Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2212
Peer-review started: August 12, 2023
First decision: September 12, 2023
Revised: September 25, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: December 15, 2023
Processing time: 123 Days and 21.6 Hours
There is a lack of robust prognostic markers for upper gastrointestinal (GI) tract cancers, including esophageal, gastric, and esophagogastric junction cancers. T cell immunoglobulin and mucin-domain containing-3 (TIM3) plays a key immunomodulatory role and is linked to the prognosis of various cancers. However, the significance of TIM3 in upper GI tract tumors is still uncertain.
To investigate the prognostic value of TIM3 expression in upper GI tract tumors.
A literature search was conducted on the PubMed, Embase, and Web of Science databases for relevant studies published until June 2023. After screening and quality assessment, studies that met the criteria were included in the meta-analysis. Statistical methods were used for the pooled analysis to assess the association of TIM3 expression in upper GI tract tumors with the prognosis and clinicopathological parameters. The results were reported with the hazard ratio (HR) and 95% confidence interval (CI).
Nine studies involving 2556 patients with upper GI tract cancer were included. High TIM3 expression was associated with a worse prognosis in upper GI tract cancer (HR: 1.17, 95%CI: 1.01-1.36). Positive expression of TIM3 in gastric cancer was correlated with the T and N stage, but the difference was not statistically significant. However, TIM3 overexpression was significantly correlated with the TNM stage (odds ratio: 1.21, 95%CI: 0.63-2.33; P < 0.05). TIM3 expression showed no association with the other clinicopathological parameters.
High expression of TIM3 in the upper GI tract cancer is associated with a worse prognosis and advanced T or N stages, indicating its potential value as a prognostic biomarker. These findings may provide a basis for the personalized treatment of upper GI tract cancers.
Core Tip: Immune checkpoint receptor inhibitors have transformed cancer treatment in a wide range of tumor types, and T cell immunoglobulin and mucin-domain containing-3 (TIM3) is one of these immune checkpoint receptors. The study initially evaluated the survival-prognostic effect of TIM3 in upper gastrointestinal tract tumors by conducting a meta-analysis, aggregating data from several independent studies, and providing the latest comprehensive insights into the field. We believe that the results of this study will further advance immunotherapy and provide some value to future research.
- Citation: Yan JJ, Liu BB, Yang Y, Liu MR, Wang H, Deng ZQ, Zhang ZW. Prognostic value of T cell immunoglobulin and mucin-domain containing-3 expression in upper gastrointestinal tract tumors: A meta-analysis. World J Gastrointest Oncol 2023; 15(12): 2212-2224
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2212.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2212
The high incidence of cancer is a major public health concern worldwide[1]. An analysis of cancer statistics in 2022 revealed a declining trend in the incidence and mortality rates of malignant tumors in the United States[2]. However, upper gastrointestinal (GI) tract tumors, including esophageal cancer, gastric cancer, and esophagogastric junction cancer, continue to pose a serious threat to health. Gastric cancer is the fifth most common cancer worldwide, and the fourth highest contributor to cancer-associated mortality. Worldwide, esophageal cancer ranks seventh in terms of incidence and sixth in terms of mortality[3].
Recent data have suggested a gradual decrease in the incidence of gastric cancer in China. However, it remains the fourth most commonly occurring cancer and the third most common cause of cancer-associated mortality in China, whereas esophageal cancer is among the top five causes of cancer-related deaths[4].
A diverse range of treatment methods are presently available for upper GI tract tumors. In addition to traditional treatments (surgery, chemotherapy, and radiotherapy), targeted therapy, immunotherapy, and combination therapy have exhibited promising results in certain subsets of patients. However, despite these novel therapeutic approaches, the prognosis of patients with middle- and advanced-stage upper GI tract tumors remains unsatisfactory. In recent years, immunotherapy using immune checkpoint inhibitors has transformed the treatment landscape for various types of cancers. Combination therapy containing immunotherapy has been the first-line treatment for advanced upper GI tract tumors. Approximately 15%-20% of patients with gastric adenocarcinoma overexpress human epidermal growth factor receptor 2 (HER2). While trastuzumab was once favored for treating HER2-positive advanced gastric cancer, the DESTINY-Gastric01 study highlighted trastuzumab deruxtecan as a superior option[5]. The MOUSEION series indicates that immunotherapy benefits both men and women, but women may show a reduced response to monotherapy[6]. Moreover, combining two immune checkpoint inhibitors yields better outcomes, and while not all gastric cancer patients show a positive response, those with high microsatellite instability or Epstein-Barr virus positivity exhibit a strong response to pembrolizumab[7]. The synergy of chemotherapy and immunotherapy, especially targeting the DNA damage response pathway, offers promising prospects in gastric cancer treatment[8]. Furthermore, immune checkpoints represent a contemporary research hotspot among prognostic markers.
Immune checkpoints are inhibitory molecules that can modulate the immune system to reduce unwanted autoimmune responses, thereby helping maintain self-tolerance and regulating the timing of physiological immune responses in peripheral tissues[9]. In the cancer microenvironment, T lymphocytes play a central role in cell-mediated cytotoxicity. Activated T cells express inhibitory receptors, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). Cancer cells express ligands, such as programmed death-ligand 1 (PD-L1), which can combine with PD-1 and further weaken the antitumor effects of T cells[10,11]. The blockade of immune checkpoints can have powerful and durable antitumor effects.
In March 2011, the United States Food and Drug Administration approved ipilimumab, a monoclonal CTLA-4 antibody, for the treatment of patients with late-stage melanoma[12]. PD-1/PD-L1 blockers are among the most successful checkpoint inhibitors[13]. PD-1 or PD-L1 inhibitors have been approved as first- or second-line treatment for several cancers, such as non-small cell lung cancer, melanoma, and gastric cancer[14]. Therefore, immune checkpoint inhibitors are presently an important component of comprehensive cancer therapy.
At present, the most commonly used immune checkpoint molecular targets are PD-1, CTLA-4, lymphocyte activation gene-3 (LAG-3), and T cell immunoglobulin-3 (TIM3). Among these, TIM3, also referred to as hepatitis A virus-cell receptor 2, is a transmembrane protein that negatively regulates type 1 immunity and plays an important regulatory role in carcinogenesis and development[15]. This suppresses the activity of T cells and other immune cells by binding to its ligand galectin-9 (Gal-9), thereby regulating the intensity and duration of the immune response[16].
Recent studies have demonstrated high TIM3 expression in upper GI tract tumor tissues, and its close association with overall survival (OS). For example, studies have reported a correlation between TIM3 expression level and the prognosis of gastric[17-20] and esophageal[21-25] cancers. However, the prognostic value of TIM3 in gastroesophageal junction cancer has not been reported. The results suggest the important role of TIM3 in these tumors.
Therefore, a meta-analysis was conducted to comprehensively assess the relationship between TIM3 expression level and the prognosis and clinicopathological parameters of upper GI tract tumors.
A literature search was performed in the PubMed, Web of Science, and Embase databases for studies published as of June 30, 2023. The keywords used were “TIM3,” “gastric cancer,” and “esophageal cancer.” No studies have investigated TIM3 expression in gastroesophageal junction cancer. In addition, to prevent the omission of relevant literature, especially focused literature, we used a new tool, Reference Citation Analysis (https://www.referencecitationanalysis.com), to analyze the articles. Next, a systematic approach was used to select eligible studies and extract relevant data. The reference lists of the selected articles were screened to identify additional studies that met the criteria for inclusion in the present meta-analysis. Only publicly available published data were used for the present study, precluding any concerns related to personal privacy or ethical issues. The present meta-analysis is registered on the PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/; Registration No. CRD42023438756).
Studies that met the following criteria were eligible for inclusion: (1) Case-control studies, cohort studies, or clinical trials; (2) patients with upper GI cancer; (3) diagnosis confirmed by histopathology; (4) detection of TIM3 expression by immunohistochemistry (IHC); and (5) variables reported of TIM3 expression levels and cancer prognosis-related outcomes (such as OS).
The exclusion criteria were: (1) In vitro studies or animal experiments; (2) inability to extract the required data from the published results; (3) non-reporting of survival outcomes; and (4) patients who received chemotherapy or radiotherapy before the assessment of TIM3 expression.
Two researchers independently extracted the data pertaining to the following variables from each study: Study design, sample size, demographic characteristics (including age, sex, nationality, and ethnicity), clinicopathological parameters (T stage, lymph node metastasis, tumor-node-metastasis [TNM] stage, and grade of differentiation), and prognostic indices (such as OS). The specifics of the TNM staging were meticulously documented. The methods and thresholds for determining TIM3 expression, primarily through IHC, were outlined. Using the Newcastle-Ottawa Scale (NOS) for quality assessment, only studies that scored between 6 to 9 points, denoting high quality, were included in the meta-analysis.
Pooled analyses of data obtained from the included studies were performed using STATA statistical software, version 15.2. P < 0.05 was considered statistically significant for all analyses. The pooled hazard ratios (HRs) and 95% confidence interval (CIs) were calculated to assess the association between TIM3 expression and the prognosis and clinicopathological parameters (sex, age, T stage, lymph node metastasis, TNM stage, and grade of differentiation) of GI tract cancer. The individual HR estimates were pooled into a summary HR. The assumption of homogeneity was tested by performing Cochran’s Q-statistic and I2 test for heterogeneity. P < 0.05 in the Q-test or I2 > 50% was considered indicative of significant heterogeneity. In case of significant heterogeneity, the random-effects model was used for the meta-analysis; otherwise, the fixed-effects model was used. HR or odds ratio (OR) > 1 (indicating worse prognosis for cases with TIM3 expression positivity) was considered statistically significant when the 95%CI did not overlap 1. In case of significant heterogeneity among the included studies, subgroup analysis or sensitivity analysis was performed to identify the source of heterogeneity and explore the impact of potential factors on the results. The results were presented as forest plots. The effect of potential publication bias on the results of the meta-analysis was assessed by drawing funnel plots, and performing the Begg’s rank correlation test.
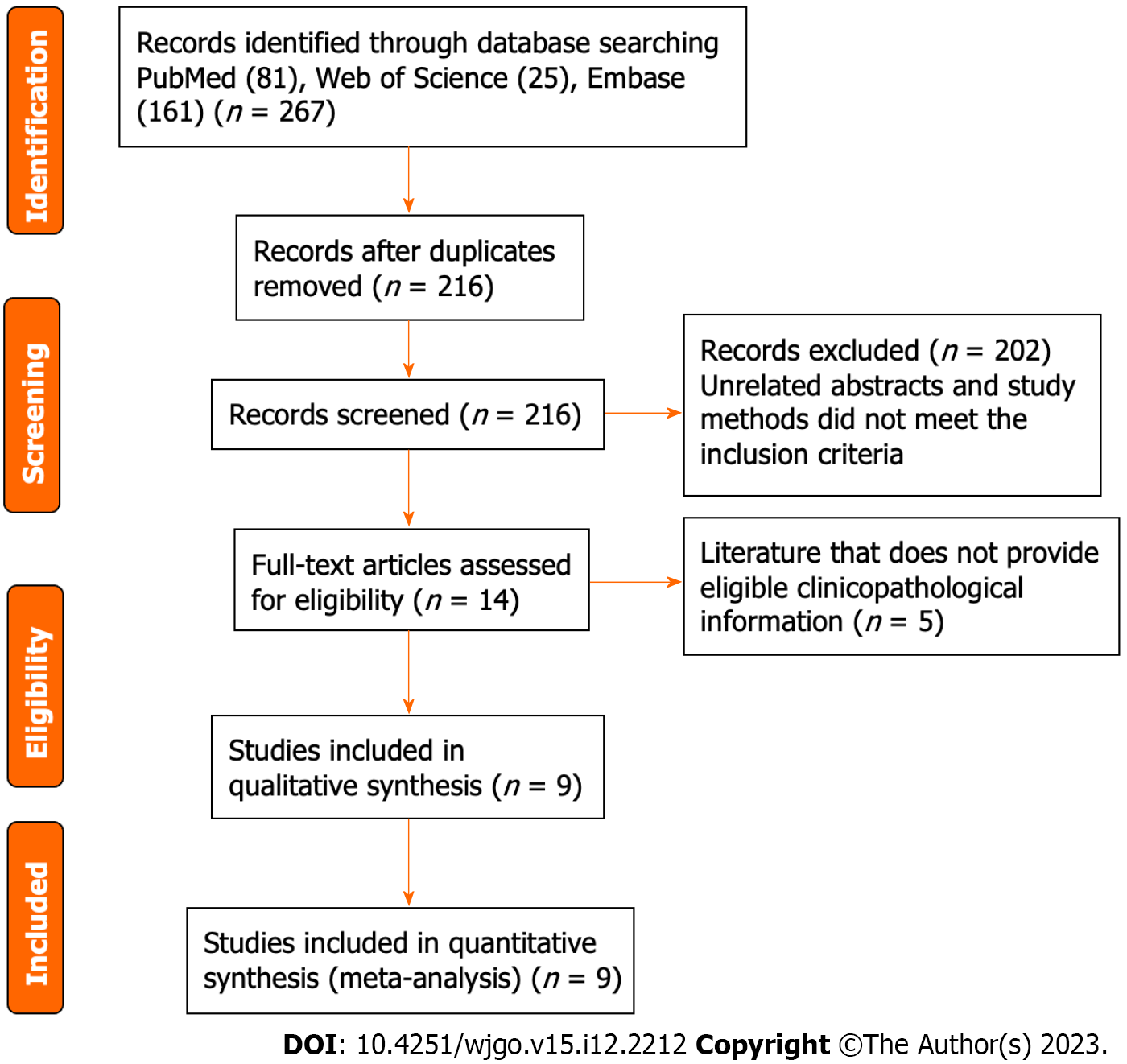
The flow diagram illustrating the study selection process and criteria is presented in Figure 1. A total of 267 studies that investigated the association of TIM3 expression in upper GI tract cancer with both prognosis and clinicopathological parameters were retrieved during the database search. Among these studies, 51 duplicate publications were excluded. Of the remaining 216 articles, 202 were excluded after the review of the abstracts and study methods, since these did not meet the inclusion criteria. Subsequently, the full texts of the remaining 14 articles were reviewed. Of these 14 studies, 5 that did not provide qualifying clinicopathological information were excluded. Finally, the remaining nine studies, which enrolled 2556 patients, were included in the pooled analysis. These studies were published between 2013 and 2022. In each of the eligible studies, TIM3 expression in upper GI tract cancer was reported to be associated with OS. All studies were considered high quality based on the NOS assessment (average score: 8). Table 1 summarizes the characteristics of the selected studies.
| Reference | Yr | Country | Ethnicity | Age | Method | Study design | Stage | Cancer type | Cut-off values | n (E +) | Follow-up time in mo (range) | OS (HR with 95%CI) | NOS |
| Jiang et al[17] | 2013 | China | Asian | 60 | IHC | Retrospective | 1-4 | Gastric cancer | HSCORE > 0 | 305 (60.0%) | Median 40 (3-135) | 0.60 (0.35–1.01) | 9 |
| Wang et al[18] | 2018 | China | Asian | 61.6 | IHC | Retrospective | 1-4 | Gastric cancer | Median number of stained cells | 587 (49.9%) | Median 48 (1-117) | 1.71 (1.327–2.203) | 9 |
| Park et al[19] | 2021 | Korea | Asian | 59 | IHC | Retrospective | 2-3 | Gastric cancer | Immunostaining 5% | 385 (61.6%) | NA | 0.30 (0.13–0.68) | 7 |
| Chen et al[20] | 2022 | China | Asian | 65 | IHC | Retrospective | 2-3 | Gastric cancer | Median number of stained cells | 496 (49.0%) | Median 37 (4-76) | 2.236 (1.427–3.504) | 7 |
| Shan et al[21] | 2016 | China | Asian | 55 | IHC | Retrospective | 1-4 | ESCC | 3 points | 64 (85.9%) | Median 31 (7-105) | 1 (0.932-1.072) | 8 |
| Hou et al[22] | 2017 | China | Asian | 58 | IHC | Retrospective | 2-3 | ESCC | 3 points | 45 (22.2%) | < 100 | 1.102 (0.292–4.157) | 7 |
| Duan et al[23] | 2018 | China | Asian | 58 | IHC | Retrospective | 1-4 | ESCC | 1% | 95 (37.9%) | Median 32 (3-84) | 0.405 (0.148–1.103) | 9 |
| Hong et al[24] | 2019 | Korea | Asian | 64 | IHC | Retrospective | 1-4 | ESCC | 1% | 396 (50.8%) | Median 24.8 (0.5-210.0) | 1.60 (1.13–2.27) | 8 |
| Zhao et al[25] | 2020 | China | Asian | 65 | IHC | Retrospective | 1-4 | ESCC | HSCORE > 3 | 183 (47.5%) | NA | 2.620 (1.569–4.375) | 8 |
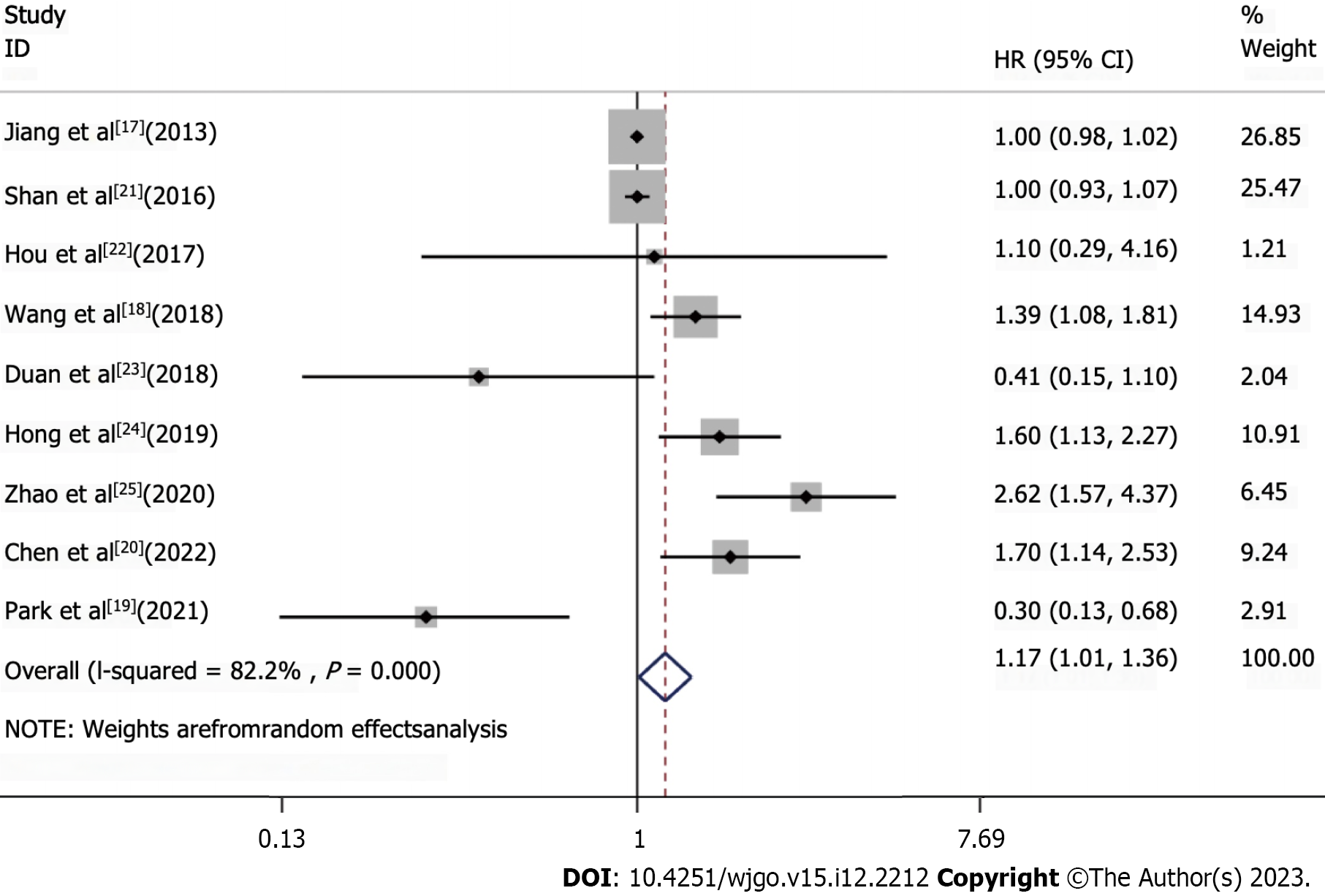
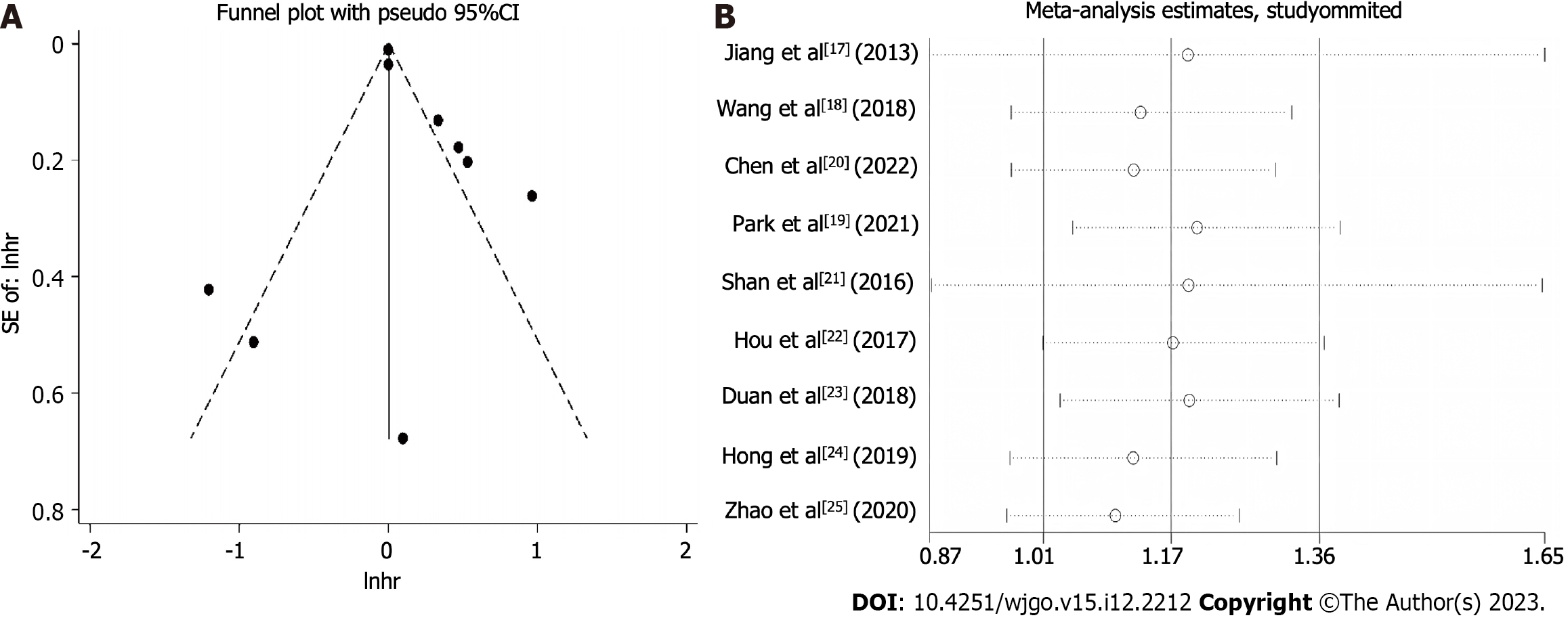
Nine studies were included in the pooled analysis of the association between TIM3 expression and OS (Figure 2). It was noteworthy that no significant positive results were obtained from the literature data provided by Wang et al[18] and Park et al[19]. Nonetheless, these results revealed that high TIM3 expression was associated with a worse prognosis of upper GI tract tumors, with a pooled HR of 1.17 (95%CI: 1.01-1.36). To further investigate the prognostic significance of TIM3 in different types of upper GI tumors, we performed the analyses grouped by cancer type (Supplemen
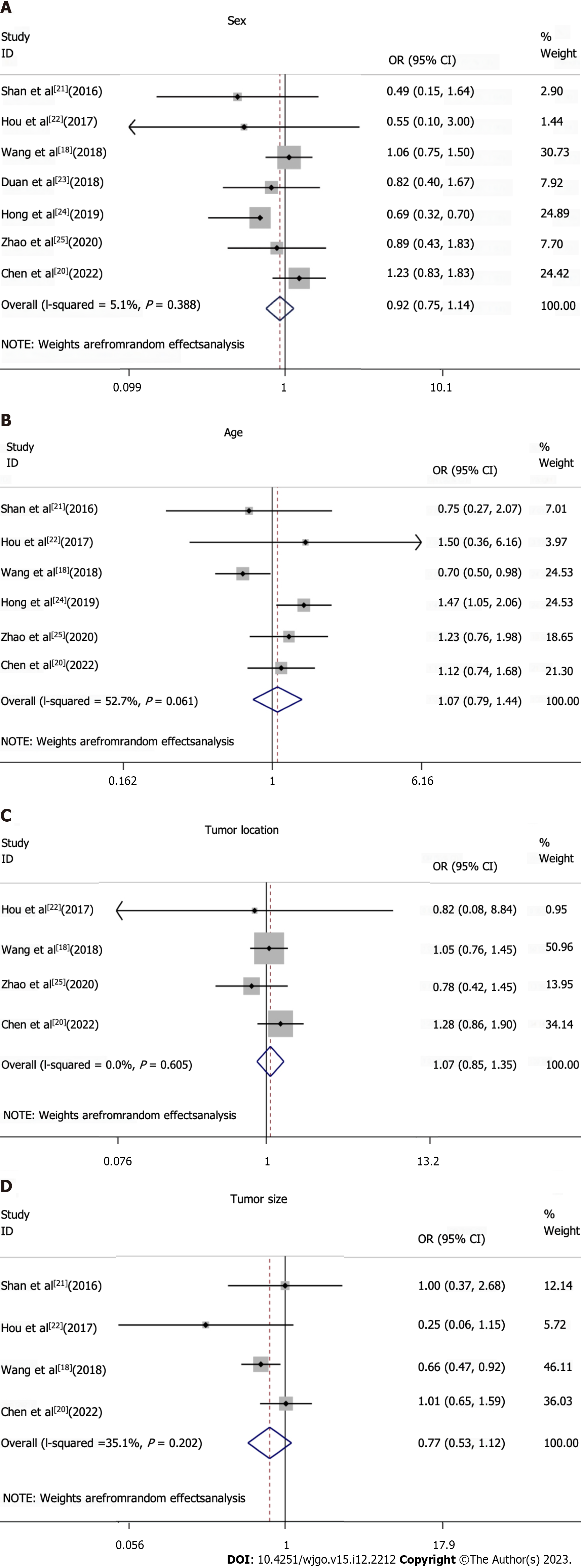
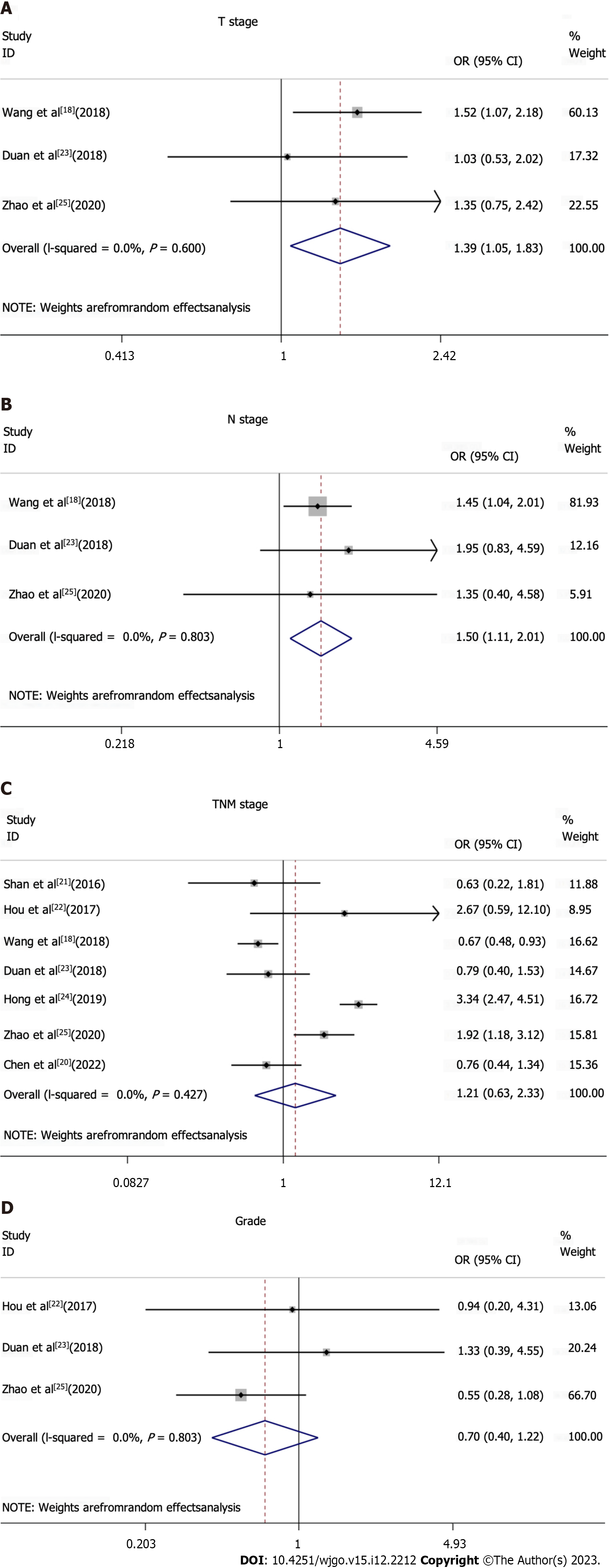
All studies included in the meta-analysis had analyzed data on TIM3 expression, and at least one clinicopathological parameter for upper GI tract tumors (Figures 3 and 4, Table 2). TIM3 expression was associated with the T and N stage, and was significantly correlated with the TNM stage. However, among the other clinicopathological parameters, TIM3 expression was not associated with sex, age, tumor location, size, or grade.
| Subgroups | OR (95%CI) | P value | Studies, n | Patients, n |
| Sex: Female vs male | 0.92 (0.75-1.14) | 0.388 | 7 | 1866 |
| Age: Elderly vs non-elderly | 1.07 (0.79-1.44) | 0.061 | 6 | 1771 |
| Tumor location: Top and middle vs bottom | 1.07 (0.85-1.35) | 0.605 | 4 | 1311 |
| Tumor size: 4 vs < 4 | 0.77 (0.53-1.12) | 0.202 | 4 | 1192 |
| T stage: T1-2 vs T3-4 | 1.39 (1.06-1.83) | 0.6 | 3 | 865 |
| N stage: N0-1 vs N2-3 | 1.5 (1.11-2.01) | 0.803 | 3 | 865 |
| TNM Stage: III/IV vs I/II | 1.21 (0.63-2.33) | < 0.001 | 7 | 1866 |
| Grade: G1 vs G2 + G3 | 0.7 (0.40-1.22) | 0.427 | 3 | 323 |
On analysis of the T stage for assessing the degree of primary tumor invasiveness, TIM3 expression was found to be associated with the T stage (T1-2 vs T3-4, OR: 1.39, 95%CI: 1.06-1.83; fixed effects model). Furthermore, TIM3 expression was higher in the T3-4 group compared to the T1-2 group, although the difference was not statistically significant (P > 0.05). This result suggests the potential role of TIM3 expression in the depth of tumor invasion of upper GI tract tumors. The analysis of the distribution of involved lymph nodes (stage N) revealed that TIM3 expression was greater in the N2-3 group compared to the N0-1 group (N0-1 vs N2-3, OR: 1.5, 95%CI: 1.11–2.01; random effects model), although the difference was not statistically significant (P > 0.05). Furthermore, the results suggested that TIM3 expression may be correlated with the N stage in upper GI tract tumors. However, further studies are required to explore this association. In further analyses, TIM3 overexpression was found to be correlated with the TNM stage, since TIM3 expression was higher in the III/IV stage of gastric cancer compared to the I/II stage (I/II vs III/IV, OR: 1.21, 95%CI: 0.63–2.33, random-effects model). Therefore, TIM3 overexpression may play an important role in the development of upper GI tract tumors.
In the sensitivity analysis, the pooled analysis results remained stable and consistent across the different statistical models and methods, reinforcing the reliability of the results. Furthermore, the funnel plot and Begg’s rank correlation test revealed no significant effect of potential publication bias on the results of the meta-analysis (Figure 5).
Upper GI tract tumors are a group of malignant tumors with a high incidence and associated mortality. The pathogenic mechanism of these tumors is complex, and intricately linked to the immune system. The immune system plays a crucial role in the initiation, advancement, and spread of cancer[26,27]. As cancer advances, exosomes released by the tumor cells not only facilitate tumor immune evasion but also serve as biomarkers of autoimmune response[28]. Notably, serum pepsinogen, a biomarker, is widely believed to reflect the pathological changes in the stomach[29]. Biomarkers are indispensable in cancer diagnosis and treatment, aiding in early diagnosis, evaluating therapeutic efficacy, predicting disease progression, and offering vital information for personalized treatment. The immunomodulatory molecule TIM3, which is an important immune checkpoint[30,31], plays a key role in tumor immune evasion and treatment resistance, and is expressed in both serum and tumors. Furthermore, TIM3 can suppress tumor immune responses by modulating the function of T helper 1 CD4 T cells and cytotoxic CD8 T cells[32]. Studies conducted in the context of melanoma, and head and neck cancers have revealed that TIM3 can limit the antitumor immunity[33,34]. TIM3 enhances the growth of hepatocellular carcinoma by inducing the functional blockade of natural killer cells[35]. Furthermore, TIM3 has been identified as a marker of microsatellite-stable colorectal carcinoma with immune failure and distinct clinicopathological features[36]. Moreover, TIM3, which acts with its ligand CEA cell adhesion molecule 1, can suppress T cells, thereby downregulating antitumor immunity[37,38]. Of note, the serum levels of TIM3 and Gal-9 are significantly elevated in patients with systemic mastocytosis, suggesting the potential value of TIM3 in the diagnosis and treatment of this condition[39]. However, the prognostic significance of TIM3 in upper GI tract tumors remain inconsistent. Therefore, we conducted a meta-analysis of the available evidence to comprehensively evaluate the potential prognostic value of TIM3 in patients with upper GI tract tumors. These findings may provide insights for guiding clinical treatment decisions and individualized therapy.
A total of nine independent studies, which enrolled 2556 patients with upper GI tract tumors, were included. In the pooled analysis, high TIM3 expression was associated with worse prognosis. Specifically, a poorer prognosis was observed in patients with high TIM3 expression, with an HR of 1.17 (95%CI: 1.01-1.36). This suggests that high TIM3 expression may be a predictor of poor prognosis, and is associated with shorter survival and higher risk of recurrence in these patients. Of note, as shown in Figure 5, the results were not very good. Hence, the investigators searched for the reason, and identified some deviations on the data obtained from two studies (Wang et al[18] and Park et al[19]). A graph was drawn to determine the effects of deleting these two articles, and it was observed that the results were very relevant to the drawn conclusions. Thus, it would be worthwhile to further investigate TIM3. It was notable that previous studies have identified the combination of TIM3, LAG-3, and others as potential biomarkers[40].
In the subgroup analysis, high expression of TIM3 was associated with a worse prognosis of patients with esophageal, gastric, and combined esophagogastric cancers. This highlights the potential role of TIM3 in the development and progression of these tumor types, and underlines its clinical relevance as a potential marker for prognostic assessment and treatment selection.
High expression of TIM3 may suggest immune escape and an immunosuppressive milieu in the tumor microenvironment. In this study, we found a significant correlation between high expression of TIM3 and the TNM staging of upper GI tumors. Given the pivotal role of TIM3 in tumor immune evasion and treatment resistance, we hypothesize that high expression of TIM3 may be intricately linked with the TNM staging of the tumor. TIM3 inhibits T cell activation and function, and induces immune tolerance by binding to its ligand Gal-9. Gal-9 promotes the persistence of PD-1 + TIM3 + T cells by binding to PD-1, and impairing Gal-9/TIM3-induced cell death[41]. Furthermore, TIM3 and Gal9 have exhibited the potential as predictive biomarkers in different cancers. In-depth characterization of the TIM3/Gal9 signaling in cancers, and its underlying mechanisms can help identify patients who are likely to respond to the blockade of this pathway, and facilitate the design of combination therapies[42]. High expression of TIM3 may lead to the suppression of immune response, and diminishing host immune clearance of tumors, thereby promoting tumor growth and proliferation. This may explain the association between high TIM3 expression and poor prognosis of upper GI tract tumors. This further emphasizes the significance of TIM3 as a potential therapeutic target and prognostic biomarker.
Through a meta-analysis approach, our study comprehensively assessed the prognostic significance of TIM3 in upper GI tract tumors. Our findings suggest that TIM3 might be a biomarker warranting further attention. It not only offers a novel reference for classifying and staging upper GI tract tumors but may also shed light on the potential immunotherapeutic strategies for these tumors. Especially as immunotherapy becomes a focal point of cancer treatment, TIM3, as one of the immune checkpoints and a regulator of T-cell responses in the tumor microenvironment, may become an important test similar to PD-1/PD-L1. Such checkpoints are commonly associated with immune escape in cancer patients, and therefore, therapies targeting them may provide significant clinical benefits.
Before embarking on this research, the existing literature presented conflicting views regarding the role of TIM3 in upper GI tract tumors. At present, we do not fully understand the specific role of TIM3 in various upper GI tract tumors and its potential interactions with other immune checkpoints, such as PD-1/PD-L1. We aim to consolidate a stronger evidence base in this area by systematically conducting meta-analyses covering a wider range of literature, employing state-of-the-art statistical techniques, and taking into account a variety of potential confounding factors. Recognizing the role of TIM3 as an immunomodulatory molecule and its expression trends in different tumors, we believe that studies around TIM3 will increase in the future. The benefits of TIM3-targeted therapeutic strategies may not be limited to upper GI tract tumors but also to other cancer types. In addition, TIM3-focused combination therapies, such as those paired with PD-1/PD-L1 inhibitors, may become a hot research topic. As more studies unravel the function of TIM3 and its interrelationships with other immune checkpoints, it may become an important target in cancer therapy. TIM3 is expected to serve as a biomarker to predict prognosis and provide guidance for immunotherapy and even chemotherapy.
Although our study provides strong evidence for the role of TIM3 in upper GI tract tumors, it also has its limitations. First, the studies included in the meta-analysis were conducted only in Asian populations. Thus, our conclusions may not be entirely generalizable to other ethnic groups. Second, the studies included in the meta-analysis were somewhat heterogeneous in terms of study design, sample source, and experimental method. Moreover, the experimental method used was IHC, which is a less accurate method for quantitative analysis compared to other protein analysis methods. In addition, TIM3-related clinical drugs are not widely available, and further drug development is needed.
In conclusion, the meta-analysis results suggest that high TIM3 expression in upper GI tract tumors is strongly associated with poor prognosis. The findings underline the role of TIM3 as a potential prognostic marker, which may provide guidance in the development of individualized treatment and immunotherapy strategies. However, more studies are needed to explore the prognostic value of TIM3 expression in upper GI tract tumors. Furthermore, unraveling its underlying mechanisms may provide a theoretical basis for the development of therapeutic strategies that target TIM3.
T cell immunoglobulin and mucin-domain containing-3 (TIM3) is an immune checkpoint molecule. The prognostic value of TIM3 expression in upper gastrointestinal (GI) tract tumors has not been comprehensively analyzed.
The study clarifies the prognostic value of TIM3 in upper GI tumors and assesses whether TIM3 could be used as a research target to guide future clinical treatment.
The study investigates the immunohistochemical expression of TIM3 in upper GI tract tumors and assesses its clinical and prognostic value.
The PubMed, Web of Science, and Embase databases were searched for subject terms, including “TIM3,” “gastric cancer,” and “esophageal cancer,” Data analyses were performed using STATA 15.2. The results were expressed as hazard ratio (HR) and odds ratio (OR), with the respective 95% confidence interval (CI). Heterogeneity was assessed using the I2 statistic.
High TIM3 expression was associated with a worse prognosis in upper GI tract cancers (HR: 1.17, 95%CI: 1.01-1.36). TIM3 overexpression was significantly correlated with the TNM stage (OR: 1.21, 95%CI: 0.63-2.33; P < 0.05). TIM3 expression showed no association with other clinicopathological parameters.
High expression of TIM3 in upper GI tract cancers is associated with a poorer prognosis and advanced T or N stage.
None of the studies included in the meta-analysis evaluated the prognostic value of TIM3 expression in esophagogastric junction cancer. In addition, the small sample size of the studies was a limitation and the effect of confounding factors on the results cannot be ruled out. However, the results revealed a significant association between high expression of TIM3 and poor survival outcomes in upper GI tract tumors. Our analysis suggests that TIM3, as an immune checkpoint receptor, may be a useful prognostic marker and a potential therapeutic target worthy of further research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang B, China; Rizzo A, Italy S-Editor: Li L L-Editor: Filipodia P-Editor: Zhang XD
Zhang XD
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9952] [Article Influence: 4976.0] [Reference Citation Analysis (2)] |
| 2. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2208] [Article Influence: 736.0] [Reference Citation Analysis (1)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64686] [Article Influence: 16171.5] [Reference Citation Analysis (177)] |
| 4. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1-9. |
| 5. | Ricci AD, Rizzo A, Rojas Llimpe FL, Di Fabio F, De Biase D, Rihawi K. Novel HER2-Directed Treatments in Advanced Gastric Carcinoma: AnotHER Paradigm Shift? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, Battelli N, Massari F. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 7. | Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, Monteiro FSM, Soares A, Molina-Cerrillo J, Grande E, Battelli N, Massari F. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72:1365-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 8. | Ricci AD, Rizzo A, Brandi G. DNA damage response alterations in gastric cancer: knocking down a new wall. Future Oncol. 2021;17:865-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10353] [Article Influence: 796.4] [Reference Citation Analysis (34)] |
| 10. | Li B, Chan HL, Chen P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr Med Chem. 2019;26:3009-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 11. | Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 294] [Reference Citation Analysis (0)] |
| 12. | Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958-6962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 13. | Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 526] [Cited by in RCA: 818] [Article Influence: 272.7] [Reference Citation Analysis (0)] |
| 14. | Chang E, Pelosof L, Lemery S, Gong Y, Goldberg KB, Farrell AT, Keegan P, Veeraraghavan J, Wei G, Blumenthal GM, Amiri-Kordestani L, Singh H, Fashoyin-Aje L, Gormley N, Kluetz PG, Pazdur R, Beaver JA, Theoret MR. Systematic Review of PD-1/PD-L1 Inhibitors in Oncology: From Personalized Medicine to Public Health. Oncologist. 2021;26:e1786-e1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1292] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 16. | Zheng S, Song J, Linghu D, Yang R, Liu B, Xue Z, Chen Q, Liu C, Zhong D, Hung MC, Sun L. Galectin-9 blockade synergizes with ATM inhibition to induce potent anti-tumor immunity. Int J Biol Sci. 2023;19:981-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia Z, Wang YP, Suo J, Cao X. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One. 2013;8:e81799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 18. | Wang Y, Zhao E, Zhang Z, Zhao G, Cao H. Association between Tim3 and Gal9 expression and gastric cancer prognosis. Oncol Rep. 2018;40:2115-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Park Y, Seo AN, Koh J, Nam SK, Kwak Y, Ahn SH, Park DJ, Kim HH, Lee HS. Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. Oncoimmunology. 2021;10:1954761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Chen K, Gu Y, Cao Y, Fang H, Lv K, Liu X, He X, Wang J, Lin C, Liu H, Zhang H, He H, Xu J, Li H, Li R. TIM3(+) cells in gastric cancer: clinical correlates and association with immune context. Br J Cancer. 2022;126:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Shan B, Man H, Liu J, Wang L, Zhu T, Ma M, Xv Z, Chen X, Yang X, Li P. TIM-3 promotes the metastasis of esophageal squamous cell carcinoma by targeting epithelial-mesenchymal transition via the Akt/GSK-3β/Snail signaling pathway. Oncol Rep. 2016;36:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Hou N, Ma J, Li W, Zhao L, Gao Q, Mai L. T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: Potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients. Oncol Lett. 2017;14:8007-8013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Duan J, Xie Y, Qu L, Wang L, Zhou S, Wang Y, Fan Z, Yang S, Jiao S. A nomogram-based immunoprofile predicts overall survival for previously untreated patients with esophageal squamous cell carcinoma after esophagectomy. J Immunother Cancer. 2018;6:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Hong MH, Shin SJ, Shin SK, Kim DJ, Zo JI, Shim YM, Lee SE, Cho BC, Park SY, Choi YL, Kim HR. High CD3 and ICOS and low TIM-3 expression predict favourable survival in resected oesophageal squamous cell carcinoma. Sci Rep. 2019;9:20197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Chen D, Wang W, Zhao T, Wen J, Zhang F, Duan S, Chen C, Sang Y, Zhang Y, Chen Y. Significance of TIM-3 Expression in Resected Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2020;109:1551-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Aragon-Sanabria V, Kim GB, Dong C. From Cancer Immunoediting to New Strategies in Cancer Immunotherapy: The Roles of Immune Cells and Mechanics in Oncology. Adv Exp Med Biol. 2018;1092:113-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 28. | Jiang J, Li J, Zhou X, Zhao X, Huang B, Qin Y. Exosomes Regulate the Epithelial-Mesenchymal Transition in Cancer. Front Oncol. 2022;12:864980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Qin Y, Geng JX, Huang B. Clinical value of serum pepsinogen in the diagnosis and treatment of gastric diseases. World J Gastrointest Oncol. 2023;15:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (2)] |
| 30. | Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 31. | Borcherding N, Kolb R, Gullicksrud J, Vikas P, Zhu Y, Zhang W. Keeping Tumors in Check: A Mechanistic Review of Clinical Response and Resistance to Immune Checkpoint Blockade in Cancer. J Mol Biol. 2018;430:2014-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Sabins NC, Chornoguz O, Leander K, Kaplan F, Carter R, Kinder M, Bachman K, Verona R, Shen S, Bhargava V, Santulli-Marotto S. TIM-3 Engagement Promotes Effector Memory T Cell Differentiation of Human Antigen-Specific CD8 T Cells by Activating mTORC1. J Immunol. 2017;199:4091-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Pagliano O, Morrison RM, Chauvin JM, Banerjee H, Davar D, Ding Q, Tanegashima T, Gao W, Chakka SR, DeBlasio R, Lowin A, Kara K, Ka M, Zidi B, Amin R, Raphael I, Zhang S, Watkins SC, Sander C, Kirkwood JM, Bosenberg M, Anderson AC, Kuchroo VK, Kane LP, Korman AJ, Rajpal A, West SM, Han M, Bee C, Deng X, Schebye XM, Strop P, Zarour HM. Tim-3 mediates T cell trogocytosis to limit antitumor immunity. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H, Zhang WF, Sun ZJ. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res. 2018;37:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Tan S, Xu Y, Wang Z, Wang T, Du X, Song X, Guo X, Peng J, Zhang J, Liang Y, Lu J, Gao C, Wu Z, Li C, Li N, Gao L, Liang X, Ma C. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020;80:1130-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Klapholz M, Drage MG, Srivastava A, Anderson AC. Presence of Tim3(+) and PD-1(+) CD8(+) T cells identifies microsatellite stable colorectal carcinomas with immune exhaustion and distinct clinicopathological features. J Pathol. 2022;257:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 544] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 38. | Yu S, Ren X, Meng F, Guo X, Tao J, Zhang W, Liu Z, Fu R, Li L. TIM3/CEACAM1 pathway involves in myeloid-derived suppressor cells induced CD8(+) T cells exhaustion and bone marrow inflammatory microenvironment in myelodysplastic syndrome. Immunology. 2023;168:273-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Konantz M, Williams M, Merkel T, Reiss A, Dirnhofer S, Meyer SC, Valent P, George TI, Tzankov A, Hartmann K. Increased TIM-3 and galectin-9 serum levels in patients with advanced systemic mastocytosis. J Allergy Clin Immunol. 2023;152:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 41. | Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, Yan M, Chang WC, Hsu JM, Cha JH, Hsu JL, Chou CW, Sun X, Deng Y, Chou CK, Yu D, Hung MC. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12:832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 373] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 42. | Kandel S, Adhikary P, Li G, Cheng K. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021;510:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |