Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.205
Peer-review started: November 5, 2022
First decision: November 23, 2022
Revised: November 24, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 15, 2023
Processing time: 66 Days and 3.6 Hours
Melanoma is the most aggressive form of skin cancer, with a tendency to metastasize to any organ. Malignant melanoma is the most frequent cause of skin cancer-related deaths worldwide. Small intestine cancers especially small intestine metastases are relatively rare. Small intestine metastases are seldom described and likely underdiagnosed. Intussusception is most common in pediatric age, and in adults are almost 5% of all cases.
A 75-year-old man with a history of acral malignant melanoma was admitted to the Gastroenterology Department of our hospital, complaining of intermittent melena for 1 mo. Magnetic resonance enterography showed partial thickening of the jejunal wall and formation of a soft tissue mass, indicating a neoplastic lesion with jejunojejunal intussusception. The patient underwent partial small bowel resection. Pathological findings and immunohistochemical staining indicated small intestine metastatic melanoma. The patient refused further anti-tumor treatment after the surgery. Ten months after the first surgery, the patient presented with melena again. Computed tomography enterography showed the anastomotic stoma was normal without thickening of the intestinal wall, and routine conservative treatment was given. Three months later, the patient developed melena again. The patient underwent a second surgery, and multiple metastatic melanoma lesions were found. The patient refused adjuvant anti-tumor treatment and was alive at the latest follow-up.
Small intestine metastatic melanoma should be suspected in any patient with a history of malignant melanoma and gastrointestinal symptoms.
Core Tip: Malignant melanoma is one of the most aggressive forms of skin cancer, with a high metastatic potential and poor prognosis. We report a patient who presented with intermittent melena and history of acral malignant melanoma. Abdominal imaging showed a neoplastic lesion of the small intestine with intussusception formation. Postoperative pathology confirmed small intestine metastatic melanoma. The patient refused further comprehensive treatment including immunotherapy and chemotherapy and experienced disease relapse 1 year later. The patient underwent a second surgery, which revealed multiple small intestine metastatic melanoma lesions. The patient was alive at last follow-up without receiving adjuvant anti-tumor therapy.
- Citation: Fan WJ, Cheng HH, Wei W. Surgical treatments of recurrent small intestine metastatic melanoma manifesting with gastrointestinal hemorrhage and intussusception: A case report. World J Gastrointest Oncol 2023; 15(1): 205-214
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/205.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.205
Malignant melanoma is one of the most aggressive forms of skin cancer which is derived from melanocytes[1]. Although melanoma accounts for only 4% of all skin cancers, it causes the greatest number of skin cancer-related deaths worldwide[2]. Malignant melanoma is characterized by its high metastatic potential and poor prognosis, with a 5-year survival rate of 9%-13%[3]. Although modern therapeutic interventions for melanoma metastases have significantly improved in the last decade, survival rates for stage IV melanoma are still relatively low[4].
Small intestine cancers constitute 5% of all gastrointestinal tumors, and most are carcinoids (45%)[5]. Even though small intestine metastases are a rare and life-threatening clinical entity, they occur more frequently than primary small intestine tumors[6]. A recent study found that tumors are the primary causes of intussusception in adults[7]. Intussusception is the invagination of a segment of the gastrointestinal tract into an immediately adjacent area. It is more common in pediatric patients and only occurs in adults in 5% of all cases[8].
Herein, we present a patient with recurrent small intestine metastatic melanoma manifesting with gastrointestinal hemorrhage and intussusception.
On January 31, 2021, a 75-year-old male presented at the Gastroenterology Department of our hospital complaining of intermittent melena with epigastric discomfort for 1 mo.
The patient reported that melena occurred several times a day and was accompanied by epigastric discomfort and fatigue, without dizziness or severe abdominal pain.
The patient detected a plantar nodule on the right side in November 2016. It gradually grew and developed an ulceration. Biopsy results showed melanoma. He underwent an extended resection of the melanoma on the right plantar. Postoperative pathological diagnosis was acral malignant melanoma. He received interferon α2b (1800 WU three times per week) and continued maintenance therapy of a decreased dose of interferon α2b. He had no history of other chronic diseases.
The patient had no history of smoking or drinking. He denied a history of allergies, and his family history was unremarkable.
At admission, the patient’s temperature was 36.5 °C, heart rate was 70 beats per min, respiratory rate was 20 breaths per min, and blood pressure was 140/70 mmHg. Abdominal examination found soft tenderness in the left quadrant, without rebound tenderness. Lung and heart examinations were normal.
Routine blood tests revealed a normal white blood cell count (7.5 × 109 cells/L), moderate anemia (hemoglobin of 75.0 g/L; normal range: 130.0-175.0 g/L), hypoproteinemia (31.0 g/L; normal range: 35.0-52.0 g/L), and elevated levels of creatinine (136 µmol/L; normal range: 59-104 µmol/L). Transaminase, amylopsin and lipase levels were normal, and coagulation function was normal.
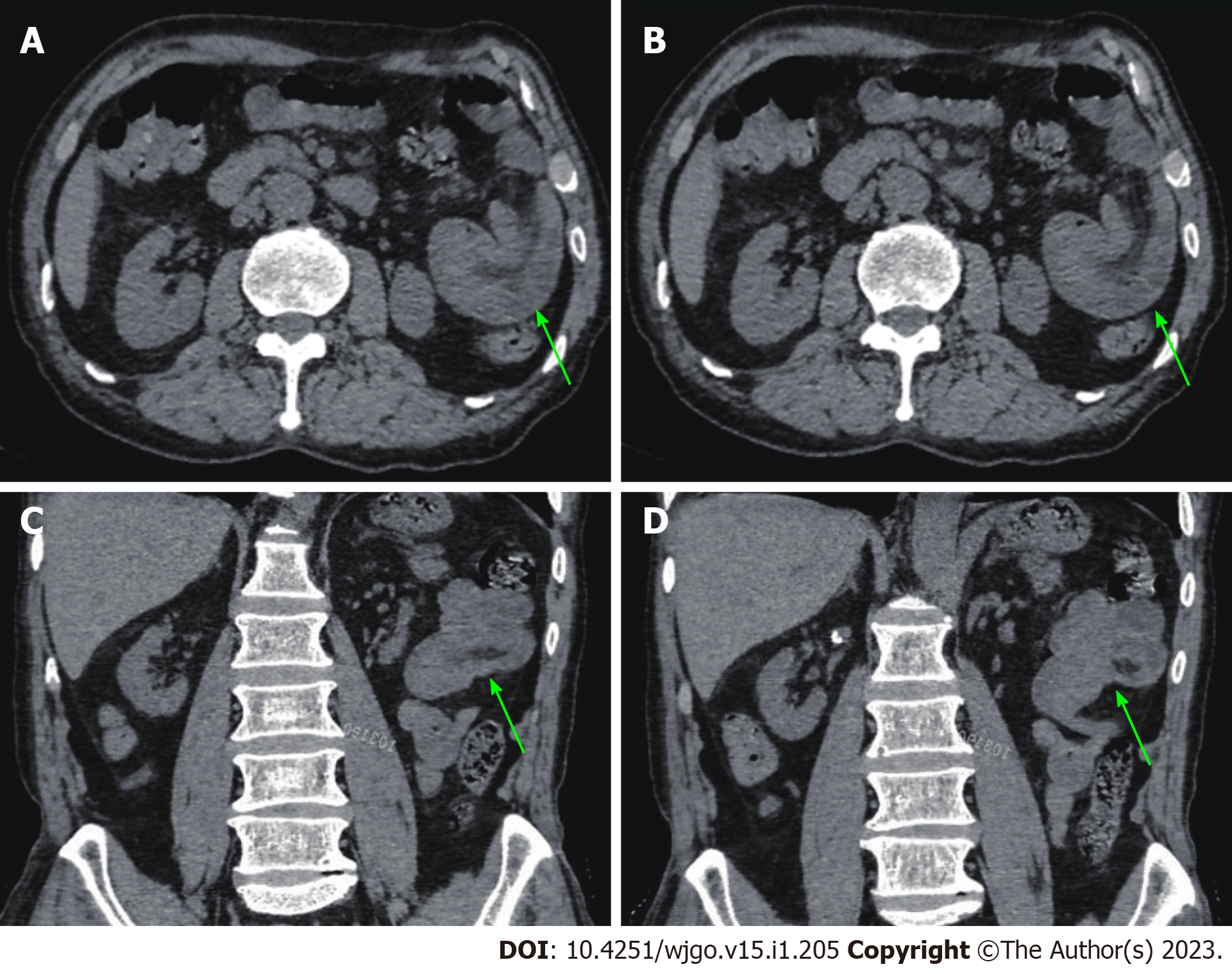
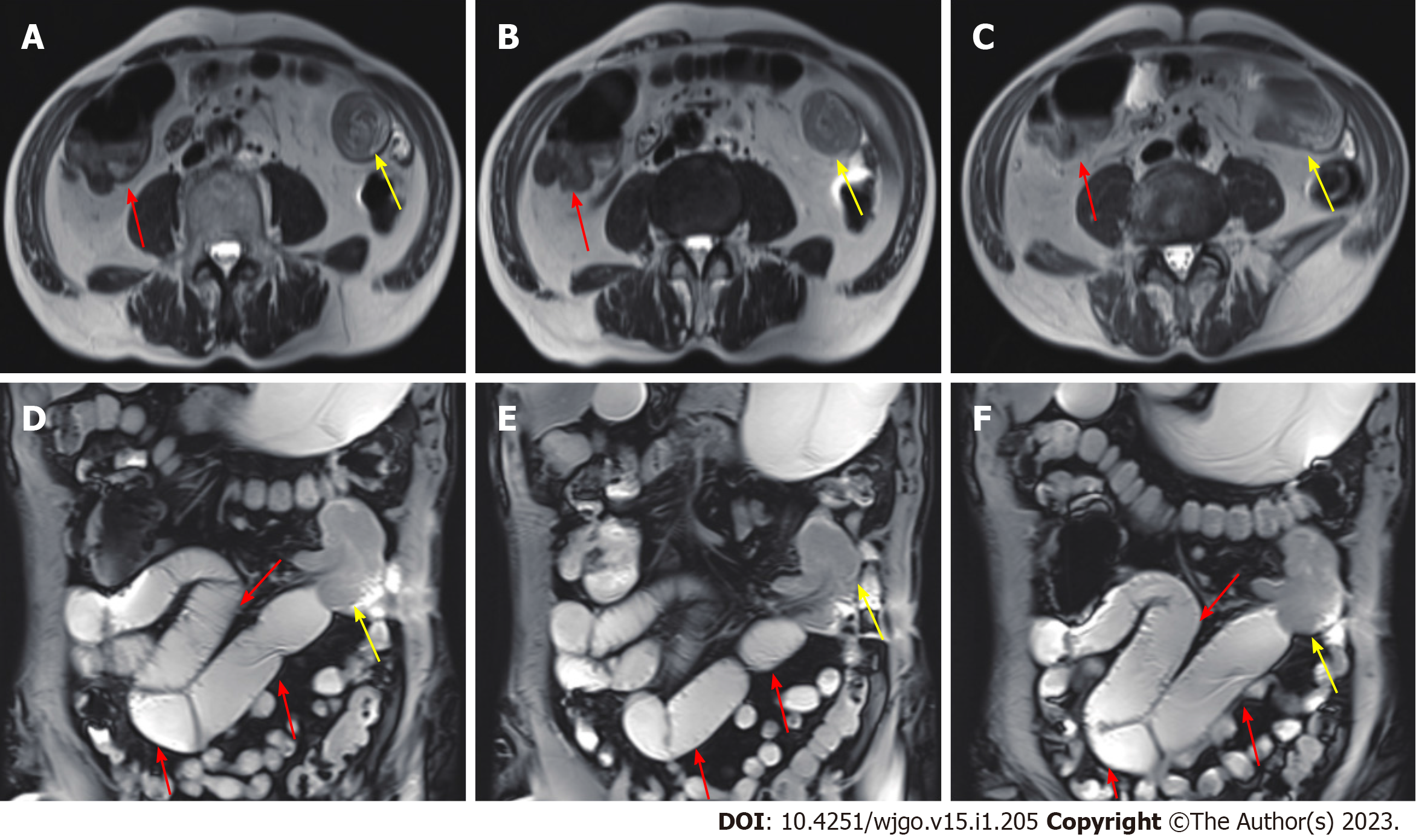
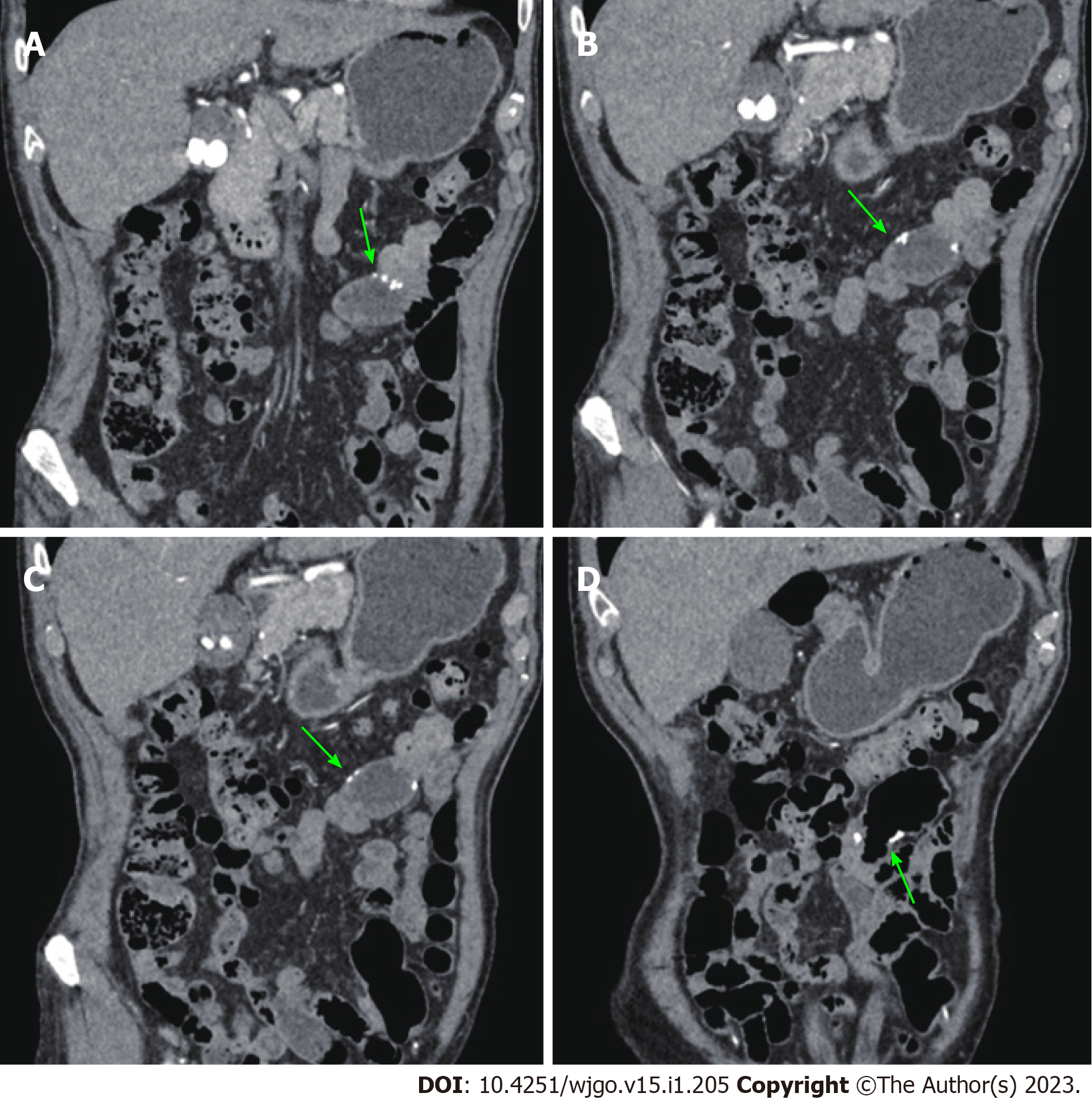
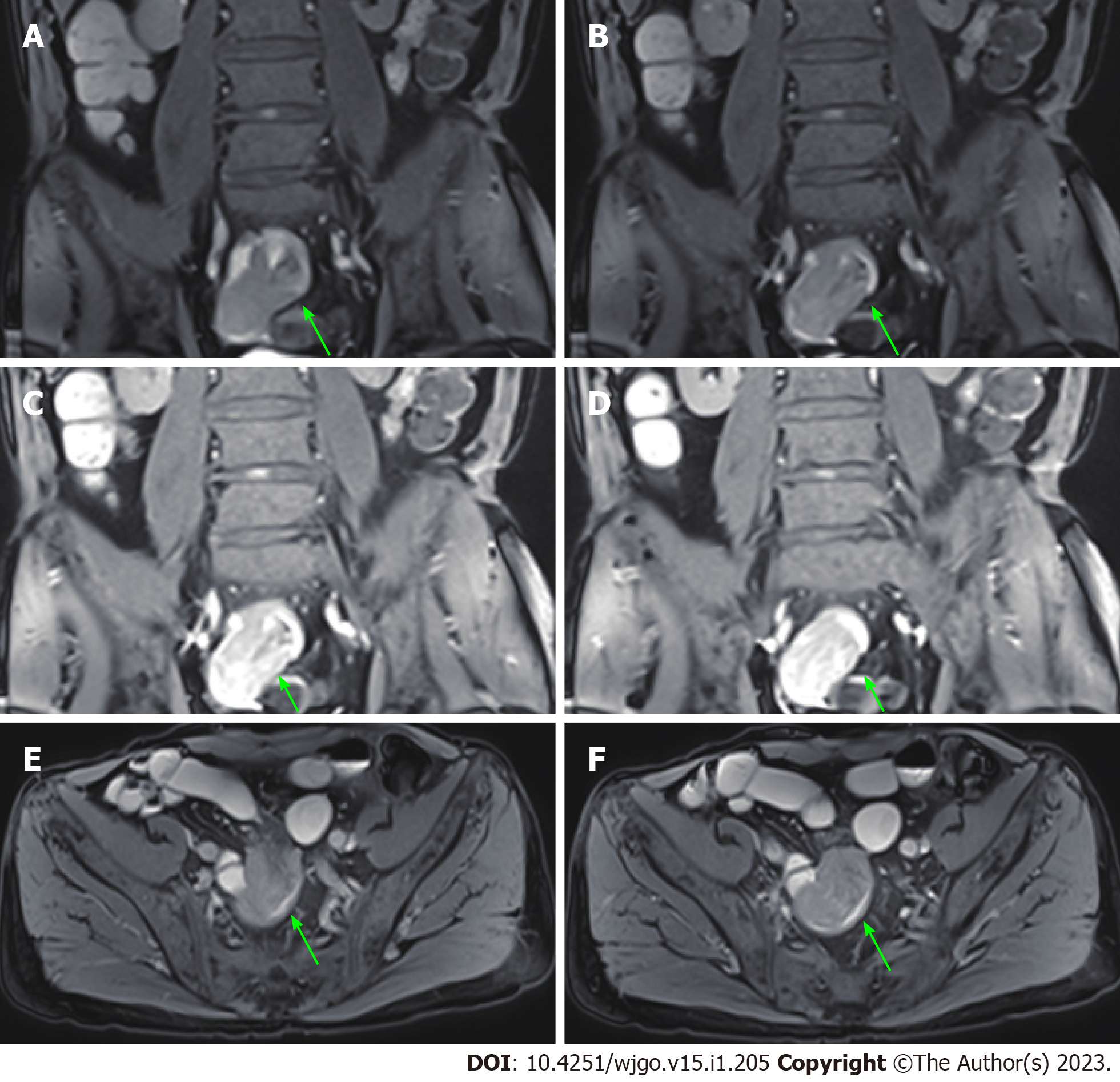
An abdominal computed tomography (CT) scan (January 30, 2021) showed an irregular shape of the jejunum in the left upper quadrant and shadows of fat, which indicated intestinal intussusception (Figure 1, green arrows). Multiple gallstones were also found. Upper gastrointestinal endoscopy (February 2, 2021) showed multiple linear ulcers in the antrum, superficial gastritis, and small polyps. Magnetic resonance enterography (February 3, 2021) showed partial thickening of the jejunal wall in the left upper quadrant and formation of a soft tissue mass (32 mm × 24 mm), indicating a neoplastic lesion (Figure 2, yellow arrows). The mass showed increased signal intensity in diffusion weighted imaging with intussusception of the distal jejunum and mesentery (jejunojejunal intussusception), and intestinal lumen expansion with air and fluid accumulation (Figure 2, red arrows). Chest CT (February 7, 2021) showed a nodule in the superior lobe of the left lung (7 mm) and tiny nodules in the right lung (2-4 mm).
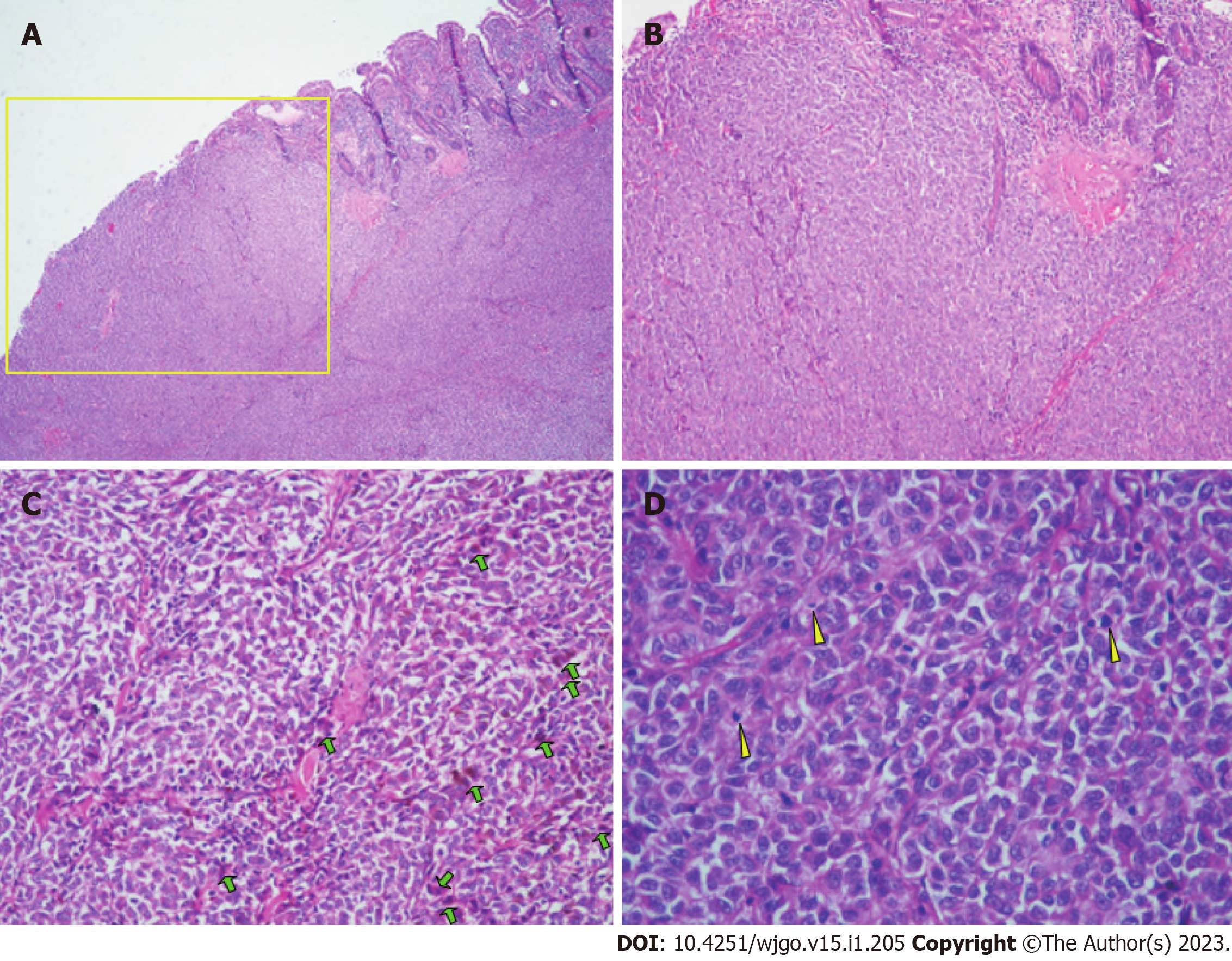
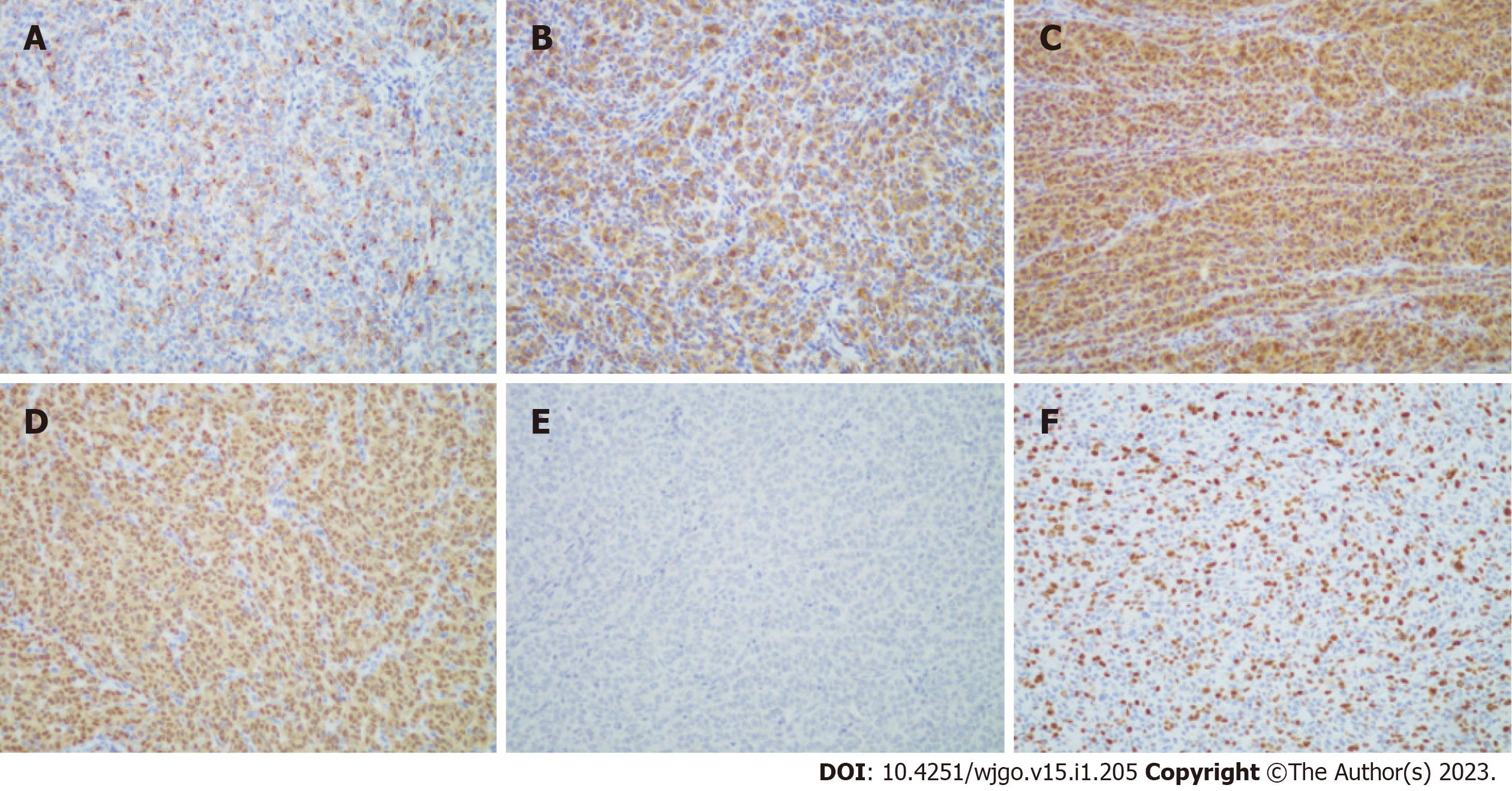
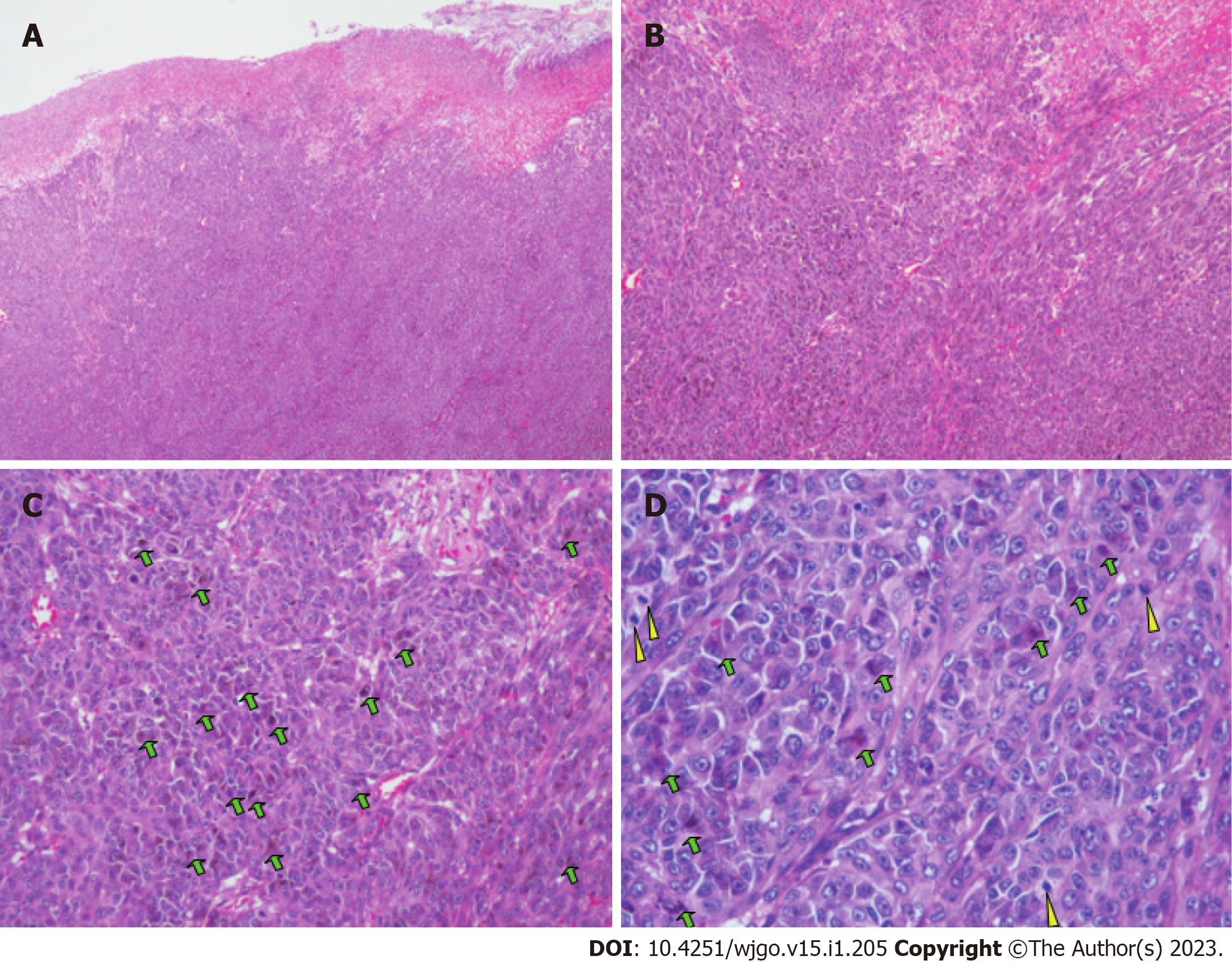
The patient underwent partial small bowel resection. Pathological findings revealed a 4.0 cm × 3.0 cm × 2.5 cm protrusion tumor. Histopathology showed diffuse growth of tumor tissue, with the majority located in the submucosa and nearly invading the mucosa (Figure 3A). Tumor cells presented with patchy distribution, large nuclei, prominent nucleoli (Figure 3B-D), and red cytoplasm (Figure 3C and D). Some tumor cells showed pigment granules in the cytoplasm (Figure 3C). Epithelioid tumor cells contained mitotic figures (Figure 3D). Immunohistochemical staining showed HMB45 (+), Melan-A (+), S-100 (+), SOX10 (+), Braf (-), PCK (-), LCA (-), Ki-67 (about 50%) (Figure 4). Lymph node metastasis was detected in one of the five lymph nodes biopsied. Combined with the patient’s history of past illness, the pathologic diagnosis was small intestine metastatic melanoma.
Small intestine metastatic melanoma, gastrointestinal hemorrhage, intussusception, superficial gastritis, gastric polyps, chronic renal insufficiency, cholecystolithiasis, moderate anemia, and hypoproteinemia.
Before small bowel resection, the patient received an esomeprazole sodium injection (80 mg continuous intravenous pumping Q12H) and a vitamin K1 injection (20 mg intravenous drip QD). Esomeprazole dose was decreased (40 mg intravenous drip QD) on the following day.
The patient was transferred to the Department of Gastrointestinal Surgery on February 5, 2021. Partial small bowel resection and side-to-side anastomosis was performed. During the surgery, a small intestinal mass (4 cm × 4 cm) was located 120 cm below the Treitz ligament, which affected the serosa with proximal expansion of the lumen and intussusception. Multiple small mesenteric lymph nodes were enlarged. There were no other lesions in the pelvis, liver, mesentery of the small intestine, or omentum majus. Cefoperazone sodium and tazobactam sodium injection (2.25 mg BID) and ornidzole and sodium chloride injection (1000 mg QD) were given as anti-infective treatment. Other nutrition support therapy included compound amino acid injection (500 mL QD), human albumin (20 g QD), and a multivitamin.
Test for Braf gene mutation was negative, indicating that the patient was not eligible for targeted therapy. Comprehensive treatment including immunotherapy (pembrolizumab) and chemotherapy was recommended. However, the patient refused further anti-tumor treatment.
On November 19, 2021, the patient developed melena with fatigue and was admitted to the Gastroenterology Department of our hospital. The patient looked ill with an anemic appearance. However, vital signs were normal. Blood tests revealed hemoglobin of 55.0 g/L. After a red blood cell transfusion, the hemoglobin was 64.0 g/L. Blood biochemistry showed hypoproteinemia (33.8 g/L) and elevated levels of creatinine (123 µmol/L).
Upper gastrointestinal endoscopy (November 20, 2021) showed superficial gastritis with bile regurgitation and small polyps. CT enterography (November 24, 2021) showed postoperative intestinal changes. The anastomotic stoma was normal without thickening of the intestinal wall (Figure 5). Head CT scan (December 19, 2021) showed lacunar infarction in the bilateral basal ganglia regions.
The patient received esomeprazole sodium injection (40 mg intravenous drip BID), compound amino acid injection (500 mL QD), and other nutritional support therapy. Because the abdominal contrast CT and upper gastrointestinal endoscopy did not find lesions accounting for gastrointestinal hemorrhage, the patient refused further examinations. The patient was discharged when the melena resolved.
On February 17, 2022, the patient again developed melena with fatigue and was admitted to the Gastroenterology Department of our hospital. The patient looked ill with an anemic appearance. However, vital signs were normal. Hemoglobin was 54.0 g/L. After a red blood cell transfusion, the hemoglobin was 80.0 g/L. Blood biochemistry again showed hypoproteinemia (30.7 g/L) and elevated levels of creatinine (139 µmol/L).
Chest CT (February 18, 2022) showed a nodule in the middle lobe of the right lung (9 mm) and tiny nodules in the right lung (2-4 mm). Magnetic resonance enterography (February 23, 2022) showed a soft tissue mass (24 mm × 21 mm) in the small intestine near the right pelvis (Figure 6) with obvious restricted diffusion on diffusion weighted imaging, indicating a small intestinal tumor with intussusception. In addition, local jejunum in the left upper quadrant showed light restricted diffusion on diffusion weighted imaging.
The patient received hemocoagulase bothrops atrox injection (2 U QD) and other nutritional support therapy including lipid emulsion, compound amino acid, human albumin, and multivitamins.
On February 26, 2022, the patient was transferred to the Department of Gastrointestinal Surgery. Partial small bowel resection and side-to-side anastomosis was performed again. During the surgery, the whole small intestine was explored, and two tumors were found. One was located 50 cm below the Treitz ligament (3 cm × 3 cm), which involved the whole layer of the intestinal wall. Another tumor was located 90 cm above the ileocecal valve. Multiple small mesenteric lymph nodes were enlarged. There were no other lesions in the pelvis, liver, mesentery of the small intestine, or omentum majus. The patient again received anti-infective treatment after surgery.
Pathology showed a 3.0 cm × 2.0 cm × 1.5 cm grey and black polyp and a 2.0 cm × 1.0 cm × 0.7 cm grey and black polyp. Histopathology showed that tumors affected the subserous layer. The histopathological and immunohistochemical finding (Figure 7) were similar to the results after the first surgery. However, Ki-67 staining had increased to approximately 70%.
We diagnosed the patient with small intestine metastatic melanoma relapse. Comprehensive treatment including immunotherapy (pembrolizumab) and chemotherapy was recommended. However, the patient again refused further anti-tumor treatment because of his age and economic reasons. At the follow-up appointment in September 2022, the patient reported that melena had not occurred again. No abdominal imaging examinations were performed.
Malignant melanoma is the cause of 1%-3% of global neoplastic disease and tends to metastasize to any organ[9]. Human cutaneous melanoma is the most common cancer to metastasize to the small intestine compared to other solid tumors. The small intestine is the most common location for melanoma metastasis (35%-97%), followed by the stomach, duodenum (5%-50%), and colon (5%-32%)[10]. The high incidence of melanoma metastases to the small intestine may be due to the presence of CCR9, a chemokine, on human melanoma cells that acts as a “homing receptor.” CCL25 is the ligand for CCR9 and is strongly expressed in the small intestine[11]. The reported average time from diagnosis of primary melanoma to the occurrence of metastasis is 29-52 mo[12,13]. Our patient was diagnosed with metastasis 51 mo after the primary diagnosis. Small intestine metastases usually reflect advanced disease with a dismal outcome. The median survival is 6-9 mo[12,13].
Gastrointestinal metastases are typically undetectable in the early stages of disease. Approximately 70% of the patients with gastrointestinal melanoma metastasis present with at least one gastrointestinal symptoms, whereas 30% of patients remain clinically asymptomatic[14]. Symptoms are primarily evident when the tumor reaches a certain size or erodes the mucosa, which causes complications. The diagnosis of small intestine metastatic melanoma remains challenging due to its vague clinical presentation and limited endoscopic access. Most lesions are identified during surveillance imaging or incidentally in patients having routine or urgent scans for other reasons. There were also reports of metastatic involvement in other gastroenteric tracts like duodenal metastasis by breast cancer[15] and renal cell carcinoma[16].
Although up to 60% of patients who die from metastatic melanoma have intestinal metastases, gastrointestinal tract involvement is clinically diagnosed in less than 5% of all cases[13]. Common gastrointestinal symptoms include abdominal pain, nausea, vomiting (60%), obstruction/intussusception (27%), and melena/bleeding (26%)[13]. Our patient presented with melena and intussusception. Tumors are the primary cause of intussusception in adults[7]. The tumors causing this abnormality are primarily benign. However, when the tumors are malignant, it is typically from metastatic disease, like melanoma, in the small intestine[17].
Diagnosis of intestinal melanoma metastasis is difficult in the preoperative period. Our patient was admitted to our department due to melena 10 mo after the first surgery. CT enterography showed that the anastomotic stoma was normal, and the patient did not receive further interventions. However, 3 mo later, magnetic resonance enterography revealed two small intestine lesions. These lesions were undetectable on the CT scan from the second admission. A recent study reported that masses were not detected preoperatively on routine surveillance CT scans in 3 patients, although gross pathology determined that they were at least 1 cm in size[18]. Another study found that contrast-enhanced CT revealed 32 of 48 small intestinal masses (sensitivity 66%)[19]. Of the 6 cases of malignant polyposis, none were identified using CT[19], suggesting CT provides inadequate preoperative detection of small lesions (though large lesions are detectable). Thus, further work is needed to optimize the detection of metastases to the small intestinal mucosa on CT because this will have a significant clinical impact. In addition, routine surveillance scans of the small intestine in patients with a history of melanoma can provide beneficial information regarding metastasis of melanoma, as metastases to the small bowel tend to occur years after the initial diagnosis of malignancy or even after years of remission. Small intestine melanoma metastasis should be suspected in any patient with a history of malignant melanoma who develops gastrointestinal symptoms or chronic anemia.
The median survival of patients with distant metastatic melanoma is 7.5 mo, and the 5-year survival rate is less than 5%[20]. Our current patient has been alive for 20 mo after two successful partial small bowel resections. It is important to accurately determine the location and number of metastases preoperatively when treating small intestinal melanoma, as malignant melanoma tends to metastasize to small intestine simultaneously and multiply. In addition to metastatic tumors, primary tumors of the gastrointestinal tract might also present synchronously. Corvino et al[21] has reported a patient with multiple primary malignancies including a primary adenocarcinoma of large bowel and three primary adenocarcinomas of small bowel (ileum). Surgical resection remains the mainstay of treatment in patients with resectable metastatic melanoma, which provides symptomatic control and leads to improved survival. The median survival period after complete surgical resection of gastrointestinal metastases is 48.9 mo[22].
There are no standardized guidelines for the management of small intestine metastatic melanoma, which leads to individualized treatment for each case. Emerging evidence supports that early diagnosis and treatment improves survival rates. Most patients with completely resected melanoma metastases will experience disease relapse like our patient. Consequently, developing adjuvant therapeutic strategies to prevent recurrence is an area warranting further research. Completely resected metastatic melanoma is a potential target for immune therapy. Because the bulk of the tumor is surgically removed, it is hypothesized that stimulating the immune system will enable elimination of microscopic disease. Unfortunately, our patient refused immune therapy after the first surgery and experienced disease relapse.
The small intestine is the most common site of cutaneous malignant melanoma metastasis. Early stages of small intestine metastases can be clinically silent, and the diagnosis is difficult. Small intestine melanoma metastasis should be suspected in any patient with gastrointestinal symptoms and a history of malignant melanoma. Surgical resection is the main treatment for patients with small intestine metastatic melanoma, and it can increase the survival of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A, Italy; Li Y, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23 Suppl 8:viii10-viii14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1612] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 3. | Ishihara K, Saida T, Otsuka F, Yamazaki N; Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Ugurel S, Röhmel J, Ascierto PA, Becker JC, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Livingstone E, Long GV, Lorigan P, McArthur GA, Ribas A, Robert C, Zimmer L, Schadendorf D, Garbe C. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition - Update 2019. Eur J Cancer. 2020;130:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 5. | Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, Locher C, Afchain P. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Zoumpos A, Ho NAH, Loeschhorn-Becker R, Schuppert F. Haemorrhagic small bowel melanoma metastasis: a clinical rarity. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Su T, He L, Zhou T, Wu M, Guo Y, Wang Q, Jiang J, Cao X. Most Adult Intussusceptions are Caused by Tumors: A Single-Centre Analysis. Cancer Manag Res. 2020;12:10011-10015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Marinis A, Yiallourou A, Samanides L, Dafnios N, Anastasopoulos G, Vassiliou I, Theodosopoulos T. Intussusception of the bowel in adults: a review. World J Gastroenterol. 2009;15:407-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 428] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (2)] |
| 9. | Lens M, Bataille V, Krivokapic Z. Melanoma of the small intestine. Lancet Oncol. 2009;10:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Wysocki WM, Komorowski AL, Darasz Z. Gastrointestinal metastases from malignant melanoma: report of a case. Surg Today. 2004;34:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res. 2008;14:638-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Cananzi FC, Dalgleish A, Mudan S. Surgical management of intraabdominal metastases from melanoma: role of the neutrophil to lymphocyte ratio as a potential prognostic factor. World J Surg. 2014;38:1542-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Patel K, Ward ST, Packer T, Brown S, Marsden J, Thomson M, Ismail T. Malignant melanoma of the gastro-intestinal tract: a case series. Int J Surg. 2014;12:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kawashima A, Fishman EK, Kuhlman JE, Schuchter LM. CT of malignant melanoma: patterns of small bowel and mesenteric involvement. J Comput Assist Tomogr. 1991;15:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Campanile F, Maurea S, Mainenti P, Corvino A, Imbriaco M. Duodenal involvement by breast cancer. Breast J. 2012;18:615-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Munir A, Khan AM, McCarthy L, Mehdi S. An Unusual Case of Renal Cell Carcinoma Metastasis to Duodenum Presenting as Gastrointestinal Bleeding. JCO Oncol Pract. 2020;16:49-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Marsicovetere P, Ivatury SJ, White B, Holubar SD. Intestinal Intussusception: Etiology, Diagnosis, and Treatment. Clin Colon Rectal Surg. 2017;30:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Lee MH, Zaheer A, Voltaggio L, Johnson PT, Fishman EK. Clinical time course and CT detection of metastatic disease to the small bowel. Abdom Radiol (NY). 2019;44:2104-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Bender GN, Maglinte DD, McLarney JH, Rex D, Kelvin FM. Malignant melanoma: patterns of metastasis to the small bowel, reliability of imaging studies, and clinical relevance. Am J Gastroenterol. 2001;96:2392-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193-201. [PubMed] |
| 21. | Corvino A, Corvino F, Radice L, Catalano O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: report of a case and review of literature. Clin Imaging. 2015;39:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975-979; 979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |