Published online Mar 15, 2023. doi: 10.4251/wjgo.v15.i3.533
Peer-review started: November 13, 2022
First decision: January 11, 2023
Revised: February 9, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 15, 2023
Processing time: 121 Days and 13.6 Hours
Increasingly extranodal marginal B-cell lymphoma of mucosa-associated ly
To create an effective survival nomogram for patients with primary gastric GML.
All data of patients with primary GML from 2004 to 2015 were collected from the SEER database. The primary endpoint was OS. Based on the LASSO and COX regression, we created and further verified the accuracy and effectiveness of the survival nomogram model by the concordance index (C-index), calibration curve and time-dependent receiver operating characteristic (td-ROC) curves.
A total of 2604 patients diagnosed with primary GML were selected for this study. A total of 1823 and 781 people were randomly distributed into the training and testing sets at a ratio of 7:3. The median follow-up of all patients was 71 mo, and the 3- and 5-year OS rates were 87.2% and 79.8%, respectively. Age, sex, race, Ann Arbor stage and radiation were independent risk factors for OS of primary GML (all P < 0.05). The C-index values of the nomogram were 0.751 (95%CI: 0.729-0.773) and 0.718 (95%CI: 0.680-0.757) in the training and testing cohorts, respectively, showing the good discrimination ability of the nomogram model. Td-ROC curves and calibration plots also indicated satisfactory predictive power and good agreement of the model. Overall, the nomogram shows favorable performance in discriminating and predicting the OS of patients with primary GML.
A nomogram was developed and validated to have good survival predictive performance based on five clinical independent risk factors for OS for patients with primary GML. Nomograms are a low-cost and convenient clinical tool in assessing individualized prognosis and treatment for patients with primary GML.
Core Tip: Increasingly extranodal marginal B-cell lymphoma of mucosa-associated lymphoid tissue, known as mucosa-associated lymphoid tissue (MALT) lymphoma, is a type of non-Hodgkin’s lymphoma. The prognosis of primary gastric MALT (GML) patients can be affected by many factors. The available data are mainly focused on epidemiology; in contrast, few studies have investigated the prognostic variables for overall survival (OS) in patients with primary GML. Based on the realities above, we searched a large amount of data on patients diagnosed with primary GML in the Surveillance, Epidemiology and End Results database. The aim was to develop and verify a survival nomogram model that can predict the OS prognosis of primary GML by combining prognostic and determinant variables. In this manuscript, our results demonstrated that Nomogram is developed and validated to have a good survival predictive performance, basing on five clinical independent risk factors of OS for primary GML patients. Nomogram will be a low cost and convenient clinical tool in assessing individualized prognosis and treatment for primary GML patients.
- Citation: Wang D, Shi XL, Xu W, Shi RH. Nomogram model predicting the overall survival for patients with primary gastric mucosa-associated lymphoid tissue lymphoma. World J Gastrointest Oncol 2023; 15(3): 533-545
- URL: https://www.wjgnet.com/1948-5204/full/v15/i3/533.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i3.533
Increasingly extranodal marginal B-cell lymphoma of mucosa-associated lymphoid tissue, known as mucosa-associated lymphoid tissue (MALT) lymphoma, is a type of non-Hodgkin’s lymphoma[1,2]. Approximately one-third of cases of MALT lymphoma and 85% of gastrointestinal MALT lymphomas present in the stomach as the affected site[3,4]. Primary gastric MALT lymphoma (GML) has a rare incidence and only accounts for approximately 5% of all primary gastric neoplasms[5].
This disease demands high attention. Researchers have observed that GML patients had significant risk of atrophic gastritis, intestinal metaplasia[6,7] and secondary tumors[8]. Compared to the healthy population, the incidence of gastric adenocarcinoma in GML patients was 6 times higher in the GML population[9,10].
The prognosis of primary GML patients can be affected by many factors. Clinical risk factors, including age, type of therapy, sex, stage and family hematologic malignancy history, also have significant effects on the development of the disease[11-14]. Meanwhile, previous studies demonstrated a good prognosis of the disease, with 5-year survival rates of up to 99%. However, more than 95% of studies were only based on stage I/II patients with a small sample size, and few of them even used different staging standards[15-19]. Matysiak-Budnik et al[11] conducted a multicenter study in France, and 416 GML patients were retrospectively enrolled. They surprisingly found that 25% of subjects diagnosed at stage III/IV and 11% of patients obtained missed or false diagnoses, which was similar to other studies[20-22].
Furthermore, the available data are mainly focused on epidemiology; in contrast, few studies have investigated the prognostic variables for overall survival (OS) in patients with primary GML. Based on the realities above, we searched a large amount of data on patients diagnosed with primary GML in the Surveillance, Epidemiology and End Results (SEER) database. The aim was to develop and verify a survival nomogram model that can predict the OS prognosis of primary GML by combining prognostic and determinant variables.
The data for our study were extracted from the SEER database (username: 12262-November 2019, software version: SEER * Stat 8.3.6). Due to the openness of the database, our research was exempted from the need for approval by the Ethics Committee of Zhongda Hospital, the Affiliated Hospital of Southeast University.
All patients diagnosed with primary GML from 2004 to 2015 were ultimately included in this study. Subjects meeting the following conditions were excluded: (1) Hospitalized death and autopsy source patients; (2) patients with tumor history; (3) patients who were not followed up or who were lost follow-up; (4) age at diagnosis < 20; (5) unknown data (race, stage, and cause of death); and (6) survival months < 1.
A total of 2604 selected GML patients were randomly assigned to the training and validation sets with a ratio of 7:3. There were 1823 and 781 people in the two groups, respectively.
The clinical covariates included sex, age, race, primary site, Ann Abor stage, surgery, chemotherapy, and radiation. Data on the survival month and vital status of patients were also analyzed. The primary endpoint was OS.
In the training set, the least absolute shrinkage and selection operator (LASSO) and multivariate Cox proportional hazard regression were combined to identify the significantly correlated prognostic factors that influenced OS. The nomogram model was established based on the above results. Meanwhile, primary GML patients were divided into low- and high-risk groups at the cutoff point of the risk score. Scatter plots, forest plots and Kaplan-Meier curves were generated to visually compare the OS times of patients in the two different risk groups.
Internal validation was performed on the patients in the validation set. The discriminatory per
Statistical analysis was performed by IBM SPSS version 22.0 (Chicago, IL, United States) and R version 4.0.2. Continuous variables were grouped and transformed into categorical variables and are expressed as frequency and percentage values, n (%). The chi-square test was used to compare categorical variables between groups. Two-sided P values < 0.05 were considered statistically significant.
The specific clinical and pathological characteristics of all enrolled patients and the training and testing groups are presented in Table 1.
| Variable | All cohort | Training cohort | Validation cohort | P valuea |
| Total | 2604 | 1823 | 781 | |
| Age | 0.423 | |||
| <45 | 234(8.98) | 168 (71.79) | 66(28.21) | |
| 45-65 | 1040(39.94) | 739 (71.06) | 301(28.94) | |
| ≥65 | 1330(51.08) | 916 (68.87) | 414(31.13) | |
| Sex | 0.262 | |||
| Male | 1250(48.00) | 862 (68.96) | 388(31.04) | |
| Female | 1354(52.00) | 961 (70.97) | 393(29.03) | |
| Race | 0.699 | |||
| White | 2059(79.07) | 1434 (69.65) | 625(30.35) | |
| Black | 284(10.91) | 201 (70.77) | 83 (29.23) | |
| Other | 261(10.02) | 188 (72.03) | 73 (27.97) | |
| Location | 0.734 | |||
| Upper third | 314(12.06) | 212 (67.52) | 102(32.48) | |
| Middle third | 642(24.65) | 455 (70.87) | 187(29.13) | |
| Low third | 425(16.32) | 291 (68.47) | 134(31.53) | |
| Stomach, NOS | 1020(39.17) | 723 (70.88) | 297(29.12) | |
| Overlapping lesion | 203(7.80) | 142 (69.95) | 61 (30.05) | |
| of stomach | 0.516 | |||
| Stage (Ann Arbor) | 1452 (69.54) | 636(30.46) | ||
| I | 2088(80.18) | 187 (73.91) | 66 (26.09) | |
| II | 253(9.71) | 47 (72.31) | 18 (27.69) | |
| III | 65(2.50) | 137 (69.19) | 61 (30.81) | |
| IV | 198(7.60) | 0.332 | ||
| Surgery | 1700 (70.25) | 720(29.75) | ||
| No/unknown | 2420(92.93) | 123 (66.85) | 61 (33.15) | |
| Yes | 184(7.07) | 0.82 | ||
| Chemotherapy | 1440 (69.90) | 620(30.10) | ||
| No/unknown | 2060(79.11) | 383 (70.40) | 161(29.60) | |
| Yes | 544(20.89) | 0.36 | ||
| Radiation | 1231 (70.58) | 513(29.42) | ||
| No/unknown | 1744(66.97) | 592 (68.84) | 268(31.16) | |
| Yes | 860(33.03) |
Initially, 4475 GML patients were searched in the SEER database. The following populations were excluded: One primary tumor = NO (n = 1408), age < 20 (n = 9), unknown race (n = 74), unknown stage (n = 317), unknown COD (n = 16), and survival month < 1 (n = 47). In total, 2604 primary GML patients were included in our study. The median survival time was 71 mo.
All patients (median age: 65 years old) were divided into three subgroups (age < 45, 45-65, and > 65; Table 1). Compared to patients < 45 years old (8.98%), the 45-65 years (39.94%) group had a higher proportion (P = 0.001, HR 4.29, 95%CI: 1.881-9.815). The proportion of patients > 65 years old was 51.08%. In total, 1250 (48.00%) were male, and 2059 (79.07%) were white. In addition, primary GML could be located in all parts of the stomach and was mostly diagnosed at stage I (2088/80.18%) according to the Ann Arbor staging system[24]. The numbers of patients who received surgery, chemotherapy and radiation were 184 (7.07%), 544 (20.89%) and 860 (33.03%), respectively. The 3- and 5-year OS rates of primary GML were 87.2% and 79.8%, respectively.
To establish the nomogram model, 1823 subjects were randomly assigned to the training cohort, while 781 were assigned to the validation cohort. No significant difference in variables was observed between the two groups (all P > 0.05).
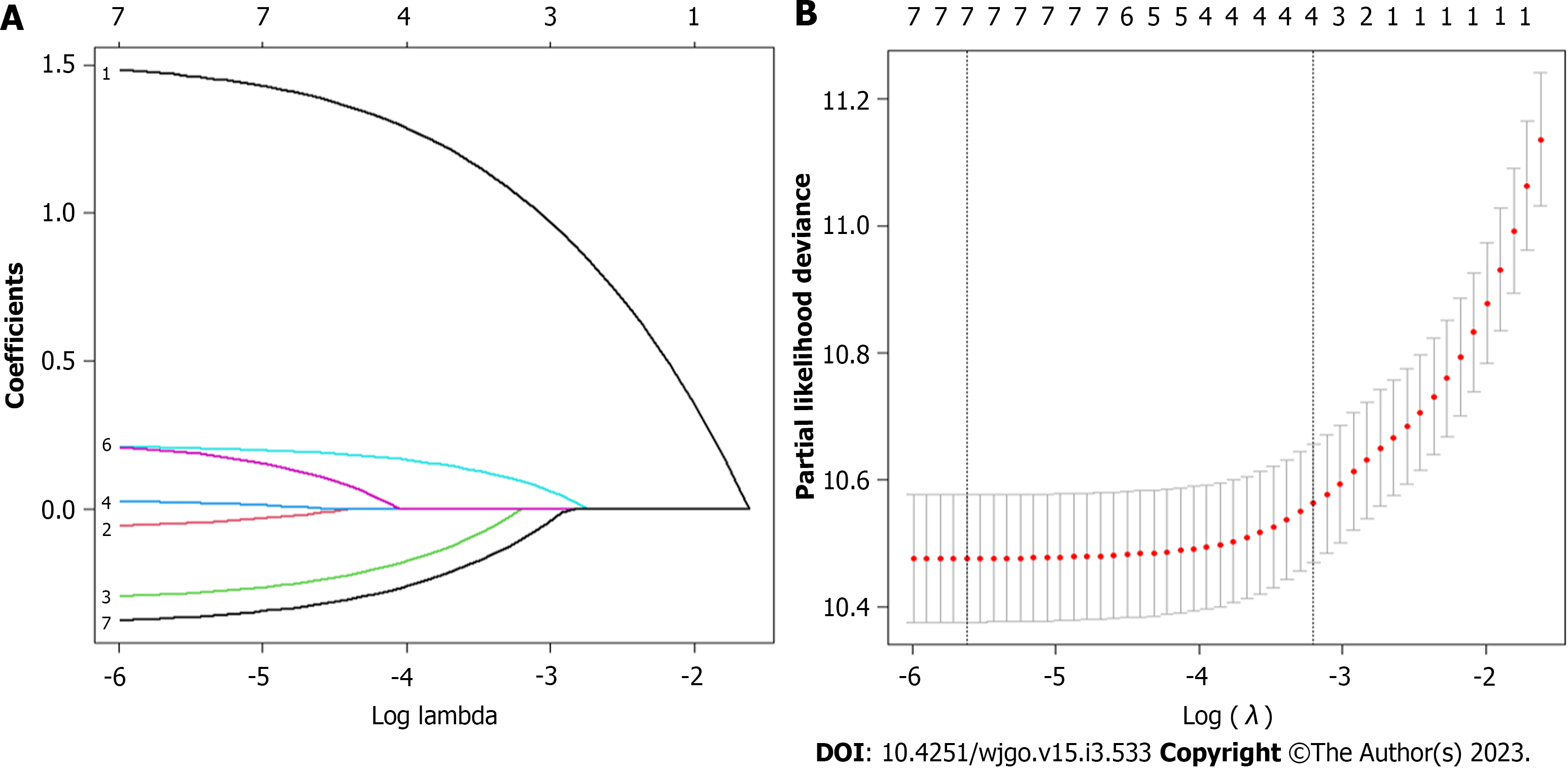
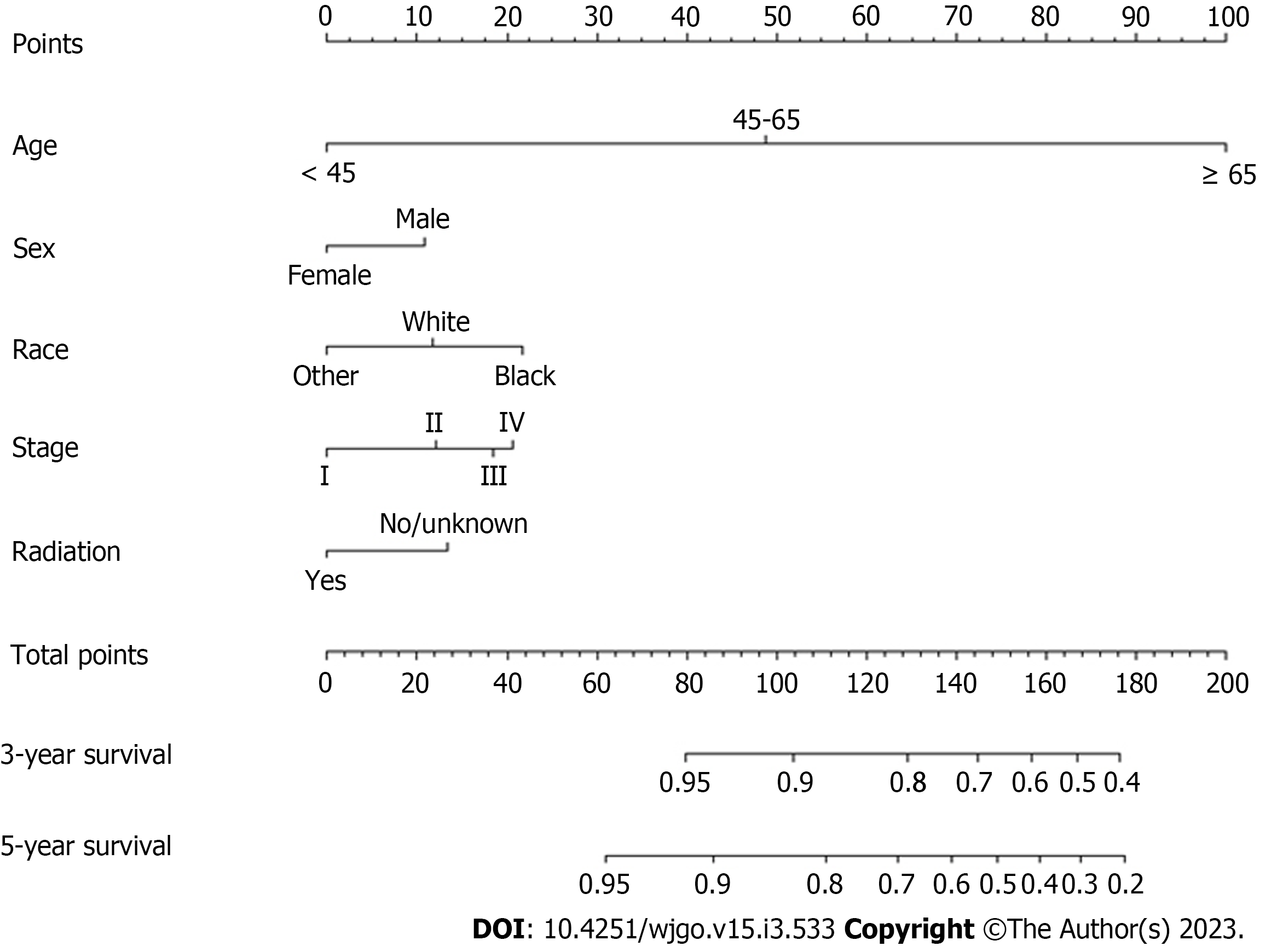
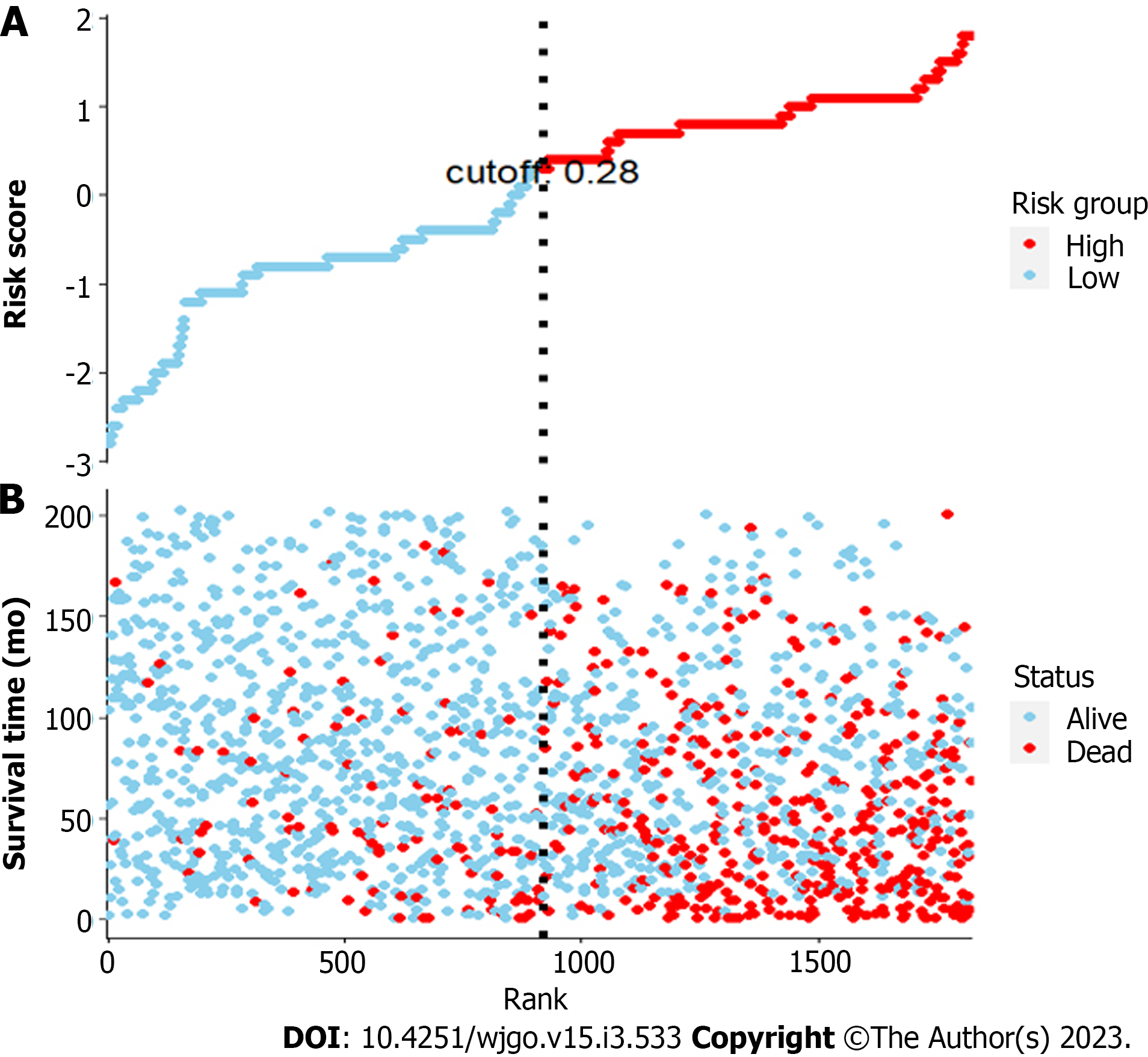
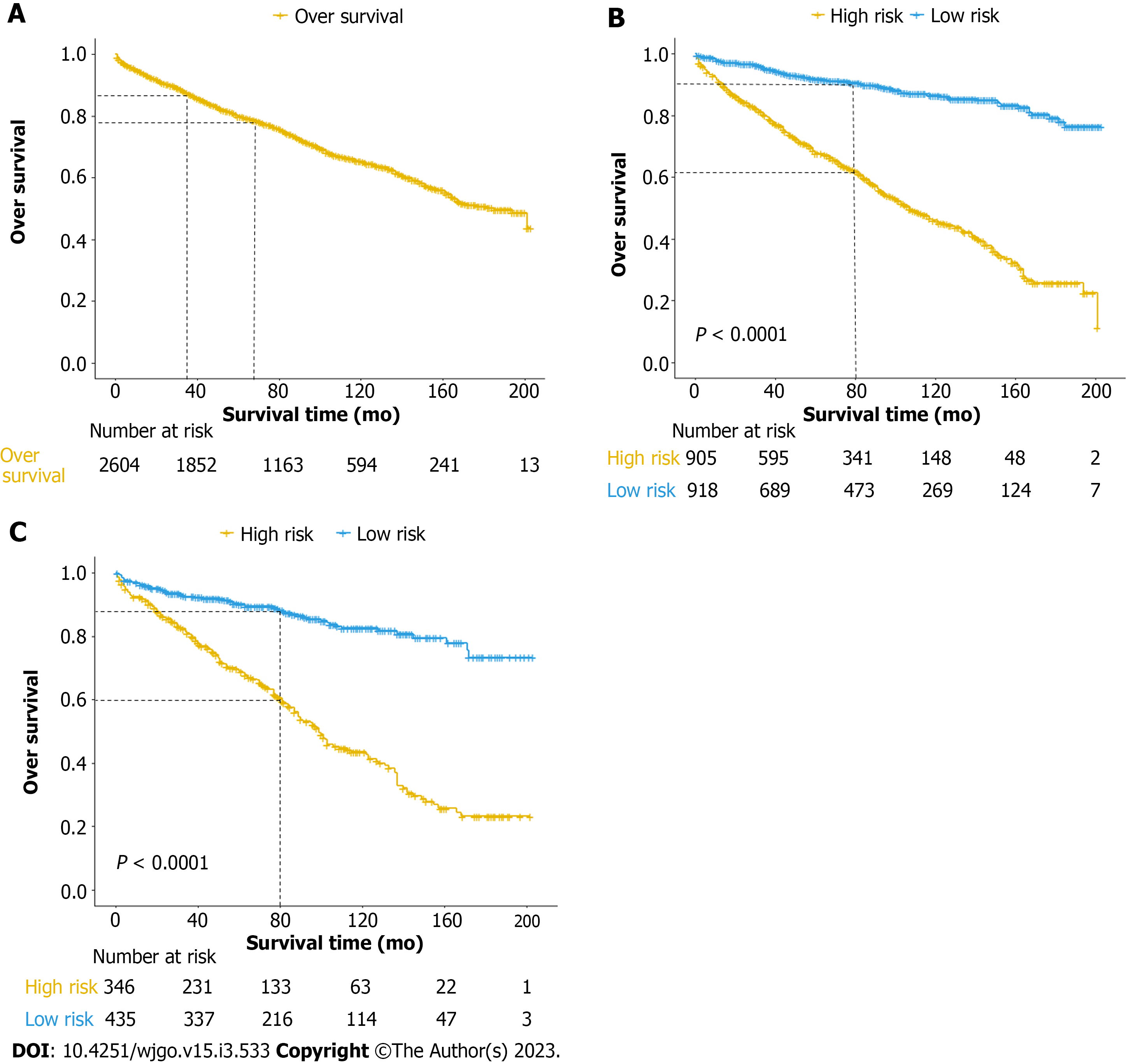
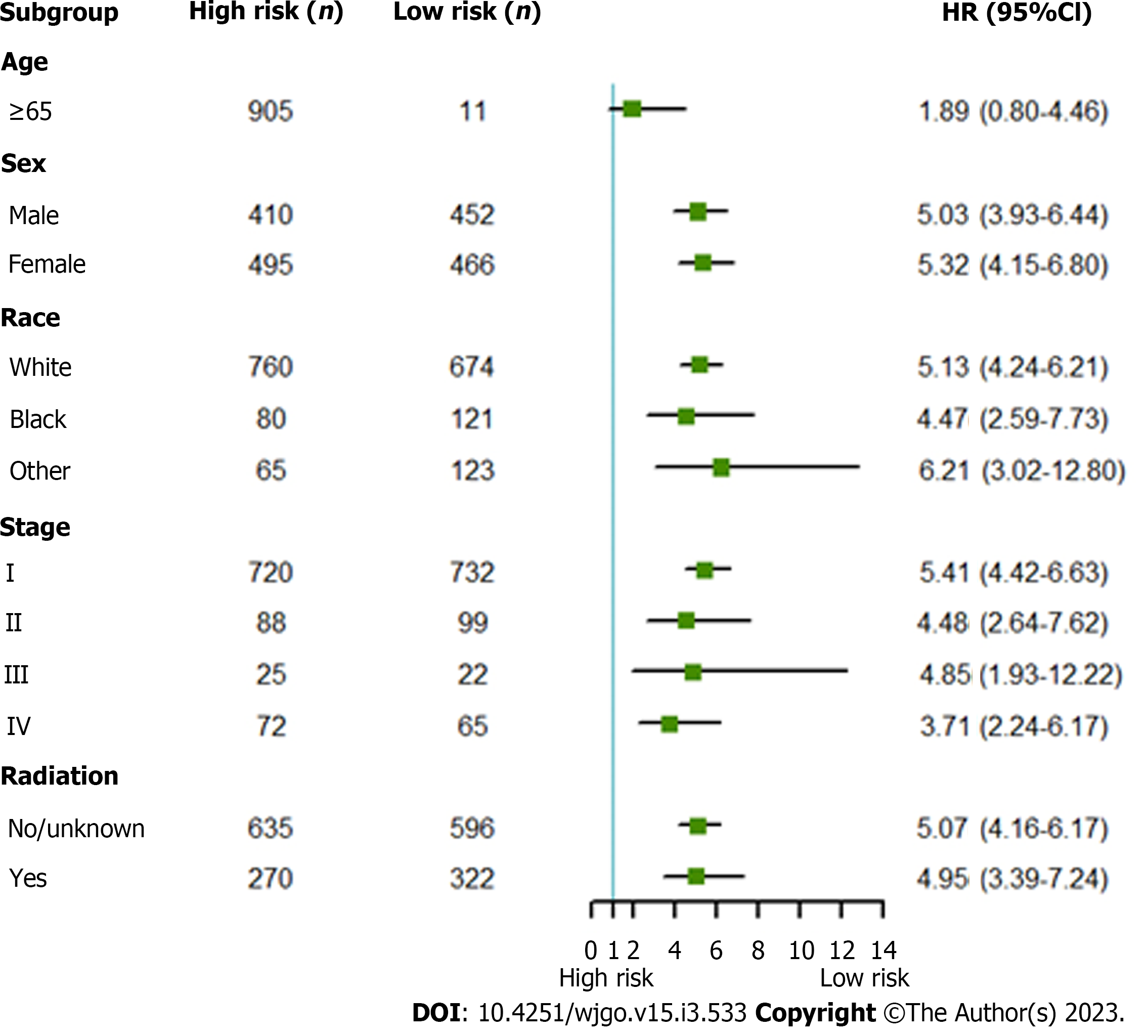
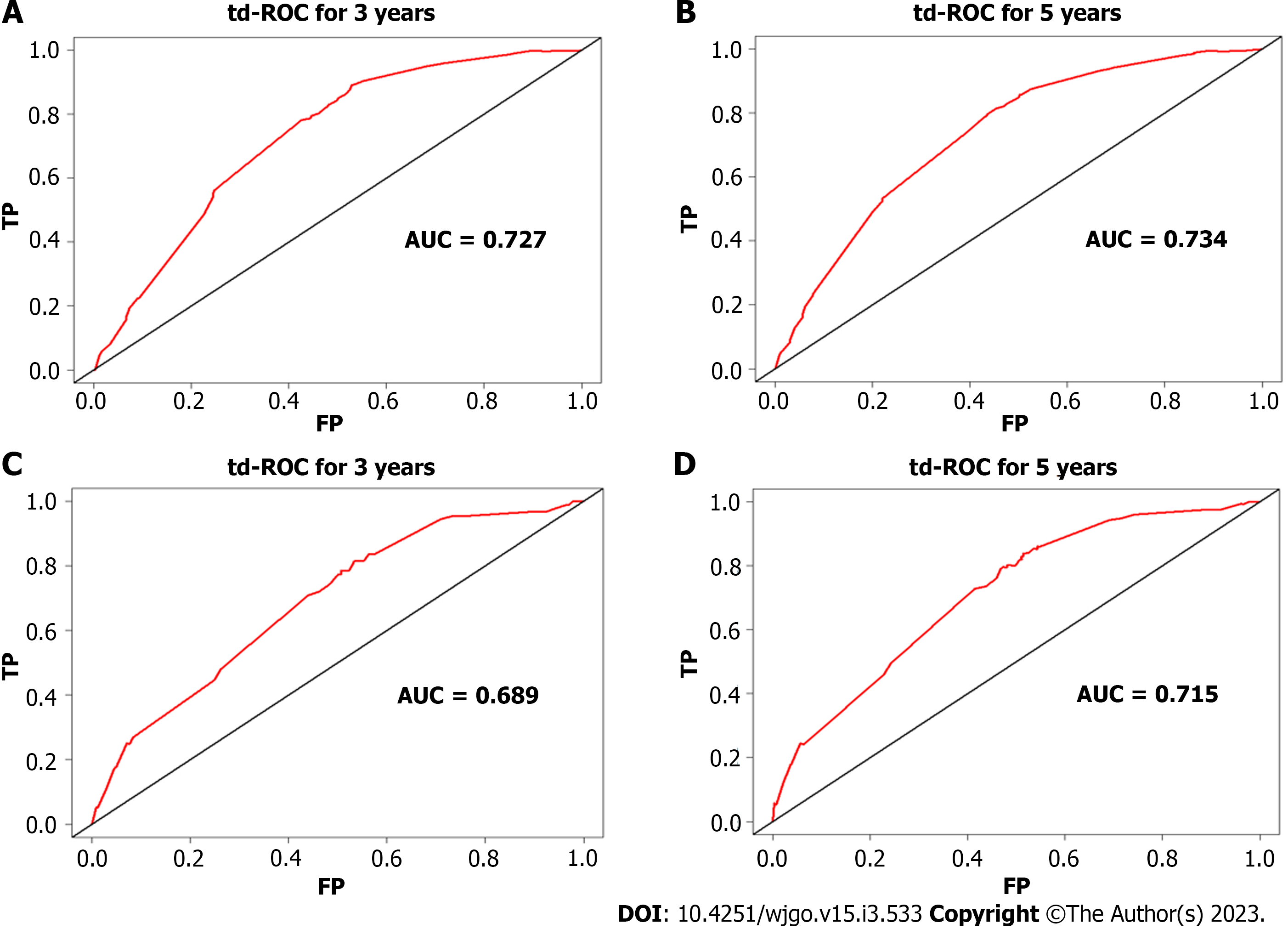
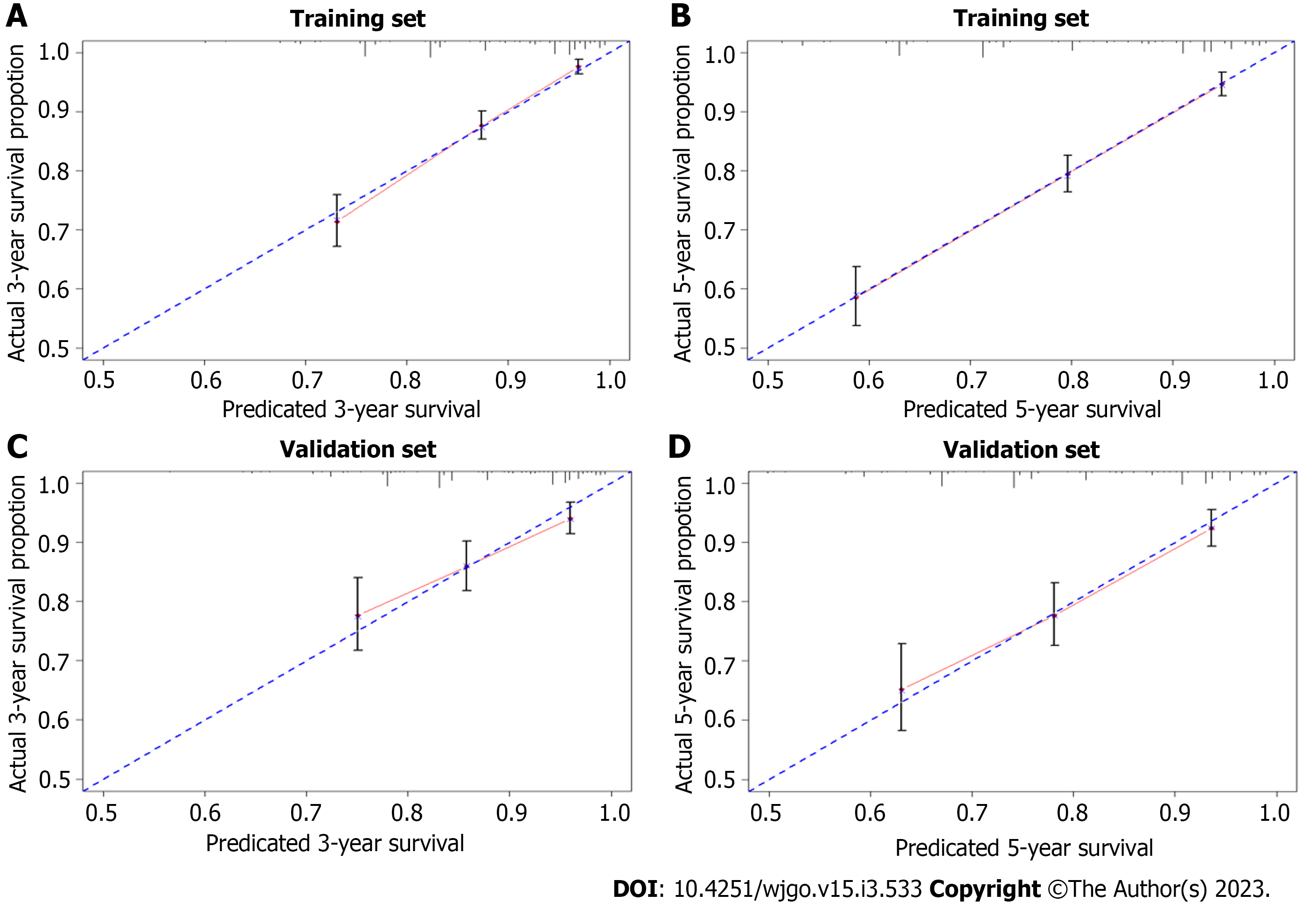
Multivariate risk factor analysis and establishment of the nomogram. In the training set, the LASSO Cox regression model was adopted to filter risk factors for OS. The results revealed that 5 out of 8 factors were significantly associated with 3- and 5-year OS, and the specific information is listed in Figure 1 and Table 2. Increased age, male sex, black race and higher disease stage were inversely correlated with survival, while radiation treatment showed a positive correlation with survival (all P < 0.05). Based on the five variables above, we established an efficient survival nomogram to precisely calculate the probability of 3- and 5-year OS in primary GML patients (Figure 2). In the nomogram, the C-index value was 0.751 (95%CI: 0.729-0.773) and demonstrated satisfactory discrimination ability. Patients were separated into high- and low-risk groups according to the median risk score (cutoff: 0.28; Figure 3A). A scatter plot (Figure 3B) visually showed a shorter survival time and a higher mortality rate of high-risk patients. The Kaplan-Meier curve (Figure 4A and B) clearly revealed that, compared to the high-risk population, more low-risk patients had a better OS (all P < 0.0001, HR 0.190, 95%CI: 0.153-0.236), which is consistent with the results shown in Figure 5 (all HR > 2). Td-ROC curves also showed good predictive power of the nomogram assessing the 3- and 5-year OS. The areas under the curve (AUCs) were 0.727 (Figure 6A) and 0.734 (Figure 6B), respectively. Moreover, another analysis, the calibration curves of the model, shown in Figure 7A and B, confirmed a high agreement between actual and predictive survival proportions. All the above results indicate the good discrimination and predictive capacities of the model.
| Variable | Multivariate | Analysis | ||
| HR | 95%CI of HR | P value | ||
| Age | ||||
| < 45 | Reference | |||
| 45-65 | 4.290 | 1.881-9.815 | 0.001b | |
| ≥ 65 | 19.843 | 8.843-44.528 | < 0.001c | |
| Sex | ||||
| Male | ||||
| Female | 0.722 | 0.606-0.860 | < 0.001c | |
| Race | ||||
| White | Reference | |||
| Black | 1.374 | 1.020-1.779 | 0.036a | |
| Other | 0.704 | 0.493-1.003 | 0.052 | |
| Stage (Ann Arbor) | ||||
| I | Reference | 1.437 | 1.084-1.904 | 0.012a |
| II | 1.732 | 1.076-2.786 | 0.024a | |
| III | 1.844 | 1.401-2.428 | < 0.001c | |
| IV | ||||
| Radiation | Reference | |||
| No/unknown | ||||
| Yes | 0.670 | 0.542-0.829 |
To better validate the nomogram model, we carried out the relevant analysis in the validation set. The C-index of validation patients was 0.718 (95%CI: 0.680-0.757), indicating good predictive accuracy of the nomogram model. There were significant survival differences between the low- and high-risk groups on Kaplan-Meier curves (HR 0.233, 95%CI: 0.174-0.312, P < 0.0001; Figure 4C). As shown in Figure 6C and D, the AUC values of the 3- and 5-year OS td-ROC curves were 0.689 and 0.715, respectively, which were similar to the results in the training set, further confirming the reliable predictive ability of the model. The same phenomenon was also observed between the calibration plots in the training and validation groups (Figure 7C and D). In conclusion, the survival nomogram model displayed a favorable performance to discriminate and predict the OS of primary GML patients in 2 sets.
Primary GML is confirmed as a low-grade, rare incidence rate lesion, and the main risk for the disease is a histological transformation to diffuse large B-cell lymphoma[19,25]. Studies mostly focus on epidemiology[26,27] and the prognosis affected by different treatment methods of the disease[14,22,28,29]. Few large studies have reported the relationship between clinical variables and prognostic survival in primary GML. Until now, no available survival model has been established for predicting the prognosis of patients with primary GML. Our study successfully constructed and validated an effective nomogram model to predict the overall survival of the disease based on clinical and pathological risk factor analysis. We demonstrated that age, gender, race, Ann Arbor stage and radiation therapy (RT) were independent risk factors for OS of primary GML. The nomogram was proven to have good predictive ability for disease prognosis.
Considering the indolent natural development of the disease, long-term and very large follow-up clinical datasets are needed. In our study, all of the data were obtained from the SEER database. This is a national cancer database gathering a large amount of data from different hospitals in the United States, and patient information is strictly managed and reliable[30]. Meanwhile, we developed and validated a survival nomogram. In recent years, nomograms have been widely used as an effective tool for cancer prognosis[31-33]. Tailored to the specific information of every patient, the nomogram can visually analyze and present the disease event (such as OS) with a single numerical probability[34]. In summary, on the basis of high-quality data and validation analysis methods, our model has good clinical prediction ability and can be applied to clinical work.
The 3- and 5-year OS rates calculated in our study were 87.2% and 79.8%, respectively. In 2019, the first national study was conducted on the general population in France. They confirmed that the 5-year OS of all populations was 79% (95%CI: 75-83)[11]. This rate is similar to published studies and may reflect a better prognostic outcome of GML disease than other gastric malignancies.
In 2017, Thieblemont et al[35] generated a novel MALT lymphoma prognostic index (MALT-IPI), including age ≥ 70 years, elevated LDH levels and Ann Arbor stage III or IV. They concluded that this index would be an effective method to predict poor outcome for MALT lymphoma. A similar conclusion was found in other studies[11,14,19,29]. Matysiak-Budnik et al[11] conducted a multiple retrospective study in French, including 416 cases of GML. They found that 5-year OS was better for patients < 67 years old (93.6%) than for those with an older age (93.6% vs 68.5%, P < 0.0001). Another multicenter cohort follow-up study of 420 patients found that age (each incremental year) was an independent prognostic factor for OS (P = 0.024)[14]. In our study, age had the highest risk and showed a significant correlation with the prognosis of primary GML (P < 0.0001, HR 19.843). The nomogram obviously indicated that increased age, especially > 65 years old, had a negative impact on the OS of the disease. Although several researchers found no association between them[20,29], an insufficient number of subjects in these studies need to be considered. Therefore, we still insist that age and prognosis are closely related. Regardless of the specific cutoff point of age, it is recognized that advanced age means an increased risk of primary GML[13]. Multiple analyses also indicated that Ann Arbor stage was a significant independent prognostic factor for the disease. In our study, most patients presented with stage I-II disease (89.9%), and as the severity of the disease increased, the prognosis of the disease worsened (all P < 0.05). The proportion of patients with localized and advanced disease at diagnosis varies among reported series[11,14,36], and we have a consensus that the prognosis of these patients is different[13].
The percentage of female patients was higher than that of male patients (52.0% vs 48.0%), and more people were white (79.07%). Statistically significant correlations were found between male sex (P < 0.001), black race (P = 0.036) and primary GML overall survival. Whether sex is related to the disease remains controversial. Some studies have reported that males have a 2-3 times higher incidence rate of development and a worse prognosis than females[37,38], while other studies have not[11,35]. Until now, no study has focused on the relationship between race and primary GML. SEER provided us with detailed race data and indicated that black individuals are more likely to develop primary GML (HR: 1.374, 95%CI: 1.020-1.779). We still need more studies to further investigate prognostic factors in primary GML.
Management and treatment guidelines for MALT lymphomas have been extremely heterogeneous until the last few years. Over the past 2-3 decades, eradiation of Helicobacter pylori (H. pylori) has been the preferred choice for GML regardless of the histological status of H. pylori[21,39]. Chemotherapy and RT were only suggested to be second-line therapies for nonresponders or advanced patients[39]. In contrast, ESMO guidelines suggested that RT might be the first option for GML patients with localized stages, and chemotherapy was an effective method in patients with all stages[40]. RT alone was also reported to have excellent treatment effects on GML with a total dose of 24-30 Gy[29,41]. Compared with other therapy methods, surgery showed no advantage over treatments in other trials[42]. In our study, no survival difference was found between patients with medication and surgical treatment. Multivariate analysis showed that only RT was significantly associated with better disease prognosis (P < 0.001). These data are consistent with previous studies of RT for gastric MALT lymphoma. In total, radiotherapy is a good choice for primary GML disease.
The limitations of this study are very obvious. First, this is a retrospective analysis. All information is from 2004-2015, and part of the data was recorded before the publication of guidelines for primary GML. This may cause heterogeneity in the clinical management of patients, resulting in data bias. Second, SEER cannot provide us with specific details of helicobacter pylori treatment and this is very important for primary GML. Some data even show unknown labels. Third, we have no external verification data. We collected a few cases of primary GML, but they are not worth analyzing con
In conclusion, a nomogram was developed and validated to have good survival predictive performance based on five clinical independent risk factors for OS for primary GML patients. Nomograms are a low-cost and convenient clinical tool in assessing individualized prognosis and treatment for patients with primary GML.
Extranodal marginal B-cell lymphoma of mucosa-associated lymphoid tissue, known as MALT lymphoma, is a type of non-Hodgkin’s lymphoma. The prognosis of primary gastric MALT (GML) patients can be affected by many factors. Few studies have investigated the prognostic variables for overall survival (OS) in patients with primary GML. We searched a large amount of data on patients diagnosed with primary GML in the Surveillance, Epidemiology and End Results (SEER) database.
In this study, we investigated the significant risk factors of primary GML and build an effective survival nomogram model for primary GML patients.
To develop and verify a survival nomogram model that can predict the OS prognosis of primary GML by combining prognostic and determinant variables.
All data of patients with primary GML were collected from the SEER database. Based on the LASSO and COX regression, we created and further verified the accuracy and effectiveness of the survival nomogram model by the concordance index (C-index), calibration curve and time-dependent receiver operating characteristic (td-ROC) curves.
A total of 2604 patients diagnosed with primary GML were selected for this study. A total of 1823 and 781 people were randomly distributed into the training and testing sets at a ratio of 7:3. The median follow-up of all patients was 71 mo, and the 3- and 5-year OS rates were 87.2% and 79.8%, respectively. Age, sex, race, Ann Arbor stage and radiation were independent risk factors for OS of primary GML (all P < 0.05). The C-index values of the nomogram were 0.751 (95%CI: 0.729-0.773) and 0.718 (95%CI: 0.680-0.757) in the training and testing cohorts, respectively, showing the good discrimination ability of the nomogram model. Td-ROC curves and calibration plots also indicated satisfactory predictive power and good agreement of the model. Overall, the nomogram shows favorable performance in discriminating and predicting the OS of patients with primary GML.
A nomogram was developed and validated to have good survival predictive performance based on five clinical independent risk factors for OS for primary GML patients.
Nomograms are a low-cost and convenient clinical tool in assessing individualized prognosis and treatment for patients with primary GML.
We are grateful for all authors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dey T, India; Esmat SM, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5418] [Article Influence: 602.0] [Reference Citation Analysis (0)] |
| 2. | Chiu BC, Weisenburger DD. An update of the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Thieblemont C, Felman P, Callet-Bauchu E, Traverse-Glehen A, Salles G, Berger F, Coiffier B. Splenic marginal-zone lymphoma: a distinct clinical and pathological entity. Lancet Oncol. 2003;4:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Cook JR, Isaacson PG. Extranodal marginal zone lymphoma of lymphoma of- mucosa-associated lymphoid tissue (MALT) lymphoma. In: Swerdlow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC, 2017: 259-262. [DOI] [Full Text] |
| 5. | Müller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Rentien AL, Lévy M, Copie-Bergman C, Gagniere C, Dupuis J, Le Baleur Y, Belhadj K, Sobhani I, Haioun C, Delchier JC, Amiot A. Long-term course of precancerous lesions arising in patients with gastric MALT lymphoma. Dig Liver Dis. 2018;50:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Capelle LG, den Hoed CM, de Vries AC, Biermann K, Casparie MK, Meijer GA, Kuipers EJ. Premalignant gastric lesions in patients with gastric mucosa-associated lymphoid tissue lymphoma and metachronous gastric adenocarcinoma: a case-control study. Eur J Gastroenterol Hepatol. 2012;24:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Tajika M, Matsuo K, Ito H, Chihara D, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Matsumoto K, Nakamura T, Yatabe Y, Yamao K, Niwa Y. Risk of second malignancies in patients with gastric marginal zone lymphomas of mucosa associate lymphoid tissue (MALT). J Gastroenterol. 2014;49:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Capelle LG, de Vries AC, Looman CW, Casparie MK, Boot H, Meijer GA, Kuipers EJ. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Copie-Bergman C, Locher C, Levy M, Chaumette MT, Haioun C, Delfau-Larue MH, Leroy K, Gaulard P, Delchier JC. Metachronous gastric MALT lymphoma and early gastric cancer: is residual lymphoma a risk factor for the development of gastric carcinoma? Ann Oncol. 2005;16:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Matysiak-Budnik T, Jamet P, Ruskoné-Fourmestraux A, de Mascarel A, Velten M, Maynadié M, Woronoff AS, Trétarre B, Marrer E, Delafosse P, Ligier K, Lapôtre Ledoux B, Daubisse L, Bouzid L, Orazio S, Cowppli-Bony A, Monnereau A. Gastric MALT lymphoma in a population-based study in France: clinical features, treatments and survival. Aliment Pharmacol Ther. 2019;50:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Wöhrer S, Troch M, Streubel B, Zwerina J, Skrabs C, Formanek M, Hauff W, Hoffmann M, Müllauer L, Chott A, Raderer M. MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course. Leukemia. 2007;21:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 15. | Abe S, Oda I, Inaba K, Suzuki H, Yoshinaga S, Nonaka S, Morota M, Murakami N, Itami J, Kobayashi Y, Maeshima AM, Saito Y. A retrospective study of 5-year outcomes of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Jpn J Clin Oncol. 2013;43:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Goda JS, Gospodarowicz M, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Tsang RW. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010;116:3815-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Jäger G, Neumeister P, Quehenberger F, Wöhrer S, Linkesch W, Raderer M. Prolonged clinical remission in patients with extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type treated with cladribine: 6 year follow-up of a phase II trial. Ann Oncol. 2006;17:1722-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Stathis A, Chini C, Bertoni F, Proserpio I, Capella C, Mazzucchelli L, Pedrinis E, Cavalli F, Pinotti G, Zucca E. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol. 2009;20:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Wang YG, Zhao LY, Liu CQ, Pan SC, Chen XL, Liu K, Zhang WH, Yang K, Chen XZ, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine (Baltimore). 2016;95:e4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Hu Q, Zhang Y, Zhang X, Fu K. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection: a review of current diagnosis and management. Biomark Res. 2016;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Todorovic M, Balint B, Jevtic M, Suvajdzic N, Ceric A, Stamatovic D, Markovic O, Perunicic M, Marjanovic S, Krstic M. Primary gastric mucosa associated lymphoid tissue lymphoma: clinical data predicted treatment outcome. World J Gastroenterol. 2008;14:2388-2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965-970. [RCA] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 24. | Lalitha N. Clinical staging of adult non-Hodgkin's lymphoma. Oncology. 1990;47:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Maeshima AM, Taniguchi H, Toyoda K, Yamauchi N, Makita S, Fukuhara S, Munakata W, Maruyama D, Kobayashi Y, Tobinai K. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol. 2016;174:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Palmela C, Fonseca C, Faria R, Baptista RB, Ribeiro S, Ferreira AO. Increased risk for metachronous gastric adenocarcinoma following gastric MALT lymphoma-A US population-based study. United European Gastroenterol J. 2017;5:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Luminari S, Cesaretti M, Marcheselli L, Rashid I, Madrigali S, Maiorana A, Federico M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2010;21:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, Raderer M, Savio A; EGILS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Wirth A, Gospodarowicz M, Aleman BM, Bressel M, Ng A, Chao M, Hoppe RT, Thieblemont C, Tsang R, Moser L, Specht L, Szpytma T, Lennard A, Seymour JF, Zucca E. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol. 2013;24:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | National Cancer Institute, Surveillance Research Program. Seer is an authoritative source for cancer statistics in the United States. August 15, 2016. Available from: http://wwwseercancergov. |
| 31. | Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 32. | Tang X, Zhou X, Li Y, Tian X, Wang Y, Huang M, Ren L, Zhou L, Ding Z, Zhu J, Xu Y, Peng F, Wang J, Lu Y, Gong Y. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann Surg Oncol. 2019;26:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Hirabayashi S, Kosugi S, Isobe Y, Nashimoto A, Oda I, Hayashi K, Miyashiro I, Tsujitani S, Kodera Y, Seto Y, Furukawa H, Ono H, Tanabe S, Kaminishi M, Nunobe S, Fukagawa T, Matsuo R, Nagai T, Katai H, Wakai T, Akazawa K. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol. 2014;25:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 2304] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 35. | Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, Laszlo D, Coiffier B, Lopez Guillermo A, Torri V, Cavalli F, Johnson PW, Zucca E. A MALT lymphoma prognostic index. Blood. 2017;130:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 36. | Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, Molina T, Rambaud JL. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Cogliatti SB, Schmid U, Schumacher U, Eckert F, Hansmann ML, Hedderich J, Takahashi H, Lennert K. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology. 1991;101:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 268] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S. Primary Gastric Lymphoma, Epidemiology, Clinical Diagnosis, and Treatment. Cancer Control. 2018;25:1073274818778256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 40. | Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi144-vi148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Tsang RW, Gospodarowicz MK. Radiation therapy for localized low-grade non-Hodgkin's lymphomas. Hematol Oncol. 2005;23:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Koch P, Probst A, Berdel WE, Willich NA, Reinartz G, Brockmann J, Liersch R, del Valle F, Clasen H, Hirt C, Breitsprecher R, Schmits R, Freund M, Fietkau R, Ketterer P, Freitag EM, Hinkelbein M, Heinecke A, Parwaresch R, Tiemann M. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol. 2005;23:7050-7059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |