Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1784
Peer-review started: June 15, 2023
First decision: August 7, 2023
Revised: August 21, 2023
Accepted: September 18, 2023
Article in press: September 18, 2023
Published online: October 15, 2023
Processing time: 116 Days and 16.6 Hours
The Khorana risk score (KRS) has poor predictive value for cancer-associated thrombosis in a single tumor type but is associated with early all-cause mortality from cancer. Evidence for the association between KRS and all-cause mortality in Japanese patients with gastric and colorectal cancer is limited.
To investigate whether KRS was independently related to all-cause mortality in Japanese patients with gastric and colorectal cancer after adjusting for other covariates and to shed light on its temporal validity.
Data from Dryad database were used in this study. Patients in the Gastroenterology Department of Sapporo General Hospital, Sapporo, Japan, were enrolled. The starting and ending dates of the enrollment were January 1, 2008 and January 5, 2015, respectively. The cutoff date for follow-up was May 31, 2016. The inde
Men and patients with Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 2 displayed a higher 2-year risk of death than women and those with ECOG PS 0-1 in the intermediate/high risk group for KRS. The higher the score, the higher the risk of early death; however, the relevance of this independent prediction decreased with longer survival. The overall survival of each patient was recorded via real-world follow-up and retrospective observations, and this study yielded the overall relationship between KRS and all-cause mortality.
The prechemotherapy baseline of KRS was independently associated with all-cause mortality within 2 years; however, this independent predictive relationship weakened as survival time increased.
Core Tip: The Khorana risk score (KRS) has poor predictive value for cancer-associated thrombosis in a single tumor type but is associated with early all-cause mortality from cancer. In Japanese patients with gastric and colorectal cancer, the prechemotherapy baseline of KRS was independently associated with all-cause mortality within 2 years. The concept of time-sensitive management needs to be established for clinicians and community workers as well. The earlier the stratified intervention for patients with intermediate/high KRS, the more likely long-term survival benefit will be achieved.
- Citation: Zhang YF, Wang GD, Huang MG, Qiu ZQ, Si J, Xu MY. Association between the Khorana risk score and all-cause mortality in Japanese patients with gastric and colorectal cancer: A retrospective cohort study. World J Gastrointest Oncol 2023; 15(10): 1784-1795
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1784.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1784
In recent years, the incidence of and mortality associated with gastric and colorectal cancers have reached the top five positions in Japan[1]. Of late, the cure rate of tumors has been immensely improved owing to advancements in chemotherapy, targeted therapy, radiotherapy, immunotherapy, surgery and other therapeutic modalities. However, at the same time, several treatment-related complications have emerged. Cancer-associated thrombosis (CAT) is one of the most dangerous complications and is directly related to patient prognosis[2]. CAT includes arterial embolic events, such as stroke and myocardial infarction; venous embolic events, such as deep vein thrombosis; pulmonary embolism; and visceral venous thrombosis. The Khorana risk score (KRS) is a risk scoring tool developed by Khorana et al[3] and has been internally and externally validated for stratifying thrombotic risks in patients with cancer. The 2019 revision of the American Society of Clinical Oncology (ASCO) thrombosis guidelines also recommend the use of KRS[4]. Nevertheless, a 2018 systematic review observed that the score exhibited poor predictive power for individual tumor types, and unexpectedly, higher scores were associated with a higher risk of early death[5]. Some prospective studies have demonstrated its ability to predict early death in lung and colorectal cancers[6,7]. However, studies on the relationship between KRS and all-cause mortality are limited. In addition, investigations in Asian populations are especially lacking, and the follow-up observation time for predicting early mortality is not long, which does not exclude the possibility that KRS possesses the ability to predict long-term survival. Therefore, this study aimed to determine whether the KRS is independently associated with all-cause mortality in Japanese patients with gastric and colorectal cancer and to show its temporal validity.
Patient's KRS obtained at baseline prior to chemotherapy served as the independent variable, and all-cause mortality (dichotomous variable: death = 1; survival = 0) served as the dependent (target) variable. The overall survival (OS) time of each patient was recorded as of May 31, 2016.
Data from the Dryad database were used in this study[8]. Patients in the Gastroenterology Department of Sapporo General Hospital, Sapporo, Japan, were enrolled. The starting and ending dates of the enrollment were January 1, 2008 and January 5, 2015, respectively. The cutoff date for follow-up was May 31, 2016. Complete inclusion/exclusion criteria, collection of patient history, and diagnostic methods for CAT have been described in the study by Aonuma et al[9]. The flowchart for the selection of the study cohort is depicted in Figure 1. The requirement for informed consent was waived owing to the retrospective nature of the study. The institutional review board of Affiliated Hospital of Jiaxing University approved this study.
The KRS at baseline before chemotherapy was obtained and recorded for stratification of categorical variables. The KRS is a predictive scoring system to determine the risk of venous thromboembolic events (VTE) in patients receiving chemotherapy and comprises five parameters: primary cancer site, platelet count, hemoglobin and/or erythropoietin use, white blood cell count, and body mass index (BMI). Patients were classified into three risk categories based on the total risk model: low-risk group (score = 0), intermediate-risk group (score = 1-2), and high-risk group (score = ≥ 3).
The following were selected as covariates: (1) Demographic data; (2) variables affecting the KRS or all-cause mortality have been reported in previous studies; and (3) variables based on our clinical experience. The full adjustment model was constructed using the following variables: (1) Continuous variables: age (obtained at baseline); (2) categorical variables: sex, CAT, arterial thromboembolism (ATE), Eastern Cooperative Oncology Group Performance Status (ECOG PS), cancer type [gastric cancer (GC); colorectal cancer (CRC)], pathological type, primary site surgery, adjuvant chemotherapy, single or multiple primary tumor, active cancer (AC), opportunity for diagnosis, central venous catheter (CVC) placement.
AC was defined as unresectable advanced gastric and colorectal tumors that recur during or after the completion of adjuvant chemotherapy and/or other unrelated malignancies. The opportunity for diagnosis was defined as the final clinical diagnosis of a patient based on the presentation of symptoms associated with CAT.
Based on the results of the retrospective and follow-up observations, the outcome variables for all-cause mortality (dichotomous variables) and OS were obtained. The term "all-cause mortality" refers to deaths due to any cause.
Patients diagnosed with GC and CRC were treated according to the then-current ASCO or National Comprehensive Cancer Network guidelines, and who developed CAT were administered anticoagulation therapy.
Categorical variables were expressed as frequency or percentage. Chi-squared (categorical variables, normal distribution) or Kruskal-Wallis H test (skewed distribution) were used to test for differences among different KRS groups (clinical cut point). Step 1: To examine the association between KRS and all-cause mortality, univariate and multivariate Cox proportional hazards models were employed. Four models were constructed: crude model, no covariates were adjusted; model 1: Only adjusted for sociodemographic data; model 2: Model 1 + those considerable covariates (P < 0.10 or having significant clinical significance); model 3: All covariates. To ensure the robustness of the experimental results, a sensitivity analysis was simultaneously performed by converting the KRS to categorical variables and calculating the trend in P-value. Step 2: Subgroup analyses were performed using the hierarchical Cox proportional hazards model. Continuous variables were initially converted to categorical variables according to the clinical cut point, and subsequently, an interaction test was performed. Tests for effect modification of subgroup indicators were followed by the likelihood ratio test. Step 3: The OS time of each group was recorded, and Kaplan-Meier (KM) survival curves were plotted to compare the median survival time of each group. Step 4: The multivariate Cox proportional hazards model was employed to calculate the risk ratios over a given number of years, and a trend graph was plotted. All analyses were performed using the statistical software packages R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7. A two-tailed test was performed and P < 0.05 was considered statistically significant.
A total of 500 participants were selected for the final data analysis (Figure 1 for the flow chart). Their median follow-up time was 22.0 mo. The baseline characteristics of these participants are listed in Table 1 based on the clinical grouping of the KRS. Their average age was 68.9 (62.5 ± 75.9) years, and 38.8% were women. There were 194 participants in the KRS low-risk group, 218 in the moderate-risk group, and 88 in the high-risk group. There were group differences among the three KRS groups in terms of cancer type, pathological type, primary site surgery, and CVC placement (P < 0.001); however, there were no statistically significant differences in terms of additional covariates (all P values > 0.05). Furthermore, it was observed that the number of patients with CVC placement (n = 55), primary site surgery (n = 43), well and mod pathological type (n = 28), and cancer type (CRC, n = 5) was lower in the KRS high-risk group than in the other groups. The final diagnosis of CAT was made in 70 (14%) of the 500 patients, of which 11 (2.2%) were diagnosed with ATE.
| Variables | Total, n = 500 | Low-risk group, n = 194 | Intermediate-risk group, n = 218 | High-risk group, n = 88 | P value |
| Age, median (IQR) | 68.9 (62.5, 75.9) | 69.1 (62.9, 75.2) | 68.6 (62.6, 76.2) | 69.0 (61.4, 76.8) | 0.93 |
| Sex, n (%) | 0.459 | ||||
| Male | 306 (61.2) | 117 (60.3) | 130 (59.6) | 59 (67) | |
| Female | 194 (38.8) | 77 (39.7) | 88 (40.4) | 29 (33) | |
| CAT, n (%) | 0.254 | ||||
| Non | 430 (86.0) | 161 (83) | 190 (87.2) | 79 (89.8) | |
| All CAT | 70 (14.0) | 33 (17) | 28 (12.8) | 9 (10.2) | |
| ATE | 11 (2.2) | 0 (0) | 6 (2.8) | 5 (5.7) | |
| ECOG PS, n (%) | 0.053 | ||||
| 0-1 | 449 (89.8) | 181 (93.3) | 194 (89) | 74 (84.1) | |
| ≥ 2 | 51 (10.2) | 13 (6.7) | 24 (11) | 14 (15.9) | |
| Cancer type, n (%) | < 0.001 | ||||
| GC | 206 (41.2) | 0 (0) | 123 (56.4) | 83 (94.3) | |
| CRC | 294 (58.8) | 194 (100) | 95 (43.6) | 5 (5.7) | |
| Adjuvant chemotherapy, n (%) | 0.069 | ||||
| No | 306 (61.2) | 111 (57.2) | 132 (60.6) | 63 (71.6) | |
| Yes | 194 (38.8) | 83 (42.8) | 86 (39.4) | 25 (28.4) | |
| Active cancer (AC), n (%) | 0.201 | ||||
| Non-AC | 141 (28.2) | 57 (29.4) | 66 (30.3) | 18 (20.5) | |
| AC | 359 (71.8) | 137 (70.6) | 152 (69.7) | 70 (79.5) | |
| Single or multiple primary tumor, n (%) | 0.95 | ||||
| Single | 450 (90.0) | 174 (89.7) | 196 (89.9) | 80 (90.9) | |
| Multiple | 50 (10.0) | 20 (10.3) | 22 (10.1) | 8 (9.1) | |
| Pathological type, n (%) | < 0.001 | ||||
| Well and mod | 317 (63.4) | 169 (87.1) | 120 (55) | 28 (31.8) | |
| Others | 169 (33.8) | 19 (9.8) | 95 (43.6) | 55 (62.5) | |
| Unknown | 14 (2.8) | 6 (3.1) | 3 (1.4) | 5 (5.7) | |
| Primary site surgery, n (%) | < 0.001 | ||||
| No | 122 (24.4) | 19 (9.8) | 58 (26.6) | 45 (51.1) | |
| Yes | 378 (75.6) | 175 (90.2) | 160 (73.4) | 43 (48.9) | |
| CVC placement, n (%) | < 0.001 | ||||
| No | 168 (33.6) | 46 (23.7) | 89 (40.8) | 33 (37.5) | |
| Yes | 332 (66.4) | 148 (76.3) | 129 (59.2) | 55 (62.5) | |
| Opportunity for Diagnosis, n (%) | 0.714 | ||||
| Asymptomatic | 495 (99.0) | 193 (99.5) | 215 (98.6) | 87 (98.9) | |
| Symptomatic | 5 (1.0) | 1 (0.5) | 3 (1.4) | 1 (1.1) | |
| Thrombosis treatment, n (%) | 0.424 | ||||
| No | 470 (94.0) | 179 (92.3) | 207 (95) | 84 (95.5) | |
| Yes | 30 (6.0) | 15 (7.7) | 11 (5) | 4 (4.5) |
Results of the univariate analysis for mortality within 2 years are presented in Table 2. The univariate Cox proportional hazards model, revealed that sex, CAT, ATE, single or multiple primary tumor, thrombosis treatment, and opportunity for diagnosis were not associated with all-cause mortality. Moreover, cancer type, primary site surgery, and adjuvant chemotherapy were negatively associated with all-cause mortality (P < 0.001). In contrast, univariate analysis indicated that age (P = 0.034), KRS intermediate/high-risk group, ECOG PS, pathological type (others vs well and mod), AC and CVC placement were positively correlated with all-cause mortality (P < 0.001).
| Variables | HR (95%CI) | P value |
| Age (≥ 65 yr vs < 65 yr) | 1.22 (1.02-1.47) | 0.034 |
| KRS (intermediate vs low) | 1.60 (1.21-2.13) | 0.001 |
| KRS (high vs low) | 2.67 (1.91-3.73) | < 0.001 |
| Sex (female vs male) | 1.02 (0.79-1.30) | 0.900 |
| CAT (yes vs no) | 1.01 (0.71-1.43) | 0.965 |
| ATE (yes vs no) | 1.36 (0.60-3.05) | 0.481 |
| ECOG PS (≥ 2 vs 0-1) | 4.05 (2.93-5.61) | < 0.001 |
| Cancer type (CRC vs GC) | 0.60 (0.47-0.76) | < 0.001 |
| Pathological type (others vs well and mod) | 1.53 (1.19-1.96) | < 0.001 |
| Pathological type (unknown vs well and mod) | 1.45 (0.68-3.09) | 0.338 |
| Primary site surgery (yes vs no) | 0.30 (0.23-0.39) | < 0.001 |
| Adjuvant chemotherapy (yes vs no) | 0.28 (0.21-0.38) | < 0.001 |
| Active cancer (yes vs no) | 4.28 (2.94-6.24) | < 0.001 |
| Multiple primary vs single primary | 0.94 (0.62-1.44) | 0.784 |
| CVC placement (yes vs no) | 1.92 (1.44-2.55) | < 0.001 |
| Thrombosis treatment (yes vs no) | 0.92 (0.56-1.53) | 0.761 |
| Opportunity for diagnosis (symptomatic vs asymptomatic) | 1.57 (0.50-4.91) | 0.436 |
In this study, four models were constructed to analyze the independent effects of KRS on all-cause mortality within 2 years (univariate and multivariate Cox proportional hazards model). The effect sizes [hazard ratios (HRs) and their 95% confidence intervals (CIs)] are listed in Table 3. In the unadjusted model (crude mode), the model-based effect size can be explained as the difference in each group of KRS associated with risk of death. For example, in the unadjusted model, the effect size for all-cause mortality denotes the strength of the correlation between the KRS and the risk of death (KRS, intermediate vs low, HR: 1.6; 95%CI: 1.21-2.13; P = 0.001; KRS, high vs low, HR: 2.67; 95%CI: 1.91-3.73; P < 0.001). In the minimum-adjusted model (model 1), compared with the low-risk group, the medium-risk group demonstrated a 60% increased risk of all-cause death (95%CI: 1.20-2.12; P = 0.001), whereas the high-risk group exhibited a 2.64-fold increase (95%CI: 1.89-3.69; P < 0.001). Similar results were obtained for model 2 (adjusting for significant covariates) and model 3 (full adjustment), which indicated a 45% increased risk of death in the intermediate risk group compared with the low-risk group (95%CI: 1.02-2.06; P = 0.041). On the contrary, the high-risk group showed a two-fold increase (95%CI: 1.26-3.24; P = 0.004). For sensitivity analysis, the KRS was converted from a continuous variable to a categorical variable (clinical grouping of KRS). The p value of the trend test for the different models was < 0.05, which suggesting the same trend effect and stable study results (Table 3).
| Variable | Crude mode | Multivariable-adjusted model 1 | Multivariable-adjusted model 2 | Multivariable-adjusted model 3 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Trend test1 | 1.63 (1.38-1.93) | < 0.001 | 1.62 (1.37-1.92) | < 0.001 | 1.39 (1.10-1.76) | 0.005 | 1.42 (1.12-1.8) | 0.004 |
| KRS, low-risk group | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||
| KRS, intermediate-risk group | 1.6 (1.21-2.13) | 0.001 | 1.6 (1.20-2.12) | 0.001 | 1.43 (1.00-2.04) | 0.047 | 1.45 (1.02-2.06) | 0.041 |
| KRS, high-risk group | 2.67 (1.91-3.73) | < 0.001 | 2.64 (1.89-3.69) | < 0.001 | 1.95 (1.22-3.12) | 0.005 | 2.02 (1.26-3.24) | 0.004 |
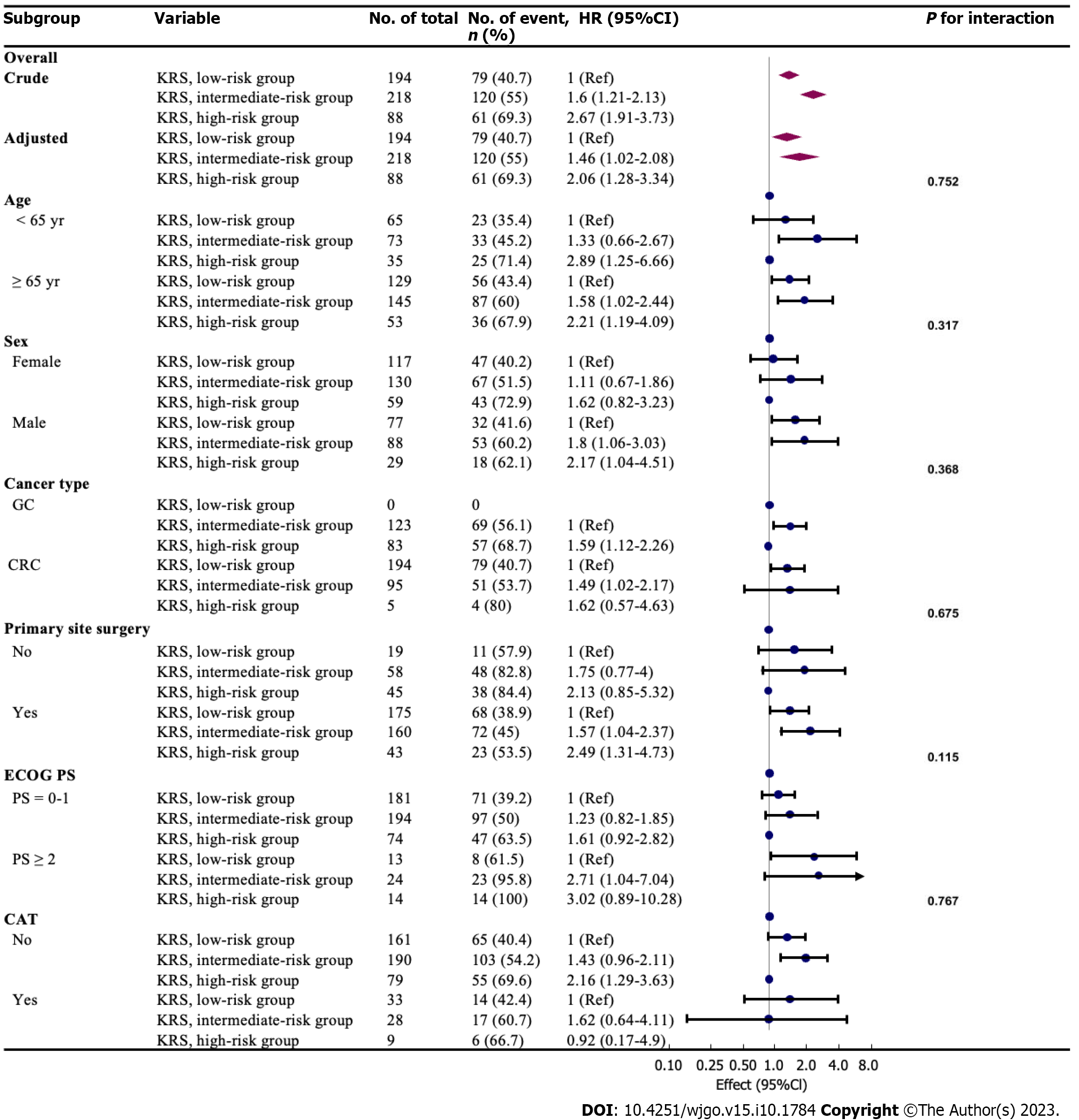
Age, sex, cancer type, primary site surgery, ECOG PS, CVC placement, CAT were used as stratification variables to examine the trend of effect sizes in these variables (Figure 2). No interactions were seen in these variables based on our a priori specification (all P values for interaction < 0.05). In this study, a stronger association was detected in men (KRS, intermediate vs low, HR: 1.8; 95%CI: 1.06-3.03; KRS, high vs low, HR: 2.17; 95%CI: 1.04-4.51), and ECOG PS ≥ 2 (KRS, intermediate vs low, HR: 2.71; 95%CI: 1.04-7.04; KRS, high vs low, HR: 3.02; 95%CI: 0.89-10.28). In contrast, a weaker association was perceived in women (ECOG PS 0-1). Patients in the intermediate-risk group aged < 65 years exhibited a lower 2-year relative risk of death (HR: 1.33, 95%CI: 0.66-2.67) than those aged ≥ 65 years and other intermediate/high-risk groups, with a mortality rate of 45.2%. The KRS high-risk group showed a higher mortality rate regardless of cancer type (68.7% in GC and 80% in CRC). In addition, the risk of death was more than two times higher in the high-risk group than in the low-risk group for KRS regardless of surgeries in the primary tumor site (HR: 2.49; 95%CI: 1.31-4.73 in the operated group; HR: 2.13; 95%CI: 0.85-5.32 in the non-operated group). However, the risk of death was not higher with CAT in the KRS high-risk group than in the low-risk group (HR: 0.92, 95%CI: 0.17-4.9).
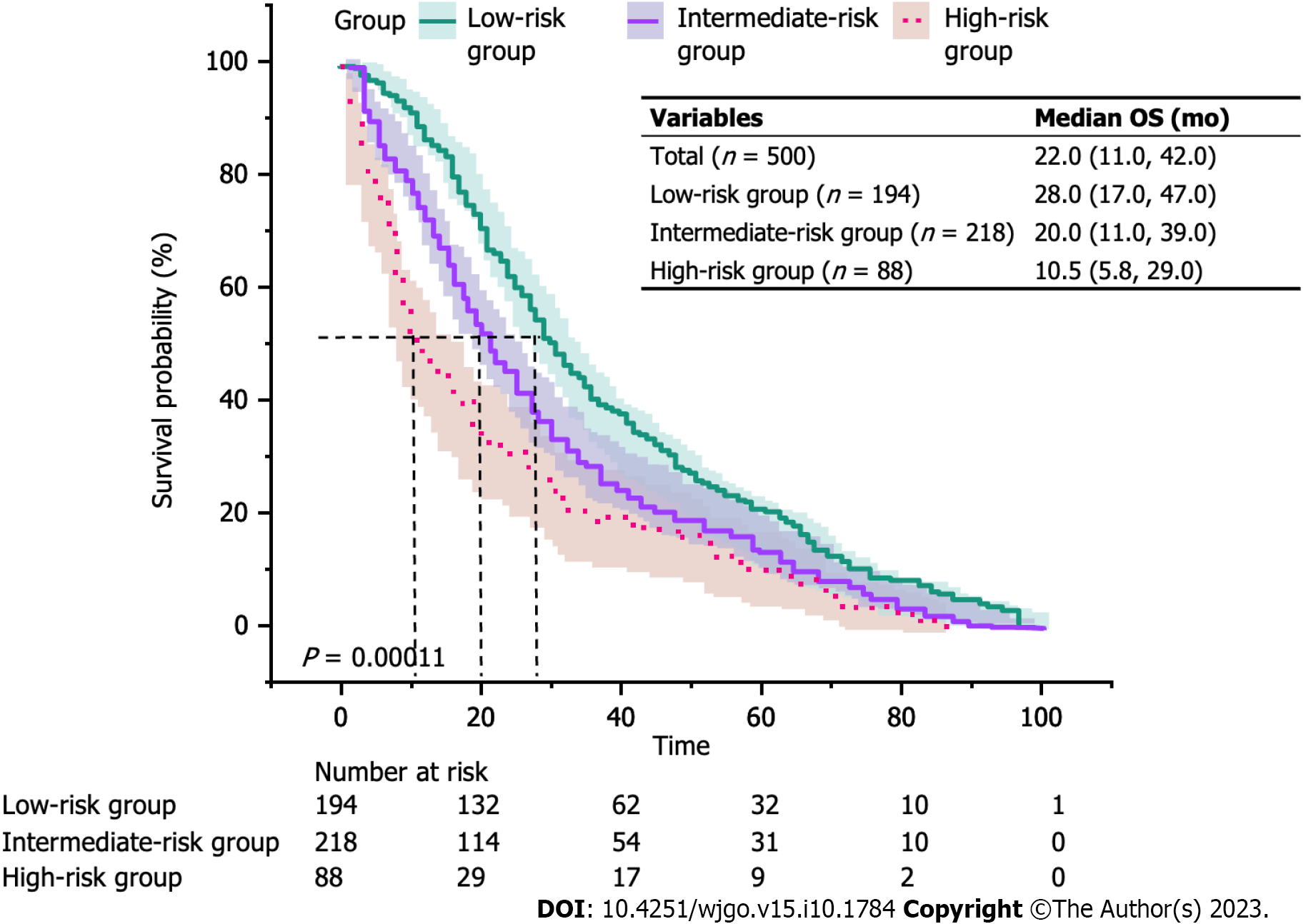
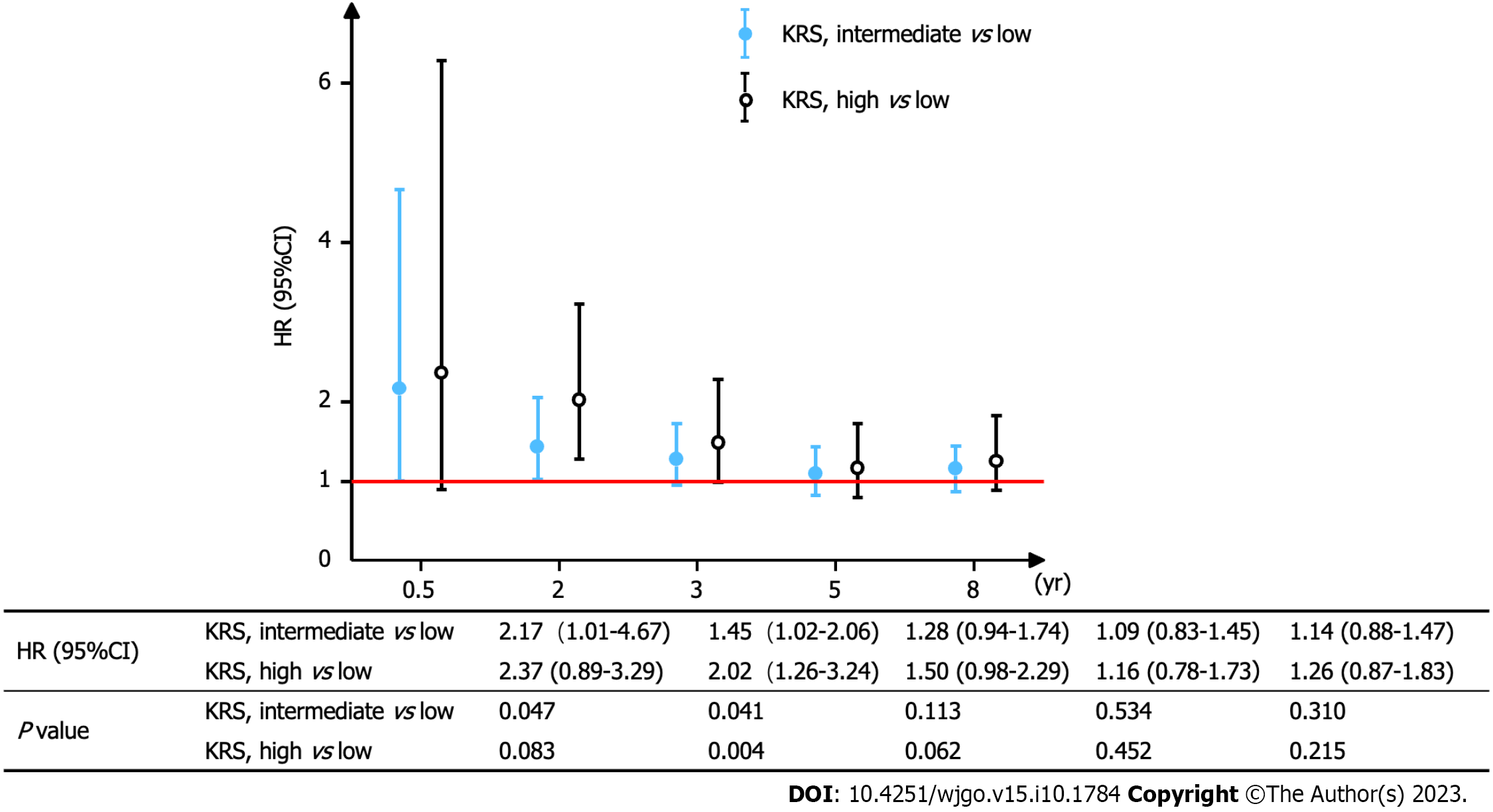
Figure 3 depicts the KM curves of OS for different risk groups. The median OS for the three groups was 28.0 mo in the low-risk group, 20.0 mo in the intermediate-risk group, and 10.5 mo in the high-risk group (P < 0.001). Furthermore, the mortality was higher in the intermediate/high-risk group with KRS in the early/middle period. Nevertheless, all three curves converged as the survival time increased, which suggested that the relationship between KRS and all-cause mortality was unknown at later times. To further test this idea, the OS time was categorized into specific periods, and a separate multivariate Cox proportional hazards model was constructed to plot the trend of risk ratio (Figure 4). The findings indicated that the risk of death within 6 mo was 2.17 times higher in the KRS intermediate-risk group than in the low-risk group (95%CI: 1.01-4.67; P = 0.047) and 2.37 times higher in the KRS high-risk group than in the low-risk group (95%CI: 0.89-3.29; P = 0.083). At the same time, the risk of death within 2 years was 1.45 times higher in the intermediate-risk group than in the low-risk group (95%CI: 1.02-2.06; P = 0.041) and 2.02 times higher in the high-risk group than in the low-risk group (95%CI: 1.26-3.24; P = 0.004). Subsequent risk ratios decreased gradually over 3, 5, and 8 years and at P > 0.05.
The findings from this study indicated that the KRS was independently associated with all-cause mortality within 2 years in Japanese patients with GC and CRC before receiving chemotherapy. Subgroup analysis aided in better understanding the trend of KRS and all-cause mortality in different populations. Men and patients with ECOG PS ≥ 2 displayed a higher 2-year risk of death than women and those with ECOG PS 0-1 in the intermediate/high risk group for KRS. Hence, the higher the score, the higher the risk of early death; however, the relevance of this independent prediction decreased with longer survival. The OS of each patient was recorded via real-world follow-up and retrospective observations, and this study yielded the overall relationship between KRS and all-cause mortality, which provides a good guide for future prospective studies.
A multivariate Cox proportional hazards model was constructed based on various factors associated with the prognosis of patients with GC and CRC, including age, CAT, cancer type, ECOG PS, primary site surgery, adjuvant chemotherapy, active cancer and CVC placement. The findings pointed to the presence of an independent predictive relationship between baseline KRS before chemotherapy and death within 2 years in patients with GC and CRC. This result is comparable to a global prospective study by Sohal et al[7], which observed that KRS predicted mortality within 6 mo in patients with CRC treated using chemotherapy. Moreover, similar findings have been reported for different tumor types in studies by Shibata et al[10], Kuderer et al[11], Mansfield et al[12], and Vathiotis et al[13]. Without emphasizing the length of the observations, their conclusions agree with the findings from this study. However, all of their follow-up observations were short or had limited sample sizes, and therefore none of the results indicated the dynamic trends in baseline KRS and mortality in patients with tumors.
Using univariate regression analysis, a study by Salazar Adum et al[14] showed that KRS predicted death in various cancer types, but additional Cox multifactorial analysis indicated the lack of an independent correlation between the two (with a maximum observation period of 25 mo). This parallel comparison confirmed our question about the time frame in which KRS predicts death. Another study demonstrated that KRS did not accurately identify patients with lung cancer who were at an elevated risk for VTE but predicted lung cancer mortality. This study noted a predictive relationship between KRS and long-term survival (180 mo) using KM survival curves but failed to perform additional multivariate Cox regression analysis. Merely based on the trend of KM survival curves, the study found that KRS was significantly associated with death within 2 years, and the grouping curves converged as the survival time increased[12]. Another study that analyzed a large population from the NHIS-HEALS database observed that maintaining hemoglobin levels in the normal range was associated with a reduction in all-cause mortality[15]. It is therefore hypothesized that the possible cause of the time effect is the survival benefit offered by early and timely intervention in the intermediate/high risk group of KRS.
Subgroup analysis performed in this study revealed that men and those with ECOG PS ≥ 2 for GC and CRC belonging to the intermediate/high risk group of KRS exhibited a higher risk of mortality. This elevated risk may be due to the higher number of smokers among men, which has been shown to exacerbate the risk of CRC mortality by 9.8% compared with nonsmokers in a large case–control study[16]. It is well known that lower ECOG PS signifies shorter survival for patients with tumor. However, ECOG PS ≥ 2 was also an unfavorable factor for survival in the GC and CRC population in this study (ECOG PS ≥ 2 vs 0-1, HR: 4.05; 95%CI: 2.93-5.61, P < 0.001), which might exert a dual effect with intermediate/high KRS, implying that this population requires special attention from clinicians for early intervention. Further analysis revealed that primary site surgery did not alleviate the 2-year risk of death in the KRS intermediate/high-risk group (KRS, intermediate vs low, HR: 1.57; 95%CI: 1.04-2.37; KRS, high vs low, HR: 2.49; 95%CI: 1.31-4.73; P for interaction = 0.675). This finding is related to the five parameters comprising the KRS.
A large retrospective study by the Japanese Association of Clinical Cancer Centers reported a higher 5-year survival rate of 72.2% for patients with colon cancer (5054 patients) than the rate of 68.7% for those with GC (15353 patients)[17], which is consistent with our findings (cancer type CRC vs GC, HR: 0.60; 95%CI: 0.47-0.76, P < 0.001). In addition, the KRS was higher for GC, which suggests that this score predicts death and CAT shares the same pathophysiological features. Several studies have proved that anemia is associated with local recurrence-free survival, recurrence-free survival, and OS not only in GC[18] and CRC[19] but also in other cancers, such as lung, breast, head and neck, and bladder cancer[20-25]. Furthermore, leukocytosis and thrombocytosis, which imply a physiologic inflammatory response, are associated with lower survival in patients with CRC, lung and cervical cancers[19,26-28]. The second World Cancer Research Fund/American Institute for Cancer Research indicated that CRC is strongly associated with obesity[29]. Another meta- analysis that pooled several prospective studies observed that class II/III obesity (BMI ≥ 35 kg/m2) was linked to significantly increased all-cause mortality from CRC[30].
Interestingly, our study did not identify a correlation between the occurrence of CAT and OS in this population (CAT yes vs no, HR: 1.22; 95%CI: 0.95-1.58, P = 0.119). This discrepancy could be attributed to limitations in screening equipment and follow-up, which make it impossible to confirm the diagnosis in all patients who developed CAT in the clinic, which resulted in an underestimation of its incidence. This finding is in contrast to the study by Fuentes et al[31], which signified that VTE was an independent predictor of mortality in patients with GC (112 cases). However, because their results were not subjected to additional multivariate Cox regression analysis and sensitivity analysis, further validation is required. In another study, the incidence of CAT in patients with CRC was highest in the first 6 mo after diagnosis and declined rapidly thereafter. CAT reduces survival in patients with local or regional disease[32]. Not coincidentally, in a prospective multi-cancer study involving 2488 patients in the United States, CAT was associated with lower survival rates in different KRS subgroups[33]. Overall, the relationship between CAT and mortality in gastrointestinal tumors needs to be investigated further.
The clinical values of this study are as follows: (1) To the best of our knowledge, the first independent correlation and time sensitivity between KRS and all-cause mortality was observed in Japanese patients with stomach and colorectal cancer.; (2) It may guide the follow-up time issue in relevant prospective studies and improve the economic efficacy; (3) It will be helpful for health care professionals working in the clinic to give stratified management of cancer patients in a specific time period and to establish a time-efficient management concept, i.e., the earlier the intervention for blood picture and BMI, the higher the survival benefit is likely to be; and (4) The results of this study will contribute to additional research on what survival benefits this intervention provides to patients with stomach and colorectal cancer, as well as the development of future all-cause mortality prediction models.
This study has several advantages: (1) The sample size was larger compared with previous similar studies; (2) This study observed and recorded the OS of each patient with GC and CRC in Japan and analyzed it entirely as well as by time period; (3) This study is the first to explain the temporal validity of KRS at the baseline in predicting cancer-related mortality; and (4) The effect modifier factor analysis enhanced the use of data and yielded stable conclusions in different models and subgroups.
However, there are certain limitations in this study: (1) This research was a retrospective observational cohort study with selection bias and bias for unknown confounders, which might have affected the findings; (2) The study population comprised Japanese patients with gastrointestinal tract tumors. Therefore, generalizability and extrapolation of the results are somewhat lacking; (3) Regarding the time effect of KRS in predicting mortality, only the approximate period could be derived and not the exact time; and (4) As patients in whom CAT occurred > 1 mo before the start of chemotherapy were excluded, the results cannot be applied to these individuals.
In Japanese patients with GC and CRC, the prechemotherapy baseline of KRS was independently associated with all-cause mortality within 2 years; however, this independent predictive relationship decreased as survival time increased.
The incidence of and mortality associated with gastric and colorectal cancers have reached the top five positions in Japan. Cancer-associated thrombosis is one of the most dangerous complications and is directly related to patient prognosis. The Khorana risk score (KRS) is a risk scoring tool and has been internally and externally validated for stratifying thrombotic risks in patients with cancer.
Studies on the relationship between KRS and all-cause mortality are limited. In addition, investigations in Asian populations are especially lacking, and the follow-up observation time for predicting early mortality is not long, which does not exclude the possibility that KRS possesses the ability to predict long-term survival.
We performed a retrospective analysis to investigate whether KRS was independently related to all-cause mortality in Japanese patients with gastric and colorectal cancer after adjusting for other covariates and to shed light on its temporal validity.
This retrospective study was conducted using data from the Dryad database. Patient's KRS obtained at baseline prior to chemotherapy served as the independent variable, and all-cause mortality (dichotomous variable: Death = 1; survival = 0) served as the dependent (target) variable. The KRS was categorized into three groups: low-risk group, intermediate-risk group, and high-risk group. All analyses were performed using the statistical software packages R 3.3.2 and Free Statistics software version 1.7.
In our study, a total of 500 participants were selected for the final data analysis . Their median follow-up time was 22.0 mo. The average age was 68.9 (62.5 ± 75.9) years, and 38.8% were women. There were 194 participants in the KRS low-risk group, 218 in the moderate-risk group, and 88 in the high-risk group. The risk of death within 6 mo was 2.17 times higher in the KRS intermediate-risk group than in the low-risk group (95%CI: 1.01-4.67; P = 0.047) and 2.37 times higher in the KRS high-risk group than in the low-risk group (95%CI: 0.89-3.29; P = 0.083). At the same time, the risk of death within 2 years was 1.45 times higher in the intermediate-risk group than in the low-risk group (95%CI: 1.02-2.06; P = 0.041) and 2.02 times higher in the high-risk group than in the low-risk group (95%CI: 1.26-3.24; P = 0.004). Men and patients with Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 2 displayed a higher 2-year risk of death than women and those with ECOG PS 0-1 in the intermediate/high risk group for KRS.
The overall survival of each patient was recorded via real-world follow-up and retrospective observations, and this study yielded the overall relationship between KRS and all-cause mortality. In Japanese patients with gastric and colorectal cancer, the prechemotherapy baseline of KRS was independently associated with all-cause mortality within 2 years. The higher the score, the higher the risk of early death; however, the relevance of this independent prediction decreased with longer survival.
A concept of time-sensitive management needs to be established for clinicians and community workers as well, i.e., the earlier the stratified intervention for patients with intermediate/high KRS, the more likely long-term survival benefit will be achieved. Further study with large sample size and more comprehensive prognostic information is desired to verify our findings.
The authors thank all the staff members of our institution. We are grateful to Dr. Liu L of Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for his contribution to the statistical support, study design consultations, and comments regarding the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lieto E, Italy; Toyoshima O, Japan S-Editor: Yan JP L-Editor: A P-Editor: Zhang XD
| 1. | Foundation for Promotion of Cancer Research. Cancer statistics in Japan-2021. [cited 2022 Nov 27]. Available from: http://ganjoho.jp/reg_stat/statistics/stat/summary.html. |
| 2. | Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 1169] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 3. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1530] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 4. | Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 948] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 5. | Khorana AA, Francis CW. Risk prediction of cancer-associated thrombosis: Appraising the first decade and developing the future. Thromb Res. 2018;164 Suppl 1:S70-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Kuderer NM, Poniewierski MS, Culakova E, Lyman GH, Khorana AA, Pabinger I, Agnelli G, Liebman HA, Vicaut E, Meyer G, Shepherd FA. Predictors of Venous Thromboembolism and Early Mortality in Lung Cancer: Results from a Global Prospective Study (CANTARISK). Oncologist. 2018;23:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Sohal DPS, Kuderer NM, Shepherd FA, Pabinger I, Agnelli G, Liebman HA, Meyer G, Kalady MF, McCrae K, Lyman GH, Khorana AA. Clinical Predictors of Early Mortality in Colorectal Cancer Patients Undergoing Chemotherapy: Results From a Global Prospective Cohort Study. JNCI Cancer Spectr. 2017;1:pkx009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Aonuma AO, Nakamura M, Sakamaki K, Murai T, Matsuda C, Itaya K, Sone T, Yagisawa M, Koike Y, Endo A, Tsukuda Y, Ono Y, Nagasaka A, Nishikawa S, Yamanaka T, Sakamoto N. Data from: Incidence of cancer-associated thromboembolism in Japanese gastric and colorectal cancer patients receiving chemotherapy: a single-institutional retrospective cohort analysis (Sapporo CAT study) [Dataset]. Dryad. 2019. Available from: https://doi.org/10.5061/dryad.84s01sv. |
| 9. | Aonuma AO, Nakamura M, Sakamaki K, Murai T, Matsuda C, Itaya K, Sone T, Yagisawa M, Koike Y, Endo A, Tsukuda Y, Ono Y, Nagasaka A, Nishikawa S, Yamanaka T, Sakamoto N. Incidence of cancer-associated thromboembolism in Japanese gastric and colorectal cancer patients receiving chemotherapy: a single-institutional retrospective cohort analysis (Sapporo CAT study). BMJ Open. 2019;9:e028563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Shibata K, Tokushige A, Imamura M, Ikeda Y, Ohishi M. Evaluating the Khorana risk score of gastrointestinal cancer patients during initial chemotherapy as a predictor of patient mortality: A retrospective study. J Cardiol. 2022;79:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Kuderer NM, Culakova E, Lyman GH, Francis C, Falanga A, Khorana AA. A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality. Oncologist. 2016;21:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14:1773-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Vathiotis I, Dimakakos EP, Boura P, Ntineri A, Charpidou A, Gerotziafas G, Syrigos K. Khorana Score: Νew Predictor of Early Mortality in Patients With Lung Adenocarcinoma. Clin Appl Thromb Hemost. 2018;24:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Salazar Adum JP, Diaz Quintero L, Fuentes HE, Lind BB, Caprini JA, Tafur AJ. Predictors of active cancer thromboembolic outcomes: mortality associated with calf deep vein thrombosis. Int Angiol. 2017;36:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Lee G, Choi S, Kim K, Yun JM, Son JS, Jeong SM, Kim SM, Park SM. Association of Hemoglobin Concentration and Its Change With Cardiovascular and All-Cause Mortality. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Hou L, Jiang J, Liu B, Nasca PC, Wu Y, Zou X, Han W, Chen Y, Zhang B, Xue F, Pang H, Li J. Association between smoking and deaths due to colorectal malignant carcinoma: a national population-based case-control study in China. Br J Cancer. 2014;110:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Okamoto N, Saruki N, Mikami H, Yamashita K, Maruyama Y, Yano T, Imamura Y, Kaneko S, Tanaka H. 5-year survival rates for primary cancer sites at cancer-treatment-oriented hospitals in Japan. Asian Pac J Cancer Prev. 2006;7:46-50. [PubMed] |
| 18. | Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J Surg Oncol. 2005;91:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Qiu MZ, Yuan ZY, Luo HY, Ruan DY, Wang ZQ, Wang FH, Li YH, Xu RH. Impact of pretreatment hematologic profile on survival of colorectal cancer patients. Tumour Biol. 2010;31:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Yovino S, Kwok Y, Krasna M, Bangalore M, Suntharalingam M. An association between preoperative anemia and decreased survival in early-stage non-small-cell lung cancer patients treated with surgery alone. Int J Radiat Oncol Biol Phys. 2005;62:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Chen Y, Chen D, Jiang Y, Huang W, Ouyang H, Xing W, Zeng M, Xie X, Zeng W. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer. 2014;14:844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Zhu W, Xu B. Association of Pretreatment Anemia with Pathological Response and Survival of Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Population-Based Study. PLoS One. 2015;10:e0136268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Dietl B, Marienhagen J, Schäfer C, Kölbl O. The prognostic value of anaemia at different treatment times in patients with locally advanced head and neck cancer treated with surgery and postoperative radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Gorphe P, Bouhir S, Garcia GCTE, Alali A, Even C, Breuskin I, Tao Y, Janot F, Bidault F, Temam S. Anemia and neutrophil-to-lymphocyte ratio in laryngeal cancer treated with induction chemotherapy. Laryngoscope. 2020;130:E144-E150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Chen C, Hu L, Li X, Hou J. Preoperative Anemia as a Simple Prognostic Factor in Patients with Urinary Bladder Cancer. Med Sci Monit. 2017;23:3528-3535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;29:2687-2690. [PubMed] |
| 27. | Kang S, Wu J, Li J, Hou Q, Tang B. Prognostic Significance of Clinicopathological Factors Influencing Overall Survival and Event-Free Survival of Patients with Cervical Cancer: A Systematic Review and Meta-Analysis. Med Sci Monit. 2022;28:e934588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Holgersson G, Sandelin M, Hoye E, Bergström S, Henriksson R, Ekman S, Nyman J, Helsing M, Friesland S, Holgersson M, Lundström KL, Janson C, Birath E, Mörth C, Blystad T, Ewers SB, Löden B, Bergqvist M. Swedish lung cancer radiation study group: the prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. Med Oncol. 2012;29:3176-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 652] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 30. | Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20:517-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Fuentes HE, Oramas DM, Paz LH, Wang Y, Andrade XA, Tafur AJ. Venous Thromboembolism Is an Independent Predictor of Mortality Among Patients with Gastric Cancer. J Gastrointest Cancer. 2018;49:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Khorana AA, Kuderer NM, McCrae K, Milentijevic D, Germain G, Laliberté F, MacKnight SD, Lefebvre P, Lyman GH, Streiff MB. Cancer associated thrombosis and mortality in patients with cancer stratified by khorana score risk levels. Cancer Med. 2020;9:8062-8073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |