PDAC, the pancreatic duct usually has smooth contours, with a short stenosis or abrupt amputation at the tumor site; when located at the head of the pancreas, it may also affect the CBD, and a double duct sign [dilated CBD and main pancreatic duct (MPD)] can be observed.
Vascular involvement is often observed in late stages of PDAC, but AIP may have a similar picture when it presents with retroperitoneal fibrosis affecting local vasculature[61].
Collateral information may aid in the differential diagnosis; the presence of pancreatic calcifications and cysts might suggest MFCP or late phases of AIP, while other organ involvement may suggest AIP[74,75].
Overall, CECT has 59% [95% confidence interval (CI), 41%-75%] sensitivity and 99% (95%CI: 88%-100%) specificity to differentiate AIP from PDAC[76].
Extrapancreatic abnormalities such as renal involvement with bilateral patchy lesions and lymph node or parotid gland involvement are associated with IgG4 systemic disease[74,75].
On MRI, AIP’s diffuse or focal enlargement of the pancreas is observed as a hypointense gland in the T1 sequence and hyperintense in the T2 sequence; however, this is similar to how PDAC is observed.
Additionally, both AIP and PDAC show a similar diffusion restriction pattern on DWI, appearing as a hyperintense gland, but unlike PDAC, AIP shows greater hypointensity in ADC.
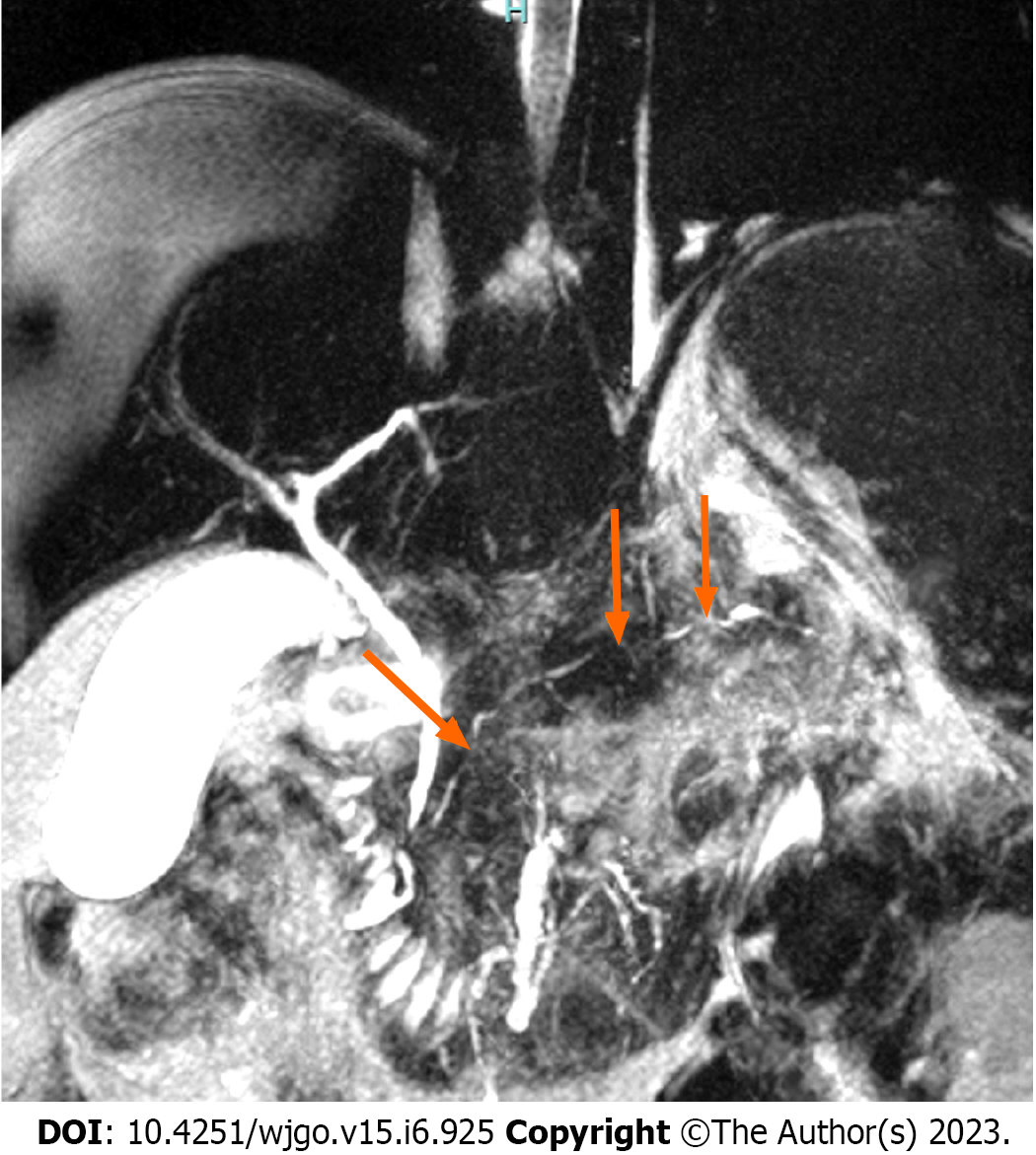
A hypointense peripheral pancreatic halo in both sequences[77] and extensive irregular stenosis (≥ 3 mm in length) in the affected pancreatic segment without upstream ductal dilation suggest AIP[26,78,79]. The presence of multiple stenoses has been reported in up to 61.5% of cases with AIP[26] (Figure 3). The penetrating duct sign (which is better observed during MRCP with secretin) in the affected area also favors AIP over PDAC[80,81]. MRI diagnostic yield has a low sensitivity (28.6%-44.4%) but high specificity (100%)[82].
Frequently, after exhaustive diagnostic work-up, atypical forms of IgG4 RD AIP and type 2 AIP remain undiagnosed, and biopsy is needed, which may confirm the diagnosis or distinguish them from MFCP and PDAC.
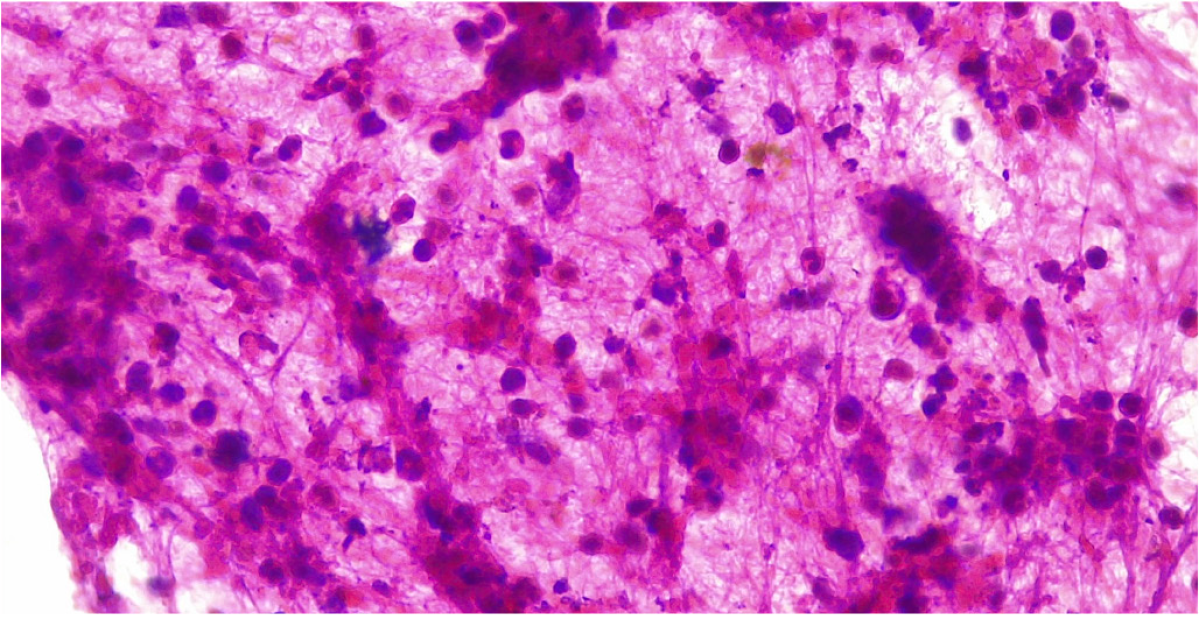
Histologic findings of AIP differ depending on whether it is type 1 or type 2. IgG4-related AIP (type 1 AIP) is characterized by T-lymphocyte infiltration, lymphoplasmacytic infiltrate with IgG4-positive plasma cells (IgG4+), storiform fibrosis, and obliterative phlebitis[78]. It has been reported that > 40% of IgG+ plasma cells and > 10 IgG4+ plasma cells per high power field (HPF) for puncture specimens or > 50 IgG4+ HPF cells for surgical specimens are present in most AIP type 1[83].
Type 2 AIP histology shows a granulocytic epithelial lesion[22,82], neutrophil and lymphocyte infiltrate and different extents of fibrosis. The pancreatic duct is narrowed by periductal fibrosis and lymphoplasmacytic infiltration, but the ductal epithelium is usually preserved[70] (Figure 4).
PDAC can also show infiltration by IgG4+ plasma cells, but to a lesser extent and unlike AIP, it does not present storiform fibrosis or obliterative phlebitis. When EUS-guided biopsy is unavailable, endoscopic biopsy of the ampulla of Vater may show a lymphoplasmacytic infiltrate with IgG4-positive plasma cells, placing it as a good surrogate in the diagnosis of type 1 AIP[84].
Some recently developed techniques might improve our capability to differentiate benign from malignant pancreatic masses.
This is a modality that seems to differentiate between MFCP and PDAC. Yadav et al[85] and Aslan et al[86] assessed the characteristics of histology-proven PDAC and MFCP on perfusion CT. Blood flow (BF) and blood volume (BV) were the best parameters that differentiated both entities from each other. Although both MFCP and PDAC presented low values in BF and BV compared with normal pancreatic parenchyma, the lowest values were more frequent in PDCA. A cut off value of 19 mL/100 mL/min for BF had 92% sensitivity and 68% specificity to differentiate PDAC from MFCP, and for a value of 5 mL/100 mL in BV, the reported sensitivity and specificity were 100% and 73%, respectively.
These results are encouraging, but they still need to be replicated and validated in larger studies.
These techniques have become the modality of choice for pancreatic cancer imaging and have shown good performance in detecting < 2 cm or isoattenuating lesions.
These methods allow a more precise characterization of the solid or cystic nature/components of a given pancreatic lesion as well as a better visualization of the pancreatic duct and surrounding vascularity[14,85,86].
Low voltage generated images increase the probability of detecting a hypodense lesion embedded in the normal pancreatic parenchyma compared with those obtained with high voltage equipment, and such distinction becomes more evident during the portal contrasted phase[87]. Low voltage CT has higher sensitivity in diagnosing PDCA compared to high voltage imaging using iodine contrast[86,87].
It has limited and low diagnostic yield when discriminating between benign inflammatory masses and malignant ones[5,87].
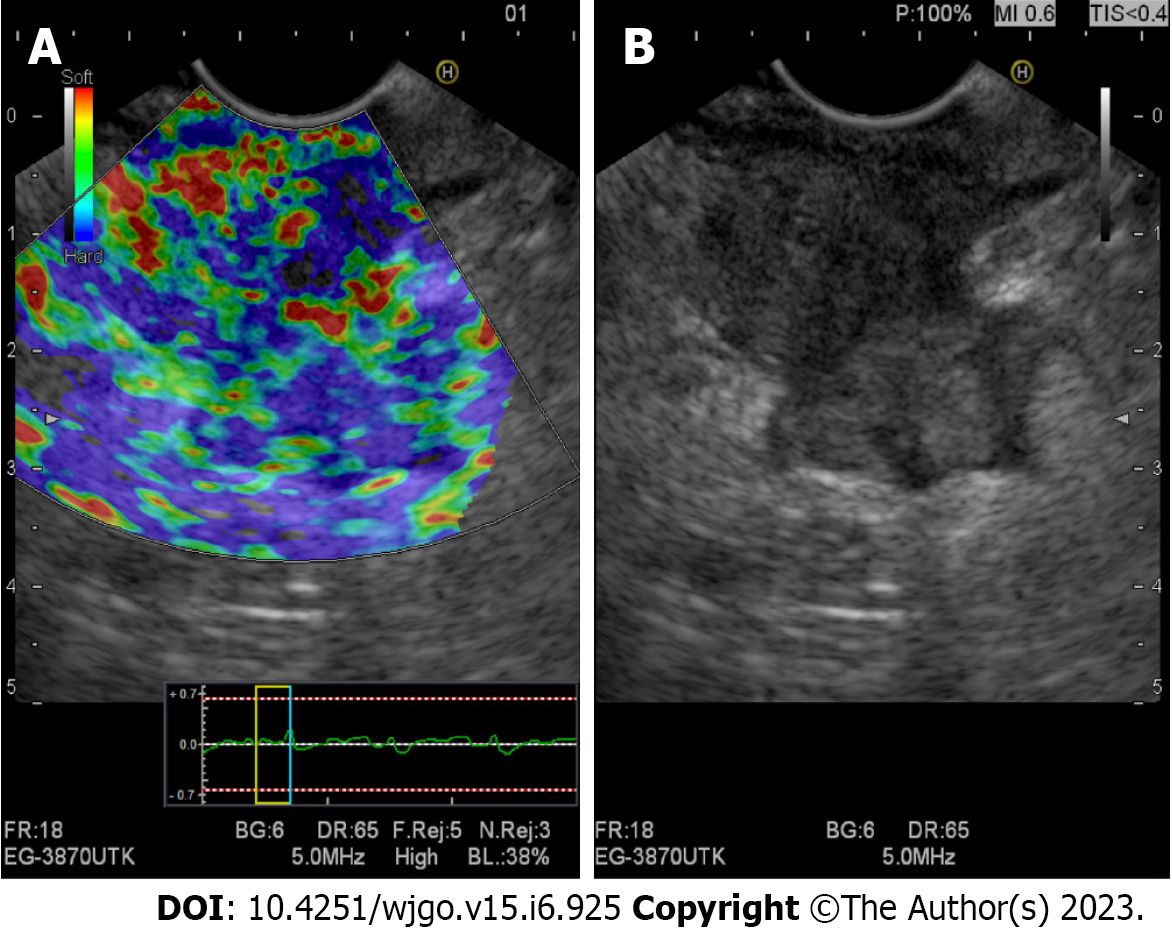
By assessing and comparing the tissue rigidity related to either an inflammatory process or a malignant process, this new tool has a high diagnostic accuracy for differentiating PDAC from AIP but still requires further study.
Shi et al[88] reported differences in tissue stiffness in AIP, PDAC, and healthy volunteers. AIP cases showed higher stiffness values [2.67 kPa (2.24-3.56 kPa) than healthy pancreas 1.24 kPa (1.18-1.24 kPa)] but significantly lower stiffness values than PDAC [3.78 kPa (3.22-5.11 kPa)] (P < 0.05).
Either qualitative or quantitative, it might discriminate benign from malignant masses based on their particular stiffness rates. A distortion ratio cutoff point > 10 or a value < 50 in the distortion histogram suggests malignancy[89-91] (Figure 5).
Giovannini et al[92] reported EUS elastography findings in 121 cases with pancreatic masses. The sensitivity, specificity, positive predictive value, and negative predictive value for differentiating benign and malignant pancreatic masses were 92.3%, 80.0%, 93.3%, and 77.4%, respectively.
Diagnosing based solely on these parameters is still far from possible; currently, these technological advances have helped in selecting the biopsy site, identifying viable tissue and avoiding necrotic areas within the mass.
Liquid biopsy identifies circulating tumoral DNA, microRNA, and cells. It has been shown to be feasible and efficient in diagnosing different malignant neoplasms at early stages (e.g., lung, breast, colon and liver cancer). It has also been suggested that it could be a reliable confirmatory test and possibly replace the need for tissue biopsy[93]. Information on pancreatic cancer is scarce but promising; it could aid in diagnosis and provide information related to potential therapeutic targets as well as prognosis[94-98].
Its reported sensitivity and specificity in diagnosing PDAC range between 33%-100% and 27%-81%, respectively[94]. Liquid biopsy could also be applied in the study and diagnosis of benign conditions such as AIP and CP.
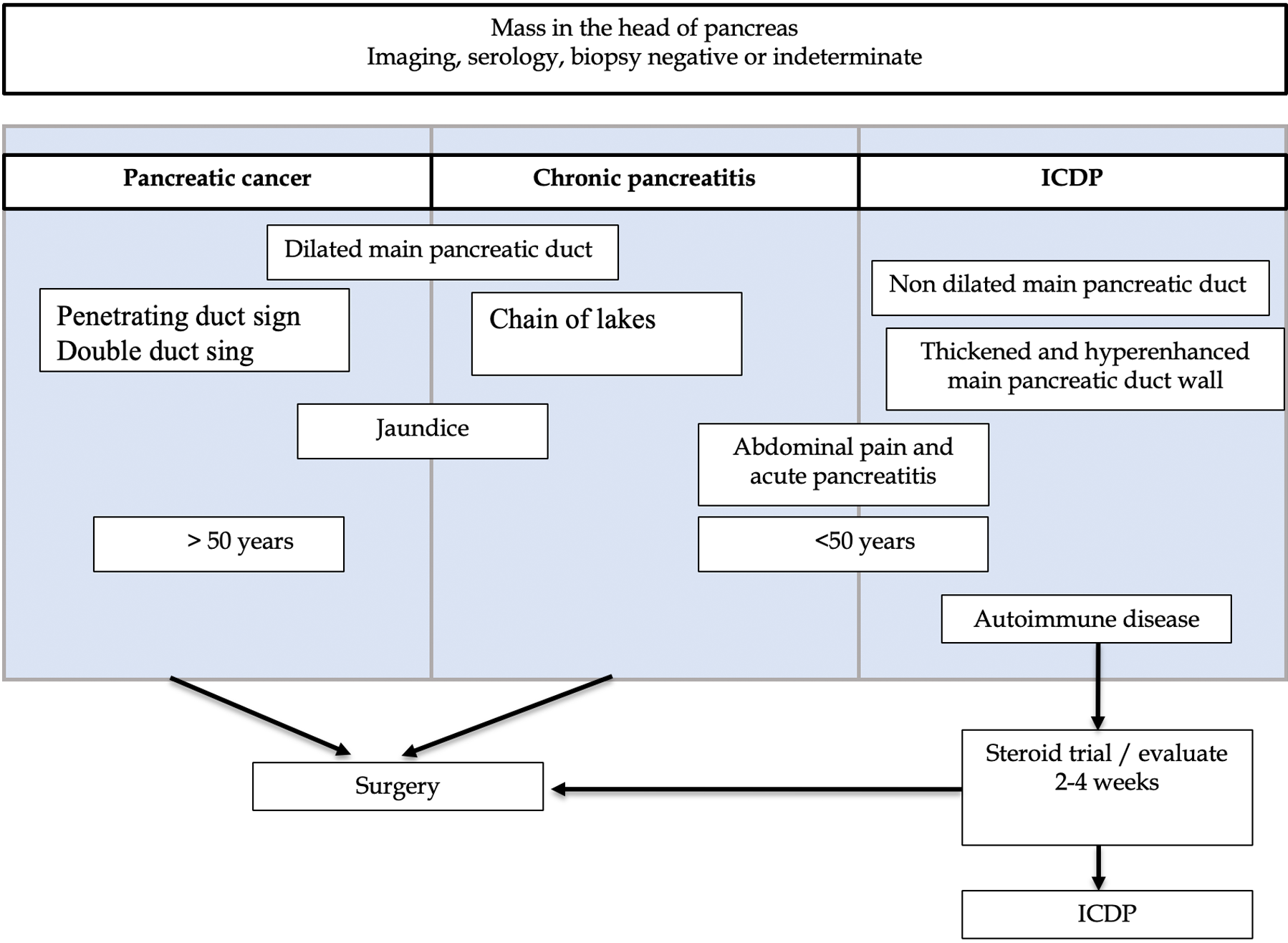
Attention to specific clinical and imaging details that might be disease specific can be highly valuable during the diagnostic work-up of a pancreatic mass (Figure 6).
PDAC can present with migratory thrombophlebitis, acute pancreatitis, hypoglycemia, and hypercalcemia; although DM related to the exocrine pancreas has distinct clinical characteristics that differentiate it from type 1 and 2-DM, it may not be an easy task to differentiate that related to PDCA from that associated with CP[44].
MFCP may show signs of longstanding overt or subclinical exocrine dysfunction in the form of steatorrhea, malnutrition, and vitamin deficiencies. Although no single serum marker is available for CP diagnosis, nutritional serum markers have been used to adjust the pancreatic enzyme supplementation dose, and in some cases, those markers may help in the diagnosis of early CP[99].
We previously reported that age of presentation, history of abdominal pain, acute pancreatitis, presence of other autoimmune diseases, pancreatic duct caliber and other clinical and imaging characteristics might help to differentiate benign from malignant masses[67].
After comparing the clinical and imaging characteristics of resected focal type 2 AIP, CP, and PDAC, the characteristics that favored a benign over a malignant mass were abdominal pain (OR 0.18; 95%CI: 0.07-0.55; P < 0.001) and a history of acute pancreatitis (OR 0.48; 95%CI: 0.01-0.16; P = 0.002).
In favor of PDAC were obstructive jaundice (OR 28.5; 95%CI: 8.18-79.49; P < 0.0001) and main pancreatic duct dilation (OR 5.21; 95%CI: 1.93-14.62; P < 0.001). Patients with PDAC were also older than nonmalignant patients (P < 0.001).
In the same group of patients, comparing AIP vs non-AIP cases (PC and PDAC), abdominal pain (OR 8.75; 95%CI: 1.83-41.75; P = 0.002), history of acute pancreatitis (OR 10.28; 95%CI: 3.29-32.12; P = 0.001), concurrent autoimmune disease (OR 20; 95%CI: 4.38-91.28; P = 0.006) and lack of main pancreatic duct dilation (OR 9.30; 95%CI: 3.05-28.69; P < 0.0001). AIP cases were younger (P < 0.001).
Features such as mass morphology, pancreatic calcification distribution, presence of duct-penetrating sign, duct stenosis, pancreatic or bile duct wall thickness, and contrast uptake are some disease-specific characteristics that can help to differentiate PDAC, AIP, and MFCP (Table 1).
| Imaging studio | AIP | MFCP | PDAC |
| Ultrasound | |||
| Conventional | Hypoechoic | Hypoechoic | Hypoechoic |
| CEUS/CE-EUS | Homogeneous enhancement | Homogeneous enhancement | No enhancement |
| Eltasography by EUS | Predominantly blue heterogeneous pattern | Heterogeneous pattern with a predominance of green color and blue stippling | Heterogeneous pattern with a predominance of blue color and green stippling |
| CT | |||
| Simple | Hypodense; peripheral halo | Hypodense Intraparenchymal calcifications or within the pancreatic duct | Hypodense; parenchymal atrophy |
| Contrasted | Hyperattenuation (compared to the spleen) in the portal venous phase; presence of extrapancreatic involvement | Heterogeneous hyperattenuation; absence of extrapancreatic involvement | No enhancement; vascular invasion |
| Perfusion | Low BF and BV values compared to normal pancreatic parenchyma but higher than PDAC | Low BF and BV values compared to MFCP | |
| Dual-energy | Appears as a hypodense mass in low voltage imaging (especially during portal phase); in the iodine mapping the mass shows enhancement | ||
| MRI | |||
| T1 | Hypointense; hypointense peripheral halo | Hypointense | Hypointense |
| T2 | Hyperintense; hypointense peripheral halo | Hyperintense: Early stage; hypointense: Advanced stage | Hyper/hypointense |
| DWI | Hyperintense | Hyperintense | Hyperintense |
| ADC | Higher hypointensity than MFCP and PDAC | Hypointense | Hypointense |
| Elastography | Higher stiffness compared to normal parenchyma but lesser compared to PDAC; multiple scattered lesions | Higher stiffness compared to AIP; single, isolated nodular lesions | |
CT and MRI allow the visualization and assessment of the main pancreatic duct and bile duct morphology, diameter, and other characteristics that can be disrupted by the presence of a pancreatic mass[100].
Although CP and PDAC may be associated with duct stenosis as well as upstream ductal irregularity and dilation, the presence of calcifications, the characteristics of the stenosis, and other extrapancreatic and clinical features can differentiate benign from malignant conditions.
In MFCP, the pancreatic duct lateral branches are usually dilated and deformed, which in MRCP is called the “chain of lakes”[101]. In PDAC, dilation of the MPD is usually more prominent as a result of abrupt narrowing caused by the centrifugal ductal growth pattern of the cancer cells, which is usually followed by significant atrophy of the pancreatic parenchyma.
In AIP, it is typical to observe stenotic areas without prestenotic dilation[83], although few cases of focal AIP may present upstream dilation of the main pancreatic duct. This is called the “icicle sign” (the MPD could penetrate the mass without complete occlusion, and the pancreatic duct stenosis may taper within the mass), which is caused by periductal fibrosis causing extrinsic compression and ductal narrowing in the affected part of the pancreas. This sign has a specificity of up to 95% and an accuracy of 88% for diagnosing AIP[102,103].
We found that in the presence of a pancreatic mass in the head of the pancreas, the absence of dilation of the main pancreatic duct favors the diagnosis of AIP with a sensitivity of 31% but a specificity of 81% (OR 9.30; 95%CI: 3.05-28.69; P < 0.0001)[67].
The CBD and the MPD converge at the major papilla, and any mass located at the head of the pancreas may compress or engulf either or both, causing subsequent prestenotic ductal dilatation. The radiological image of dilatation of both ductal systems is referred to as the double duct sign[76]. Although not pathognomonic of PDAC, it has been reported to be present in up to 80% of cases. It can be present but less frequently in AIP and MFCP[3].
MFCP showing a double duct sign can show ductal strictures alternated with dilated areas, producing a beaded appearance, although in some cases, the pancreatic duct may be dilated through its entire extension.
In AIP, the pancreatic duct caliber is usually normal, with few cases presenting slight dilation. The ductal stenosis length (either biliary or pancreatic) tends to be longer in MFCP and AIP and shorter or punctual in PDCA[75].
This sign is defined as a nonobstructed, nonstenotic, or normal-appearing MPD running into a pancreatic mass and is usually seen on MRCP images[100-103]. This sign has a sensitivity of 85% and specificity of 96% to discern an inflammatory pancreatic mass (MFCP and AIP) from PDAC[80].
The pathophysiological basis of the duct-penetrating sign is that the dense fibrotic infiltrate of the PDAC causes an abrupt and complete narrowing of the duct, whereas in inflammatory pancreatic masses, the narrowing is subtle and tends to taper down, and the duct can still be visualized in the affected area in the MRPC[3].
Parenchymal calcifications are not unique to CP; they can be present in PDAC and some cystic neoplasms of the pancreas, such as neuroendocrine tumors, serous cystadenoma, and intraductal papillary mucinous neoplasm.
Diffuse parenchymal calcifications with ductal calcifications, parenchymal atrophy, and cystic lesions are specific to CP[86]. In PDAC, calcifications are usually neither diffuse nor intraductal.
The presence of calcifications may not completely rule out or confirm a malignant or benign mass, since long-standing CP cases may develop PDAC. In such a clinical scenario, should a prior CT image be available, a change in the calcification distribution as a result of a new mass displacing them may be observed, which in turn may favor the presence of PDAC in a patient with preexisting CP.
A pancreatic mass with blood vessel involvement is not unique to malignancies. Although advanced stages of PDAC present with soft tissue involving the celiac trunk and the superior mesenteric and splenic vessels, similar findings can be found in chronic inflammatory processes such as retroperitoneal fibrosis that can be associated with IgG4 systemic disease[62,75].
Some studies have looked for imaging surrogates to differentiate AIP from PDAC and MFCP, highlighting the diagnostic potential of perfusion CT scans as well as EUS.
In addition to the classical imaging features of AIP and PDAC, long pancreatic duct stenosis without upstream dilation, subtle bile, and/or pancreatic abnormalities can be of significant aid.
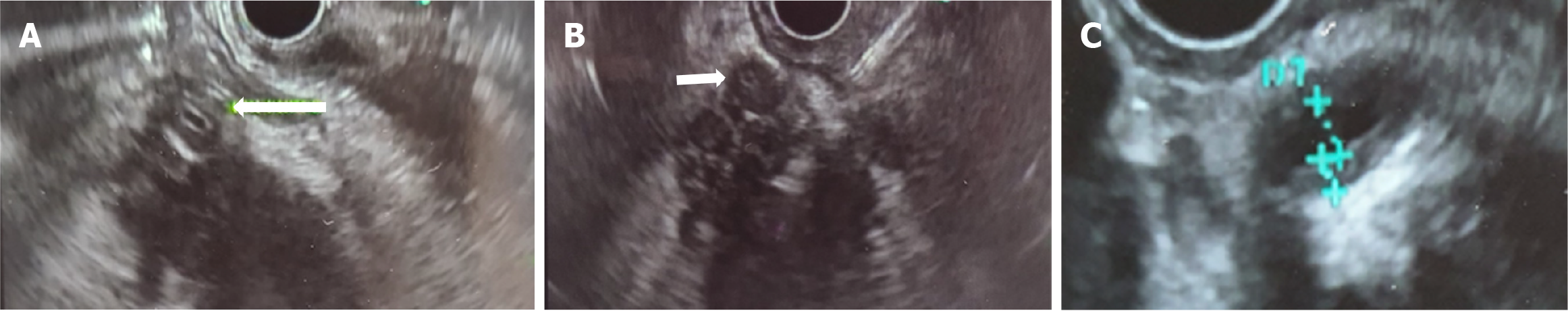
The presence of irregular narrowing of the main pancreatic duct in association with duct wall thickening during EUS has a diagnostic accuracy for type 1 AIP of 98.3%. Hyperechoic parietal thickening is also more frequent in AIP (93%) than in PDAC or CP (23%) (Figure 7).
On CT/MRI, diffuse and homogeneous ductal wall hyperenhancement is present in 47% of AIP and 22% of PDAC cases and has been reported to be highly predictive of AIP[104-107].
As has been explained throughout this review, the differential diagnosis between focal pattern AIP and PDAC is extremely difficult since the clinical picture, imaging, and serological methods may provide questionable results[71,77-79].
Although some of the clinical and imaging diagnostic difficulties can be overcome with a biopsy; AIP, MFCP, and PDAC can share similar histological findings[108], and the associated inflammation or fibrosis, sampling error, bloody aspirates, and errors in cytologic interpretation may provide equivocal results.
Some have suggested that in highly selected cases with diagnostic uncertainty after a thorough diagnostic approach in experienced centers, a systemic steroid trial may be beneficial[26,74,76,77,109].
On average, a 2- to 4-wk interval is needed to evaluate the response with a radiological study (CECT or MRI) since the clinical response is not a reliable parameter. It is important to keep in mind that patients with pancreatic cancer may show improvement due to the reduction in peritumoral inflammation, which can create confusion[108,109]. There is usually significant radiological improvement in all cases of AIP[77], while the absence of response rules out AIP[26].
Even after a thorough diagnostic approach, up to 5% of resected masses under suspicion of malignancy are benign. Uncertain pancreatic masses require a multidisciplinary approach, preferably in referral centers, by highly experienced specialists (radiologist, gastroenterologist, surgeons, and pathologists).
Due to the distinctive therapeutic and prognostic features of AIP, MFCP, and PDAC, a precise diagnosis is of the utmost relevance. The initial approach to a pancreatic mass must include a detailed clinical exam exploring relevant personal, non-personal and family history, exposure to risk factors, blood tests including IgG4 and Ca19-9 levels, and high-quality imaging (CECT). In uncertain cases after an initial approach, MRI, EUS and tissue examination may provide auxiliary concluding diagnostic information. Newer imaging and molecular techniques are promising tools, but further research is still needed. Currently, in the differential diagnosis of uncertain pancreatic masses, all available collateral clinical, imaging, and histological information remains the cornerstone for an accurate diagnosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy; Asociación Mexicana de Gastroenterología; American Pancreatic Association, International Association of Pancreatology
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu C, China; Solimando AG, Italy; Tan CL, China S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Poddighe D. Autoimmune pancreatitis and pancreatic cancer: Epidemiological aspects and immunological considerations. World J Gastroenterol. 2021;27:3825-3836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 3. | Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic Pancreatitis or Pancreatic Tumor? Radiographics. 2019;39:1965-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [PubMed] [DOI] [Full Text] |
| 5. | Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 200] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (5)] |
| 6. | Tokar JL, Walia R. Diagnostic evaluation of solid pancreatic masses. Curr Gastroenterol Rep. 2013;15:347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Sugumar A, Chari ST. Distinguishing pancreatic cancer from autoimmune pancreatitis: a comparison of two strategies. Clin Gastroenterol Hepatol. 2009;7:S59-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Elsherif SB, Virarkar M, Javadi S, Ibarra-Rovira JJ, Tamm EP, Bhosale PR. Pancreatitis and PDAC: association and differentiation. Abdom Radiol (NY). 2020;45:1324-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology. 2010;256:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Safi F, Beger HG, Bittner R, Büchler M, Krautzberger W. CA 19-9 and pancreatic adenocarcinoma. Cancer. 1986;57:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 352] [Reference Citation Analysis (3)] |
| 12. | Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 362] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 13. | Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864-7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (4)] |
| 14. | Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, Kim SJ, Baek JH, Kim SH, Lee JY, Han JK, Choi BI. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Best LM, Rawji V, Pereira SP, Davidson BR, Gurusamy KS. Imaging modalities for characterising focal pancreatic lesions. Cochrane Database Syst Rev. 2017;4:CD010213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Francis IR. Pancreatic adenocarcinoma: diagnosis and staging using multidetector-row computed tomography (MDCT) and magnetic resonance imaging (MRI). Cancer Imaging. 2007;7 Spec No A:S160-S165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Burk KS, Lo GC, Gee MS, Sahani DV. Imaging and Screening of Pancreatic Cancer. Radiol Clin North Am. 2017;55:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 779] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 20. | Maccioni F, Martinelli M, Al Ansari N, Kagarmanova A, De Marco V, Zippi M, Marini M. Magnetic resonance cholangiography: past, present and future: a review. Eur Rev Med Pharmacol Sci. 2010;14:721-725. [PubMed] |
| 21. | Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Tamm EP, Bhosale PR, Vikram R, de Almeida Marcal LP, Balachandran A. Imaging of pancreatic ductal adenocarcinoma: State of the art. World J Radiol. 2013;5:98-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, Nakajo M. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology. 2012;263:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, Hammond NA, Yaghmai V, Nikolaidis P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Jia H, Li J, Huang W, Lin G. Multimodel magnetic resonance imaging of mass-forming autoimmune pancreatitis: differential diagnosis with pancreatic ductal adenocarcinoma. BMC Med Imaging. 2021;21:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Lopes Vendrami C, Shin JS, Hammond NA, Kothari K, Mittal PK, Miller FH. Differentiation of focal autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2020;45:1371-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P. Groove pancreatitis: a diagnostic challenge. Eur Radiol. 2009;19:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Blasbalg R, Baroni RH, Costa DN, Machado MC. MRI features of groove pancreatitis. AJR Am J Roentgenol. 2007;189:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Zhang TT, Wang L, Liu HH, Zhang CY, Li XM, Lu JP, Wang DB. Differentiation of pancreatic carcinoma and mass-forming focal pancreatitis: qualitative and quantitative assessment by dynamic contrast-enhanced MRI combined with diffusion-weighted imaging. Oncotarget. 2017;8:1744-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Levy MJ, Wiersema MJ, Chari ST. Chronic pancreatitis: focal pancreatitis or cancer? Endoscopy. 2006;38 Suppl 1:S30-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Gonzalo-Marin J, Vila JJ, Perez-Miranda M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J Gastrointest Oncol. 2014;6:360-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Cho MK, Moon SH, Song TJ, Kim RE, Oh DW, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Contrast-Enhanced Endoscopic Ultrasound for Differentially Diagnosing Autoimmune Pancreatitis and Pancreatic Cancer. Gut Liver. 2018;12:591-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | D'Onofrio M, De Robertis R, Barbi E, Martone E, Manfrin E, Gobbo S, Puntel G, Bonetti F, Pozzi Mucelli R. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol. 2016;26:1801-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36; quiz 751, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | DeWitt J, McGreevy K, Sherman S, LeBlanc J. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Suzuki R, Lee JH, Krishna SG, Ramireddy S, Qiao W, Weston B, Ross WA, Bhutani MS. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointestin Liver Dis. 2013;22:183-187. [PubMed] |
| 39. | Mitchell RA, Stanger D, Shuster C, Telford J, Lam E, Enns R. Repeat Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Patients with Suspected Pancreatic Cancer: Diagnostic Yield and Associated Change in Access to Appropriate Care. Can J Gastroenterol Hepatol. 2016;2016:7678403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1346] [Article Influence: 149.6] [Reference Citation Analysis (2)] |
| 41. | Apte MV, Wilson JS. The desmoplastic reaction in pancreatic cancer: an increasingly recognized foe. Pancreatology. 2007;7:378-379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, Shimosegawa T. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 43. | Al-Hawary MM, Kaza RK, Azar SF, Ruma JA, Francis IR. Mimics of pancreatic ductal adenocarcinoma. Cancer Imaging. 2013;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Ren S, Chen X, Cui W, Chen R, Guo K, Zhang H, Chen S, Wang Z. Differentiation of chronic mass-forming pancreatitis from pancreatic ductal adenocarcinoma using contrast-enhanced computed tomography. Cancer Manag Res. 2019;11:7857-7866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Tong GX, Geng QQ, Chai J, Cheng J, Chen PL, Liang H, Shen XR, Wang DB. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15:5029-5034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Al Zahrani H, Kyoung Kim T, Khalili K, Vlachou P, Yu H, Jang HJ. IgG4-related disease in the abdomen: a great mimicker. Semin Ultrasound CT MR. 2014;35:240-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Javadi S, Menias CO, Korivi BR, Shaaban AM, Patnana M, Alhalabi K, Elsayes KM. Pancreatic Calcifications and Calcified Pancreatic Masses: Pattern Recognition Approach on CT. AJR Am J Roentgenol. 2017;209:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Oshikawa O, Tanaka S, Ioka T, Nakaizumi A, Hamada Y, Mitani T. Dynamic sonography of pancreatic tumors: comparison with dynamic CT. AJR Am J Roentgenol. 2002;178:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | D'Onofrio M, Barbi E, Dietrich CF, Kitano M, Numata K, Sofuni A, Principe F, Gallotti A, Zamboni GA, Mucelli RP. Pancreatic multicenter ultrasound study (PAMUS). Eur J Radiol. 2012;81:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 51. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 52. | Nicaud M, Hou W, Collins D, Wagh MS, Chauhan S, Draganov PV. The utility of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. Gastroenterol Res Pract. 2010;2010:268290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Löhr JM, Vujasinovic M, Rosendahl J, Stone JH, Beuers U. IgG4-related diseases of the digestive tract. Nat Rev Gastroenterol Hepatol. 2022;19:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 54. | Martín-Nares E, Hernández-Molina G, Baenas DF, Paira S. IgG4-Related Disease: Mimickers and Diagnostic Pitfalls. J Clin Rheumatol. 2022;28:e596-e604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Cao Z, Tian R, Zhang T, Zhao Y. Localized Autoimmune Pancreatitis: Report of a Case Clinically Mimicking Pancreatic Cancer and a Literature Review. Medicine (Baltimore). 2015;94:e1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020;115:322-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 57. | Storz P, Crawford HC. Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2020;158:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 58. | Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, Okazaki K; Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. 2020;55:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 59. | Kennedy T, Preczewski L, Stocker SJ, Rao SM, Parsons WG, Wayne JD, Bell RH, Talamonti MS. Incidence of benign inflammatory disease in patients undergoing Whipple procedure for clinically suspected carcinoma: a single-institution experience. Am J Surg. 2006;191:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Pelaez-Luna M, Soriano-Rios A, Lira-Treviño AC, Uscanga-Domínguez L. Steroid-responsive pancreatitides. World J Clin Cases. 2020;8:3411-3430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 61. | Soriano Rios A, Paredes H, Hernández-Calleros J, Uscanga-Domínguez L, Peláez-Luna M. Retroperitoneal fibrosis. Steroid treatment response seems to depend on its association to IgG4 related disease. Med Hypotheses. 2019;122:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 62. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, Tsubota K, Yoshino T, Kawa S, Suzuki R, Takegami T, Tomosugi N, Kurose N, Ishigaki Y, Azumi A, Kojima M, Nakamura S, Inoue D; Research Program for Intractable Disease by Ministry of Health, Labor and Welfare (MHLW) Japan G4 team. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 63. | Dite P, Novotny I, Dvorackova J, Kianicka B, Blaho M, Svoboda P, Uvirova M, Rohan T, Maskova H, Kunovsky L. Pancreatic Solid Focal Lesions: Differential Diagnosis between Autoimmune Pancreatitis and Pancreatic Cancer. Dig Dis. 2019;37:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Culver EL, Sadler R, Simpson D, Cargill T, Makuch M, Bateman AC, Ellis AJ, Collier J, Chapman RW, Klenerman P, Barnes E, Ferry B. Elevated Serum IgG4 Levels in Diagnosis, Treatment Response, Organ Involvement, and Relapse in a Prospective IgG4-Related Disease UK Cohort. Am J Gastroenterol. 2016;111:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | Kiyama K, Yoshifuji H, Kandou T, Hosono Y, Kitagori K, Nakashima R, Imura Y, Yukawa N, Ohmura K, Fujii T, Kawabata D, Mimori T. Screening for IgG4-type anti-nuclear antibodies in IgG4-related disease. BMC Musculoskelet Disord. 2015;16:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Aparisi L, Farre A, Gomez-Cambronero L, Martinez J, De Las Heras G, Corts J, Navarro S, Mora J, Lopez-Hoyos M, Sabater L, Ferrandez A, Bautista D, Perez-Mateo M, Mery S, Sastre J. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut. 2005;54:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 67. | Peláez-Luna M, Medina-Campos C, Uscanga-Domínuez L, Hernandez-Calleros J, Chan-Nuñez C, Negrete E, Angeles A. A Nondilated Main Pancreatic Duct Predicts Type 2 Autoimmune Pancreatitis: Comparative Study of Resected Pancreatic Head Masses. Digestion. 2020;101:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 68. | Ráty S, Sand J, Nordback I, Rinta-Kiikka I, Vasama K, Hagström J, Nordling S, Sirén J, Kiviluoto T, Haglund C. Tumor-like Chronic Pancreatitis Is Often Autoimmune Pancreatitis. Anticancer Res. 2015;35:6163-6166. [PubMed] |
| 69. | Dickerson LD, Farooq A, Bano F, Kleeff J, Baron R, Raraty M, Ghaneh P, Sutton R, Whelan P, Campbell F, Healey P, Neoptolemos JP, Yip VS. Differentiation of Autoimmune Pancreatitis from Pancreatic Cancer Remains Challenging. World J Surg. 2019;43:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 71. | Takuma K, Kamisawa T, Gopalakrishna R, Hara S, Tabata T, Inaba Y, Egawa N, Igarashi Y. Strategy to differentiate autoimmune pancreatitis from pancreas cancer. World J Gastroenterol. 2012;18:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Chang WI, Kim BJ, Lee JK, Kang P, Lee KH, Lee KT, Rhee JC, Jang KT, Choi SH, Choi DW, Choi DI, Lim JH. The clinical and radiological characteristics of focal mass-forming autoimmune pancreatitis: comparison with chronic pancreatitis and pancreatic cancer. Pancreas. 2009;38:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Kwon JH, Kim JH, Kim SY, Byun JH, Kim HJ, Lee MG, Lee SS. Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase. Eur Radiol. 2019;29:5763-5771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Treadwell JR, Zafar HM, Mitchell MD, Tipton K, Teitelbaum U, Jue J. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma: A Meta-Analysis. Pancreas. 2016;45:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 76. | Naitoh I, Nakazawa T, Hayashi K, Okumura F, Miyabe K, Shimizu S, Kondo H, Yoshida M, Yamashita H, Ohara H, Joh T. Clinical differences between mass-forming autoimmune pancreatitis and pancreatic cancer. Scand J Gastroenterol. 2012;47:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31:1379-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 78. | Carbognin G, Girardi V, Biasiutti C, Camera L, Manfredi R, Frulloni L, Hermans JJ, Mucelli RP. Autoimmune pancreatitis: imaging findings on contrast-enhanced MR, MRCP and dynamic secretin-enhanced MRCP. Radiol Med. 2009;114:1214-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Löhr JM, Beuers U, Vujasinovic M, Alvaro D, Frøkjær JB, Buttgereit F, Capurso G, Culver EL, de-Madaria E, Della-Torre E, Detlefsen S, Dominguez-Muñoz E, Czubkowski P, Ewald N, Frulloni L, Gubergrits N, Duman DG, Hackert T, Iglesias-Garcia J, Kartalis N, Laghi A, Lammert F, Lindgren F, Okhlobystin A, Oracz G, Parniczky A, Mucelli RMP, Rebours V, Rosendahl J, Schleinitz N, Schneider A, van Bommel EF, Verbeke CS, Vullierme MP, Witt H; UEG guideline working group. European Guideline on IgG4-related digestive disease - UEG and SGF evidence-based recommendations. United European Gastroenterol J. 2020;8:637-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 80. | Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Chamokova B, Bastati N, Poetter-Lang S, Bican Y, Hodge JC, Schindl M, Matos C, Ba-Ssalamah A. The clinical value of secretin-enhanced MRCP in the functional and morphological assessment of pancreatic diseases. Br J Radiol. 2018;91:20170677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Hsu WL, Chang SM, Wu PY, Chang CC. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J Int Med Res. 2018;46:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Backhus J, Seufferlein T, Perkhofer L, Hermann PC, Kleger A. IgG4-Related Diseases in the Gastrointestinal Tract: Clinical Presentation, Diagnosis and Treatment Challenges. Digestion. 2019;100:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Kawakami H, Zen Y, Kuwatani M, Eto K, Haba S, Yamato H, Shinada K, Kubota K, Asaka M. IgG4-related sclerosing cholangitis and autoimmune pancreatitis: histological assessment of biopsies from Vater's ampulla and the bile duct. J Gastroenterol Hepatol. 2010;25:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Yadav AK, Sharma R, Kandasamy D, Pradhan RK, Garg PK, Bhalla AS, Gamanagatti S, Srivastava DN, Sahni P, Upadhyay AD. Perfusion CT - Can it resolve the pancreatic carcinoma versus mass forming chronic pancreatitis conundrum? Pancreatology. 2016;16:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Aslan S, Nural MS, Camlidag I, Danaci M. Efficacy of perfusion CT in differentiating of pancreatic ductal adenocarcinoma from mass-forming chronic pancreatitis and characterization of isoattenuating pancreatic lesions. Abdom Radiol (NY). 2019;44:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Macari M, Spieler B, Kim D, Graser A, Megibow AJ, Babb J, Chandarana H. Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol. 2010;194:W27-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 88. | Shi Y, Cang L, Zhang X, Cai X, Wang X, Ji R, Wang M, Hong Y. The use of magnetic resonance elastography in differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma: A preliminary study. Eur J Radiol. 2018;108:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Zhu L, Guo J, Jin Z, Xue H, Dai M, Zhang W, Sun Z, Xu J, Marticorena Garcia SR, Asbach P, Hamm B, Sack I. Distinguishing pancreatic cancer and autoimmune pancreatitis with in vivo tomoelastography. Eur Radiol. 2021;31:3366-3374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. New Imaging Techniques: Endoscopic Ultrasound-Guided Elastography. Gastrointest Endosc Clin N Am. 2017;27:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y, Walter S, Hanz S, Carl S, Christoph D, Pierre E, Jean-Luc VL, Jacques D, Peter V, Andrian S. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 93. | Hou J, Li X, Xie KP. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol Cancer. 2021;20:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 94. | Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202-10207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 95. | Yan TB, Huang JQ, Huang SY, Ahir BK, Li LM, Mo ZN, Zhong JH. Advances in the Detection of Pancreatic Cancer Through Liquid Biopsy. Front Oncol. 2021;11:801173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Javadrashid D, Baghbanzadeh A, Derakhshani A, Leone P, Silvestris N, Racanelli V, Solimando AG, Baradaran B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 97. | Sherman MH, Beatty GL. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu Rev Pathol. 2023;18:123-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 210] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 98. | Luchini C, Capelli P, Scarpa A. Pancreatic Ductal Adenocarcinoma and Its Variants. Surg Pathol Clin. 2016;9:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 99. | Valdez-Hernández P, Pérez-Díaz I, Soriano-Rios A, Gómez-Islas V, García-Fong K, Hernández-Calleros J, Uscanga-Dominguez L, Pelaez-Luna M. Pancreatogenic Diabetes, 2 Onset Forms and Lack of Metabolic Syndrome Components Differentiate It From Type 2 Diabetes. Pancreas. 2021;50:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Hadidi A. Pancreatic duct diameter: sonographic measurement in normal subjects. J Clin Ultrasound. 1983;11:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Kamat R, Gupta P, Rana S. Imaging in chronic pancreatitis: State of the art review. Indian J Radiol Imaging. 2019;29:201-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Shankar A, Srinivas S, Kalyanasundaram S. Icicle sign: autoimmune pancreatitis. Abdom Radiol (NY). 2020;45:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 103. | Hur BY, Lee JM, Lee JE, Park JY, Kim SJ, Joo I, Shin CI, Baek JH, Kim JH, Han JK, Choi BI. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. J Magn Reson Imaging. 2012;36:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 104. | Campisi A, Brancatelli G, Vullierme MP, Levy P, Ruszniewski P, Vilgrain V. Are pancreatic calcifications specific for the diagnosis of chronic pancreatitis? Clin Radiol. 2009;64:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Negrelli R, Manfredi R, Pedrinolla B, Boninsegna E, Ventriglia A, Mehrabi S, Frulloni L, Pozzi Mucelli R. Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. Eur Radiol. 2015;25:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Zaheer A, Singh VK, Akshintala VS, Kawamoto S, Tsai SD, Gage KL, Fishman EK. Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J Comput Assist Tomogr. 2014;38:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 107. | Palazzo M, Palazzo L, Aubert A, Fabre M, Couvelard A, Vullierme MP, Maire F, Lévy P, Ruszniewski P. Irregular narrowing of the main pancreatic duct in association with a wall thickening is a key sign at endoscopic ultrasonography for the diagnosis of autoimmune pancreatitis. Pancreas. 2015;44:211-215. [PubMed] |
| 108. | Bang SJ, Kim MH, Kim DH, Lee TY, Kwon S, Oh HC, Kim JY, Hwang CY, Lee SS, Seo DW, Lee SK, Song DE, Jang SJ. Is pancreatic core biopsy sufficient to diagnose autoimmune chronic pancreatitis? Pancreas. 2008;36:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Berger Z. [Focal autoimmune pancreatitis versus pancreatic cancer: value of steroid treatment in the diagnosis]. Rev Med Chil. 2014;142:413-417. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |