Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.902
Peer-review started: January 26, 2023
First decision: March 8, 2023
Revised: March 13, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 15, 2023
Processing time: 105 Days and 19.3 Hours
After the failure of second-line standard therapy, effective treatment options for metastatic colorectal cancer are limited, and the duration of remission cannot meet clinical needs. In addition, associated drug toxicity may lead to treatment interruption that may affect patient outcomes. Therefore, more safe, effective and convenient treatments are urgently needed.
Here, we describe a patient with advanced colorectal cancer with multiple metastases in both lungs. Oxaliplatin combined with 5-fluorouracil or cape
This case report detailed preliminary evidence showing that the combination of fruquintinib with tegafur-gimeracil-oteracil potassium chemotherapy double oral therapy may result in longer progression-free survival in patients with advanced colorectal cancer.
Core Tip: After the failure of second-line standard therapy, effective treatment options for metastatic colorectal cancer (CRC) are limited. Here, we describe a patient with CRC with multiple lung metastases. Disease progression occurred after oxaliplatin + 5-fluorouracil or capecitabine as first-line treatment and bevacizumab + irinotecan as second-line treatment. The patient received targeted therapy with fruquintinib and responded well for 12 mo. Progression-free survival was again achieved over 13.5 mo by continuing fruquintinib in combination with tegafur-gimeracil-oteracil potassium chemotherapy. This case detailed preliminary evidence showing that the combination of fruquintinib with tegafur-gimeracil-oteracil potassium resulted in longer progression-free survival in patients with CRC.
- Citation: Qu FJ, Wu S, Kong Y. Oral fruquintinib combined with tegafur-gimeracil-oteracil potassium for advanced colorectal cancer to obtain longer progression-free survival: A case report. World J Gastrointest Oncol 2023; 15(5): 902-910
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/902.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.902
Colorectal cancer (CRC) is one of the most common cancers worldwide and the second most common cause of cancer-related deaths worldwide[1,2]. The most common cause of death in patients with CRC is distant metastasis, with approximately 25%-40% of patients with CRC already having metastases at the time of initial diagnosis[3,4].
Treatment recommendations for metastatic CRC (mCRC) include single or combination chemotherapy [5-fluorouracil (5-FU), capecitabine, oxaliplatin, and irinotecan] with or without targeted therapy (bevacizumab and cetuximab)[5]. These regimens are generally used for first-line and second-line treatments. The median overall survival for advanced CRC has now reached 30 mo by rational drug distribution[6]. For patients who do not respond to first-line and second-line standard therapies, the drugs approved for third-line treatment of mCRC in China currently include regorafenib, fruquintinib and garcia-carbonero (TAS-102), but the efficacy of these three regiments is limited[7,8,10].
5-FU combined with anti-vascular therapy showed synergistic efficacy and increased antitumor activity in refractory mCRC. The toxicity of the combined regimen was considered tolerable[11]. However, the use of bevacizumab needs to be carried out in the hospital. If the combination of two oral drugs is selected, it may reduce the inconvenience of repeated visits to the hospital and improve patient compliance and quality of life.
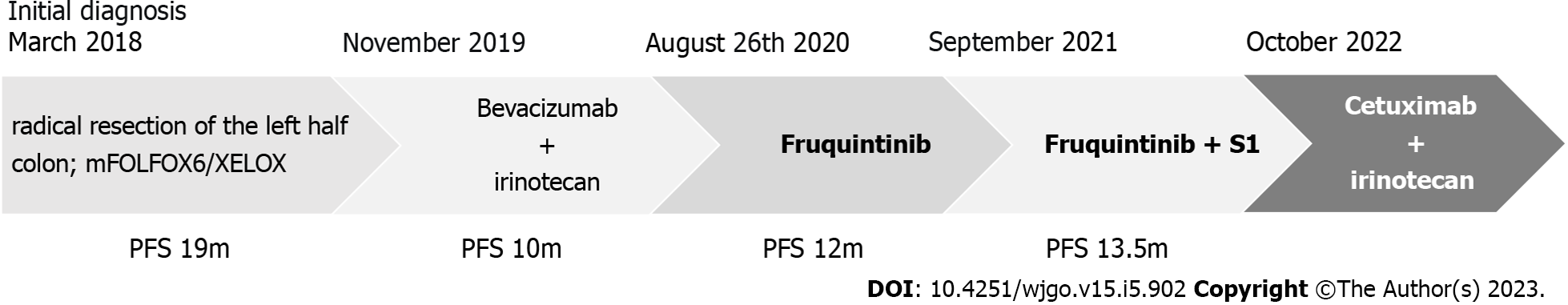
A 58-year-old male presented for treatment in March 2018 due to continuous increased frequency of defecation. Colonoscopy and biopsy revealed sigmoid adenocarcinoma, while chest computed tomography (CT) during the same period indicated multiple pulmonary nodules, which was considered metastasis.
He underwent laparoscopic radical resection of the left half colon on March 28, 2020. The postoperative pathological examination showed: (Sigmoid lesion) moderately differentiated adenocarcinoma; tumor size: 6.5 cm × 4.5 cm; invasion of the whole layer; and 19 lymph nodes around the intestine were negative for cancer metastasis. Immunohistochemistry examinations revealed the following: MSH2 (+), MLH1 (+), PMS2 (+), MSH6 (+), and suggestive pMMR. Genetic testing revealed KRAS, NRAS, BRAF wildtype, microsatellite stable, and UGT1A1 GG type.
He had a history of hypertension and paroxysmal atrial fibrillation for 3 years.
In April 2018, reexamination by chest CT 1 mo after surgery revealed that the pulmonary nodules were larger than before, and abdomen CT showed no active findings.
The patient was diagnosed with stage IV sigmoid adenocarcinoma with multiple lung metastases.
The patient refused to use bevacizumab and cetuximab because of concerns about adverse events such as hypertension and rash associated with targeted drug therapy. Instead starting in April 2018, the patient received six cycles of first-line treatment with mFOLFOX6 regimen (oxaliplatin 165 mg IV on day 1 + leucovorin 0.6 g IV on day 1 + 5-FU 0.75 g IV on day 1 + 5-FU 4.5 g CIV 46 h q2w), during which the intrapulmonary metastases were stable. In August 2018, the patient voluntarily stopped taking the mFOLFOX6 regimen due to personal reasons and did not receive maintenance treatment. Follow-up CT examination during drug withdrawal indicated slow progression of lung lesions.
In February 2019, the patient began the XELOX protocol (oxaliplatin 200 mg IV on day 1 +capecitabine 1.5 g PO bid on days 1–14 q3w) for five cycles of intermittent treatment. After the lung lesions were stable for a period of time, the patient stopped treatment again in July 2019. CT reexamination in October 2019 indicated that the lesions in the lung were enlarged. We judged the efficacy as progressive disease, and the progression-free survival (PFS) time of first-line treatment was 19 mo.
The major adverse events during treatment were grade 2 nausea and vomiting after chemotherapy, myelosuppression (grade 1 leukopenia and neutropenia and grade 2 thrombocytopenia), grade 1 liver dysfunction, and grade 1 numbness of the hands and feet. These adverse reactions and the inconvenience of repeated visits to the hospital were the main reasons the patient decided to stop treatment many times. The patient had no coughing and wheezing during treatment and maintained an Eastern Cooperative Oncology Group performance score of 0.
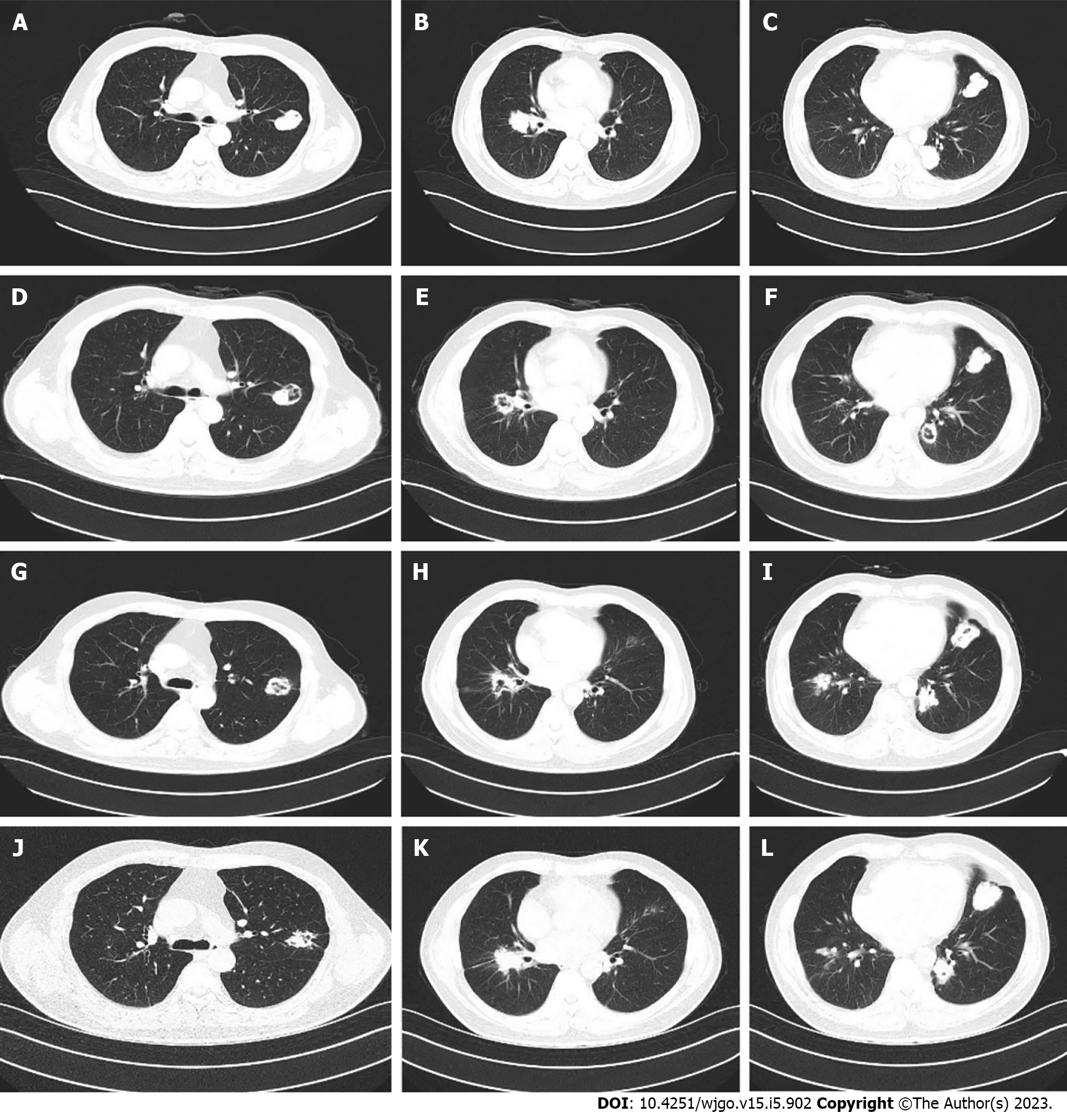
The patient was still unable to receive cetuximab due to anxiety about the rash. Second-line bevacizumab combined with irinotecan was initiated in November 2019 for a total of eight cycles. Bevacizumab (500 mg) and irinotecan (350 mg) were given every 3 wk. During second-line treatment, the patient’s treatment was interrupted for 2 mo due to the coronavirus disease 2019 pandemic. The treatment evaluation was stable disease. However, grade 2 nausea and vomiting and grade 1 diarrhea occurred repeatedly during the treatment, and the patient could not tolerate these adverse events. Second-line treatment continued until July 2020. Due to no signs of disease progression (Figure 1A-C), the patient again requested that the treatment be discontinued and replaced with oral medication.
Therefore, treatment with oral fruquintinib [5 mg once a day (qd) on days 1–21 every 4 wk] was administrated as a third-line treatment starting on August 26, 2020. CT reexamination after 3 mo of treatment showed no significant change in the size of the metastatic lesions in both lungs, but the cavity in the lesions increased (Figure 1D-F). Subsequent follow-up and CT examination of the lung lesions remained stable (Figure 1G-I), while the main toxicity experienced by the patient during this period was hypertension, which could be well managed.
After 12 mo, some lesions in the lung slowly enlarged again (Figure 1J-L). After communication with the patient, the patient refused to receive intravenous therapy. Considering the slow growth of the lesions and no new metastatic lesions after comprehensive evaluation, our treatment group suggested that the patient should continue fruquintinib in combination with another oral chemotherapy drug: tegafur-gimeracil-oteracil potassium capsules (S-1). After repeated consultations with the patient and the signing of informed consent, the regimen of fruquintinib + S-1 was initiated as fourth-line treatment in September 2021. Fruquintinib (5 mg) was given once daily, 2 wk on and 1 wk off; S-1 (60 mg) was given twice daily, 2 wk on and 1 wk off.
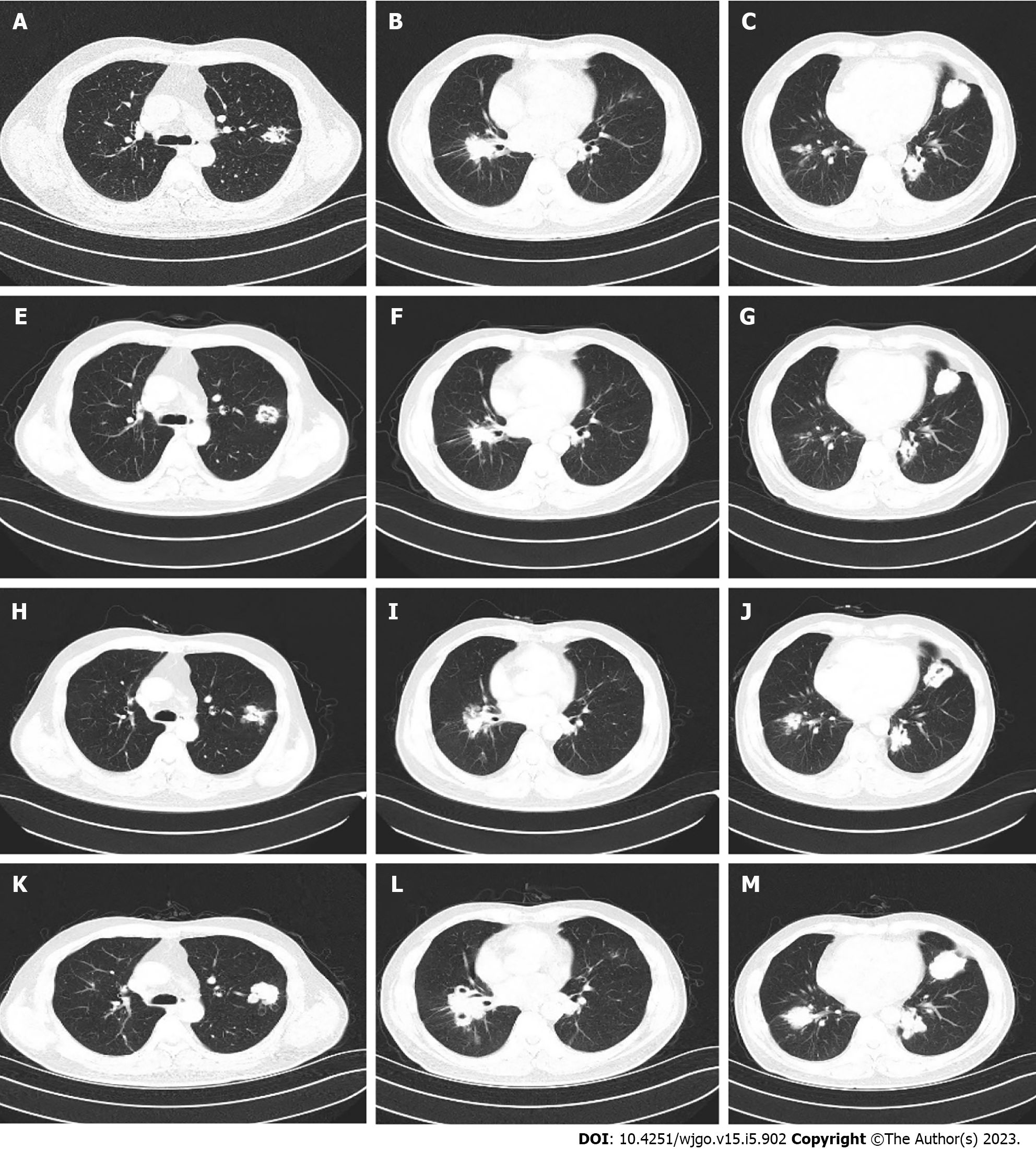
During administration, the patient experienced grade 2 leukocyte decline and grade 1 diarrhea. Considering the patient’s high quality of life requirements, we decided to adjust the dose of S-1 based on toxicity 9graded according to National Cancer Institute CTCAE V.4.0) and possible benefits. S-1 was adjusted from 60 mg twice daily to 40 mg in the morning and 60 mg in the evening. At the same time, nifedipine sustained release tablets and skin moisturizing care were given regularly to monitor the adverse reactions (grade I hypertension, grade I hand-foot syndrome). Follow-up during medication was regular, and chest CT examination showed stable lung metastases until August 2022 (Figure 2). The patient maintained good quality of life, and the Eastern Cooperative Oncology Group score was 0.
Unfortunately, another chest CT examination in October 2022 revealed the enlargement of pulmonary metastases and the appearance of multiple new lesions (Figure 2), which was judged to be disease progression. In addition, abdomen CT still showed no active findings. PFS of 13.5 mo was achieved by fruquintinib S-1.
The patient achieved a continuous survival benefit from continued treatment with fruquintinib monotherapy after diagnosis of mCRC and subsequent combination therapy with S-1, including at least 25.5 mo of PFS and 30 mo of overall survival (Figure 3).
Considering that the second-line treatment of irinotecan had not shown resistance combined with the genetic test results of the patient, we started the cetuximab plus irinotecan regimen as the fifth-line treatment starting in October 2022. The efficacy of this regimen was not available by the submission date.
In this case report, informed consent was obtained before each treatment. The therapeutic effect was evaluated by the Response Evaluation Criteria in Solid Tumors criteria. The nature and severity of adverse events were evaluated according to the National Cancer Institute CTCAE 4.0.
Informed consent for treatment was signed by the patient before each treatment, and written informed consent was obtained from the patient in order to publish relevant clinical and imaging data. These situations are in line with the ethical standards to be implemented in research involving human participants by institutions and/or national research councils as well as the Declaration of Helsinki (2013 revision).
Angiogenesis plays a critical role in the neoplastic growth, progression, and metastasis of CRC[12]. It was reported that vascular endothelial growth factor (VEGF) secreted by tumor cells can stimulate endothelial cell survival and proliferation, causing changes in vascular permeability and promoting neo-angiogenesis[12]. Anti-angiogenetic therapy is an important strategy for mCRC treatment. Anti-angiogenic agents include the small molecule inhibitors that block the activity of VEGF receptor kinases and the neutralization antibody of VEGF (monoclonal antibodies), which inhibit angiogenesis, cause vascular degeneration, and normalize tumor blood vessels[14]. Bevacizumab is the most successful antibody for neutralizing VEGF, and it has been authorized for mCRC first-line and second-line treatment combined with chemotherapy[5,15,16]. Nonetheless, there are some disadvantages associated with the use of bevacizumab as a monoclonal antibody, including immunogenicity and intravenous administration.
Fruquintinib is a highly selective and potent small-molecule inhibitor against VEGF receptor 1, 2, and 3[17]. It originated and was developed by Hutchison MediPharma and was authorized for third-line treatment in mCRC in China. Fruquintinib is a highly selective inhibitor of the VEGF signaling pathway, which can decrease cell proliferation of human umbilical vein endothelial cells and human lymphatic endothelial cells. It also decreases tumor angiogenesis and lymphangiogenesis, effectively reduces blood vessel density, inhibits tumor proliferation and migration, and has strong antitumor activity[17].
In clinical studies, fruquintinib has shown a survival benefit in mCRC patients. In the phase III FRESCO trial, the fruquintinib group had significantly prolonged median overall survival compared with the placebo group [9.3 mo; 95% confidence interval (CI): 8.2-10.5 vs 6.6 mo; 95%CI: 5.9-8.1]. The median PFS of fruquintinib was also significantly increased (3.7 mo; 95%CI: 3.7-4.6 vs 1.8 mo; 95%CI: 1.8-1.8)[8]. Fruquintinib also showed an acceptable safety and tolerability profile[8,9].
Our patient chose fruquintinib after drug resistance and intolerance of first-line and second-line treatments. He achieved 12 mo of PFS with monotherapy while maintaining a good quality of life. However, fruquintinib also faced the challenge of drug resistance.
In addition to molecular targeted therapy drugs, chemotherapy is still the main treatment for patients with advanced CRC, among which 5-FU based chemotherapy combined with oxaliplatin or irinotecan is the preferred standard treatment for patients with advanced or recurrent CRC[18]. However, if a patient does not respond to chemotherapy or has a toxic reaction, there are few remaining options.
In 2017, another oral drug, trifluridine-tipiracil (TAS-102), was approved by the United States Food and Drug Administration, which finally offered another potential option for patients with mCRC[19]. TAS-102 has shown a significant overall survival benefit compared with placebo in patients with chemorefractory mCRC in the phase III RECOURSE trial, with a longer median overall survival (7.1 mo vs 5.3 mo) and a longer median PFS (2.0 mo vs 1.7 mo) in the TAS-102 group[10]. Unfortunately, neither fruquintinib nor TAS-102 gave patients with refractory mCRC a satisfactory PFS[8,10].
Considering that targeted anti-angiogenic drugs combined with chemotherapy play a synergistic antitumor role by inhibiting neovascularization, inducing vascular normalization, and enhancing cytotoxic drug delivery, anti-angiogenic therapy combined with chemotherapy drugs have been explored for refractory CRC. Pfeiffer et al[11] conducted a phase II clinical study after previous studies achieved good results and continued to explore the safety and effectiveness of the combination of bevacizumab and TAS-102. The median PFS was 2.6 mo in the TAS-102 group and 4.6 mo in the TAS-102 + bevacizumab group. TAS-102 combined with bevacizumab showed significant clinically relevant improvement in PFS with tolerable toxicity compared to TAS-102 monotherapy.
Based on these results, continuous anti-vascular targeted therapy combined with cytotoxic drugs may have clinical significance in patients with refractory advanced CRC. This combination therapy may change practice in the future, but we do not yet understand the characteristics of patients most suitable for second-line post-combination therapy. At present, the price of TAS-102 is relatively high in China, and it is not covered by medical insurance. Meanwhile, bevacizumab requires intravenous infusion. Therefore, a safe, effective, and more convenient and economical treatment plan is urgently needed in clinic.
S-1, a novel oral 5-FU derivative developed by Taiho Pharmaceuticals Company (Japan), has been widely used in the treatment of gastrointestinal malignancies[20]. S-1 monotherapy has an effectiveness rate of 19%-39% in the treatment of advanced CRC[21,22] and has been shown to be as effective as 5-FU and capecitabine[23,24]. In these studies, the toxicity of S-1 treatment was primarily hematological, while hand-foot syndrome was more common in the capecitabine group. However, there was no statistically significant difference between the two groups. Therefore, S-1 is also an option for the treatment of advanced CRC[22,23,24].
Based on the above information, it is reasonable to conclude that fruquintinib combined with S-1 may be superior to fruquintinib or S-1 alone in some patients with refractory CRC who do not respond to standard therapy. The therapeutic mechanism of this combination regimen may be associated with the synergistic antitumor action in addition to the respective antitumor effects of fruquintinib and S-1.
In this case, some lung metastases slowly enlarged after 12 mo of monotherapy with fruquintinib. Combined with the results of the above related studies and after repeated communication with the patient and signing the informed consent, the patient began treatment with the combination of fruquintinib and cytotoxic drug S-1. The patient gained 13.5 mo of PFS, and the lung metastases were again well controlled. Only grade 2 leukopenia, grade 1 diarrhea, grade 1 hypertension, and grade 1 hand-foot syndrome were observed during the treatment period.
In the phase III CORRECT, FRESCO, and RECOURSE trials, the median PFS of patients in the regorafenib group, fruquintinib group, and TAS-102 group were 1.9 mo, 3.7 mo, and 2.0 mo, respectively[7,8,10]. Considering that the absolute PFS benefit time of third-line treatment was shorter in previous clinical trials, we hypothesize that the combination of fruquintinib and S-1 caused the longer survival benefit. At the same time, the combination of the two oral drugs reduced the inconvenience of repeated visits to the hospital and improved the patient’s quality of life. This treatment combination may have potential clinical significance in future salvage treatment of advanced CRC. Since China is still a developing country, we also need to consider the factors of pharmacoeconomics to reduce the economic burden of patients as much as possible[25]. The strategy of using oral drugs also reduced the risk of exposure to coronavirus disease 2019 during the pandemic. To our knowledge, this is the first time that fruquintinib combined with S-1 in the treatment of advanced CRC has been reported with a good efficacy.
In conclusion, the prognosis of CRC after advanced multiline therapy is extremely poor, and survival after existing third-line and post-third-line therapies is limited. Therefore, it is very important to explore suitable therapies for patients with refractory advanced CRC to prolong the overall survival time of patients and enhance the quality of life of patients as much as possible.
Although fruquintinib combined with S1 is not currently the standard treatment for mCRC, this case provides evidence for its antitumor activity and safety. Given that only 1 patient was observed in this report and the clinical data is very limited, further investigation and accumulation of more experience are needed. Further clinical studies can be conducted to demonstrate the efficacy and safety of this combination regimen.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Bordonaro M, United States S-Editor: Ma YJ L-Editor: Filipodia A P-Editor: Wu RR
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11940] [Article Influence: 2985.0] [Reference Citation Analysis (4)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64673] [Article Influence: 16168.3] [Reference Citation Analysis (176)] |
| 3. | Shah MA, Renfro LA, Allegra CJ, André T, de Gramont A, Schmoll HJ, Haller DG, Alberts SR, Yothers G, Sargent DJ. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J Clin Oncol. 2016;34:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Xu R, Wang W, Zhu B, Lin X, Ma D, Zhu L, Zhao Q, Nie Y, Cai X, Li Q, Fang W, Li H, Wang N, Chen Y, Peng C, Fang H, Shen L. Disease characteristics and treatment patterns of Chinese patients with metastatic colorectal cancer: a retrospective study using medical records from China. BMC Cancer. 2020;20:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Cao R, Zhang S, Ma D, Hu L. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol. 2015;32:325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a andomized, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1331] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 7. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, andomized, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2118] [Article Influence: 176.5] [Reference Citation Analysis (0)] |
| 8. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 9. | Li J, Guo W, Bai Y, Deng Y, Yang L, Chen Z, Zhong H, Xu R, Pan H, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Xu J, Chen D, Li W, Sun S, Yu Z, Cao P, Shen L, Chen H, Wang S, Wang H, Fan S, Guo X, Wang N, Han R, Zhang B, Qin S. Safety Profile and Adverse Events of Special Interest for Fruquintinib in Chinese Patients with Previously Treated Metastatic Colorectal Cancer: Analysis of the Phase 3 FRESCO Trial. Adv Ther. 2020;37:4585-4598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1010] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 11. | Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, Poulsen LØ, Winther SB, Thomsen KG, Qvortrup C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, andomized, phase 2 trial. Lancet Oncol. 2020;21:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 12. | Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1757] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 13. | Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368-4380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1170] [Cited by in RCA: 1152] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 14. | Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs. 2016;25:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O’Neil BH, Atkins JN, Berry S, Polite BN, O’Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 16. | Moehler M, Sprinzl MF, Abdelfattah M, Schimanski CC, Adami B, Godderz W, Majer K, Flieger D, Teufel A, Siebler J, Hoehler T, Galle PR, Kanzler S. Capecitabine and irinotecan with and without bevacizumab for advanced colorectal cancer patients. World J Gastroenterol. 2009;15:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Sun Q, Zhou J, Zhang Z, Guo M, Liang J, Zhou F, Long J, Zhang W, Yin F, Cai H, Yang H, Gu Y, Ni L, Sai Y, Cui Y, Zhang M, Hong M, Sun J, Yang Z, Qing W, Su W, Ren Y. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15:1635-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 19. | Marcus L, Lemery SJ, Khasar S, Wearne E, Helms WS, Yuan W, He K, Cao X, Yu J, Zhao H, Wang Y, Stephens O, Englund E, Agarwal R, Keegan P, Pazdur R. FDA Approval Summary: TAS-102. Clin Cancer Res. 2017;23:2924-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602-2606. [PubMed] |
| 21. | Shin SJ, Jeong JH, Park YS, Lee KH, Shim BY, Kim TW, Oh DY, Lee MA, Kim YT, Kim YH, Zang DY, Roh JK, Ahn JB. Phase II trial of S-1 monotherapy in elderly or frail patients with metastatic colorectal cancer. Invest New Drugs. 2011;29:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H, Satoh A. Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer. 2004;100:2355-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, Chung IJ, Cho SH, Lee KH, Shin SJ, Kang HJ, Shin DB, Jo SJ, Lee JW. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a andomized, non-inferiority phase 3 trial. Lancet Oncol. 2012;13:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Liu H, Wang Y, Li G, Song W, Wang R. Clinical study of tegafur-gimeracil-oteracil potassium capsule (s-1) and oxaliplatin combination chemotherapy in advanced colorectal cancer. J Cancer Res Ther. 2015;11:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Guan X, Li H, Xiong X, Peng C, Wang N, Ma X, Ma A. Cost-effectiveness analysis of fruquintinib versus regorafenib as the third-line therapy for metastatic colorectal cancer in China. J Med Econ. 2021;24:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |