Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.19
Peer-review started: September 16, 2022
First decision: October 19, 2022
Revised: November 3, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: January 15, 2023
Processing time: 115 Days and 17.4 Hours
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer and the fourth leading cause of cancer-related deaths in the world. HCC has a reported recurrence rate of 70%-80% after 5 years of follow-up. Controlling tumor recurrence is the most critical factor associated with HCC mortality. Conventional salvage therapies for recurrent HCC include re-hepatectomy or liver trans
Core Tip: Studies report a recurrence rate for hepatocellular carcinoma of up to 70%-80% after 5 years of follow-up. Controlling tumor recurrence is the most critical factor associated with hepatocellular carcinoma (HCC) mortality. Although, conventional salvage therapies, including re-hepatectomy, transcatheter arterial chemoembolization, target therapy, immunotherapy have yet to achieve favorable outcomes. Complementary therapies as an adjuvant treatment modality may strengthen and augment conventional therapies. We herein discuss the molecular mechanisms underlying complementary therapies and the interactions with conventional therapy in recurrent HCC related to augmenting the local control, inhibiting epithelial-mesenchymal transition, migration, invasion, metastasis and cancer stem cells, and by ameliorating drug resistance.
- Citation: Lai HC, Lin HJ, Jeng LB, Huang ST. Roles of conventional and complementary therapies in recurrent hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(1): 19-35
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/19.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.19
Worldwide, hepatocellular carcinoma (HCC) is the fifth most common type of cancer and the fourth leading cause of cancer-related deaths. The highest prevalence rates of HCC are reported in East Asia, while the incidence rates are approximately 6.7/100000 among the age-adjusted population, 2.6% in nonalcoholic steatohepatitis cirrhosis patients, and 0.13% in nonalcoholic fatty liver disease patients[1,2]. The annual incidence rate increased by 2% to 3% between 2007 and 2016, while HCC notably has the second poorest 5-year survival rate of all cancer types (18%)[3]. Early-stage treatments for HCC include resection, liver transplantation, and radiofrequency ablation (RFA), while transcatheter arterial chemoembolization (TACE), chemotherapy, molecular target therapy, immunotherapy with immune checkpoint inhibitors may commonly be applied in later stages. Even in resected HCC, the recurrence rate remains over 10% after 1 year, and 70%-80% after 5 years[4]. Thus, controlling tumor recurrence is a primary concern to reduce HCC mortality rates.
Salvage therapies for recurrent HCC include re-hepatectomy or liver transplantation, TACE, Y-90, target therapy, and immunotherapy. Meanwhile, studies have reported that complementary therapies such as traditional Chinese medicine (TCM), acupuncture, and dietary supplements have demonstrated notable anti-tumor effects[5,6]. These complementary therapies affect multiple biological mechanisms such as promoting tumor cell apoptosis, autophagy, cell cycle arrest, anti-metastasis, anti-angiogenesis, anti-proliferation, anti-epithelial-mesenchymal transition (EMT), and control of cancer stem cell (CSC) proliferation[5,6]. In addition, TCM has been noted to prevent drug resistance and act to facilitate conventional therapies in cases of recurrence.
However, systemic reviews related to complementary therapies in recurrent HCC are limited. The aim of this review is to introduce and discuss the molecular mechanisms underlying the effects of complementary therapies in recurrent HCC.
Patients graded as Child-Pugh class A or B, presenting with three or fewer tumors of < 3 cm are commonly recommended local control methods such as hepatectomy and RFA[7]. Although hepatic resection has a lower reported rate of recurrence as compared to RFA or TACE, the recurrence rate remains relatively high (70%-80%)[4]. This could be due to incomplete treatment of the primary tumor related to poor tumor location or surgical factors. Studies have indicated two critical mechanisms which come into play after local control.
The first mechanism involves the congestion status, including increased intratumoral pressure or portal hypertension causing microrupture and tunnel seeding in the operation process, thereby increasing the risk of metastasis[8]. Patients presenting with a hepatic venous pressure gradient over 10 mmHg have a 5-year survival rate of approximately 50%, while that rate increases to approximately 70% in patients with a hepatic venous pressure gradient less than 10 mmHg[9]. In this regard, TCM characterizes high hepatic venous pressure as “blood stasis”, while liver and spleen “stiffness” have been associated with late HCC recurrence[10]. Thus, herbs promoting blood circulation could act to improve portal hypertension, thereby reducing the risk of HCC recurrence. Salviae miltiorrhizae (Danshen) is noted for effectively treating angina pectoris and ischemic stroke. Recent studies have demonstrated that Salviae miltiorrhizae or its derivates lower portal hypertension by inhibiting nitric oxide production, the RhoA signaling pathway and downstream myosin phosphatase target subunit 1 phosphorylation[11-14]. Furthermore, the reported anti-cancer effects exhibited by Salviae miltiorrhizae in liver cancer[15] likewise act to decrease HCC recurrence[16,17]. Wan et al[18] demonstrated that tetrandrine (1 mL/0.1 kg) gavage in a Sprague-Dawley (SD) rat model could inhibit nitric oxide production and ameliorate cirrhosis and portal hypertension[18], whereby HCC recurrence risks may be reduced. Additionally, Aconiti Lateralis Radix Praeparata and Fructus Aurantii used for 14 consecutive days reduced portal pressure in an SD rat model[19,20].
Acupuncture has been applied to treat liver diseases for centuries. The role of acupuncture in HCC is to regulate the ying and yang, as well as improve body circulation. Recent studies have indicated acupuncture protects against liver injury caused by carbon tetrachloride, and reverses fibrogenesis accompanied with decreasing hyaluronic acid, laminin and procollagen III[21,22]. Of note, the most commonly chosen acupoints used when treating chronic liver diseases are ST36, LR3, SP6, BL18, GB34, and RN12[23]. A randomized controlled trial of 90 patients who received acupuncture on ST36, LR3 and SP6 reported a decrease in the liver fibrosis grade after 3 mo of treatment[24]. In addition, low-frequency electroacupuncture (2 Hertz) at ST36, lowered portal pressure by attenuating tumor necrosis factor (TNF)-α, nitric oxide, and 6-keto-prostaglandin F1 alpha overproduction[25]. Furthermore, one study using a rat model reported that moxibustion on BL18 once every 3 d for 10 wk decreased HCC progression and concurrently increased cluster of differentiation (CD) 3+ and CD 4+ T cell levels and reduced CD 8+ T levels[26]. Meanwhile, a randomized controlled trial by Wei et al[27] studied 72 cases who received acupuncture on RN 8 and RN 12, reporting improved portal circulation[27]. Taken together, these studies indicate that acupuncture may enhance circulation in the liver and portal area and decrease HCC progression.
The second mechanism involves multicentric tumor occurrence in the liver which could lead to recurrence. To this end, TCM offers multiple compounds presenting anti-tumor effects, such as flavonoids[28], phenylpropanoids, quinones, and alkaloids[5]. Alkaloids act to induce autophagy and apoptosis to inhibit HCC proliferation[5]. Piperidine alkaloids have been reported to induce mitochondrial fission and to regulate the mammalian sterile 20-like kinase 1-c-Jun N-terminal kinase (JNK) pathway, the extracellular signal-regulated kinase (ERK) signaling pathway and the phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/ protein kinase B (AKT) pathway[5]. In addition, isoquinoline alkaloids have been shown to affect the AKT pathway, the AKT/Forkhead box O (FoxO) 3a/S-phase kinase-associated protein 2 (Skp2) axis, the phosphatidylinositol 3-kinase (PI3K)/AKT mammalian target of rapamycin (mTOR) pathway, and the Wnt/β-catenin-mediated pathway, thereby hindering HCC cell growth[5]. Further studies have indicated that terpenoid alkaloids, including perillyl alcohol, geraniol and paclitaxel are effective antitumor agents[29]. In a study involving Hep G2 and BEL-7402 cell lines, terpenoid alkaloids regulated AKT, p53, caspase-3, mitogen-activated protein kinase (MAPK) and Ras which induced apoptosis and cell cycle arrest, and effectively inhibited proliferation[5]. Meanwhile, indole alkaloids have been noted to influence the nucleotide-binding oligomerization domain 1 pathway, the AKT pathway, and the WW domain-containing oxidoreductase-dependent pathway to induce apoptosis and cell cycle arrest, and thereby inhibit HCC proliferation[5]. In terms of TCM, herbs containing steroidal alkaloids include Solanaceae, Apocynaceae, and Liliaceae[30]. Steroidal alkaloids may induce necroptosis, apoptosis, and cell cycle arrest to inhibit cell proliferation. Yin et al[31] reported that the compound solamargine induced autophagy and apoptosis by affecting the microRNA (miR)-192-5p/CYR61/Akt signaling pathways[31]. Additionally, in a study involving a HepG2 cell line, quinoline alkaloids were reported to induce necroptosis and apoptosis[5]. Of further note, flavonoids have been shown to offer notable anti-HCC effects. Wogonin, one type of flavonoid, acts to induce apoptosis and cell cycle arrest by activating the MOB1-LATS1 signal pathway and over-expressing phospho-glycogen synthase kinase (GSK) 3beta Tyr216[32,33]. Additionally, wogonin has been reported to inhibit HCC proliferation by affecting nuclear factor kappa B (NF-κB)/B-cell lymphoma 2 (Bcl-2), epidermal growth factor receptor (EGFR) and the EGFR downstream ERK/AKT signal pathway[5]. Furthermore, baicalein has been reported to inhibit cancer progression by inducing autophagy, apoptosis and cell cycle arrest in HCC cell lines[34]. Similarly, the long non-coding RNAs (lncRNAs)-hsa-miR-4443-AKT1 pathway responds positively to baicalein treatment[35]. Studies have further revealed that silibinin induces autophagy through the adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway and induces apoptosis by up-regulated p21/cyclin-dependent kinases (CDK) 4 and p27/CDK4 complexes, and down-regulated Rb-phosphorylation and E2F1/DP1 complex[36,37]. Lee and Kwon[38] demonstrated that luteolin causes ER stress in p53-wild type HepG2 cells and Hep3B cells[38]. It has further been shown that luteolin induces apoptosis and cell cycle arrest by transforming the growth factor (TGF)-β1, p53, and Fas/Fas-ligand signaling pathway and increasing the BCL2-associated X protein (Bax)/Bcl-XL ratio[39,40]. Similarly, studies have reported that kaempferol induces autophagy by activating the AMPK signaling pathway[41]. Moreover, the combination of luteolin and kaempferol has been shown to increase caspase-3 and reactive oxygen species (ROS) reactions and induce apoptosis in a rat model[42]. Additionally, quercetin has been noted to inhibit cell proliferation by decreasing ROS and downregulating the PI3K pathway and induce apoptosis and autophagy by modulation of the PI3K/Akt/mTOR, Wnt/-catenin and MAPK/ERK1/2 pathways[43,44]. Additionally, studies have reported that phenylpropanoid (chlorogenic acid and 16-O-caffeoyl-16-hydroxylhexadecanoic acid) and quinone (thymoquinone and juglanthraquinone C) induce apoptosis in HCC cell lines[45-48]. Separate studies have demonstrated that 4-acetylantrocamol LT3 induces autophagy by activation of the AMPK pathway[49], while aloin and andrographolide induce apoptosis[50,51], and plantamajoside and sanguisorba Officinalis L. decrease proliferation in HCC cell lines[52,53]. Curcumin also offers antioxidant, apoptotic, and anti-inflammatory effects, and is thus applied in the treatment of HCC[54]. Meanwhile, several herbs associated with TCM have been reported to inhibit HCC proliferation by targeting miRNAs, these include Coptidis rhizoma (miR21 and miR23a), berberine (miR-23a), ginsenoside (miR-491), camptothecin (miR-122), and matrine (miR-21)[55]. Yang et al[56] reported on a randomized control trial of 291 patients who received the Fuzheng Jiedu Xiaoji formula and consequently exhibited a reduced mortality rate by the effective inhibition of liver cancer cell proliferation and migration via modulated AKT/CyclinD1/p21/p27 pathways[56]. The compounds associated with reducing multicentric tumor occurrence are shown in Table 1.
| Compound or Chinese herbal medicine | Cell line | Molecular mechanism | Effect | Ref. |
| Piperidine alkaloids | HepG2, Hep3B | Modulate Mst1-JNK pathway, ERK pathway, PINK1/Parkin axis and PTEN/AKT pathway | ↑Autophagy, apoptosis, mitochondrial fission. ↓Proliferation | Liu et al[5] |
| Isoquinoline alkaloids | SMMC-7721, HCCLM9, Huh7, HepG2 | Modulate AKT pathway, AKT/FoxO3a/Skp2 axis, PI3K/AKT-mTOR pathway, Wnt/β-catenin-mediated pathway and anthranilic acid metabolic pathway | ↑Autophagy, apoptosis, cell cycle arrest. ↓Proliferation | Liu et al[5] |
| Indole alkaloids | HepG2, SMMC-7721, Hepa1-6, BEL-7404, Hep3B, Huh7 | Modulate NOD1 pathway, AKT pathway and WWOX-dependent pathway | ↑Apoptosis, cell cycle arrest. ↓Proliferation | Liu et al[5] |
| Terpenoids alkaloids | HLE, L-02, BEL-7402, HepG2 | Modulate AKT, p53, caspase-3, MAPK, AFP, Ras | ↑Apoptosis, cell cycle arrest. ↓Proliferation | Liu et al[5] |
| Steroidal alkaloids | HepG2, SMMC-7721, Hep3B | ↑Gene expression of human TNFR I | ↑Necroptosis, apoptosis, cell cycle arrest. ↓Proliferation | Liu et al[5] |
| Quinoline alkaloids | HepG2, L-02, QGY-7703 | Modulate MMP-9, PCNE, ANT3 and VEGF | ↑Necroptosis, apoptosis | Liu et al[5] |
| Solamargine | HepG2, Huh7 | Modulate miR-192-5p/CYR61/Akt pathway | ↑Autophagy, apoptosis. ↓Proliferation | Yin et al[31] |
| Wogonin | HepG2, BEL-7402 | Modulate NF-κB/Bcl-2, EGFR and EGFR/ERK/AKT pathway | ↓Proliferation | Liu et al[5] |
| SMMC-7721, HCCLM3 | ↑MOB1-LATS1 pathway. ↓YAP, WW domain–containing transcription regulator 1, and expression of Claspin | ↑Apoptosis, cell cycle arrest | Wu et al[32] | |
| MHCC97-L, HepG2 | ↑Phospho-GSK-3β Tyr216. ↓Cyclin D1 | ↑Cell cycle arrest. ↓Proliferation | Hong et al[33] | |
| Baicalein | Human HCC tissues | Modulate lncRNAs-hsa-miR-4443-AKT1 pathway | ↓Proliferation | Zhao et al[35] |
| Silibinin | HepG2, Hep3B | Modulate AMPK pathway | ↑Autophagy. ↓Glycolysis | Yang et al[36] |
| Huh7, HepG2, Hep3B, PLC/PRF/5 human hepatoma cells | ↑p21/CDK4 and p27/CDK4 complexes. ↑Caspase-3 and -9. ↓Rb-phosphorylation and E2F1/DP1 complex | ↑Apoptosis. ↓Proliferation | Lah et al[37] | |
| luteolin | p53-wild type HepG2 cells, Hep3B | ↑Endoplasmic reticulum stress | ↑Autophagy, apoptosis | Lee and Kwon[38] |
| HepG2 | Modulate TGF-β1, p53, Fas/Fas ligand pathway | ↑Apoptosis, cell cycle arrest | Yee et al[39] | |
| HepG2, SK-Hep-1, PLC/PRF/5, Hep3B, HA22T/VGH | ↑Bax/Bcl-XL ratio. ↑Caspase-3 | ↑Cell cycle arrest | Chang et al[40] | |
| Kaempferol | HepG2, Huh7, BEL-7402, SMMC | ↑AMPK pathway. ↑Melanoma antigen 6, AMPK ubiquitin ligase, AMPKα1 | ↑Autophagy | Han et al[41] |
| Luteolin and Kaempferol | DEN and 2-AAF induced rat model | ↑Caspase-3 and ROS reaction | ↑Apoptosis | Seydi et al[42] |
| Quercetin | HepG2 | ↑p53, BAX. ↓ROS, PI3K, COX-2, PKC | ↓Proliferation | Maurya and Vinayak[43] |
| Chlorogenic acid | Hep-G2, Huh7 | ↑BH3-only protein Bcl-2 binding component 3. ↓Noncanonical NF-κB pathway | ↑Apoptosis | Jiang et al[45] |
| Thymoquinone | Thioacetamide (TAA)-induced HCC, Sprague Dawley rats | ↓Oxidative stress. ↓TGF-β1 | ↑Apoptosis | Helmy et al[47] |
| Juglanthraquinone C | HepG2, BEL-7402 | ↑Akt/Fox O pathway. ↑Intracellular ROS level | ↑Apoptosis | Hou et al[48] |
| 4-acetylantrocamol LT3 | HepG2 | ↑AMPK pathway | ↑Autophagy | Chen et al[49] |
| Aloin | -- | Modulate circ_0011385/miR-149-5p/WT1 axis | ↑Apoptosis and autophagy | Fu et al[50] |
| Andrographolide | Hep G2 | ↓EphB4 | ↑Apoptosis | Duan et al[51] |
| Sanguisorba Officinalis L. | HepG2 cells | Modulate EGFR, PI3K/AKT, NF-κB and MAPK pathways | ↓Proliferation | Jiang et al[52] |
| Plantamajoside | Huh7, PLC/PRF 5, THLE-2 | ↓NF-κB and Cox-2 | ↓Proliferation | Luo et al[53] |
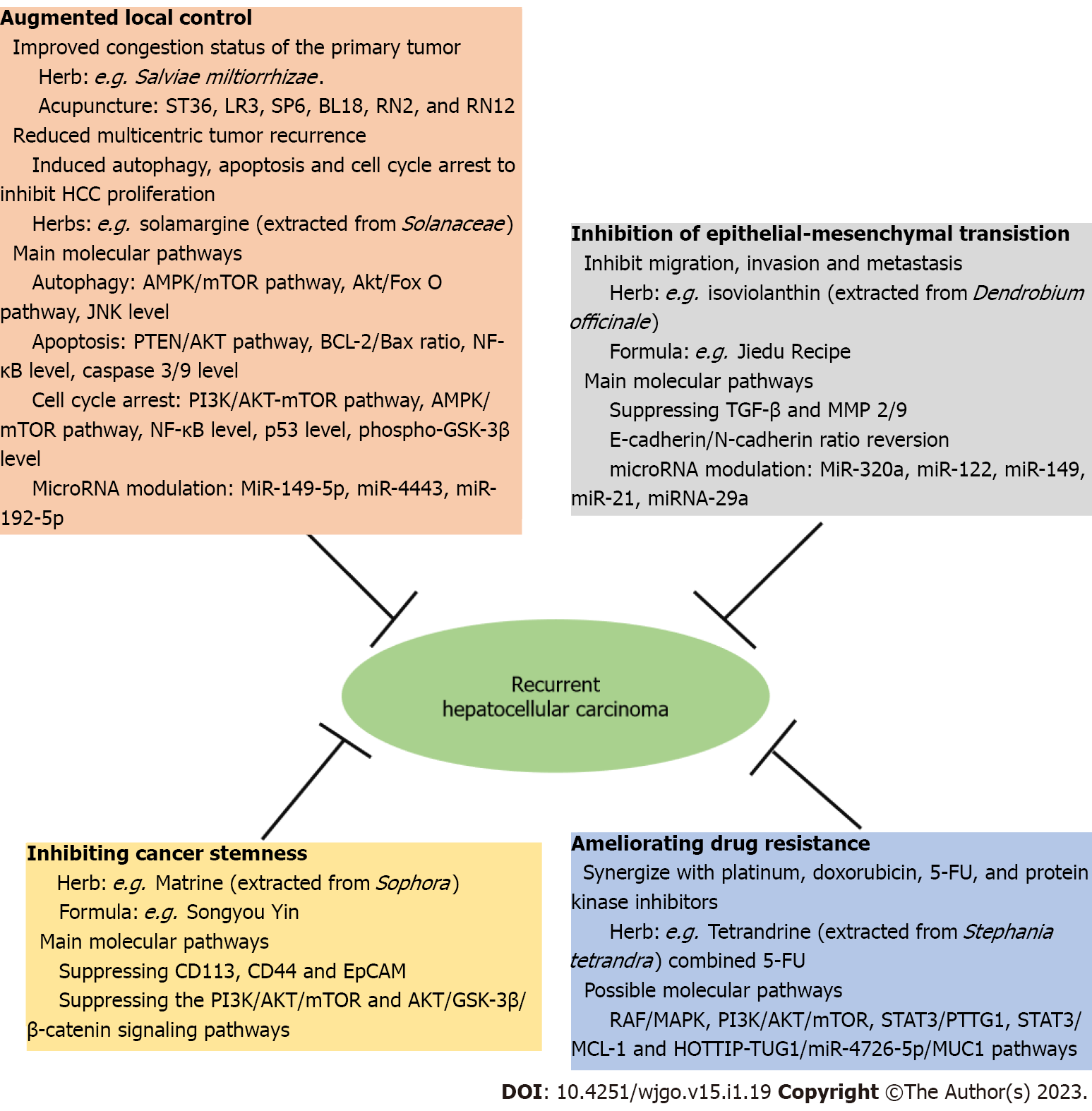
In summary, TCM and acupuncture treatments have been reported to augment the local control by improving the congestion status of the primary tumor. The molecular mechanisms are related to attenuating nitric oxide production and inhibiting fibrosis progression (via reducing procollagen III), thereby effectively preventing HCC recurrence. Meanwhile, various anti-tumor compounds have been reported to reduce multicentric tumor occurrence by inducing autophagy, apoptosis and cell cycle arrest to inhibit HCC proliferation. With regards to the pathways associated with autophagy, multiple herbs and their derivates have been shown to primarily affect the AMPK/mTOR pathway and the Akt/Fox O pathway, and to regulate the JNK level. Additionally, several herbs and their derivates have been reported to induce apoptosis mainly via regulating the PTEN/AKT pathway, the BCL-2/Bax ratio and the NF-κB level, and by increasing the caspase 3/9 level. As for cell cycle arrest, several herbs and their derivates affect the PI3K/AKT-mTOR pathway, the AMPK/mTOR pathway and the NF-κB level, and act to up-regulate levels of p53 and phospho-GSK-3β (Figure 1).
The liver has a sinus structure, abundant blood flow, an immunosuppressive microenvironment, and is involved in regulating blood circulation and the lymphatic system[57]. The migration, invasion, and metastasis associated with HCC recurrence significantly influence mortality rates. HCC is prone to metastasis to the lungs (47%), lymph nodes (45%), bones (37%), and adrenal glands (12%). The prognosis of HCC patients presenting with extrahepatic metastasis is poor[58], and likewise linked to a poor survival rate. Of note, anti-metastasis drugs, including sorafenib, lenvatinib, and a combination of protocols (e.g., sintilimab plus bevacizumab) have not demonstrated favorable outcomes in metastatic HCC patients[59,60].
Research indicates that the progression of cancer metastasis involves a series of steps[61]. First, EMT occurs in the early stages of tumor-cell metastasis, which allows epithelial phenotypic cells to convert into mesenchymal-like cells[62]. EMT studies have observed the involvement of epithelial proteins (E-cadherin, claudins, occludins, and α-catenin) as well as mesenchymal phenotypic proteins (N-cadherin, β-catenin, and vimentin). There are various pathways, including Wnt/β-catenin, mesenchymal-epithelial transition factor (c-Met)/hepatocyte growth factor (HGF)/Snail, neurogenic locus notch homolog protein 1/NF-κB, TGF-β/suppressor of mothers against decapentaplegic (SMAD), and basic fibroblast growth factor (FGF)-related signaling which play roles in EMT[62]. A coordinated sequence of invasion and metastasis subsequently occurs, which involves parenchymal, nonparenchymal and immune cells related to cytokines, histone methyltransferase/demethylase [e.g., enhancer of zeste homolog 2, SETDB1 (KMT1E) and euchromatic histone lysine methyltransferase 2 (G9a, EHMT2)], and non-coding RNAs[63,64].
Multiple TCM herbs and their derivatives have been shown to possess inhibitory effects against EMT as related to HCC. Scutellariae baicalensis, and its derivative baicalin, have recognized hepatoprotective effects, acting to modulate the TGF-β/SMAD, MAPK and NF-κB pathways, and inhibit matrix metalloproteinase (MMP)-1 to hinder EMT in HCC[65]. In a study involving Huh7 and MHCC97-H cell lines, astragaloside IV modulated the Akt/GSK-3β/β-catenin pathway and inhibited EMT[66]. Additionally, camptothecin, a topoisomerase inhibitor, has been shown to inhibit EMT by upregulating the expressions of zonula occludens protein-1, E-cadherin, and claudin-1[57]. Isoviolanthin has been shown to inhibit TGF-β1, associated with the downregulation of the TGF-β/SMAD and PI3K/Akt/mTOR signaling pathways, which resulted in the inhibition of EMT[67]. 18β-Glycyrrhetinic Acid, an ingredient of Glycyrrhiza glabra L. root (licorice), has been found to inhibit EMT and metastasis by suppressing the Src (Sarcoma) homology 2 domain phosphatase (SHP)1&SHP2/signal transducer and activator of transcription 3 (STAT3)/Snail pathway[68]. One study revealed that Echinacea purpurea regulated the PI3K/Akt signaling pathway to inhibit EMT[69]. Separately, tetrandrine impeded the Wnt/β-catenin signaling pathway and decreased metastatic tumor antigen 1 expression in Huh7 and Hep3B cell lines, leading to the inhibition of EMT, invasion, and migration[70]. Other studies have reported that scorpion and myricetin regulated the epithelial/mesenchymal proteins ratio and inhibited EMT[71,72]. More recently, miRNA and lncRNA have been associated with impacting the EMT process and drug resistance in HCC. Hydroxygenkwanin (upregulation in miR320a)[73], oleanolic acid (upregulation in miR-122)[74], aloin (regulation in circ_0011385/miR-149-5p/WT1 axis)[50], and puerarin (regulation in miR-21/PTEN/EMT axis)[75] inhibited EMT, invasion and migration in HCC cell lines. Both in vivo and in vitro studies by Chen et al[76] demonstrated that corylin, a flavonoid compound extracted from Psoralea corylifolia L., upregulated lncRNA growth arrest-specific transcript 5 to inhibit EMT and decrease tumor size[76].
Researchers have revealed that TCM offers multiple herbs and formulas found to inhibit migration and invasion and may therefore be applied to prevent HCC recurrence. Both kaempferol and dulcitol have been noted to decrease MMP to impede migration[77,78]. Sanguisorba officinalis has been shown to modulate the PI3K/AKT, NF-κB and MAPK signaling pathways to inhibit HepG2 cell migration and invasion[52]. Zanthoxylum avicennae augemented PP2Acα, GSK-3β, adenomatous polyposis coli protein and β-transducin repeat-containing protein levels, and diminished β-catenin, p-GSK-3β, T-Box Transcription Factor 3 and interleukin (IL)-8 proteins to prevent metastasis[79]. A study by Feng et al[80] reported that bufalin upregulated tank-binding kinase 1 and the interferon regulatory factor 3 and NF-κB pathways to hinder migration and invasion[80]. In terms of specific TCM formulas linked to EMT inhibition, QHF [consisting of HuaChanSu, 20(R) ginseng saponin Rg3, notoginseng total saponin, and lentinan] activated p38/JNK/MAPK pathway and inactivated ERK pathway to inhibit migration and invasion in a study using HepG2 cells[81]. The main ingredients in QHF, including cinobufotalin, ginsenoside Rg3, panax notoginsenosides, and lentinan, act to downregulate the HGF/c-Met signaling pathway to prevent metastasis and invasion[82]. In addition, the Biejiajian pill and Jiedu recipe have separately been found to prevent EMT by suppressing the Akt/GSK-3β/Snail signaling cascade and modulating E-cadherin/N-cadherin ratio, respectively[83,84]. In a study involving multiple HCC cell lines, the Xiaoai Jiedu recipe regulated the miRNA-29a/transcription 3 axis and decreased metastasis[85]. The TCM compounds and formulas associated with reducing HCC recurrence via inhibition of EMT, migration, invasion and metastasis are summarized in Table 2.
| Compound or Chinese herbal medicine or formula | Cell line/animal/human | Molecular mechanism | Effect and outcome | Ref. |
| Astragaloside IV | Huh7, MHCC97-H | Modulate Akt/GSK-3β/β-catenin pathway. ↑E-cadherin. ↓N-cadherin, vimentin, α-Smooth Muscle Actin, Slug | ↓EMT, invasion, migration | Qin et al[66] |
| Camptothecin | Huh7 | Modulate ZO-1, E-cadherin, claudin-1 | ↓EMT, metastasis | Liu et al[57] |
| Isoviolanthin | HepG2, BEL-7402 | ↓TGF-β1. ↓TGF-β/SMAD and PI3K/Akt/mTOR pathway. ↓MMP-2 and -9 | ↓EMT | Xing et al[67] |
| 18β-Glycyrrhetinic Acid | BEL-7402, LM3 | Modulate. SHP1&SHP2/STAT3/Snail pathway. ↓Phosphorylation of STAT3. ↑SHP1 and SHP2 | ↓EMT and metastasis | Jie et al[68] |
| Echinacea purpurea | Hepa1-6, HepG2, L-02 | Modulate PI3K/Akt pathway | ↓EMT | Xu et al[69] |
| Tetrandrine | Huh7, Hep3B | ↓Wnt/β-catenin pathway. ↓Metastatic tumor antigen 1 | ↓EMT, invasion, migration | Zhang et al[70] |
| Scorpion | Hepa1-6/Sprague-Dawley rats (6-wk, male, 0.63 g/200 g, every day for 4 wk) | ↑E-cadherin. ↓N-cadherin | ↓EMT, migration, invasion | Yan et al[71] |
| Myricetin | MHCC97-H | ↑E-cadherin expression. ↓N-cadherin | ↓Migration, invasion | Ma et al[72] |
| Hydroxygenkwanin | HepG2 and Huh7/nude mice (6-wk, male, 1 mg/kg for 3 times per week) | ↑miR-320a, ↓Forkhead box protein M1 | ↓EMT, invasion, migration. ↓Tumor size | Chou et al[73] |
| Oleanolic acid | HepG2, SK-Hep-1 | ↑miR-122, E-cadherin. ↓β-catenin, N-cadherin, vimentin | ↓EMT, migration, invasion | He et al[74] |
| Aloin | -- | Modulate circ_0011385/miR-149-5p/WT1 axis | ↓Invasion | Fu et al[50] |
| Puerarin | BEL-7402, Huh7, L-02 | ↑PTEN. Modulate miR-21/PTEN/EMT axis | ↓EMT, migration, invasion | Zhou et al[75] |
| Corylin | Hep G2, Huh7/nude mice (BALB/cAnN-Foxnlnu/CrlNarl, 6-wk, male, 60 mg/kg, 3 times per week) | ↑GAS5 | ↓EMT. ↓Tumor size | Chen et al[76] |
| Kaempferol | Huh7, SK-Hep-1 | ↓MMP-9 and Akt pathway | ↓Migration | Ju et al[77] |
| Dulcitol | HepG2 | ↓MMP-2, uPA, MMP-9. ↑E-cadherin | ↓Migration and invasion | Lin et al[78] |
| Sanguisorba officinalis | HepG2 | Modulate EGFR, PI3K/AKT, NF-κB and MAPK pathway | ↓Migration, invasion | Jiang et al[52] |
| Zanthoxylum avicennae | HA22T | ↑PP2Acα, GSK-3β, APC, β-TrCP/HOS. ↓β-catenin, p-GSK-3β, TBX 3, IL-8. ↓Nuclear and cytosolic β-catenin | ↓Metastasis | Wu et al[79] |
| Bufalin | MHCC97-H | ↑TBK1, IRF3 and NF-κB pathway | ↓Migration, invasion | Feng et al[80] |
| QHF (consisting of HuaChanSu, 20(R)ginseng saponin Rg3, notoginseng total saponin and lentinan) | HepG2 | ↑p38, JNK, MAPK pathway. ↓ERK pathway | ↓Migration, invasion | Chen et al[81] |
| QHF (consisting of cinobufotalin, ginsenoside Rg3, panax notoginsenosides, lentinan) | HCCLM3, HepG2/SPF BALB/c mice (20 g, male, 0.2 ml/mice, once every other day for 4 wk) | ↓p-c-Met protein. ↓HGF/c-Met pathway | ↓Metastasis, invasion | Yuan et al[82] |
| Biejiajian pill | MHCC-97H, SMMC-7721/BALB/c nude mice (4-5 wk, female, 1.1 g/kg, daily for 4 wk) | ↓Akt/GSK-3β/Snail pathway | ↓EMT, metastasis | Sun et al[83] |
| Jiedu Recipe | SMMC-7721, Huh7 | ↑E-cadherin. ↓p-Smad2/3, Smad2/3. ↓TGF-β1, vimentin, N-cadherin, MMP2/9 | ↓EMT, invasion, migration | Liang et al[84] |
| Xiaoai Jiedu Recipe | Male nude mice (BALB/c (nu/nu), 4–5 wk, male, 10 g/kg, 4 consecutive days). 40 HCC patients and 40 volunteer controls | Modulate miRNA-29a signal transducer ↑Transcription 3 Axis | ↓Metastasis | Shi et al[85] |
Collectively, multiple investigations have demonstrated that TCM and its derivative compounds act to prevent HCC recurrence by inhibiting EMT, migration, invasion and metastasis. The possible mechanisms involved in this prevention include suppressing TGF-β and MMP-2/9, E-cadherin/N-cadherin ratio reversion, and microRNA modulation (Figure 1).
CSCs may be characterized as possessing features of self-renewal, differentiation potential, and colony-forming. Research indicates that CSCs may be a major cause of tumorigenesis, metastasis and antitumor agent resistance, and are thus a primary culprit in tumor relapse after therapy. Dai et al[86] suggested that HCC CSCs create an immunosuppressive microenvironment through both intrinsic and extrinsic mechanisms to escape immune surveillance[86]. In HCC cell lines, CSCs are primarily identified as CD133[87]; while other surface markers include epithelial cell adhesion molecule (EpCAM), CD44, CD13, CD90, CD24, CD47, oval cell marker OV6, K19, c-kit, breast cancer resistant protein, and aldehyde dehydrogenases[88]. Meanwhile, signaling pathways including the Wnt/β-catenin, AKT/GSK-3β/β-catenin, ERK/Snail, AKT/PKB, AKT/mTOR, and TGF-β pathways have been recognized in CSC formation[88].
With regards to TCM treatments associated with CSC hindrance, investigations have revealed several notable findings. Antrodia cinnamomea, a fungus species, has well-documented anti-HCC effects, and has been found to hinder CD133+ CSC and downregulating onco-miRNAs in glioblastoma multiforme 8401 and breast adenocarcinoma (MDA-MB-231) cell lines[89]. Tiliroside, a compound isolated from Tribulus terrestris L., decreased CD133+ and carbonic anhydrases XII expressing while concurrently activated E2F1/E2F3/Caspase-3 axis in Hep3B and SNU-449 cell lines[90]. In addition, 8-bromo-7-methoxychrysin decreased expressions of CD133, CD44 and IL-6, and inhibited self-renewal of SMMC-7721- and MHCC97H-derived liver cancer stem-like cells[91]. Curcumin decreased expressions of several CSC markers (c-KIT, EpCAM, CD133, RING finger protein 51, and NANOG) and inhibited the oncogenic NF-κB signaling pathway[92]. Sophocarpine decreased expressions of CD133, CD90, and EpCAM as well as TGF-β to inhibit both EMT and CSC[93]. Matrine, extracted from Sophora flavescens, reduced the EpCAM+/CD133+ in HCC cells by inactivating the PI3K/AKT/mTOR and AKT/GSK-3β/β-catenin signaling pathways[94]. In addition, Brucea javanica has been found to decrease expressions of CD133, NANOG and EpCAM, subsequently inducing apoptosis and suppressing CSCs[95]. 2-Etho
| Compound or Chinese herbal medicine or formula | Cell line/animal | Molecular mechanism | Effect | Ref. |
| Antrodia cinnamomea | Hep G2 | ↓CD133+ | ↓CSC | Su et al[89] |
| Tiliroside | Hep3B, SNU-449 | ↓CD133+. ↓Carbonic anhydrases XII. ↑E2F1/E2F3/Caspase-3 axis | ↓Tumor sphere formation. ↓WInvasion and migration. ↓Stemness gene expression. ↑Apoptosis | Han et al[90] |
| 8-bromo-7-methoxychrysin | SMMC-7721, MHCC97H-derived LCSLCs | ↓CD133, CD44. ↓IL-6 | ↓CSC | Wen et al[91] |
| Curcumin | PLC/PRF5, WRL68, Huh7, KMCH, AFP-negative primary HCC cell line | ↓CD117, EpCAM, CD133, RNF51, NANOG. ↓NF-κB | ↓CSC | Marquardt et al[92] |
| Sophocarpine | HCC-LM3, MHCC-97H. BALB/c nude mice (4-wk, male, 0.4-6 g/kg, twice a week for 4 wk) | ↓TGF-β. ↓CD133, CD90 and EpCAM | ↓EMT. ↓CSC | Zhang et al[93] |
| Matrine | Hep3B, Huh7. BALB/c nude mice (-,-,10 mg/kg, daily for 3 wk) | ↓EpCAM+/CD133+ cell number. ↓PI3K/AKT/mTOR pathway, AKT/GSK-3β/β-catenin pathway | ↓Sphere formation. ↓Stem cell markers. ↑Mature hepatocyte markers | Liu et al[94] |
| Brucea javanica | HepG2 (HB-8065, wild-type p53), Hep3B (HB-8064, p53-null) | ↓CD133, NANOG, EpCAM | ↑Apoptosis. ↓Stem-like cells | Chen et al[95] |
| 2-Ethoxystypandrone | Hep3B, HepG2, Huh7, Li-7, SK-Hep-1 | ↓STAT3 activation | ↓Proliferation, ↑Apoptosis. ↓CSC | Li et al[96] |
| BRM270 | HepG2 (CD133+), SNU-398. CRJORI:CD-1-5WM (6-wk, male, 5 mg/kg/day, daily for 12 wk) | ↓CyclinD1/Bcl2 mediated c-Jun apoptotic pathway. ↓CD113 | ↓Proliferation, ↑Apoptosis. ↓CSC | Kumar et al[97] |
| Songyou Yin (consisted by Salvia miltiorrhiza, Astragalus membranaceus, Lycium barbarum, Crataegus pinnatifida and Trionyx sinensis) | MHCC97-H, Hep3B | ↓CD90, BCRP, ALDH, CD44, EpCAM, vimentin, MMP-9. ↑E-cadherin | ↑Oxaliplatin chemosensitivity. ↓Motility, invasion, and colony formation. ↓CSC | Jia et al[98] |
In summary, CSC plays an important role in HCC recurrence. In this regard, TCM and its derivative compounds could suppress CSC markers, particularly in CD113, CD44 and EpCAM, reduce TGF-β which promotes CSC properties, and suppress the PI3K/AKT/mTOR and AKT/GSK-3β/β-catenin signaling pathways (Figure 1).
In the advanced stages of HCC and in patients presenting with recurrence, molecular target therapy has become a viable alternative treatment. Target therapy agents such as sorafenib [targeting VEGFR and platelet-derived growth factor receptors (PDGFR)], ramucirumab (targeting VEGFR), regorafenib (targeting VEGFR), gefitinib (targeting EGFR), erlotinib (targeting EGFR), lenvatinib (targeting VEGFR, PDGFR, FGF receptor), and everolimus (targeting mTOR) are commonly prescribed. However, patients having received target therapy have not exhibited significant beneficial effects in terms of overall survival, while drug resistance has further limited the anticancer effect. Previous studies have shown that inflammation and fibrosis have caused sorafenib-resistance and HCC progression. TNF-α and IL-6 are key cytokines which promote intrahepatic HCC progression via STAT3 activation[101]. The combination of two or three drugs which impact multiple targets may improve treatment to control the complex cancer metabolic system, whereby TCM may serve as a multi-target adjuvant therapy in preventing HCC recurrence.
Investigations have revealed that cisplatin and oxaliplatin, platinum-based chemotherapeutic agents, cause cytotoxic effects through DNA damage. The resistance to oxaliplatin in HCC has been associated with the lysine-specific demethylase 1/long intergenic non-protein-coding RNA 1134 (LINC01134)/SP1/p62 axis or the miR-129-5p/ETS translocation variant 1 axis[102,103]. It has been reported that trametes robiniophila extract repressed the expression of Yes-associated protein and apoptosis-related proteins (Bcl-2) to sensitize the oxaliplatin effect[104]. In as separate study, falcarindiol sensitized the cisplatin anti-Huh7 and LM3 effects by downregulation of the STAT3/pituitary tumor transforming gene 1 (PTTG1) pathway expression[105]. As applied in advanced or recurrent HCC, doxorubicin has been shown to intercalate the DNA, stabilize the topoisomerase II complex and halt the DNA replication process. In addition, dihydroartemisinin has been found to decrease P-gp expression through downregulating the p53 (R248Q)-ERK1/2-NF-κB signaling pathway to augment anticancer effects in mutant p53 (R248Q)-expressing Hep3B cells (doxorubicin resistant cell line)[106]. Of note, it has been reported that Solanum nigrum enhanced cisplatin and doxorubicin’s anti-HCC effect through apoptosis and autophagy by cleavage of caspase-7 and accumulation of microtubule-associated protein-1 Light chain-3 A/1B II[107]. Meanwhile, 5-fluorouracil (5-FU) is a thymidylate synthase inhibitor which interferes with DNA replication and leads to cytotoxicity. As reported, H1 (a derivative of tetrandrine, molecular formula: C27H40N2O6Br) and bufalin increased 5-FU sensitivity in 5-FU-resistant HCC cells (BEL-7402/5-FU)[108,109]. Additionally, bufalin induced apoptosis by increasing in the Bax/Bcl-xL ratio, inhibited drug efflux pump activity via downregulation of multidrug resistance protein 1 and reduced the expression of thymidylate synthase[108]. Furthermore, H1 downregulated the STAT3/ myeloid cell leukemia 1 (MCL-1) pathway to sensitize 5-FU treatment[109]. Sorafenib is a protein kinase inhibitor which acts against VEGFR and PDGFR, and rapidly accelerates fibrosarcoma (RAF) kinases. In separate studies, artesunate and tetrandrine increased the effectiveness of sorafenib on HCC apoptosis by inhibiting the PI3K/AKT/mTOR pathway[110,111]. Artesunate has also been shown to inhibit the RAF/MAPK pathway[110]. Meanwhile, Zhai et al[112] reported that bufalin reversed sorafenib resistance via the inositol-requiring enzyme 1 pathway in HepG2 and Huh7 cell lines[112]. Furthermore, solamargine has been shown to provide a synergistic anticancer effect with sorafenib by regulating HOXA distal transcript antisense RNA (HOTTIP)–the taurine upregulated 1 (TUG1)/miR-4726-5p/mucin 1 signaling pathway[113]. The combination of 8-bromo-7-methoxychrysin and sorafenib has been reported to decrease expressions of HIF-1α and the EMT regulator Twist1 to inhibit CSC[114]. To be applied in cases of HCC recurrence or in advanced cases, icaritin has been found to enhance the effects of doxorubicin and lenvatinib in Hepa1-6 and Huh7 cells[115]. The compounds involved in reversing drug resistance are listed in Table 4.
| Compound or Chinese herbal medicine | Conventional drug | Cell line | Molecular mechanism | Ref. |
| Trametes robiniophila Murr | Oxaliplatin | BEL-7404, SMMC-7721 | ↓YAP and apoptosis related proteins | Tao et al[104] |
| Falcarindiol | Cisplatin | Huh7, LM3 | ↓STAT3/PTTG1 pathway | Hong et al[105] |
| Dihydroartemisinin | Doxorubicin | Mutant p53 (R248Q)-expressing Hep3B | Modulate p53 (R248Q)-ERK1/2-NF-κB pathway. ↓P-gp expression | Yang et al[106] |
| Solanum nigrum | Cisplatin and doxorubicin | Hep3B, HepJ5 | ↑Cleavage of caspase-7. ↑LC-3 A/B II. ↑Apoptosis, autophagy | Wang et al[107] |
| Bufalin | 5-FU | BEL-7402/5-FU | ↑Apoptosis arrested the cell cycle at the | Gu et al[108] |
| H1 (a derivative of tetrandrine, molecular formula: C27H40N2O6Br) | 5-FU | BEL-7402/5-FU | ↓STAT3/MCL-1 pathway. ↑PUMA expression | Li et al[109] |
| Artesunate | Sorafenib | SK-hep1, SMMC-7721 | ↑Apoptosis. ↓RAF/MAPK pathway. ↓PI3K/AKT/mTOR pathway | Jing et al[110] |
| Tetrandrine | Sorafenib | SMMC-7721, PLC/PRF/5 | ↓PI3K/AKT/mTOR pathway. ↓Proliferation. ↑Apoptosis | Niu et al[111] |
| Bufalin | Sorafenib | HepG2, Huh7 | ↓p-Akt. Modulate IRE1 pathway | Zhai et al[112] |
| Solamargine | Sorafenib | HepG2, Huh7 | ↓lncRNA HOTTIP and TUG1. Modulate HOTTIP-TUG1/miR-4726-5p/mucin 1 pathway | Tang et al[113] |
| 8-bromo-7-methoxychrysin | Sorafenib | SMMC-7721 | ↓Migration and invasion. ↓N-cadherin. ↑E-cadherin ↑Apoptosis in LCSLCs. ↓HIF-1α and EMT regulator Twist1 | Zou et al[114] |
| Icaritin | Doxorubicin and lenvatinib | Hepa1-6, Huh7 | ↑Mitophagy and apoptosis. ↑Immunogenic cell death | Yu et al[115] |
With regards to the benefits of acupuncture in the amelioration of drug resistance, a limited number of studies have focused directly on the antitumor and synergistic effects associated with acupuncture, electroacupuncture and moxibustion. Although, Yang et al[116] reported that electroacupuncture around a breast cancer tumor increased the local concentration of paclitaxel and decreased the tumor volume[116].
Drug resistance indeed limits the therapeutic effectiveness of drug treatments for recurrent HCC. However, investigations have demonstrated that the combination of two or three drugs impacting multiple targets may offer promising anti-HCC treatment strategies. As such, TCM has been found to provide a wide range of synergistic effects associated with platinum, doxorubicin, 5-FU, and protein kinase inhibitors. The mechanisms underlying these effects are associated with the RAF/MAPK, PI3K/AKT/mTOR, STAT3/PTTG1, STAT3/MCL-1 and HOTTIP-TUG1/miR-4726-5p/MUC1 pathways (Figure 1).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: A JD, China; Sahle Z, Ethiopia S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1212] [Article Influence: 202.0] [Reference Citation Analysis (1)] |
| 2. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1198] [Article Influence: 299.5] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15294] [Article Influence: 3058.8] [Reference Citation Analysis (4)] |
| 4. | Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol. 2011;9:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Liu C, Yang S, Wang K, Bao X, Liu Y, Zhou S, Liu H, Qiu Y, Wang T, Yu H. Alkaloids from Traditional Chinese Medicine against hepatocellular carcinoma. Biomed Pharmacother. 2019;120:109543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Li JJ, Liang Q, Sun GC. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: A focus on epithelial-mesenchymal transition. J Integr Med. 2021;19:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 567] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 8. | Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res. 2017;47:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo S, Abraldes JG, Bruix J, Bosch J, García-Pagan JC. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol. 2012;56:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M, Festi D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 11. | Tian T, Xu LM. [Effects of Salviae miltiorrhizae and salvianolic acid B on microcirculation of liver in mice with portal hypertension]. Zhong Xi Yi Jie He Xue Bao. 2009;7:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Wang H, Chen XP, Qiu FZ. Salviae miltiorrhizae ameliorates cirrhosis and portal hypertension by inhibiting nitric oxide in cirrhotic rats. Hepatobiliary Pancreat Dis Int. 2003;2:391-396. [PubMed] |
| 13. | Zhou Y, Gu J, Xu LM. [Effect and mechanism of salvianolic acid B in attenuating elevated portal pressure in a rat model of portal hypertension induced by endothelin-1]. Zhong Xi Yi Jie He Xue Bao. 2007;5:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Xu H, Zhou Y, Lu C, Ping J, Xu LM. Salvianolic acid B lowers portal pressure in cirrhotic rats and attenuates contraction of rat hepatic stellate cells by inhibiting RhoA signaling pathway. Lab Invest. 2012;92:1738-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Gao H, Sun W, Zhao J, Wu X, Lu JJ, Chen X, Xu QM, Khan IA, Yang S. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci Rep. 2016;6:33720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Chang JH, Lin CH, Shibu MA, Chou YC, Liu JY, Chou YH, Shen CY, Yeh YL, Viswanadha VP, Huang CY. Cryptotanshinone (Dsh-003) from Salvia miltiorrhiza Bunge inhibits prostaglandin E2-induced survival and invasion effects in HA22T hepatocellular carcinoma cells. Environ Toxicol. 2018;33:1254-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zhu P, Liu Z, Zhou J, Chen Y. Tanshinol inhibits the growth, migration and invasion of hepatocellular carcinoma cells via regulating the PI3K-AKT signaling pathway. Onco Targets Ther. 2019;12:87-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Wang H, Chen X. Tetrandrine ameliorates cirrhosis and portal hypertension by inhibiting nitric oxide in cirrhotic rats. J Huazhong Univ Sci Technolog Med Sci. 2004;24:385-388, 395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 19. | Lin JS, Chan CY, Yang C, Wang YH, Chiou HY, Su YC. Zhi-fuzi, a cardiotonic Chinese herb, a new medical treatment choice for portal hypertension? Exp Biol Med (Maywood). 2007;232:557-564. [PubMed] |
| 20. | Huang YT, Wang GF, Chen CF, Chen CC, Hong CY, Yang MC. Fructus aurantii reduced portal pressure in portal hypertensive rats. Life Sci. 1995;57:2011-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Zhang XP, Zhang F, Zhang ZL, Ma J, Kong DS, Ni GX, Wang AY, Chen WX, Lu Y, Zheng SZ. Acupuncture combined with curcumin disrupts platelet-derived growth factor β receptor/extracellular signal-regulated kinase signalling and stimulates extracellular matrix degradation in carbon tetrachloride-induced hepatic fibrosis in rats. Acupunct Med. 2012;30:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Zhang F, Ma J, Lu Y, Ni GX, Ni CY, Zhang XJ, Zhang XP, Kong DS, Wang AY, Chen WX, Zheng SZ. Acupuncture combined with curcumin attenuates carbon tetrachloride-induced hepatic fibrosis in rats. Acupunct Med. 2012;30:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Qi L, Li S, Xu J, Lou W, Cheng L, Zhang C. Acupuncture for the Treatment of Liver Cirrhosis: A Meta-analysis. Gastroenterol Res Pract. 2020;2020:4054781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Li Z, Cao H, Yao M, Lei X. Effect of acupuncture combined with Shenqi Yigan Decoction on liver function and T cell subsets in patients with HBV-induced liver fibrosis. Am J Transl Res. 2021;13:3409-3417. [PubMed] |
| 25. | Chen YS, Wen CK, Liu GH, Lee TY. Electroacupuncture attenuates vascular hyporeactivity in a rat model of portal hypertension induced by bile duct ligation. Acupunct Med. 2022;40:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Yan YN, Wang N, Wang ZY, Tian Y, Cheng YZ, Ma HF, Hou ZW, Ma JJ, Guan HY. [Effects of Direct Moxibustion of "Ganshu"(BL 18) on the Contents of T cells in Peripheral Blood in Rats with Precancerous Lesion of Primary Hepatocellular Carcinoma]. Zhen Ci Yan Jiu. 2016;41:321-326. [PubMed] |
| 27. | Wei YN, Li NF, Cai XY, Lu BY, Huang F, Mo SF, Zhang HC, Wang MD, Wu FS. Clinical application of fast-track surgery with Chinese medicine treatment in the devascularization operation for cirrhotic portal hypertension. Chin J Integr Med. 2015;21:784-790. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 630] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 29. | Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in Pharmacological Activities of Terpenoids. Nat Prod Commun. 2020;15:1934578X20903555. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Jiang QW, Chen MW, Cheng KJ, Yu PZ, Wei X, Shi Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med Res Rev. 2016;36:119-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Yin S, Jin W, Qiu Y, Fu L, Wang T, Yu H. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J Hematol Oncol. 2022;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 32. | Wu K, Teng M, Zhou W, Lu F, Zhou Y, Zeng J, Yang J, Liu X, Zhang Y, Ding Y, Shen W. Wogonin Induces Cell Cycle Arrest and Apoptosis of Hepatocellular Carcinoma Cells by Activating Hippo Signaling. Anticancer Agents Med Chem. 2022;22:1551-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Hong M, Almutairi MM, Li S, Li J. Wogonin inhibits cell cycle progression by activating the glycogen synthase kinase-3 beta in hepatocellular carcinoma. Phytomedicine. 2020;68:153174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Chandrashekar N, Pandi A. Baicalein: A review on its anti-cancer effects and mechanisms in lung carcinoma. J Food Biochem. 2022;46:e14230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Zhao X, Tang D, Chen X, Chen S, Wang C. Functional lncRNA-miRNA-mRNA Networks in Response to Baicalein Treatment in Hepatocellular Carcinoma. Biomed Res Int. 2021;2021:8844261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Yang J, Sun Y, Xu F, Liu W, Hayashi T, Mizuno K, Hattori S, Fujisaki H, Ikejima T. Autophagy and glycolysis independently attenuate silibinin-induced apoptosis in human hepatocarcinoma HepG2 and Hep3B cells. Hum Exp Toxicol. 2021;40:2048-2062. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13:5299-5305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Lee Y, Kwon YH. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem Biophys Res Commun. 2019;517:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Yee SB, Choi HJ, Chung SW, Park DH, Sung B, Chung HY, Kim ND. Growth inhibition of luteolin on HepG2 cells is induced via p53 and Fas/Fas-ligand besides the TGF-β pathway. Int J Oncol. 2015;47:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. Increase of Bax/ Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci. 2005;76:1883-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Han B, Yu YQ, Yang QL, Shen CY, Wang XJ. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget. 2017;8:86227-86239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Seydi E, Salimi A, Rasekh HR, Mohsenifar Z, Pourahmad J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr Cancer. 2018;70:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015;42:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Fernández-Palanca P, Fondevila F, Méndez-Blanco C, Tuñón MJ, González-Gallego J, Mauriz JL. Antitumor Effects of Quercetin in Hepatocarcinoma In Vitro and In Vivo Models: A Systematic Review. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Jiang Y, Nan H, Shi N, Hao W, Dong J, Chen H. Chlorogenic acid inhibits proliferation in human hepatoma cells by suppressing noncanonical NF-κB signaling pathway and triggering mitochondrial apoptosis. Mol Biol Rep. 2021;48:2351-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Guo R, Lin B, Shang XY, Zhou L, Yao GD, Huang XX, Song SJ. Phenylpropanoids from the fruit of Crataegus pinnatifida exhibit cytotoxicity on hepatic carcinoma cells through apoptosis induction. Fitoterapia. 2018;127:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Helmy SA, El-Mesery M, El-Karef A, Eissa LA, El Gayar AM. Thymoquinone upregulates TRAIL/TRAILR2 expression and attenuates hepatocellular carcinoma in vivo model. Life Sci. 2019;233:116673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Hou YQ, Yao Y, Bao YL, Song ZB, Yang C, Gao XL, Zhang WJ, Sun LG, Yu CL, Huang YX, Wang GN, Li YX. Juglanthraquinone C Induces Intracellular ROS Increase and Apoptosis by Activating the Akt/Foxo Signal Pathway in HCC Cells. Oxid Med Cell Longev. 2016;2016:4941623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Chen YL, Yen IC, Lin KT, Lai FY, Lee SY. 4-Acetylantrocamol LT3, a New Ubiquinone from Antrodia cinnamomea, Inhibits Hepatocellular Carcinoma HepG2 Cell Growth by Targeting YAP/TAZ, mTOR, and WNT/β-Catenin Signaling. Am J Chin Med. 2020;48:1243-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Fu D, Ji Q, Wang C, Yu L, Yu R. Aloin decelerates the progression of hepatocellular carcinoma through circ_0011385/miR-149-5p/WT1 axis. Cell Cycle. 2021;20:2476-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Duan X, Li T, Han X, Ren J, Chen P, Li H, Gong S. The antitumor effect of arsenic trioxide on hepatocellular carcinoma is enhanced by andrographolide. Oncotarget. 2017;8:90905-90915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Jiang N, Li H, Sun Y, Zeng J, Yang F, Kantawong F, Wu J. Network Pharmacology and Pharmacological Evaluation Reveals the Mechanism of the Sanguisorba Officinalis in Suppressing Hepatocellular Carcinoma. Front Pharmacol. 2021;12:618522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Luo S, Jiang X, Yin G, Liu Y, Liu Z, Meng L, Wu J, Wu H. The herbal agent plantamajoside, exerts a potential inhibitory effect on the development of hepatocellular carcinoma. Exp Ther Med. 2021;21:573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 55. | Hong M, Wang N, Tan HY, Tsao SW, Feng Y. MicroRNAs and Chinese Medicinal Herbs: New Possibilities in Cancer Therapy. Cancers (Basel). 2015;7:1643-1657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Yang X, Feng Y, Liu Y, Ye X, Ji X, Sun L, Gao F, Zhang Q, Li Y, Zhu B, Wang X. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine. 2021;87:153575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Liu F, Lou G, Zhang T, Chen S, Xu J, Xu L, Huang C, Liu Y, Chen Z. Anti-metastasis traditional Chinese medicine monomer screening system based on perinucleolar compartment analysis in hepatocellular carcinoma cells. Am J Transl Res. 2019;11:3555-3566. [PubMed] |
| 58. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 59. | Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 60. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 664] [Article Influence: 166.0] [Reference Citation Analysis (1)] |
| 61. | Fidler IJ, Kripke ML. The challenge of targeting metastasis. Cancer Metastasis Rev. 2015;34:635-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 62. | Gurzu S, Kobori L, Fodor D, Jung I. Epithelial Mesenchymal and Endothelial Mesenchymal Transitions in Hepatocellular Carcinoma: A Review. Biomed Res Int. 2019;2019:2962580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 63. | Han TS, Ban HS, Hur K, Cho HS. The Epigenetic Regulation of HCC Metastasis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 64. | Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res. 2016;22:5971-5982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 65. | Gao Y, Snyder SA, Smith JN, Chen YC. Anticancer properties of baicalein: a review. Med Chem Res. 2016;25:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH, Wang YC, Ye BG, Cao MQ, Gao DM, Tang ZY. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol Rep. 2017;37:1725-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Xing S, Yu W, Zhang X, Luo Y, Lei Z, Huang D, Lin J, Huang Y, Huang S, Nong F, Zhou C, Wei G. Isoviolanthin Extracted from Dendrobium officinale Reverses TGF-β1-Mediated Epithelial⁻Mesenchymal Transition in Hepatocellular Carcinoma Cells via Deactivating the TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Jie M, Zhang ZQ, Deng N, Liu QM, Wang C, Ge QY, Du PC, Song SS, Zhang XW, Long-Xin, Liang HF, Chu L, Zhang L, Chen XP, Chen J, Dong HH, Zhang BX. 18[Formula: see text]-Glycyrrhetinic Acid Inhibits TGF-[Formula: see text]-Induced Epithelial-to-Mesenchymal Transition and Metastasis of Hepatocellular Carcinoma by Targeting STAT3. Am J Chin Med. 2022;50:313-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Xu W, Hu B, Cheng Y, Guo Y, Yao W, Qian H. Echinacea purpurea suppresses the cell survival and metastasis of hepatocellular carcinoma through regulating the PI3K/Akt pathway. Int J Biochem Cell Biol. 2022;142:106115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Zhang Z, Liu T, Yu M, Li K, Li W. The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J Exp Clin Cancer Res. 2018;37:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Yan YQ, Xie J, Wang JF, Shi ZF, Zhang X, Du YP, Zhao XC. Scorpion inhibits epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma. Exp Biol Med (Maywood). 2018;243:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Ma H, Zhu L, Ren J, Rao B, Sha M, Kuang Y, Shen W, Xu Z. Myricetin inhibits migration and invasion of hepatocellular carcinoma MHCC97H cell line by inhibiting the EMT process. Oncol Lett. 2019;18:6614-6620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Chou LF, Chen CY, Yang WH, Chen CC, Chang JL, Leu YL, Liou MJ, Wang TH. Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression. Biomolecules. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | He Y, Liu X, Huang M, Wei Z, Zhang M, He M, Zheng Z, Dong H, Liu D. Oleanolic acid inhibits the migration and invasion of hepatocellular carcinoma cells by promoting microRNA-122 expression. Pharmazie. 2021;76:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 75. | Zhou Y, Xue R, Wang J, Ren H. Puerarin inhibits hepatocellular carcinoma invasion and metastasis through miR-21-mediated PTEN/AKT signaling to suppress the epithelial-mesenchymal transition. Braz J Med Biol Res. 2020;53:e8882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Chen CY, Chen CC, Shieh TM, Hsueh C, Wang SH, Leu YL, Lian JH, Wang TH. Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Ju PC, Ho YC, Chen PN, Lee HL, Lai SY, Yang SF, Yeh CB. Kaempferol inhibits the cell migration of human hepatocellular carcinoma cells by suppressing MMP-9 and Akt signaling. Environ Toxicol. 2021;36:1981-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Lin XL, Li K, Yang Z, Chen B, Zhang T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine. 2020;66:153112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Wu HC, Lay IS, Shibu MA, Ho TJ, Cheng SM, Lin CH, Dung TD, Jeng LB, Viswanadha VP, Huang CY. Zanthoxylum avicennae extract enhances GSK-3β to attenuate β-catenin via phosphatase 2A to block metastatic effects of HA22T cells and hepatocellular carcinoma xenografted nude mice. Environ Toxicol. 2017;32:2133-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Feng Y, Chen Y, Meng Y, Cao Q, Liu Q, Ling C, Wang C. Bufalin Suppresses Migration and Invasion of Hepatocellular Carcinoma Cells Elicited by Poly (I:C) Therapy. Oncoimmunology. 2018;7:e1426434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Chen T, Wang Q, Li Y, Huang H, Hu W. Chinese herbal formula QHF inhibits liver cancer cell invasion and migration. Exp Ther Med. 2016;11:2413-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Yuan S, Gong Y, Chen R, Du J, Zhang H, Chen T. Chinese herbal formula QHF inhibits hepatocellular carcinoma metastasis via HGF/c-Met signaling pathway. Biomed Pharmacother. 2020;132:110867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Sun J, Chen W, Wen B, Zhang M, Sun H, Yang X, Zhao W, La L, An H, Pang J, Gao L, He S. Biejiajian Pill Inhibits Carcinogenesis and Metastasis via the Akt/GSK-3β/Snail Signaling Pathway in Hepatocellular Carcinoma. Front Pharmacol. 2021;12:610158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Liang S, Zou Y, Gao J, Liu X, Lin W, Yin Z, Du J, Zhang Y, Chen Q, Li S, Cheng B, Ling C. The Chinese Medicine, Jiedu Recipe, Inhibits the Epithelial Mesenchymal Transition of Hepatocellular Carcinoma via the Regulation of Smad2/3 Dependent and Independent Pathways. Evid Based Complement Alternat Med. 2018;2018:5629304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Shi Y, Kong W, Lu Y, Zheng Y. Traditional Chinese Medicine Xiaoai Jiedu Recipe Suppresses the Development of Hepatocellular Carcinoma via Regulating the microRNA-29a/Signal Transducer and Activator of Transcription 3 Axis. Onco Targets Ther. 2020;13:7329-7342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Dai X, Guo Y, Hu Y, Bao X, Zhu X, Fu Q, Zhang H, Tong Z, Liu L, Zheng Y, Zhao P, Fang W. Immunotherapy for targeting cancer stem cells in hepatocellular carcinoma. Theranostics. 2021;11:3489-3501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 87. | Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 605] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 88. | Liu YC, Yeh CT, Lin KH. Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 89. | Su YK, Shih PH, Lee WH, Bamodu OA, Wu ATH, Huang CC, Tzeng YM, Hsiao M, Yeh CT, Lin CM. Antrodia cinnamomea sensitizes radio-/chemo-therapy of cancer stem-like cells by modulating microRNA expression. J Ethnopharmacol. 2017;207:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Han R, Yang H, Lu L, Lin L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci Rep. 2021;11:8626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Wen Q, Xu C, Zhou J, Liu NM, Cui YH, Quan MF, Cao JG, Ren KQ. 8-bromo-7-methoxychrysin suppress stemness of SMMC-7721 cells induced by co-culture of liver cancer stem-like cells with hepatic stellate cells. BMC Cancer. 2019;19:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, Galle PR, Andersen JB, Factor VM, Thorgeirsson SS. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 93. | Zhang PP, Wang PQ, Qiao CP, Zhang Q, Zhang JP, Chen F, Zhang X, Xie WF, Yuan ZL, Li ZS, Chen YX. Differentiation therapy of hepatocellular carcinoma by inhibiting the activity of AKT/GSK-3β/β-catenin axis and TGF-β induced EMT with sophocarpine. Cancer Lett. 2016;376:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Liu Y, Qi Y, Bai ZH, Ni CX, Ren QH, Xu WH, Xu J, Hu HG, Qiu L, Li JZ, He ZG, Zhang JP. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol Sin. 2017;38:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Chen JH, Kim SH, Fan PW, Liu CY, Hsieh CH, Fang K. The aqueous extract of Chinese medicinal herb Brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Des Devel Ther. 2016;10:2003-2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Li W, Zhang Q, Chen K, Sima Z, Liu J, Yu Q. 2-Ethoxystypandrone, a novel small-molecule STAT3 signaling inhibitor from Polygonum cuspidatum, inhibits cell growth and induces apoptosis of HCC cells and HCC Cancer stem cells. BMC Complement Altern Med. 2019;19:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Kumar Mongre R, Sharma N, Singh Sodhi S, Ghosh M, Kumar Singh A, Kim N, Park YH, Shin YG, Kim SJ, Jiao Jiao Z, Huynh DL, Jeong DK. Novel phyto-derivative BRM270 inhibits hepatocellular carcinoma cells proliferation by inducing G2/M phase cell cycle arrest and apoptosis in xenograft mice model. Biomed Pharmacother. 2017;87:741-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Jia QA, Ren ZG, Bu Y, Wang ZM, Zhang QB, Liang L, Jiang XM, Tang ZY. Herbal Compound "Songyou Yin" Renders Hepatocellular Carcinoma Sensitive to Oxaliplatin through Inhibition of Stemness. Evid Based Complement Alternat Med. 2012;2012:908601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Wu C, Kan H, Hu M, Liu X, Boye A, Jiang Y, Wu J, Wang J, Yang X, Yang Y. Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocarcinogenesis via modulating TGF-β/TβR and Imp7/8. Exp Ther Med. 2018;16:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Rui-Chuan C, Jin-Hua S, Gao-Liang O, Ke-Xia C, Jin-Quan L, Xiao-Guang X. Induction of differentiation in human hepatocarcinoma cells by isoverbascoside. Planta Med. 2002;68:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Jiang Y, Chen P, Hu K, Dai G, Li J, Zheng D, Yuan H, He L, Xie P, Tu M, Peng S, Qu C, Lin W, Chung RT, Hong J. Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. J Cell Mol Med. 2021;25:1568-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Chen J, Yuan D, Hao Q, Zhu D, Chen Z. LncRNA PCGEM1 mediates oxaliplatin resistance in hepatocellular carcinoma via miR-129-5p/ETV1 axis in vitro. Adv Clin Exp Med. 2021;30:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Ma L, Xu A, Kang L, Cong R, Fan Z, Zhu X, Huo N, Liu W, Xue C, Ji Q, Li W, Chu Z, Kang X, Wang Y, Sun Z, Han Y, Liu H, Gao X, Han J, You H, Zhao C, Xu X. LSD1-Demethylated LINC01134 Confers Oxaliplatin Resistance Through SP1-Induced p62 Transcription in HCC. Hepatology. 2021;74:3213-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 104. | Tao Y, Shan L, Xu X, Jiang H, Chen R, Qian Z, Yang Z, Liang B, Zheng H, Cai F, Yu Y, Ma L. Huaier Augmented the Chemotherapeutic Sensitivity of Oxaliplatin via Downregulation of YAP in Hepatocellular Carcinoma. J Cancer. 2018;9:3962-3970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Hong H, Jin Z, Qian T, Xu X, Zhu X, Fei Q, Yang J, Sui C, Xu M. Falcarindiol Enhances Cisplatin Chemosensitivity of Hepatocellular Carcinoma via Down-Regulating the STAT3-Modulated PTTG1 Pathway. Front Pharmacol. 2021;12:656697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Yang Y, He J, Chen J, Lin L, Liu Y, Zhou C, Su Y, Wei H. Dihydroartemisinin Sensitizes Mutant p53 (R248Q)-Expressing Hepatocellular Carcinoma Cells to Doxorubicin by Inhibiting P-gp Expression. Biomed Res Int. 2019;2019:8207056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 107. | Wang CK, Lin YF, Tai CJ, Wang CW, Chang YJ, Choong CY, Lin CS, Chang CC. Integrated Treatment of Aqueous Extract of Solanum nigrum-Potentiated Cisplatin- and Doxorubicin-Induced Cytotoxicity in Human Hepatocellular Carcinoma Cells. Evid Based Complement Alternat Med. 2015;2015:675270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Gu W, Liu L, Fang FF, Huang F, Cheng BB, Li B. Reversal effect of bufalin on multidrug resistance in human hepatocellular carcinoma BEL-7402/5-FU cells. Oncol Rep. 2014;31:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Li F, Wang J, Wu N, Zhang H, Li Z, Wei N. H1, a derivative of tetrandrine, enhances the efficacy of 5-FU in Bel7402/5-FU cells via suppressing STAT3/MCL-1 and inducing PUMA. Biochem Biophys Res Commun. 2019;520:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Jing W, Shuo L, Yingru X, Min M, Runpeng Z, Jun X, Dong H. Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;519:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 111. | Niu B, Wei S, Sun J, Zhao H, Wang B, Chen G. Deciphering the molecular mechanism of tetrandrine in inhibiting hepatocellular carcinoma and increasing sorafenib sensitivity by combining network pharmacology and experimental evaluation. Pharm Biol. 2022;60:75-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Zhai B, Hu F, Yan H, Zhao D, Jin X, Fang T, Pan S, Sun X, Xu L. Bufalin Reverses Resistance to Sorafenib by Inhibiting Akt Activation in Hepatocellular Carcinoma: The Role of Endoplasmic Reticulum Stress. PLoS One. 2015;10:e0138485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 113. | Tang Q, Li X, Chen Y, Long S, Yu Y, Sheng H, Wang S, Han L, Wu W. Solamargine inhibits the growth of hepatocellular carcinoma and enhances the anticancer effect of sorafenib by regulating HOTTIP-TUG1/miR-4726-5p/MUC1 pathway. Mol Carcinog. 2022;61:417-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 114. | Zou H, Cao X, Xiao Q, Sheng X, Ren K, Quan M, Song Z, Li D, Zheng Y, Zeng W, Cao J, Peng Y. Synergistic inhibition of characteristics of liver cancer stem-like cells with a combination of sorafenib and 8-bromo-7-methoxychrysin in SMMC-7721 cell line. Oncol Rep. 2016;36:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano. 2020;14:4816-4828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 116. | Yang M, Wan Y, Jiang X, Qi X, Wang L, Liu Z, Song X, Pan L, Sun W, Zhao W, Huang J, Lian Z. Electro-Acupuncture Promotes Accumulation of Paclitaxel by Altering Tumor Microvasculature and Microenvironment in Breast Cancer of Mice. Front Oncol. 2019;9:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |