Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.1835
Peer-review started: July 9, 2023
First decision: September 6, 2023
Revised: September 15, 2023
Accepted: September 27, 2023
Article in press: September 27, 2023
Published online: November 15, 2023
Processing time: 128 Days and 21.3 Hours
Cancer seriously endangers human health. Gastrointestinal cancer is the most common and major malignant tumor, and its morbidity and mortality are gradually increasing. Although there are effective treatments such as radio
Core Tip: The incidence and mortality rates of gastrointestinal tumors have been increasing steadily over the years. However, the side effects associated with conventional chemotherapy have been a major concern for patients. Ginger, a traditional herb known for its medicinal and food homology, has been found to possess anti-tumor properties against various types of gastrointestinal tumors. This article reviews the current research advancements on ginger’s role in treating gastrointestinal tumors. The findings of this review are expected to pave the way for new research directions and inspire innovative ideas for utilizing natural drugs in the treatment of gastrointestinal tumors.
- Citation: Chen GQ, Nan Y, Huang SC, Ning N, Du YH, Lu DD, Yang YT, Meng FD, Yuan L. Research progress of ginger in the treatment of gastrointestinal tumors. World J Gastrointest Oncol 2023; 15(11): 1835-1851
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/1835.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.1835
Cancer is a significant global health threat, with incidence and mortality rates on the rise. It poses a serious challenge to human health worldwide[1]. According to recent data, there were approximately 19.3 million new cases of cancer worldwide in 2020, resulting in 10 million deaths. Gastrointestinal cancer stands out as one of the primary causes of mortality[2]. Out of the top 10 cancers, gastrointestinal cancers make up six of them. These include colorectal cancer (9.4%), liver cancer (8.3%), gastric cancer (7.7%), esophageal cancer (5.5%), and pancreatic cancer (4.7%). Multimodal treatment, including chemotherapy, radiotherapy, and surgery, has become a primary option for treating gastrointestinal tumors. Although effective, the use of this treatment is often associated with significant toxic side effects, including diarrhea, nausea, vomiting, and severe malabsorption[3]. As the number of chemotherapy cycles increases, tumor cells become less sensitive to chemotherapy drugs, resulting in drug resistance. This can lead to tumor recurrence, ultimately impacting the effectiveness of treatment and the long-term survival of patients[4,5].
In recent years, there has been a growing awareness of the importance of food safety and health, leading more and more people to seek out foods with health benefits and therapeutic effects[6]. This concept is known as 'medication-food homology' in traditional Chinese medicine[7]. According to this theory, the source of Chinese medicine and food is the same, and some items can only be used to cure diseases (called medicine), others can only be consumed as food (called diet), while most items have both curative effects and can be consumed as food (called medication-food homology). The concept of homologous medicine and food can be traced back to Huangdi Neijing (Yellow Emperor's Internal Classic), which mentioned that food can also be used as medicine (such as a medicinal diet) to improve people's immunity and thereby achieve the effect of preventing or treating diseases. Since the pre-Qin period, people have found that some foods made into soup can not only meet food and clothing needs, but also treat diseases. In the Han dynasty, people began to consciously classify a variety of medicinal materials according to their functions in the Sui dynasty. Some people put forward the idea that all foods have medicinal properties and that diets should pay attention to the concept of mutual promotion and reasonable matching. Bencao Gangmu (Compendium of Materia Medica) of the Ming dynasty detailed the edible methods of medicinal and edible homologous plants, and clarified the appropriate population and usage[8]. Nowadays, with the continuous development of medicine and the accumulation of experience, people have found that medicine-food homologous substances have significant advantages in pharmacological activities such as hypoglycemic[9], lipid-lowering[10], antioxidant[11], anti-inflammatory[12], immunomodulatory[13] and anti-tumor activities[14] and have fewer adverse reactions. Related research has gradually attracted attention[15]. By 2022, a total of 110 medicinal herbs with medicinal and food homology have been listed by the relevant state departments according to the Food Safety Law of the People's Republic of China and a series of measures such as safety assessment[16].
Ginger is a Zingiber officinale roscoe in the Zingiber family, which was first found in southeast Asia and other tropical areas, and now all countries in the world are planted[17]. Ginger was first widely used as a Chinese herbal medicine in China, because it has the effects of relieving cough and asthma, clearing away heat, warming the lung and dispersing cold. It is also consumed as a spice and vegetable, and is a typical medicine-food homologous herb. Ginger is versatile and has remained popular to date. Its extensive pharmacological actions include anti-inflammatory[18], antioxidant[19], hypoglycemic and lipid-lowering[20,21] and significant antitumor activity[22,23]. Ginger and its active ingredients possess anti-tumor properties that can effectively combat various gastrointestinal tumors. Figure 1 depicts the types of tumors treated with ginger.
Searching "ginger", "cancer", "carcinoma", "tumor" and "neoplasm" as keywords in PubMed, it was found that there was no review on ginger and its active components in the treatment of gastrointestinal tumors in the past 5 years. More attention is still paid to ginger and its active ingredients in the treatment of single cancers such as breast cancer, colorectal cancer, etc, and the alleviation of nausea and vomiting caused by chemotherapy. We retrieved a review published in 2015 on the treatment of gastrointestinal tumors with ginger, which only focused on the in vitro and in vitro effects of ginger on gastrointestinal cancer, but it was a long time ago, and the time distance of the cited literature was far away, so the reference value was not great. In this review, the ethnic pharmacology and main active components of ginger were summarized, and the anti-tumor effects of each component of ginger were evaluated to reveal its mechanism of action in the prevention and treatment of gastrointestinal cancers. The specific process is shown in Figure 2.
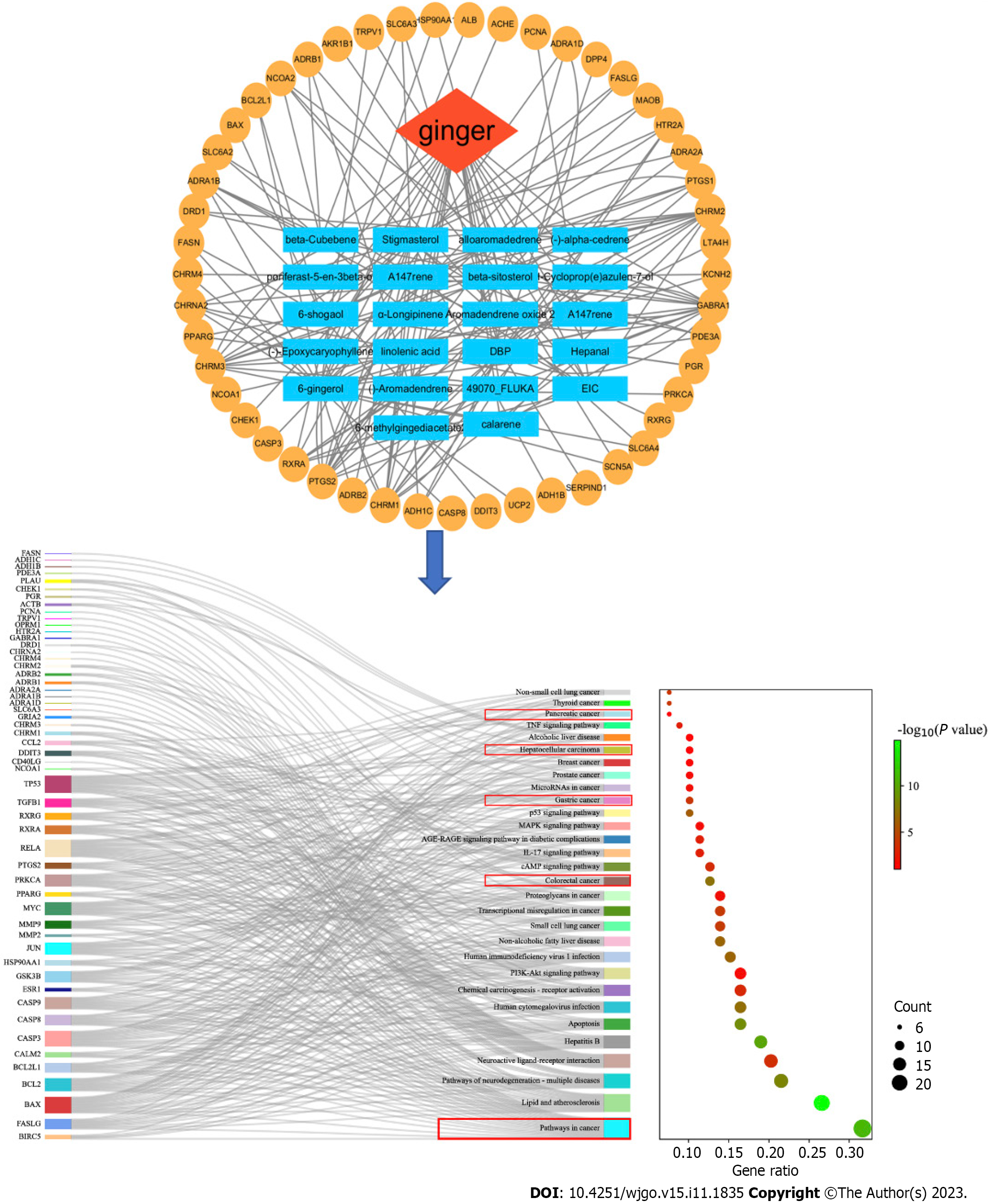
To investigate the potential anti-gastrointestinal tumor effects of ginger, a network pharmacological analysis was conducted. This analysis took into account the multi-component, multi-target, and multi-level properties of ginger. A total of 22 active ingredients and 156 related proteins were screened from the TCMSP database (https://old.tcmsp-e.com/tcmsp.php) using the criteria of oral bioavailability ≥ 30% and drug-likeness ≥ 0.10 (Supplementary Table 1). The protein name was entered into the multiple proteins section of the STRING database (https://cn.string-db.org), specifically selecting the Homo sapiens species. The gene name corresponding to the protein name was then downloaded and matched (Supplementary Table 2). To construct pological network maps of the 12 active components and 63 corresponding genes, we utilized Cytoscape software 3.9.1. In the figure, the red module represents the herb name, the blue template represents the active ingredient, and the yellow module represents the corresponding gene. The 63 genes were analyzed using the DAVID database (https://david.ncifcrf.gov), and the results were visualized using the bioinformatics online platform (http://www.bioinformatics.com.cn/). The results are shown in Figure 3, and most of the genes were enriched in the tumors. In addition, there are colorectal cancer, gastric cancer, hepatocellular carcinoma, pancreatic cancer and other related gastrointestinal cancer pathways. This provides a theoretical basis for us to study the treatment of gastrointestinal cancer with ginger.
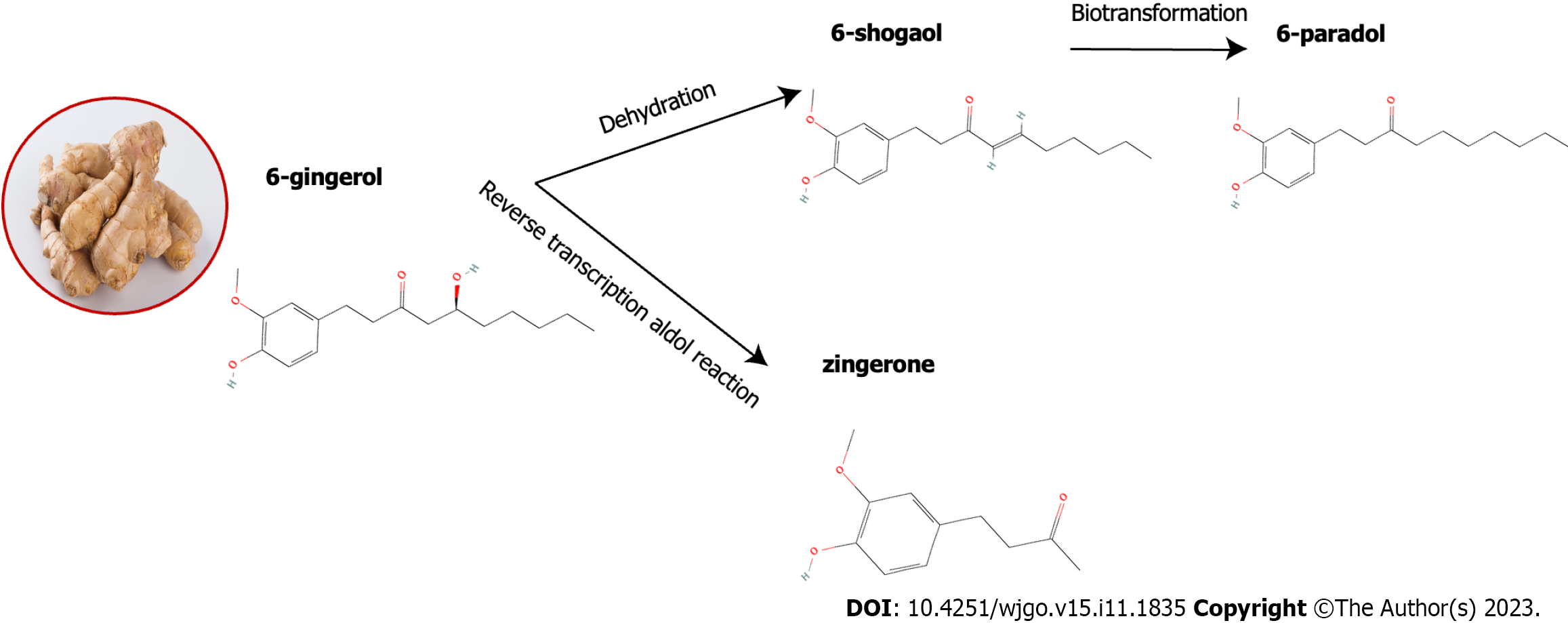
Ginger possesses a multifaceted chemical composition that comprises phenolic compounds, terpenes, polysaccharides, fibrils, etc[24]. As shown in Figure 4, the phenolic compounds in ginger are mainly gingerol, shogaol, zingerone and paradol. Gingerol can be converted to the corresponding gingerol by heating or storage. After hydrogenation, shogaol can be converted to paradol. Ginger also contains several other phenolic compounds, including zingerone, gingerdione, gingerdiol, 6-dehydrogingerol, gingerenone-A, 5-acetoxy-6-gingerol, and 6-dehydrogingeradione, etc[25-27].
Gingerol is a general term for a series of congeners with phenolic ketone functional groups and alkyl side chains connecting different numbers of carbon atoms. Gingerol has strong biological activity and pharmacological effects due to its lower solubility in water and chemical instability, and it has anti-inflammatory and anti-tumor effects on a variety of cancers[28]. Ginger has therapeutic effects that are believed to be caused by a combination of gingerol derivatives, including 6-gingerol, 8-gingerol, and 10-gingerol. These derivatives are responsible for the pungent taste associated with ginger[29,30].
6-shogaol, one of the main ingredients in ginger, is an alkylphenol compound formed by dehydration of 6-gingerol. Studies have shown that 6-shogaol is the hallmark chemical ingredient of ginger and can be used as a quality control index for ginger. 6-shogaol has demonstrated its potential as an effective agent with anticancer, anti-inflammatory, antioxidant, and neuroprotective properties[31,32]. The C3-carbonyl group and C5-hydroxyl group (i.e. β-hydroxyl ketone structure) on the hydrocarbon chain of gingerol make the chemical properties of gingerol extremely unstable. Under acidic or heated conditions, shogaol can be formed when the active hydrogen of C4 on gingerol reacts with the hydroxyl group of C5, resulting in dehydration. The conversion of 6-gingerol to 6-shogaol was dependent on p H. 6-gingerol was most stable at pH = 4, and the reversible conversion was most rapid at 100 ℃ and pH = 1[33]. Studies have shown that 6-shogaol is superior to 6-gingerol in anti-cancer, antioxidant and anti-inflammatory effects, which may be attributed to the chemical structure of 6-shogaol, which contains α, β-unsaturated carbonyl fragments (Michael receptor)[34-36].
Zingerone is a phenolic alkanone isolated from ginger that is found in up to 9.25% of ginger. Zingerone, a compound with a basic phenolic ring and a methoxy group attached to the benzene ring, has been shown to exhibit significant pharmacological effects such as antioxidant, anti-inflammatory, and anti-cancer activities[37]. It has been suggested that zingerone is mainly found in dried ginger, whereas the content of zingerone in fresh ginger is usually very low, but cooking or drying also converts gingerol to zingerone through the reverse aldol reaction[38].
According to research, dried ginger contains 6-paradol as its primary active component, which is synthesized through a biotransformation process from 6-shogaol[35]. It has a large number of pharmacological effects and has been proven to have anti-tumor and anti-proliferative effects[39,40].
In addition, the major terpene components in ginger are β-bisabolene, curcumene, α-farnesene, and β-sesquiculene[41]. The main components of ginger essential oil are known for their anti-inflammatory and antioxidant effects, as well as their ability to provide neuroprotective and anti-cancer properties[42].
Phorbol 12-myristate 13-acetate (PMA) has been shown to activate transcription factors AP-1 and NF-κB in various cancer cells[43]. In colon cancer cells treated with PMA, 6-gingerol has been found to down-regulate PMA-induced phosphorylation of ERK1/2 and JNK MAP kinases as well as the activation of AP-1 transcription factor. However, it has little effect on p38 MAP kinase phosphorylation and NF-κB activation. Moreover, 6-gingerol has been found to significantly inhibit the proliferation of colon cancer cell SW-480 induced by PMA[44].
Epidermal growth factor receptor (EGFR) belongs to the tyrosine kinase family of ERBB cell surface receptors. Upon binding to its ligands, EGFR undergoes autophosphorylation and activates downstream signaling pathways to promote cell proliferation and metastasis[45]. Hu et al[46] found that 8-gingerol inhibits the proliferation and migration of colorectal cancer cells by targeting the EGFR/STAT/ERK pathway, and its effect depends on the expression of EGFR. Jiang et al[47] found the anti-pancreatic cancer activity of 6-paradol was observed through its ability to reduce EGFR expression and inhibit phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B (PI3K/AKT) signaling activity.
Toll receptor 4 (TLR4) is strongly associated with poor prognosis in cancer patients[48,49]. Activation of Wnt/β-catenin signaling contributes to cell proliferation and metastasis in many diseases[50]. In addition, TLR4 mediates Wnt/β-catenin signaling in HCC[51]. Zhang et al[52] hold that 6-shogaol inhibits the proliferation of HepG2 hepatoma cells by mediating Wnt/β-catenin signaling through TLR4.
Cyclin D1 is a proto-oncogene that can cause uncontrolled cell proliferation and malignant transformation when overexpressed. Activation of β-catenin signaling induces cyclin D1, which in turn leads to G1/S phase progression and increased proliferation. Nonsteroidal anti-inflammatory drug-activated gene-1 (NAG-1), a cytokine with pro-apoptotic and antitumor properties, is activated by non-steroidal anti-inflammatory drugs. Lee et al[53] found that the inhibition of cyclin D1 transcription was observed upon treatment with 6-gingerol, which was achieved through the inhibition of β-catenin translocation into the nucleus. Additionally, 6-gingerol was found to increase cyclin D1 proteolysis via proteasomal degradation, leading to growth arrest in colorectal cancer cells.
The p53 gene is responsible for encoding a transcription factor that acts as a tumor suppressor. Its main function is to inhibit cell proliferation by inducing cell cycle arrest and apoptosis[54]. The Cip/Kip family of cyclin-dependent kinase inhibitors p21Cip1, p27Kip1, and p57Kip2, initially identified as cell cycle inhibitors mediating growth-inhibitory signals in upstream signaling pathways, has now emerged as a multilayered protein with cell cycle regulatory functions[55]. Studies have reported that 6-gingerol induced reactive oxygen species (ROS) generation and p53 activation in LoVo cells, inhibited degradation of p27Kip1 and p21 Cip1, and thus significantly induced cell G2/M phase arrest[56]. 6-gingerol also induces cell death in p53-expressing mutant cells and destroys them in G1 phase[57]. Qi et al[58] found that 6-shogaol induced G2/M cell cycle arrest in HCT-116 cells through p53/p21-cdc2/cdc25A crosstalk to achieve tumor effects.
Caspases are a class of cysteine proteases that are activated during apoptosis. Radhakrishnan et al[44] found that 6-gingerol could affect caspase activation and Poly(ADP-ribose) polymerase cleavage to induce apoptosis in colon cancer SW-480 cells using immunoblotting experiments.
Mahlavu cells, which are a subline of human hepatoma cells, are highly refractory to many chemotherapeutic agents and radiotherapy due to their poor differentiation and p53 mutations. Chen et al[59] found that 6-shogaol caused cell death in Mahlavu subline of human liver cancer cells through a process involving oxidative stress and activation of caspases.
Protein kinase C (PKC) is involved in intracellular signal transduction pathways and regulates various functions such as cell cycle, apoptosis and cytoskeleton. Lee et al[53] confirmed that 6-gingerol stimulates colorectal cancer cell apoptosis by upregulating NAG-1 and through the PKCε pathway.
Cathepsin D plays a crucial role in the process of apoptosis. Decreased activity of cathepsin D leads to inhibition of cytochrome c release. Mansingh et al[60] found that treatment of human gastric cancer AGS cells with 6-gingerol results in a decrease in mitochondrial membrane potential. This decrease leads to the up-regulation of cytochrome c, which in turn triggers the caspase cascade and induces cell apoptosis. Yang et al[61] conducted a study which suggests that cathepsin D may play an active role in mediating apoptosis induced by 6-gingerol in HepG2 cells. In another study, the anticancer activity of 6-shogaol in laryngeal cancer (Hep-2) cells was investigated. The study revealed that 6-shogaol induces apoptosis in Hep-2 cells through oxidative damage and the regulation of apoptotic markers[62]. Additionally, 6-shogaol has been found to induce apoptosis in COLO 205 cells through ROS production, caspase activation, and GADD 153 expression[63].
Abdel-Rasol et al[64] found that ginger extract induced the expression of apoptosis-related genes, thereby inducing apoptosis. This may be the mechanism of ginger extract's anti-HCT-116 colorectal cancer (CRC) cells and dimethylhydrazine (DMH)-induced colorectal tumor activity. In HCT116 colon cancer cells treated with zingerone, there was a significant increase in the production of reactive oxygen species, while the mitochondrial membrane potential and antioxidant levels in the cells were decreased. These results indicate that zingerone can induce oxidative stress-mediated apoptosis to achieve the purpose of anti-colon cancer[65].
MAPK is a crucial signal transmitter from the cell surface to the nucleus. It is composed of four subfamilies, namely ERK, p38, JNK, and ERK5, and plays a vital role in regulating various physiological processes such as cell growth, differentiation, and apoptosis[66]. In human colon cancer, HCT116 cells, both 10-gingerol and 6-shogaol induce apoptosis through the mitochondrial pathway. Specifically, 10-gingerol activates the MAPK pathway while 6-shogaol's induction is regulated by the bcl-2 family[58,67].
The endoplasmic reticulum (ER) plays a crucial role in regulating protein synthesis, folding and intracellular calcium levels. However, the loss of these functions can lead to ER stress, which is associated with apoptosis. Hu et al[68] conducted a study that found 6-shogaol to induce apoptosis in human hepatoma SMMC-6 and SMMC-7721 cells through ER stress-related mechanisms.
Tumor cells exhibit malignant behaviors through invasion and migration. Invasion is characterized by the occupation of adjacent host tissues by malignant tumors from primary or secondary tumors. On the other hand, metastasis is the recurrent multistep process of the primary tumor spreading to distant organs[69]. Tumor metastasis is a significant cause of cancer treatment failure and recurrence[70].
Angiogenesis is a crucial factor in tumor progression and is caused by an imbalance between pro-angiogenic and anti-angiogenic factors. This imbalance is mainly due to the excessive production of vascular endothelial growth factor (VEGF) triggered by tissue hypoxia[71]. Farombi et al[72] conducted a study on mice with colon cancer and found that 6-gingerol had an inhibitory effect on angiogenesis by reducing the concentrations of VEGF, angiopoietin-1, fibroblast growth factor and GDF-15.
Epithelial-mesenchymal transition (EMT) is a significant biological process that allows malignant tumor cells derived from epithelial tissue to gain the ability to migrate and invade surrounding tissues. Transforming growth factor beta (TGF-β) is a master regulator of EMT, which can regulate a variety of cellular functions. Kim et al[73] found that zingerone and its derivatives inhibited TGF-β1-induced EMT, thereby inhibiting the migration and invasion of SNU182 hepatoma cells.
Matrix metalloproteinases (MMPs) are crucial molecules in the regulation of tumor invasion, metastasis, proliferation, differentiation, and cell death. They are responsible for the degradation of most extracellular matrix and basement membrane protein components[74]. Tissue inhibitors of metalloproteinases (TIMP) is a natural inhibitor of MMPs by inhibiting their proteolytic activity. An imbalance between MMPs and related TIMPs may play a vital role in the aggressive phenotype of malignancies[75,76]. Weng et al[77] found that 6-shogaol and 6-gingerol may exert anti-invasion effects on hepatocellular carcinoma cells by regulating MMP-9 and TIMP-1.
Tight junctions (TJs) are complexes that help cells adhere to each other and are crucial for maintaining the integrity of epithelial and endothelial barriers[78]. These complexes are important in the gut and liver for barrier formation, but can also contribute to the development of colorectal and gastric cancers[79]. The transcription factor NF-κB plays a crucial role in the signaling pathways of metastasis and invasion[80]. The transcription factor NF-κB is involved in signaling pathways related to metastasis and invasion. 6-gingerol was found to inhibit the nuclear translocation of Snail, which is regulated by NF-κB[81]. Kim and Kim[81] put forth a novel approach to prevent cancer invasion and metastasis. They proposed using 6-gingerol to restore TJS in PANC-1 cells. The results of their experiment demonstrated that 6-gingerol can regulate TJ-related proteins and effectively inhibit the invasion and metastasis of PANC-1 cells by inhibiting the ERK pathway and NF-κB/snail pathway.
Metadherin (MTDH) is a specific tumor-associated antigen that plays a significant role in promoting cancer proliferation, invasion, stance, chemoresistance and angiogenesis[82]. Fang et al[83] found that zingerone inhibited the invasion and migration of HCC cells by inhibiting the MTDH-mediated PI3K/Akt pathway.
Inflammation is a natural response of the body to tissue damage caused by various factors such as physical injury, ischemic injury, infection, exposure to toxins, or trauma. This response triggers changes in cells and the immune system, which promote the repair of damaged tissue and cell growth at the site of injury. However, this environment can also be conducive to the development and progression of cancer[84]. Therefore, targeting inflammation is considered an effective strategy for both cancer prevention and treatment.
It is a widely accepted fact that tumor cells often exhibit excessive expression of proinflammatory mediators, which include proteases, cytokines and chemokines. Among these, cytokines such as tumor necrosis factor α (TNF-α), NF-κB, and interleukin-10 have been linked to human cancer and can either facilitate or impede tumor growth[85]. Studies have shown that ginger extract can significantly reduce the expression of NF-κB and TNF-α to play a role in anti-cancer and anti-inflammatory effects in liver cancer rats[86]. Ginger extract can also exert anti-inflammatory activity by inhibiting pro-inflammatory markers, alleviating oxidative stress and inflammatory response associated with gastric cancer[87]. Ajeigbe et al[88] discovered that 6-gingerol exhibited protective properties against the development of colorectal cancer in mice induced by azoxymethane/dextran sodium sulfate. The researchers attributed this mechanism to the anti-inflammatory and antioxidant properties of 6-gingerol.
Nuclear factor E2-related factor (Nrf-2) can respond to the generation of ROS and regulate the NF-κB transcription factor, ultimately playing a crucial anti-inflammatory role in cell protection[89,90]. Ganaie et al[91] found that zingerone showed promising chemopreventive potential in DMH-induced colon carcinogenesis by inhibiting reactive oxygen species and modulating Nrf-2 and NFκB-mediated inflammation.
Autophagy is a cellular process that involves the use of lysosomes to break down cytoplasmic proteins and damaged organelles. This process is regulated by autophagy-related genes[92]. Autophagy is a therapeutic target in cancer because of its potential ability to regulate cell death[93]. 6-Shogaol has been shown to induce the production of ROS and ER stress-related proteins, and subsequently activate autophagy in human hepatocellular carcinoma HepG2 cells[94]. Moreover, 8-parador induces mitochondrial damage, thereby inducing PTEN-induced kinase 1/Parkinson-mediated mitophagy, thereby inhibiting gastric cancer progression[95].
CINV is a common side effect of antineoplastic chemotherapy and can significantly impact the quality of life and physical function of patients during treatment[96]. Ginger, which has been used as an antiemetic in various traditional medical systems for over 2000 years, has shown promise in reducing the severity of CINV[97].
CINV can occur through relatively independent peripheral and central systems, and many neurotransmitters are involved in its pathogenesis. Some studies have shown that gingerol can significantly inhibit CINV, and its mechanism of action may be related to the inhibition of central or peripheral increases in 5-HT, substance P, and DA[98,99]. Similarly, gingerol has the ability to inhibit the increase in central or peripheral dopamine by inhibiting D2R and TH, while also accelerating dopamine transporter scan. This mechanism of action has been shown to effectively inhibit CINV in rats[100]. Tian et al[101] used two models of emesis to evaluate the antiemetic effects of gingerol. The study revealed that administering gingerol reduced the instances of kaolin consumption in rats induced by cisplatin and vomiting episodes in minks. The reduction in these instances may be attributed to the simultaneous regulation of the 5-HT, SP, and DA systems. According to Feng et al[102], 6-gingerol has been found to be just as effective as ondansetron in treating cisplatin-induced pica in rats. Some researchers have suggested that the potential impact of 6-gingerol on CINV may be attributed to its ability to regulate the TPH/MAO-A/SERT/5-HT/5-HT3 receptor system, leading to a reduction in 5-HT levels[103]. An alternative hypothesis is that 6-gingerol significantly reduced intracellular Ca2+ levels and inhibited 5-HT3Rs-mediated Ca2+/CaMKII/ERK1/2 signaling pathway in NG108-15 cells, resulting in an antiemetic effect on CINV[104].
Soliman et al[105] suggested that pretreatment with zingerone significantly reduced the cardiac histological abnormalities and cardiotoxicity indicators elevation induced by cisplatin or gamma radiation. In addition, zingerone attenuated the elevation of inflammatory markers, including TNF-α levels, myocardial peroxidase activity, and cyclooxygenase-2 protein expression. These results reveal the potential of zingerone as a therapeutic intervention for chemotherapy-and radiotherapy-induced cardiac injury. Research shows that ginger can improve cisplatin-induced myocardial fiber organization disorder, destruction and degeneration, down-regulation of P53, TNF-α immune expression, serum creatine-kinase and actate dehydrogenase levels. The results showed that ginger had a protective effect against cisplatin-induced cardiotoxicity mainly through its anti-apoptotic, antioxidant and anti-inflammatory effects[106].
Adriamycin, a commonly used anti-tumor medication, is often limited in clinical use due to its potential for causing cardiac toxicity. It has been concluded that ginger extract can reduce the cardiotoxicity of Adriamycin in mice hepatocellular carcinoma by oxidative stress, increase of VEGF and downregulation of MDR1[107]. In addition, 6-gingerol can ameliorate doxorubicin-induced cardiotoxicity through the effects of NF-κB and protein glycosylation[108]. Gingerol was also shown to prevent DOX-induced vascular injury[109].
Diethylnitrosamine (DEN) is a known carcinogen that can cause severe liver damage and liver cancer when orally administered[110]. Recent research by Alsahli et al[111] suggests that DEN induces oxidative stress in the liver and changes in hepatocytes. However, they found that 6-gingerol has a significant hepatoprotective effect on DEN-induced liver injury in rats by reducing oxidative stress and inflammation. Similarly, Mansour et al[112] demonstrated that ginger extract was also protective against early oxidative stress and inflammation in rat liver induced by the carcinogen DEN.
Nephrotoxicity is also a major side effect of cisplatin therapy, which limits the application and efficacy of cisplatin in cancer treatment[113]. The administration of 6-gingerol subsequent to cisplatin treatment resulted in the improvement of renal insufficiency and tubular injury. This was observed through reductions in serum creatinine and blood urea nitrogen levels, as well as improvements in histological abnormalities[114]. The study's findings indicate that 6-gingerol has therapeutic properties that can mitigate the effects of cisplatin-induced acute kidney injury. This is achieved through its ability to curb oxidative stress, renal tubular cell death, and inflammation. Zingerone can not only significantly reduce the level of malondialdehyde in tissue after cisplatin administration, but also significantly preserve the activities of catalase and glutathione peroxidase in renal tissue, and inhibit cisplatin-induced inflammation by reducing the level of TNF-α, thus playing a renoprotective effect[115]. Radiotherapy is an effective cancer treatment; however, its use is often limited due to the acute and chronic side effects it has on normal organs. Saberi et al[116] conducted a study which found that radiation causes kidney damage through peroxidative DNA damage and inflammation. The study also showed that pretreatment with ginger antioxidants, acting as an oxidant and anti-inflammatory agent, can alleviate these effects.
Oxaliplatin is a platinum-based anticancer drug that is widely used in the treatment and adjuvant chemotherapy of metastatic and advanced colorectal cancer. The induction of peripheral neuropathic pain shortly after oxaliplatin injection can be a severe issue, potentially leading to treatment interruption and significantly impacting the treatment of cancer patients[117]. Kim et al[118] discovered that the use of 6-shogaol was effective in reducing oxaliplatin-induced neuropathic pain in mice. This was achieved through the activation of serotonergic receptors and gamma-aminobutyric acid in the spinal cord. Ginger extract also significantly alleviated allodynia induced by oxaliplatin treatment alone, possibly by increasing the expression of 5-HT1A receptor mRNA in the spinal cord[119].
Lee et al[120] found that zingerone attenuated cisplatin-induced ototoxicity through mechanisms including oxidative stress, inflammation, and apoptosis. Radiotherapy for pelvic and abdominal tumors can lead to intestinal injury, which is a common acute complication. However, zingerone derivatives have demonstrated potential for protecting against radiation-induced intestinal damage by promoting the proliferation and differentiation of intestinal crypt stem cells. These derivatives have been shown to inhibit apoptosis and reduce DNA damage, which contributes to their protective effects[121].
The off-target testicular toxicity of anticancer drug cisplatin is a concern in the clinic. Studies have shown that fresh ginger juice can reduce cisplatin-induced testicular toxicity in rats by preventing oxidative stress, endocrine imbalance and NO/iNOS/NF-κB signaling[122].
Aspirin is used clinically to reduce pain and inflammation. There is mounting evidence that daily low-dose aspirin can reduce the incidence of colorectal cancer[123]. However, the primary drawback of aspirin usage is its potential to cause gastrointestinal ulcers and bleeding as side effects. Zhu et al[124] synthesized aspirin and 6-gingerol and found that they could exert enhanced anticancer properties in vitro without deleterious effects on the gastric mucosa. Therefore, the combination of aspirin and 6-gingerol can reduce the cardiovascular risk and the occurrence of gastrointestinal complications associated with aspirin, which may be an effective alternative method.
It is well known that the combination of traditional Chinese medicine and ginger, which are homologous to traditional medicine and food, plays a crucial role in diet and medical treatment. In the ancient Chinese books Huangdi Neijing and Jingui Yaolue, jujube and ginger are the most common drug pairs in the compatibility of traditional Chinese medicine. Wu et al[125] extracted and purified the major polysaccharide fraction of jujube as a potential anticancer compound, and further investigated the antitumor activity of the combination of jujube polysaccharide and 6-gingerol. The results showed that the combination of jujube polysaccharide and 6-gingerol had a high inhibitory effect on the proliferation of SW620 cells through apoptosis pathway and G0/G1 phase arrest mechanism. Ganoderma lucidum is also one of the most famous traditional Chinese herbs and has been used for 1000s of years. It has been found that the combination of ginger and Ganoderma lucidum extracts exhibits synergistic anti-proliferative and apoptotic effects on colorectal cancer cells[126]. Saeedifar et al[127] evaluated the anticancer activities of ginger and licorice extracts and the synergistic effects of their combinations. The results showed that their combination synergistically inhibited colon cancer cell growth and increased apoptosis. Therefore, the combination of traditional Chinese medicine and ginger can be developed into an effective anti-tumor agent for functional food and public health in the pharmaceutical industry, which is worthy of further exploration.
The combination of anti-cancer agents from natural products and ginger can help people better fight cancer and reduce adverse reactions. Prescott et al[128] investigated the potential of the combination of the natural derivative sanguinarine as a novel anticancer agent and ginger extract as an ultrasound sensitizer for sonodynamic therapy in pancreatic cancer PANC-1 cells in vitro. The results showed that all their combinations were cytotoxic in a dose-dependent manner in the presence of PANC-1 cells. Besides, higenamine, the active component of aconitum, combined with 6-gingerol has cardioprotective effect on Adriamycin-induced cardiotoxicity by activating the PI3K/Akt signaling pathway[129].
Chemotherapy resistance in colorectal cancer is a major challenge in cancer treatment. Recent studies have shown that using a combination of clinical drugs and ginger derivatives could potentially improve the development of resistance. By combining 5-fluorouracil (5-FU) and 6-shogaol, AMPK signaling is activated, leading to the restoration of the effect of SREBP-1 upregulation. This, in turn, reduces the sensitivity of CRC cells to 5-FU cytotoxicity[130]. The study conducted by Lee et al[131] and team showed that the combination of ginger extract and oxaliplatin has the potential to induce cytotoxic effects in oxaliplatin-resistant CRC cells. This effect is achieved through modulation of CXCR4 expression, indicating a synergistic effect between the two compounds. Their combination seems to provide a therapeutic strategy for the treatment of chemoresistance in CRC. Studies on gingerol and γ-tocotrienol have shown that their combination treatment can inactivate cell cycle process by interfering with cell cycle, down-regulating Wnt signaling pathway, caspase-independent programmed cell death caused by mitochondrial dysfunction, activating endoplasmic reticulum unfolded protein response, disrupting DNA repair mechanism, down-regulating FOXM1 and other major proliferation genes[132,133]. Thus, enhancing the effect of chemotherapy. These studies have promoted the clinical application of ginger in the treatment of CRC.
According to research, Gelam honey from Malaysia possesses anti-cancer properties. It has been found to inhibit cell proliferation and DNA damage, induce apoptosis, and cause cell cycle arrest in a range of cancers. Wee et al[134] proposed that the combination of Gelam honey and ginger had a dose-dependent effect on inducing apoptosis in colon cancer HT29 cells. The highest rate of apoptosis was observed with the combined treatment. Hakim et al[135] also studied that the combination of these two natural compounds had a synergistic effect in the inhibitory effect on HCT 116 CRC cells and could enhance the anticancer effect of 5-FU on HCT 116 CRC cells.
The extraction of active components from ginger holds promises for the development of modern drugs to treat gastrointestinal tumors. However, the properties of these components, including stability, solubility, irritation, and bioavailability, present challenges. To overcome these issues and expand the clinical use of ginger, innovative drug delivery technologies can be employed to address the problems faced in the drug delivery system.
The structure of liposomes is very similar to that of cell membrane, which makes it highly biocompatible and biodegradable. Therefore, liposomes can protect the drug from enzymatic degradation before reaching the lesion site. At the same time, the drug can be hidden inside the liposomes in the form of physical encapsulation, which can improve the stability of the drug, reduce the toxicity of the drug, increase the dose of the drug, and have a better therapeutic effect[136,137]. Yavari et al[138] prepared nanoliposomes containing ginger extract and investigated their effects on colorectal cancer cells. This finding further emphasizes that liposomes containing ginger extract enhanced anticancer properties by increasing the induction of apoptosis and stimulating the immune system.
The use of deep eutectic solvents (DES) as a green solvent for extracting bioactive compounds from plant materials has gained popularity due to its numerous benefits such as biodegradability, sustainability, low toxicity, and affordability[139]. Recent studies have demonstrated that fermented DES-ginger extract can enhance the cytotoxicity and therapeutic effects of oxaliplatin in drug-resistant CRC cells by inhibiting NF-κB and CXCR4[131].
Chitosan is a biodegradable polymer. Due to its good biodegradability, strong adhesion to biological mucosa, non-toxicity and histocompatibility, it is an ideal drug carrier[140,141]. Nano-microspheres prepared by chitosan can improve the stability of drugs, improve the solubility of hydrophobic drugs, change the route of administration, increase the absorption of drugs, improve the bioavailability of drugs, reduce the adverse reactions of drugs, and can also be sustained release, controlled release, or targeted release of drugs[142]. Therefore, chitosan nanospheres have great application potential as drug carriers. Abo Mansour et al[107] found that loading ginger extract into chitosan nano
The field of biomedicine has seen considerable progress with the application of nanotechnology, which has provided a new solution to overcome the challenges associated with cancer treatment. In recent times, there has been a growing focus on the synthesis of nano-ZnO materials and their potential for anti-tumor activity[143,144]. In a study by Ahamed et al[145], ZrO2-doped ZnO/reduced graphene oxide nanocomposites were synthesized using ginger rhizome extract. The ginger extract facilitated a synergistic effect between ZnO, ZrO2, and rGO, resulting in high anticancer efficacy and improved biocompatibility.
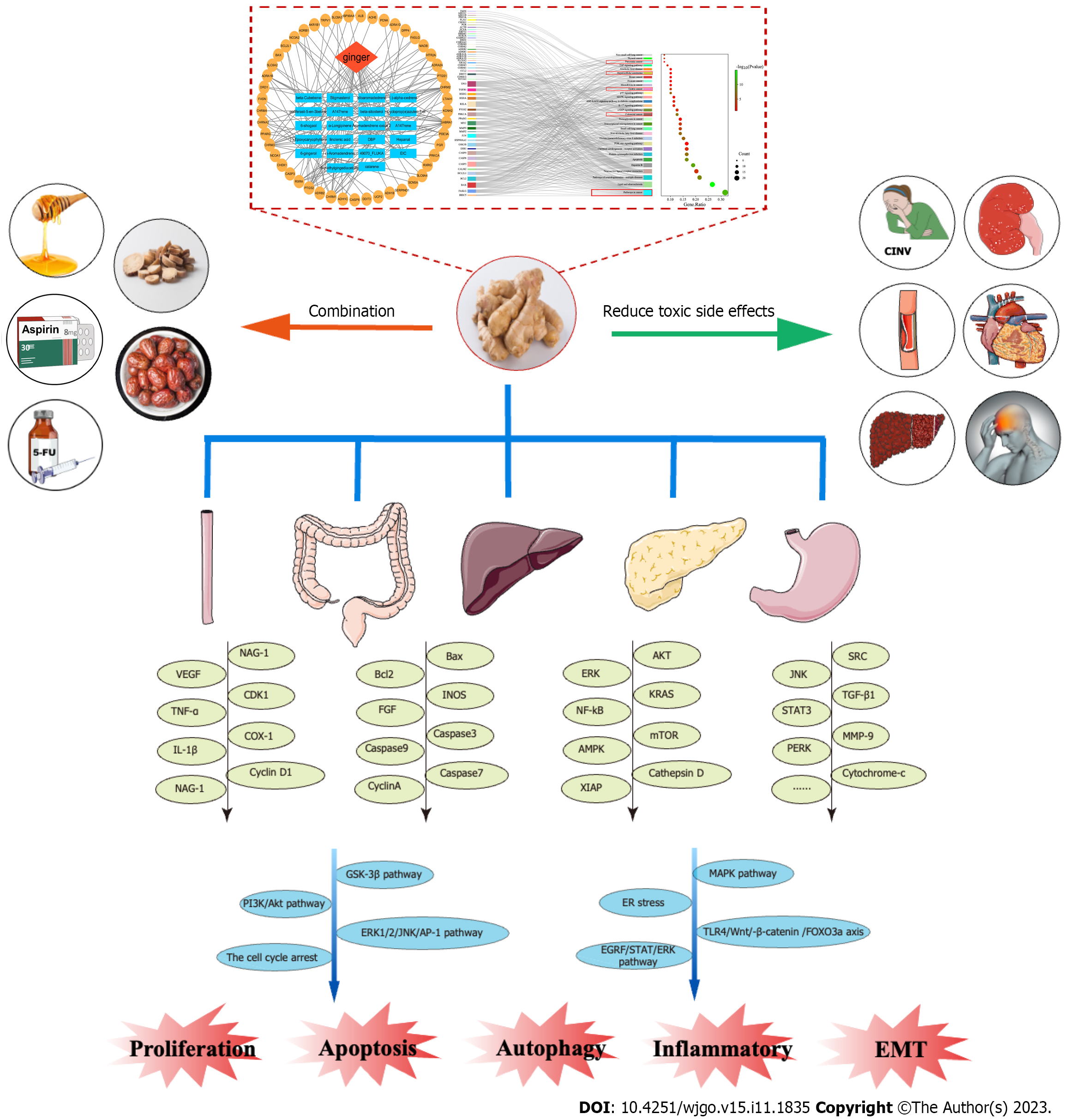
Cancer is a disease that occurs when the body cells lose their normal regulation and start to excessively proliferate, which poses a threat to human health. Ginger has been found to have significant advantages in the treatment of gastrointestinal tumors due to its wide range of pharmacological activities and various application routes.
This article reviews the application of ginger and its active components in the treatment of gastrointestinal tumors, focusing on the anti-tumor mechanism of ginger, the reduction of adverse reactions of radiotherapy and chemotherapy, the combination of ginger and modern medicine, and new drug delivery technologies, so as to inhibit the occurrence and malignant progression of tumors. Ginger's active ingredients have the ability to regulate several signaling pathways such as PI3K/Akt/mTOR, Wnt/β-catenin, EGFR, and NF-κB. This regulation is achieved through components such as 6-gingerol, 6-shogaol, zingerone, and others that can directly or indirectly act on signal targets, leading to an anti-tumor effect.
As a traditional Chinese herbal medicine with a long history of medicinal use, ginger's active ingredients and pharmacological effects are gradually being recognized and explored through available scientific research. Numerous studies have shown that its natural active ingredients have significant effects in the treatment of many diseases such as cancer and inflammation, with fewer side effects, which is of great significance in anti-cancer research. In addition, ginger is a common material, and people are paying more and more attention to health care, so the utilization of ginger's medicinal and food values will have a broad prospect.
Several experimental studies have confirmed the anti-tumor properties of ginger. However, further research is necessary to address the current deficiencies and limitations in order to fully understand and utilize its potential in cancer treatment. Furthermore, the majority of current research findings are derived from in vitro cell experiments, with fewer in vivo animal experiments and clinical trials. Therefore, it is imperative to emphasize the integration of basic experiments and clinical trials. Recent years have seen successful developments in drug delivery systems, which have tackled issues such as stability, solubility, and bioavailability. However, research on new delivery technologies for other active components of ginger remains scarce. Despite the growing recognition of the benefits of ginger combined with modern medicine, significant challenges and barriers to its widespread use persist.
Ginger contains several antitumor compounds that can improve the effectiveness of anticancer drugs and minimize the negative side effects of radiation and chemotherapy. As a result, it has the potential to be a valuable addition to cancer treatment regimens. To further investigate and develop the anti-tumor properties of ginger, it is suggested to use molecular biology techniques to explore its anti-tumor mechanism and confirm it through in vivo experiments. Additionally, comparing the anti-tumor activities of different components of ginger and investigating the optimal compatibility and advanced drug delivery system can provide further insight into the anti-tumor effect of ginger. With the potential for more active ingredients to emerge from ginger in the future, it could offer safe and effective natural drugs and preparations for treating various tumors in clinical settings.
The authors would like to acknowledge Joanna Tibenda for revising the article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Serban ED, Romania S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2172] [Article Influence: 724.0] [Reference Citation Analysis (1)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64217] [Article Influence: 16054.3] [Reference Citation Analysis (174)] |
| 3. | Grabenbauer GG, Holger G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2016;30:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Di Fiore F, Van Cutsem E. Acute and long-term gastrointestinal consequences of chemotherapy. Best Pract Res Clin Gastroenterol. 2009;23:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y, Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 6. | Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. 2020;369:m2482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 7. | Zou L, Li H, Ding X, Liu Z, He D, Kowah JAH, Wang L, Yuan M, Liu X. A Review of The Application of Spectroscopy to Flavonoids from Medicine and Food Homology Materials. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hou Y, Jiang JG. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013;4:1727-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Gong X, Ji M, Xu J, Zhang C, Li M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit Rev Food Sci Nutr. 2020;60:2303-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Xu Y, Zhang X, Yan XH, Zhang JL, Wang LY, Xue H, Jiang GC, Ma XT, Liu XJ. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int J Biol Macromol. 2019;135:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Qin T, Ren Z, Liu X, Luo Y, Long Y, Peng S, Chen S, Zhang J, Ma Y, Li J, Huang Y. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int J Biol Macromol. 2019;125:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Lu Q, Li R, Yang Y, Zhang Y, Zhao Q, Li J. Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chem. 2022;368:130610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Fu YP, Feng B, Zhu ZK, Feng X, Chen SF, Li LX, Yin ZQ, Huang C, Chen XF, Zhang BZ, Jia RY, Song X, Lv C, Yue GZ, Ye G, Liang XX, He CL, Yin LZ, Zou YF. The Polysaccharides from Codonopsis pilosula Modulates the Immunity and Intestinal Microbiota of Cyclophosphamide-Treated Immunosuppressed Mice. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Bai R, Li W, Li Y, Ma M, Wang Y, Zhang J, Hu F. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int J Biol Macromol. 2018;120:1544-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Zhao J, Ge LY, Xiong W, Leong F, Huang LQ, Li SP. Advanced development in phytochemicals analysis of medicine and food dual purposes plants used in China (2011-2014). J Chromatogr A. 2016;1428:39-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Xu J, Zhang J, Sang Y, Wei Y, Chen X, Wang Y, Xue H. Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Kiyama R. Nutritional implications of ginger: chemistry, biological activities and signaling pathways. J Nutr Biochem. 2020;86:108486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 18. | Ballester P, Cerdá B, Arcusa R, Marhuenda J, Yamedjeu K, Zafrilla P. Effect of Ginger on Inflammatory Diseases. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 19. | Morvaridzadeh M, Sadeghi E, Agah S, Fazelian S, Rahimlou M, Kern FG, Heshmati S, Omidi A, Persad E, Heshmati J. Effect of ginger (Zingiber officinale) supplementation on oxidative stress parameters: A systematic review and meta-analysis. J Food Biochem. 2021;45:e13612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Veisi P, Zarezade M, Rostamkhani H, Ghoreishi Z. Renoprotective effects of the ginger (Zingiber officinale) on Diabetic kidney disease, current knowledge and future direction: a systematic review of animal studies. BMC Complement Med Ther. 2022;22:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Carvalho GCN, Lira-Neto JCG, Araújo MFM, Freitas RWJF, Zanetti ML, Damasceno MMC. Effectiveness of ginger in reducing metabolic levels in people with diabetes: a randomized clinical trial. Rev Lat Am Enfermagem. 2020;28:e3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Mahomoodally MF, Aumeeruddy MZ, Rengasamy KRR, Roshan S, Hammad S, Pandohee J, Hu X, Zengin G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin Cancer Biol. 2021;69:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 23. | Almatroudi A, Alsahli MA, Alrumaihi F, Allemailem KS, Rahmani AH. Ginger: A Novel Strategy to Battle Cancer through Modulating Cell Signalling Pathways: A Review. Curr Pharm Biotechnol. 2019;20:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, Li HB. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 25. | Ji K, Fang L, Zhao H, Li Q, Shi Y, Xu C, Wang Y, Du L, Wang J, Liu Q. Ginger Oleoresin Alleviated γ-Ray Irradiation-Induced Reactive Oxygen Species via the Nrf2 Protective Response in Human Mesenchymal Stem Cells. Oxid Med Cell Longev. 2017;2017:1480294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Schadich E, Hlaváč J, Volná T, Varanasi L, Hajdúch M, Džubák P. Effects of Ginger Phenylpropanoids and Quercetin on Nrf2-ARE Pathway in Human BJ Fibroblasts and HaCaT Keratinocytes. Biomed Res Int. 2016;2016:2173275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Arcusa R, Villaño D, Marhuenda J, Cano M, Cerdà B, Zafrilla P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front Nutr. 2022;9:809621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Alharbi KS, Nadeem MS, Afzal O, Alzarea SI, Altamimi ASA, Almalki WH, Mubeen B, Iftikhar S, Shah L, Kazmi I. Gingerol, a Natural Antioxidant, Attenuates Hyperglycemia and Downstream Complications. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Ma RH, Ni ZJ, Zhu YY, Thakur K, Zhang F, Zhang YY, Hu F, Zhang JG, Wei ZJ. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2021;12:519-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 30. | Ali AMA, El-Nour MEM, Yagi SM. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J Genet Eng Biotechnol. 2018;16:677-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Bischoff-Kont I, Primke T, Niebergall LS, Zech T, Fürst R. Ginger Constituent 6-Shogaol Inhibits Inflammation- and Angiogenesis-Related Cell Functions in Primary Human Endothelial Cells. Front Pharmacol. 2022;13:844767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Hassan SM, Hassan AH. The possibility of using shogaol for treatment of ulcerative colitis. Iran J Basic Med Sci. 2018;21:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Bischoff-Kont I, Fürst R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, Badmaev V, Ho CT. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57:10645-10650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 36. | Kou X, Wang X, Ji R, Liu L, Qiao Y, Lou Z, Ma C, Li S, Wang H, Ho CT. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018;9:1310-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Min G, Ku SK, Lee T, Bae JS. Suppressive effects of zingerone on TGFBIp-mediated septic responses. Arch Pharm Res. 2018;41:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir Mu. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). ScientificWorldJournal. 2015;2015:816364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Nagendra chari KL, Manasa D, Srinivas P, Sowbhagya HB. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013;139:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Sapkota A, Park SJ, Choi JW. Neuroprotective Effects of 6-Shogaol and Its Metabolite, 6-Paradol, in a Mouse Model of Multiple Sclerosis. Biomol Ther (Seoul). 2019;27:152-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Sharifi-Rad M, Varoni EM, Salehi B, Sharifi-Rad J, Matthews KR, Ayatollahi SA, Kobarfard F, Ibrahim SA, Mnayer D, Zakaria ZA, Sharifi-Rad M, Yousaf Z, Iriti M, Basile A, Rigano D. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Wang X, Shen Y, Thakur K, Han J, Zhang JG, Hu F, Wei ZJ. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 43. | Mowla S, Pinnock R, Leaner VD, Goding CR, Prince S. PMA-induced up-regulation of TBX3 is mediated by AP-1 and contributes to breast cancer cell migration. Biochem J. 2011;433:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AK, Soniya EV, Anto RJ. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS One. 2014;9:e104401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 46. | Hu SM, Yao XH, Hao YH, Pan AH, Zhou XW. 8‑Gingerol regulates colorectal cancer cell proliferation and migration through the EGFR/STAT/ERK pathway. Int J Oncol. 2020;56:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Jiang X, Wang J, Chen P, He Z, Xu J, Chen Y, Liu X, Jiang J. [6]-Paradol suppresses proliferation and metastases of pancreatic cancer by decreasing EGFR and inactivating PI3K/AKT signaling. Cancer Cell Int. 2021;21:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Xiang X, Li L, Bo P, Kuang T, Liu S, Xie X, Guo S, Fu X, Zhang Y. 7‑Difluoromethyl‑5,4'‑dimethoxygenistein exerts anti‑angiogenic effects on acute promyelocytic leukemia HL‑60 cells by inhibiting the TLR4/NF‑κB signaling pathway. Mol Med Rep. 2020;21:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 49. | Daskalopoulos AG, Avgoustidis D, Chaisuparat R, Karanikou M, Lazaris AC, Sklavounou A, Nikitakis NG. Assessment of TLR4 and TLR9 signaling and correlation with human papillomavirus status and histopathologic parameters in oral tongue squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129:493-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Lin Y, Higashisaka K, Shintani T, Maki A, Hanamuro S, Haga Y, Maeda S, Tsujino H, Nagano K, Fujio Y, Tsutsumi Y. Progesterone receptor membrane component 1 leads to erlotinib resistance, initiating crosstalk of Wnt/β-catenin and NF-κB pathways, in lung adenocarcinoma cells. Sci Rep. 2020;10:4748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Zhao C, Yu T, Dou Q, Guo Y, Yang X, Chen Y. Knockout of TLR4 promotes fracture healing by activating Wnt/β-catenin signaling pathway. Pathol Res Pract. 2020;216:152766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Wang J, Qu Y, Chen Y. 6-Shogaol Suppresses the Progression of Liver Cancer via the Inactivation of Wnt/[Formula: see text]-Catenin Signaling by Regulating TLR4. Am J Chin Med. 2021;49:2033-2048. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Kanapathipillai M. Treating p53 Mutant Aggregation-Associated Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 55. | Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 853] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 56. | Lin CB, Lin CC, Tsay GJ. 6-Gingerol Inhibits Growth of Colon Cancer Cell LoVo via Induction of G2/M Arrest. Evid Based Complement Alternat Med. 2012;2012:326096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Park YJ, Wen J, Bang S, Park SW, Song SY. [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med J. 2006;47:688-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Qi LW, Zhang Z, Zhang CF, Anderson S, Liu Q, Yuan CS, Wang CZ. Anti-Colon Cancer Effects of 6-Shogaol Through G2/M Cell Cycle Arrest by p53/p21-cdc2/cdc25A Crosstalk. Am J Chin Med. 2015;43:743-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH, Lu FJ, Lin YS, Kuo SH, Chen CH. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J Agric Food Chem. 2007;55:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Mansingh DP, O J S, Sali VK, Vasanthi HR. [6]-Gingerol-induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J Biochem Mol Toxicol. 2018;32:e22206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Yang G, Wang S, Zhong L, Dong X, Zhang W, Jiang L, Geng C, Sun X, Liu X, Chen M, Ma Y. 6-Gingerol induces apoptosis through lysosomal-mitochondrial axis in human hepatoma G2 cells. Phytother Res. 2012;26:1667-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Annamalai G, Kathiresan S, Kannappan N. [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed Pharmacother. 2016;82:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Abdel-Rasol MA, El-Beih NM, Yahya SS, El-Sayed WM. The Antitumor Activity of Ginger against Colorectal Cancer Induced by Dimethylhydrazine in Rats. Anticancer Agents Med Chem. 2022;22:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Su P, Veeraraghavan VP, Krishna Mohan S, Lu W. A ginger derivative, zingerone-a phenolic compound-induces ROS-mediated apoptosis in colon cancer cells (HCT-116). J Biochem Mol Toxicol. 2019;33:e22403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Yue J, López JM. Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 762] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 67. | Ryu MJ, Chung HS. [10]-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim. 2015;51:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Hu R, Zhou P, Peng YB, Xu X, Ma J, Liu Q, Zhang L, Wen XD, Qi LW, Gao N, Li P. 6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress. PLoS One. 2012;7:e39664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Cuypers A, Truong AK, Becker LM, Saavedra-García P, Carmeliet P. Tumor vessel co-option: The past & the future. Front Oncol. 2022;12:965277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Nakhjavani M, Smith E, Townsend AR, Price TJ, Hardingham JE. Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 71. | Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 72. | Farombi EO, Ajayi BO, Adedara IA. 6-Gingerol delays tumorigenesis in benzo[a]pyrene and dextran sulphate sodium-induced colorectal cancer in mice. Food Chem Toxicol. 2020;142:111483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Kim YJ, Jeon Y, Kim T, Lim WC, Ham J, Park YN, Kim TJ, Ko H. Combined treatment with zingerone and its novel derivative synergistically inhibits TGF-β1 induced epithelial-mesenchymal transition, migration and invasion of human hepatocellular carcinoma cells. Bioorg Med Chem Lett. 2017;27:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Conlon GA, Murray GI. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2019;247:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 75. | Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997;15:61-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 333] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 76. | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1323] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 77. | Weng CJ, Wu CF, Huang HW, Ho CT, Yen GC. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res. 2010;54:1618-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 1007] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 79. | Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 80. | Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2784] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 81. | Kim SO, Kim MR. [6]-Gingerol Prevents Disassembly of Cell Junctions and Activities of MMPs in Invasive Human Pancreas Cancer Cells through ERK/NF- κ B/Snail Signal Transduction Pathway. Evid Based Complement Alternat Med. 2013;2013:761852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Dhiman G, Srivastava N, Goyal M, Rakha E, Lothion-Roy J, Mongan NP, Miftakhova RR, Khaiboullina SF, Rizvanov AA, Baranwal M. Metadherin: A Therapeutic Target in Multiple Cancers. Front Oncol. 2019;9:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Fang J, Zhu H, Xu P, Jiang R. Zingerone suppresses proliferation, invasion, and migration of hepatocellular carcinoma cells by the inhibition of MTDH-mediated PI3K/Akt pathway. J Recept Signal Transduct Res. 2022;42:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 828] [Article Influence: 165.6] [Reference Citation Analysis (0)] |
| 85. | Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 86. | Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, Yusof YA. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo). 2008;63:807-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 87. | Mansingh DP, Pradhan S, Biswas D, Barathidasan R, Vasanthi HR. Palliative Role of Aqueous Ginger Extract on N-Nitroso-N-Methylurea-Induced Gastric Cancer. Nutr Cancer. 2020;72:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Ajeigbe OF, Maruf OR, Anyebe DA, Opafunso IT, Ajayi BO, Farombi EO. 6- shogaol suppresses AOM/DSS-mediated colorectal adenoma through its antioxidant and anti-inflammatory effects in mice. J Food Biochem. 2022;46:e14422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 89. | Basak P, Sadhukhan P, Sarkar P, Sil PC. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol Rep. 2017;4:306-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | Li Y, Shen L, Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int Immunopharmacol. 2016;40:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 91. | Ganaie MA, Al Saeedan A, Madhkali H, Jan BL, Khatlani T, Sheikh IA, Rehman MU, Wani K. Chemopreventive efficacy zingerone (4-[4-hydroxy-3-methylphenyl] butan-2-one) in experimental colon carcinogenesis in Wistar rats. Environ Toxicol. 2019;34:610-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 1071] [Article Influence: 214.2] [Reference Citation Analysis (0)] |
| 93. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1943] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 94. | Wu JJ, Omar HA, Lee YR, Teng YN, Chen PS, Chen YC, Huang HS, Lee KH, Hung JH. 6-Shogaol induces cell cycle arrest and apoptosis in human hepatoma cells through pleiotropic mechanisms. Eur J Pharmacol. 2015;762:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Wang R, Lee YG, Dhandapani S, Baek NI, Kim KP, Cho YE, Xu X, Kim YJ. 8-paradol from ginger exacerbates PINK1/Parkin mediated mitophagy to induce apoptosis in human gastric adenocarcinoma. Pharmacol Res. 2023;187:106610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 96. | Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 542] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 97. | Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2013;53:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 98. | Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res. 2009;32:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Qian QH, Yue W, Chen WH, Yang ZH, Liu ZT, Wang YX. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin Med J (Engl). 2010;123:478-484. [PubMed] |
| 100. | Qian W, Cai X, Wang Y, Zhang X, Zhao H, Qian Q, Yang Z, Liu Z, Hasegawa J. Effect of Gingerol on Cisplatin-Induced Pica Analogous to Emesis Via Modulating Expressions of Dopamine 2 Receptor, Dopamine Transporter and Tyrosine Hydroxylase in the Vomiting Model of Rats. Yonago Acta Med. 2016;59:100-110. [PubMed] |
| 101. | Tian L, Qian W, Qian Q, Zhang W, Cai X. Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J Nat Med. 2020;74:353-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Feng X, Cheng Q, Meng Q, Yang Y, Nie K. Effects of ondansetron and [6]-gingerol on pica and gut microbiota in rats treated with cisplatin. Drug Des Devel Ther. 2019;13:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Cheng Q, Feng X, Meng Q, Li Y, Chen S, Wang G, Nie K. [6]-Gingerol Ameliorates Cisplatin-Induced Pica by Regulating the TPH/MAO-A/SERT/5-HT/5-HT(3) Receptor System in Rats. Drug Des Devel Ther. 2020;14:4085-4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. |
Mo Z, Xian Y, Zhang R, Dai Y, Chen W, Nie K.
6-Gingerol, a major ingredient of ginger, attenuated cisplatin-induced pica in rats |
| 105. | Soliman AF, Anees LM, Ibrahim DM. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:819-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | El-Hawwary AA, Omar NM. The influence of ginger administration on cisplatin-induced cardiotoxicity in rat: Light and electron microscopic study. Acta Histochem. 2019;121:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Abo Mansour HE, El-Batsh MM, Badawy NS, Mehanna ET, Mesbah NM, Abo-Elmatty DM. Ginger Extract Loaded into Chitosan Nanoparticles Enhances Cytotoxicity and Reduces Cardiotoxicity of Doxorubicin in Hepatocellular Carcinoma in Mice. Nutr Cancer. 2021;73:2347-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 108. | El-Bakly WM, Louka ML, El-Halawany AM, Schaalan MF. 6-gingerol ameliorated doxorubicin-induced cardiotoxicity: role of nuclear factor kappa B and protein glycation. Cancer Chemother Pharmacol. 2012;70:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Al-Abbasi FA, Alghamdi EA, Baghdadi MA, Alamoudi AJ, El-Halawany AM, El-Bassossy HM, Aseeri AH, Al-Abd AM. Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 110. | Gupta C, Vikram A, Tripathi DN, Ramarao P, Jena GB. Antioxidant and antimutagenic effect of quercetin against DEN induced hepatotoxicity in rat. Phytother Res. 2010;24:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Alsahli MA, Almatroodi SA, Almatroudi A, Khan AA, Anwar S, Almutary AG, Alrumaihi F, Rahmani AH. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediators Inflamm. 2021;2021:6661937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 112. | Mansour DF, Abdallah HMI, Ibrahim BMM, Hegazy RR, Esmail RSE, Abdel-Salam LO. The Carcinogenic Agent Diethylnitrosamine Induces Early Oxidative Stress, Inflammation and Proliferation in Rat Liver, Stomach and Colon: Protective Effect of Ginger Extract. Asian Pac J Cancer Prev. 2019;20:2551-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 113. | Ridzuan NRA, Rashid NA, Othman F, Budin SB, Hussan F, Teoh SL. Protective Role of Natural Products in Cisplatin-Induced Nephrotoxicity. Mini Rev Med Chem. 2019;19:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Gwon MG, Gu H, Leem J, Park KK. Protective Effects of 6-Shogaol, an Active Compound of Ginger, in a Murine Model of Cisplatin-Induced Acute Kidney Injury. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Alibakhshi T, Khodayar MJ, Khorsandi L, Rashno M, Zeidooni L. Protective effects of zingerone on oxidative stress and inflammation in cisplatin-induced rat nephrotoxicity. Biomed Pharmacother. 2018;105:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 116. | Saberi H, Keshavarzi B, Shirpoor A, Gharalari FH, Rasmi Y. Rescue effects of ginger extract on dose dependent radiation-induced histological and biochemical changes in the kidneys of male Wistar rats. Biomed Pharmacother. 2017;94:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 118. | Kim S, Gang J, Lee JH, Yang H, Cheon C, Ko SG, Bae H, Kim W. [6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Lee JH, Min D, Lee D, Kim W. Zingiber officinale Roscoe Rhizomes Attenuate Oxaliplatin-Induced Neuropathic Pain in Mice. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Lee CH, Lee DH, Lee SM, Kim ASY. Otoprotective Effects of Zingerone on Cisplatin-Induced Ototoxicity. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Wu J, Duan Y, Cui J, Dong Y, Li H, Wang M, Fan S, Li D, Li Y. Protective effects of zingerone derivate on ionizing radiation-induced intestinal injury. J Radiat Res. 2019;60:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Famurewa AC, Ekeleme-Egedigwe CA, Onwe CS, Egedigwe UO, Okoro CO, Egedigwe UJ, Asogwa NT. Ginger juice prevents cisplatin-induced oxidative stress, endocrine imbalance and NO/iNOS/NF-κB signalling via modulating testicular redox-inflammatory mechanism in rats. Andrologia. 2020;52:e13786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 123. | Fink SP, Yamauchi M, Nishihara R, Jung S, Kuchiba A, Wu K, Cho E, Giovannucci E, Fuchs CS, Ogino S, Markowitz SD, Chan AT. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med. 2014;6:233re2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 124. | Zhu Y, Wang F, Zhao Y, Wang P, Sang S. Gastroprotective [6]-Gingerol Aspirinate as a Novel Chemopreventive Prodrug of Aspirin for Colon Cancer. Sci Rep. 2017;7:40119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Wu Z, Gao R, Li H, Wang Y, Luo Y, Zou J, Zhao B, Chen S. New insight into the joint significance of dietary jujube polysaccharides and 6-gingerol in antioxidant and antitumor activities. RSC Adv. 2021;11:33219-33234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 126. | Saeedifar AM, Ghazavi A, Mosayebi G, Ganji A. Synergistic apoptotic effects of ethanolic extracts of ginger and Ganoderma lucidum in a colorectal cancer cell line. Biotech Histochem. 2023;98:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Saeedifar AM, Mosayebi G, Ghazavi A, Ganji A. Synergistic Evaluation of Ginger and Licorice Extracts in a Mouse Model of Colorectal Cancer. Nutr Cancer. 2021;73:1068-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 128. | Prescott M, Mitchell J, Totti S, Lee J, Velliou E, Bussemaker M. Sonodynamic therapy combined with novel anti-cancer agents, sanguinarine and ginger root extract: Synergistic increase in toxicity in the presence of PANC-1 cells in vitro. Ultrason Sonochem. 2018;40:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Chen YL, Zhuang XD, Xu ZW, Lu LH, Guo HL, Wu WK, Liao XX. Higenamine Combined with [6]-Gingerol Suppresses Doxorubicin-Triggered Oxidative Stress and Apoptosis in Cardiomyocytes via Upregulation of PI3K/Akt Pathway. Evid Based Complement Alternat Med. 2013;2013:970490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Lee KC, Wu KL, Yen CK, Chen CN, Chang SF, Huang WS. 6-Shogaol Antagonizes the Adipocyte-Conditioned Medium-Initiated 5-Fluorouracil Resistance in Human Colorectal Cancer Cells through Controlling the SREBP-1 Level. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Lee KC, Wu KL, Chang SF, Chang HI, Chen CN, Chen YY. Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Yusof KM, Makpol S, Fen LS, Jamal R, Wan Ngah WZ. Suppression of colorectal cancer cell growth by combined treatment of 6-gingerol and γ-tocotrienol via alteration of multiple signalling pathways. J Nat Med. 2019;73:745-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Yusof KM, Makpol S, Jamal R, Harun R, Mokhtar N, Ngah WZ. γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules. 2015;20:10280-10297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 134. | Wee LH, Morad NA, Aan GJ, Makpol S, Wan Ngah WZ, Mohd Yusof YA. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/β-catenin Pathways. Asian Pac J Cancer Prev. 2015;16:6549-6556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 135. | Hakim L, Alias E, Makpol S, Ngah WZ, Morad NA, Yusof YA. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pac J Cancer Prev. 2014;15:4651-4657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 136. | Khan YY, Suvarna V. LIPOSOMES CONTAINING PHYTOCHEMICALS FOR CANCER TREATMENT-AN UPDATE. International Journal of Current Pharmaceutical Research. 2016;9:20-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 137. | Mirzavi F, Barati M, Soleimani A, Vakili-Ghartavol R, Jaafari MR, Soukhtanloo M. A review on liposome-based therapeutic approaches against malignant melanoma. Int J Pharm. 2021;599:120413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 138. | Yavari M, Jaafari MR, Mirzavi F, Mosayebi G, Ghazavi A, Ganji A. Anti-tumor effects of PEGylated-nanoliposomes containing ginger extract in colorectal cancer-bearing mice. Iran J Basic Med Sci. 2022;25:890-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 139. | Ling JKU, Hadinoto K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 140. | Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 827] [Cited by in RCA: 726] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 141. | Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 142. | Matalqah SM, Aiedeh K, Mhaidat NM, Alzoubi KH, Bustanji Y, Hamad I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr Drug Targets. 2020;21:1613-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Wiesmann N, Tremel W, Brieger J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J Mater Chem B. 2020;8:4973-4989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 144. | Ayanwale AP, Estrada-Capetillo BL, Reyes-López SY. Antifungal activity and cytotoxicity study of ZrO2-ZnO bimetallic nanoparticles. Inorganic Chemistry Communications. 2021;134:108954. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 145. | Ahamed M, Lateef R, Khan MAM, Rajanahalli P, Akhtar MJ. Biosynthesis, Characterization, and Augmented Anticancer Activity of ZrO(2) Doped ZnO/rGO Nanocomposite. J Funct Biomater. 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |