Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1833
Peer-review started: April 8, 2022
First decision: May 11, 2022
Revised: June 11, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 15, 2022
Processing time: 154 Days and 8.4 Hours
Percutaneous bilateral biliary stenting is an established method for the manage
To evaluate the efficacy and safety of a novel uncovered biliary stent, specifically designed for hilar reconstruction.
This, single-center, retrospective study included 18 patients (mean age 71 ± 11 years; 61.1% male) undergoing percutaneous transhepatic Moving cell stent (MCS) placement for hilar reconstruction using the stent-in-stent technique for malignant biliary strictures, between November 2020 and July 2021. The Patients were diagnosed with cholangiocarcinoma (12/18; 66.6%), gallbladder cancer (5/18; 27.7%), and colorectal liver metastasis (1/18; 5.5%). Primary endpoints were technical (appropriate stent placement) and clinical (relief from jaundice) success. Secondary endpoints included stent patency, overall survival, compli
The technical and clinical success rates were 100% (18/18 cases). According to Kaplan-Meier analysis, the estimated overall patient survival was 80.5% and 60.4% at 6 and 12 mo respectively, while stent patency was 90.9% and 68.2% at 6 mo and 12 mo respectively. The mean stent patency was 172.53 ± 56.20 d and median stent patency was 165 d (range 83-315). Laboratory tests for cholestasis significantly improved after procedure: mean total bilirubin decreased from 15.2 ± 6.0 mg/dL to 1.3 ± 0.4 mg/dL (P < 0.001); mean γGT decreased from 1389 ± 832 U/L to 114.6 ± 53.5 U/L (P < 0.001). One periprocedural complication was reported. Stent-related complications were observed in 5 patients (27.7%), including 1 occlusion (5.5%) and 1 stent migration (5.5 %).
Percutaneous hilar bifurcation biliary stenting with the MCS resulted in excellent clinical and technical success rates, with acceptable complication rates. Further studies are needed to confirm these initial positive results.
Core Tip: This single-center, retrospective study investigated eighteen patients with unresectable malignant hilar biliary obstructions treated with a novel uncovered biliary metallic stent [Moving Cell Stent (MCS); BCM Co., Ltd., Gyeonggi-do, South Korea], specifically designed for hilar reconstruction, using stent-in-stent technique via percutaneous approach. Primary endpoints were clinical and technical success. The study results indicate that percutaneous MCS placement using stent-in-stent technique is feasible and safe. Comparison with other stents demonstrated superiority in both stent patency and technical success.
- Citation: Cortese F, Acquafredda F, Mardighian A, Zurlo MT, Ferraro V, Memeo R, Spiliopoulos S, Inchingolo R. Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction. World J Gastrointest Oncol 2022; 14(9): 1833-1843
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1833.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1833
Malignant hilar biliary obstructions (MHBO) are very difficult to treat because most patients are diagnosed at an unresectable stage[1]. Hilar Cholangiocarcinoma (HiCC) is the most frequent cause of MHBO. Other malignant strictures may be due to pancreatic, gallbladder and liver tumors, to metastatic hilar lesions or to lymphadenopathies[2]. The primary principle behind the criteria for unresectability is the requirement for biliary and vascular reconstruction options with adequate future remnant hepatic parenchyma, as well as the presence of distant metastases or comorbidity of the patient[3,4]. Since only 10% to 20% of patients are suitable for resection, most of them receive palliative treatment[5]. The main aim of palliation is to re-create a connection between the biliary system and bowel to allow phy
Due to the complexity of MHBO management, an organized multidisciplinary approach is paramount to deliver best quality care[7]. The main palliative treatments are biliary drainage and biliary stent implantation which can be performed with percutaneous or endoscopic approach, but there is no clear evidences of the superiority of one over the other. According to currently available data and the ESMO guidelines, percutaneous is the recommended approach in cases in which the endoscopic methods are not possible, commonly noted in advanced hilar Bismuth IV obstructions[8-10]. Moreover, percutaneous approach enables precise lobar selection for drainage[6].
With regard to bilateral vs unilateral drainage/stenting in cases of advanced HiCC, the goal is to drain at least 50% of the liver volume, which usually requires more than one stent when bile ducts are dissociated[8]. A self-expandable metallic stent (SEMS) rather than a plastic one is preferred in patients with unresectable cancer and a life expectancy longer than 3 mo[9].
Bilateral stent implantation can be achieved using side-by-side (SBS) or stent-in-stent (SIS) technique, but there is no large consensus concerning which procedure is better[11,12]. Some studies have shown that SIS technique may offer a lower adverse events rate[13] and longer stent patency[12]. On the other hand, some authors have found no significant differences in clinical outcomes between SIS and SBS techniques[14,15]. However, SIS procedure is technically more difficult and complex due to the necessity of introducing the second SEMS through the mesh of the previously placed SEMS[16-18]. To overcome this issue, a novel uncovered SEMS, the HILZO Moving Cell Stent (MCS) (BCM Co., Gyeonggi-do, South Korea) was created.
The purpose of the present study was to evaluate the efficacy and safety of a novel uncovered biliary stent, specifically designed for hilar reconstruction, in patients with MBHO.
This, single-center, retrospective study was conducted at “F.Miulli” Hospital in the Inteventional Radiology Unit. A total of 18 patients (mean age 71 ± 11 years; 61.1% male) with MHBO undergoing percutaneous MCS (BCM Co., Ltd., Gyeonggi-do, South Korea) placement using SIS technique were enrolled within a 12-mo period (November 2020 and November 2021). The study was approved by the ethics committee of M Hospital and the patients provided written informed consent prior to enrolment. The study protocol conformed to the ethical guidelines of the 2013 Declaration of Helsinki (most recent version).
The diagnosis of MHBO was based on standard clinical and radiological criteria [following computed tomography (CT) and/or magnetic resonance imaging (MRI)], and was confirmed by percutaneous needle biopsy or percutaneous endobiliary forceps biopsy[19]. All patients were evaluated by a multidisciplinary team including oncologists, surgeons, gastroenterologists, radiotherapists, and interventional radiologists. Inclusion criteria were: MHBO caused by a biopsy-confirmed hilar malignancy, not suitable for surgery (due to unresectability, metastatic disease or severe comorbidities) and an estimated survival of over 3 mo. Exclusion criteria were patients with uncorrectable coagulopathy (INR >1.8; Platelets < 50.000) and presence of an atrophic lobe.
In the patient group, the causes of hilar obstruction included cholangiocarcinoma (12/18; 66, 6%), gallbladder cancer (5/18; 27, 7%), and colorectal liver metastasis (1/18; 5, 5%). Patients’ baseline demographical data are outlined in Table 1.
| Characteristics | Value |
| Total number of patients, n | 18 |
| Median age, yr | 71 |
| Range age, yr | 37-84 |
| Male sex, n (%) | 11 (61.1) |
| Etiology, n (%) | |
| Cholangiocarcinoma | 12 (66.6) |
| Gallbladder carcinoma | 5 (27.7) |
| Colorectal liver metastases | 1 (5.5) |
| Chemotherapy | 17 (94.4) |
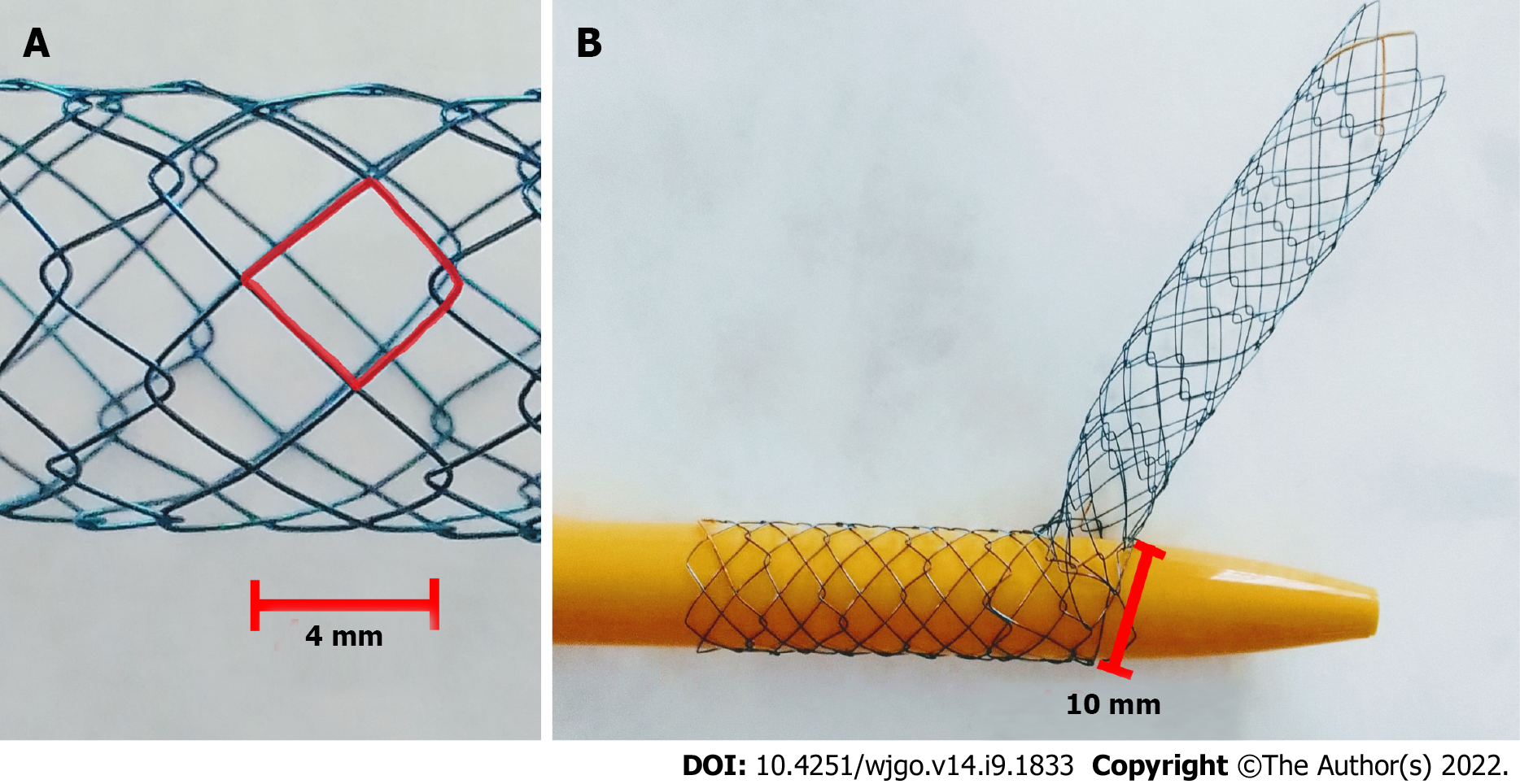
The Hilzo Biliary MCS (BCM Co., Ltd., Gyeonggi-do, South Korea) (Figure 1) is a novel uncovered metallic stent with a small cell size (4 mm) and a high radial force, dedicated for biliary SIS technique. The small cell size is expected to reduce ingrowth, and the high radial force results in higher expansion potential. The special design of this novel stent allows each cell to expand from 4 mm to 10 mm to enable a passage of the second stent through the stent struts. The MCS has radiopaque markers at each end, and two in the midsection and requires an 8Fr percutaneous access[20].
This was a two-stage procedure. The first stage was percutaneous transhepatic biliary drainage (PTBD) and the second stage was MCS placement. All procedures were performed in the angiography suite, according to the CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography, Biliary Drainage and Stenting[21] using local anesthesia (2% Lidocaine), and conscious sedation (Fentanyl and Midazolam). A single-dose of iv antibiotic prophylaxis (Cefprozil 1g) was administrated before each procedure.
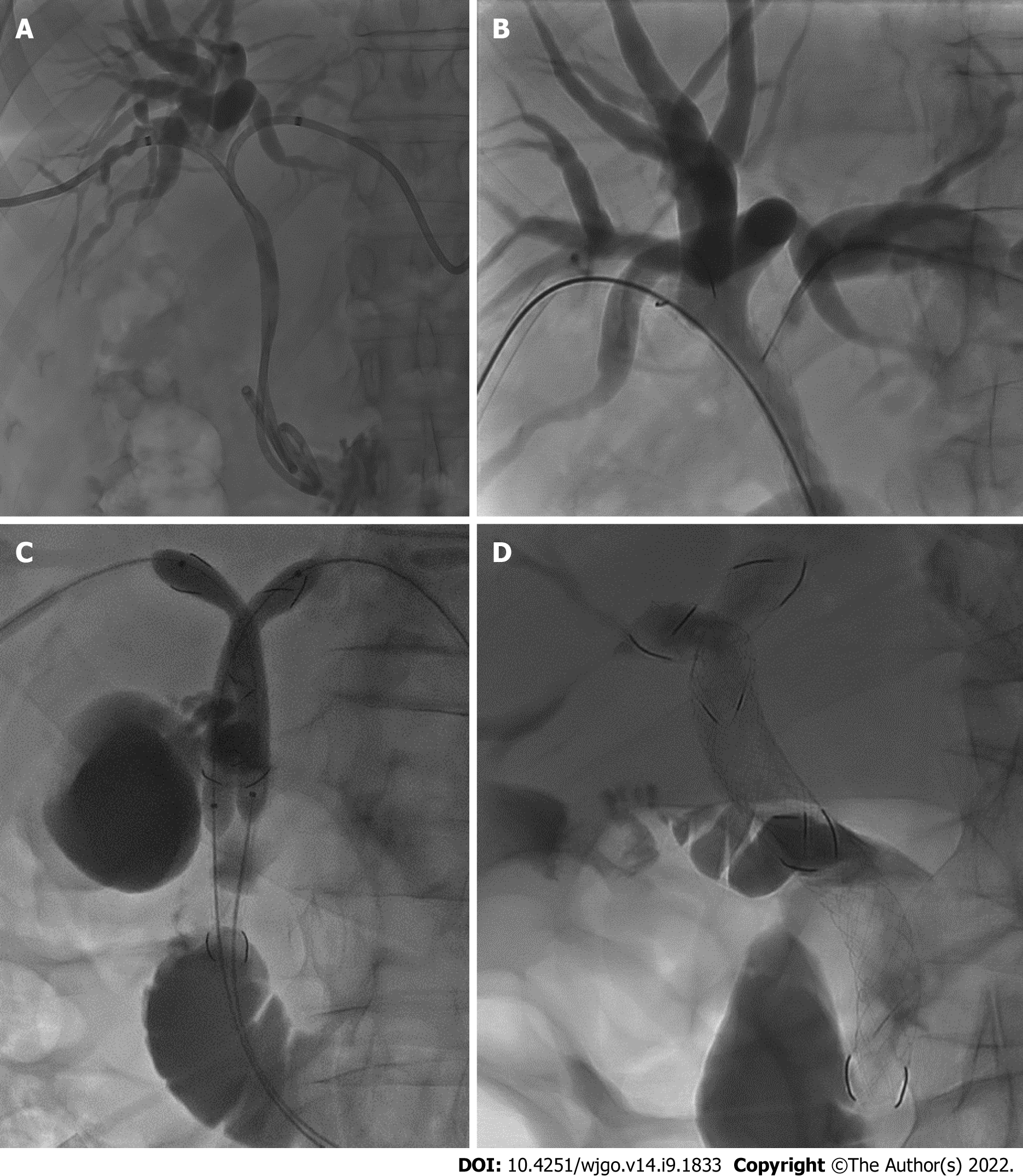
Under ultrasound guidance (Philips CX50) combined with fluoroscopy (Philips Allura FD20 Clarity), both right and left intrahepatic bile ducts were punctured with 21-gauge Chiba needles (Cook, Bloomington, IN, United States) and two 8.5-Fr drainage catheters (Cook Medical, Bloomington, IN, United States) were inserted (Figure 2A).
In 11 cases in which histological diagnosis was not already available, a percutaneous transluminal biopsy[19] was performed using a dedicated, transluminal biliary access and biopsy forceps set (Cook Medical, Bloomington, IN, United States) during the same PTBD session.
After approximately 7 to 21 d, and following improvement of obstructive jaundice symptoms, biliary stents placement was performed. Under fluoroscopic guidance, two hydrophilic guidewires (0.035 in.; Terumo Corporation, Tokyo, Japan) were introduced via the previously placed drainage catheters that were removed and two bilateral 8-Fr sheaths were placed within the biliary ducts over the hydrophilic guidewires.
Following cholangiography for the evaluation of the position and length of the biliary obstruction, the hydrophilic guidewire on one side was changed with an Amplatz Super Stiff™ 0.035 in. guidewire (Boston Scientific Corporation, Boston, MA, United States) using a 5-fr catheter KMP Beacon Tip (Cook Medical, Bloomington, IN, United States), and the corresponding type of MCS (10 or 8 mm × 10 or 8 or 6 cm) was implanted over the guidewire and dilated with a standard balloon catheter (Armada 35 PTA Catheter, Abbott Vascular, Santa Clara, CA, United States).
Analogously, on the other side, the hydrophilic guidewire was inserted through a mesh of the first MCS and exchanged (Figure 2B) with the stiff guidewire. Subsequently the second MCS (10 or 8 mm × 10 or 8 or 6 cm) was implanted and dilated. At this time, from the upper part of the first stent, the mesh of the controlateral MCS was engaged with the wire and, over the two stiff guidewires, two balloon catheters were placed inside the MCSs and a kissing balloon dilatation was performed (Figure 2C).
A final contrast check was performed to depict appropriate stent placement according to the SIS technique, thus the apex of the longest stent should be positioned within the duodenum, while the apex of the shorter stent should end within the first MCS (Figure 2D).
Pre-scheduled follow up protocol was set at 3 and 6 mo and every 6 mo thereafter and included clinical evaluation, laboratory tests and restaging CT (Figure 3).
The study’s primary endpoints were technical and clinical success. Technical success was defined as appropriate placement of a bilateral MCS using the SIS technique (as described above). Clinical success was defined as a reduction of bilirubin values to normal (< 1.3 mg/dL) or to < 50% of the pre-PTDB value within 14 d. Secondary endpoints included stent patency, overall survival, peri-procedural adverse events, procedural duration and stent-related complications. Stent patency was defined as the time between stent placement and stent dysfunction, determined by the relapse of cholestasis and/or cholangitis according to clinical, laboratory and imaging findings. Stent patency and patient survival were estimated by the Kaplan-Meier method. Adverse events were graded according to the CIRSE Classification System for Complications[22]. Procedural duration was considered as the amount of elapsed time between local anaesthesia and removal of the sheaths.
mean ± SD were used to describe continuous variables, while counts and percentages were used for categorical variables. The statistical analysis was conducted using the SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL, United States) and a P value of < 0.05 was considered significant.
The clinical outcomes of bilateral MCS placement using the SIS technique are summarized in Table 2. Technical success and clinical success were 100% (18 out of 18 patients). The median procedural duration was 81.5 min ± 32.2 min. A single (5.5%) periprocedural adverse event occurred: Hemobilia due to porto-biliary fistula, treated during the same procedure with absorbable gelatin sponge (Spongostan) injection within the affected portal branch. This complication occurred during bile duct PTBD, and not during stent placement, and was judged as grade 1 according to the CIRSE Classification System for Complications[22].
| Endpoint | Value |
| Technical success, n (%) | 18 (100) |
| Clinical success, n (%) | 18 (100) |
| Periprocedural complications, n (%) | 1 (5.5) |
| Stent-related complications, n (%) | 5 (27.7) |
| Stent occlusion, n (%) | 1 (5.5) |
| Stent migration, n (%) | 1 (5.5) |
| Mean procedural duration min | 81.5 ± 32.2 |
| Median stent patency days (range) | 169 (93-315) |
| Overall mortality, n (%) | 4 (22.2) |
The mean follow-up time was 169 d (range 83-315 d). Stent-related complications occurred in five (27.7%) patients (Table 3). Three (16.5%) patients who developed cholangitis without stent obstruction were treated with antibiotic therapy. Two patients (11%) presented with jaundice. For the first patient, the symptoms appeared 85 d after stent placement and the jaundice was caused by stent migration (5.5%) into common bile duct, treated with an additional MCS implantation. For the second patient, the jaundice appeared 151 d after stent placement and was caused by neoplastic ingrowth (5.5%). Due to the progression disease and the poor performance status of patients, it was decided to perform PTBD instead of an additional MCS placement. During the follow-up period, 4 patients (22.2%) died due to liver failure and/or progression disease.
| Age/sex | Etiology | Clinical manifestations | US findings | PTC findings | Treatment |
| 75/F | GC | Jaundice | Left intrahepatic biliary dilatation | Stent migration | Additional MCS using SIS technique |
| 77/M | CC | Jaundice | Bilateral intrahepatic biliary dilatation | Stent occlusion | PTBD |
| 68/F | CC | Cholangitis | Aerobilia and no biliary dilatation | Not performed | Antibiotic therapy |
| 81/M | CC | Cholangitis | Aerobilia and no biliary dilatation | Not performed | Antibiotic therapy |
| 75/F | CC | Cholangitis | Aerobilia and no biliary dilatation | Not performed | Antibiotic therapy |
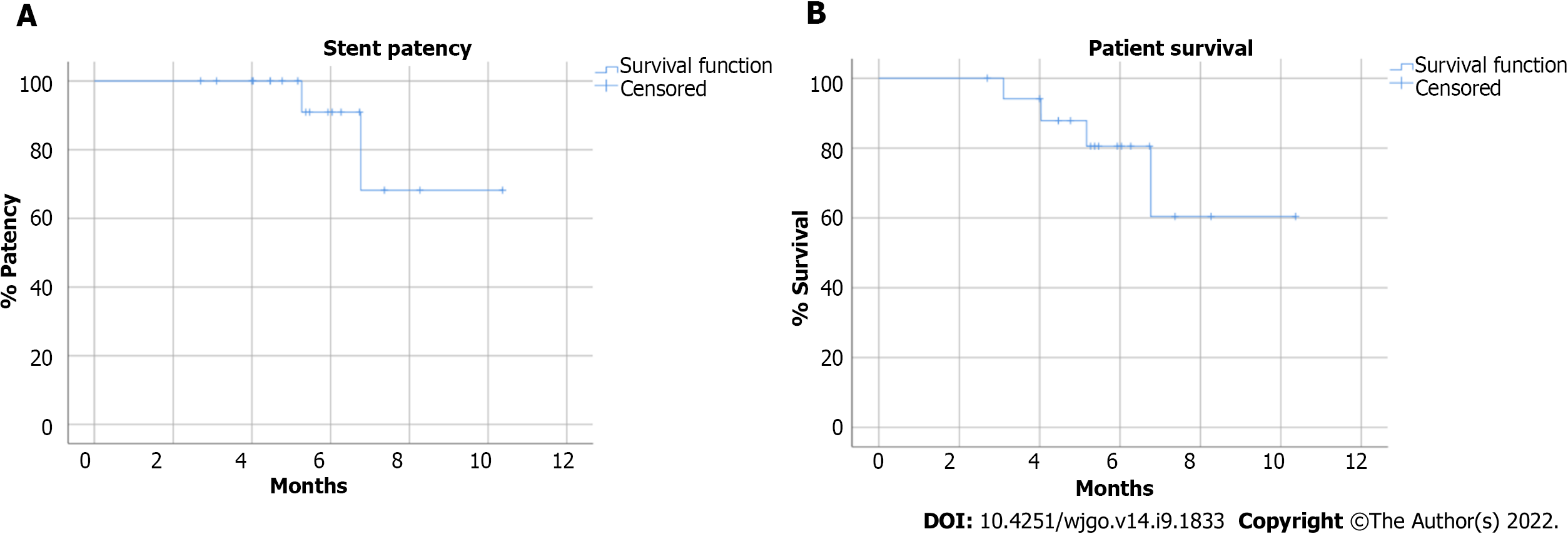
According to the Kaplan-Meier analysis, the estimated overall patient survival rate was 80.5% and 60.4% at 6 mo and 12 mo respectively, while stent patency was 90.9% and 68.2% at 6 and 12 mo respectively (Figure 4). The mean stent patency was 172.5 ± 56.2 d and median stent patency was 165 d (range 83-315). Laboratory tests for cholestasis significantly improved after procedure: mean total bilirubin decreased from 15.2 ± 6.0 mg/dL to 1.3 ± 0.4 mg/dL (P < 0.001); mean γGT decreased from 1389 ± 832 U/L to 114.6 ± 53.5 U/L (P < 0.001) (Table 4).
| PRE-PTBD | PRE-stent | POST-stent | P value | |
| Total bilirubin (mg/dL) | 15.2 ± 6.0 | 4.04 ± 1.50 | 1.31 ± 0.40 | < di 0.001 |
| Direct bilirubin (mg/dL) | 13.5 ± 5.5 | 3.32 ± 1.30 | 0.86 ± 0.30 | < di 0.001 |
| ɣGT (U/L) | 1389.2 ± 832.2 | 393.6 ± 321.7 | 114.6 ± 53.5 | < di 0.001 |
| Alkaline phosphatase (mU/mL) | 321.7 ± 250.0 | 200.3 ± 179.4 | 115.7 ± 117.8 | 0.037 |
| AST (UI/L) | 243.9 ± 136.4 | 93.5 ± 47.6 | 50.6 ± 21.8 | < di 0.001 |
| ALT (UI/L) | 319.3 ± 242.7 | 104.3 ± 53.3 | 71.7 ± 40.7 | < di 0.001 |
| WBC (10³/µL) | 10.2 ± 3.1 | 9.82 ± 4.00 | 7.16 ± 1.70 | < di 0.001 |
| PCR (mg/dL) | 3.1 ± 1.5 | 3.9 ± 6.5 | 1.2 ± 1.2 | < di 0.002 |
MHBO are often unresectable at presentation, thus palliative biliary decompression play a crucial role in improving the patients’ quality of life[6].
Although outcomes of endoscopic US-guided biliary drainage techniques for hilar obstructions are very satisfactory[23-25], bilobar drainage with Y-configured SEMS using percutaneous approach is a well-established method for the palliative management of unresectable advanced MHBO in patients with estimated lifetime of more than 3 mo[9,10].
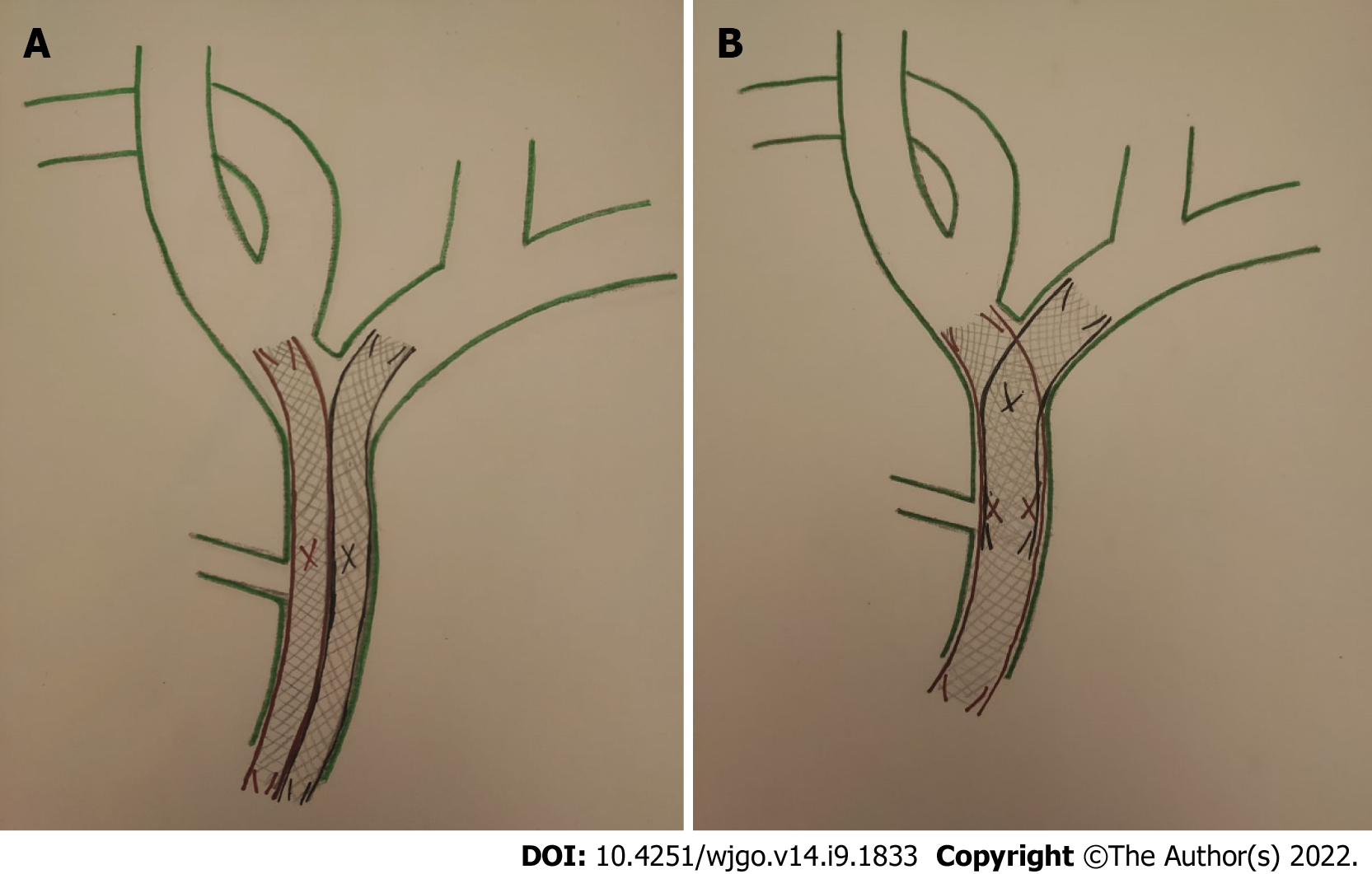
Bilateral SEMS placement can be achieved with SBS or SIS techniques (Figure 5). The SBS technique, considered technically easier[12], consists of the implantation of two parallel and close SEMS at and below the hepatic confluence, draining both hepatic lobes. Theoretically, the SBS technique has its inherent problems. The two SEMS cannot be fully expanded with major probability of partial collapse. Furthermore, the strong radial force caused by the parallel stent placement might be too strong to cause portal vein compression, bile duct rupture, or tumor ingrowth/tissue hyperplasia through the stent mesh[26,27].
On the other hand, in the SIS technique, after placing the first SEMS across the hilar stricture, a second SEMS is inserted into the contralateral hepatic duct through the mesh of first SEMS. Thereby, the single radial forces of both stents are added together opposing the biliary stricture, with a lower probability of stent migration or collapse; so the entire length of stricture is expanded by a single stent caliber[26]. Moreover, the SIS technique provides a more physiological Y-conformation stent to bile outflow, but it is still technically challenging[27].
The Hilzo Biliary MCS was designed specially for the SIS technique. According to the literature, there are only two previously published studies both investigating endoscopic bilateral Y-stenting using the MCS[17,18], therefore this is the first study investigating percutaneous placement of MCS.
The herein presented results are in accordance with those of Ogura et al[17] and Kawai et al[18] Specifically, similar technical success (100.0% vs 95.6%[17] vs 100.0%[18]), clinical success (100.0% vs 95.6 %[17] vs 89.9%[18]), periprocedural complications (5.5% vs 4.4%[17] vs 7.4%[18]) and 6-months stent patency rate (90.9% vs approx. 85.0% vs approx. 75.0%) were noted. However, dissimilar stent occlusion rates were noted [1/18 (5.5%) vs 4/23 (17.0%)[17] vs 12/27 (44.4%)[18]] The authors speculate that this discrepancy could be attributed to the only substantial technical difference: routine balloon post-dilatation was performed in all procedures in this study, whereas post-dilation was not performed in the two previously published studies. This could have contributed in the increased procedural duration noted in this study (81.5 ± 32.0 min vs 36.6 min, range 18-62[16] vs 23.7 ± 8.1 min[17]), but interestingly did not result in an increase of periprocedeural complications.
Generally, SEMS can be classified as small closed-cell, large open-cell types and mixed form of closed-cell type[16]. Closed-cell type SEMS (Wallstent, Boston Scientific Corp., Marlborough, MA, United States; Bonastent, Standard SciTech, Inc., Seoul, South Korea; Hanarostent, MI Tech Co., Seoul, Korea) have small cells to prevent ingrowth. However, characteristic of the closed-cell type hinders the deployment of a second stent or revision after stent malfunction, particularly in high-grade strictures[16], therefore they are not suitable for the SIS technique.
Open-cell type SEMS (JOSTENT SelfX, Abbott Vascular Devices, Redwood City, CA, United States; Zilver stent, Wilson-Cook Medical, Inc., Bloomington, IN, United States; Niti-S Y-type or Niti-S large cell D-type, Taewoong Medical Inc., Seoul, South Korea) facilitate the second stent implantation. Theoretically open-cell-type SEMS could be more vulnerable to tumor ingrowth and also demonstrate less radial force[16]. Although there are no published studies directly comparing outcomes of the SIS technique using these different stent types, superior stent patency rates were achieved by the MCS in this study compared to that of open-cell stents (MCS: 90.9%-68.2% vs large cell Niti-D biliary stent: 60%-20%[28] vs Sentinol stent: 65%-0%[29], at 6 mo and 12 mo; respectively).
Finally, the BONASTENT M-Hilar (Standard Sci Tech Inc., Seoul, South Korea) is a dedicate hilar reconstruction mixed form of closed-cell type stent, with a cross-wired structure only at the 25-mm-long central portion to facilitate placement of the contralateral stent[16,29]. However, the reported technical success rate was low (78.6 %), as the insertion of the second stent via the 25-mm central portion, is technical demanding unlike the MCS in which all the cells are dilatable and are therefore potential insertion sites for the second stent[30].
This study has several limitations. First, the number of patients is relatively low, so the statistical validity of the results is limited. Moreover, there was no control group, so comparative data are not available, while the single-center design limits the external validity of the results.
In conclusion, palliative treatment of patients with unresectable MHBO using percutaneous MCS placement with the SIS technique is safe and feasible and resulted in excellent clinical and technical success rates. Periprocedural and stent-related complications were acceptable. Prospective, multicentre, randomized trials are needed to verify these initial promising results.
The treatment of malignant hilar biliary obstruction is very difficult because patients are often not suitable for surgery, therefore palliative care plays a pivotal role.
According to the literature, there are only two previously published studies both investigating endoscopic bilateral Y-stenting using the, therefore this is the first study investigating percutaneous placement of Moving Cell Stent (MCS).
To evaluate the efficacy and safety of a novel uncovered biliary stent, specifically designed for hilar reconstruction in patients with unresectable malignant hilar biliary obstructions.
A retrospective, single-centre study was performed, investigating 18 patients with unresectable malignant hilar biliary obstructions treated with a novel uncovered biliary metallic stent (MCS; BCM Co., Ltd., Gyeonggi-do, South Korea), specifically designed for hilar reconstruction, using stent-in-stent technique via percutaneous approach. Primary endpoints were clinical and technical success.
The technical and clinical success rates were 100%. One periprocedural complication was reported. Stent-related complications were observed in 5 patients. According to Kaplan-Meier analysis, the estimated overall patient survival was 80.5% and 60.4% at 6 and 12 mo respectively, while stent patency was 90.9% and 68.2% at 6 mo and 12 mo respectively.
For patients with unresectable malignant hilar biliary obstruction using percutaneous placement with the stent-in-stent technique was a feasible and safe and resulted in excellent technical and clinical success rates. Periprocedural and stent-related complications were acceptable.
Since MCS is a recently introduced stent, prospective, multicentre, randomized trials are needed to verify these initial promising results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Havre RF, Norway; Piltcher-da-Silva R, Brazil; Sugimoto M, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Lee TH. Proper management of inoperable malignant hilar biliary obstruction: Endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, or percutaneous approach? Int J Gastrointest Interv. 2021;10:120-127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 4. | Rassam F, Roos E, van Lienden KP, van Hooft JE, Klümpen HJ, van Tienhoven G, Bennink RJ, Engelbrecht MR, Schoorlemmer A, Beuers UHW, Verheij J, Besselink MG, Busch OR, van Gulik TM. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg. 2018;403:289-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Gwon D II. Interventional radiologic approach to hilar malignant biliary obstruction. Int J Gastrointest Interv. 2016;5:47-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Madhusudhan KS, Gamanagatti S, Gupta AK. Imaging and interventions in hilar cholangiocarcinoma: A review. World J Radiol. 2015;7:28-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kim DT, Rahman U, Tenney RW, Roa OAC, Rastogi P, Cynamon J, Golowa Y. Multidisciplinary Approach to Malignant Biliary Obstruction. Digest Dis Intervent. 2020;4:323-333. [DOI] [Full Text] |
| 8. | Mocan T, Horhat A, Mois E, Graur F, Tefas C, Craciun R, Nenu I, Spârchez M, Sparchez Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World J Gastrointest Oncol. 2021;13:2050-2063. |
| 9. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 10. | Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, Lee JK, Ryu JK, Kim YT, Yoon YB. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Cao Q, Sun L, Li ZQ, Xia FF, Zhang JH, Song T. Bilateral stenting for hilar biliary obstruction: a meta-analysis of side-by-side versus stent-in-stent. Minim Invasive Ther Allied Technol. 2022;31:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lee TH, Moon JH, Choi JH, Lee SH, Lee YN, Paik WH, Jang DK, Cho BW, Yang JK, Hwangbo Y, Park SH. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest Endosc. 2019;90:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Naitoh I, Hayashi K, Nakazawa T, Okumura F, Miyabe K, Shimizu S, Yoshida M, Yamashita H, Ohara H, Joh T. Side-by-side versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Dig Dis Sci. 2012;57:3279-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Kim KM, Lee KH, Chung YH, Shin JU, Lee JK, Lee KT, Shim SG. A comparison of bilateral stenting methods for malignant hilar biliary obstruction. Hepatogastroenterology. 2012;59:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 15. | Hong W, Chen S, Zhu Q, Chen H, Pan J, Huang Q. Bilateral stenting methods for hilar biliary obstructions. Clinics (Sao Paulo). 2014;69:647-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Lee TH, Moon JH, Park SH. Biliary stenting for hilar malignant biliary obstruction. Dig Endosc. 2020;32:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Ogura T, Takenaka M, Shiomi H, Nishioka N, Ueno S, Miyano A, Kamiyama R, Higuchi K. Single-session multiple stent deployment using moving cell stent without dilating initial stent mesh to treat malignant hilar biliary obstruction (with videos). J Hepatobiliary Pancreat Sci. 2020;27:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kawai J, Ogura T, Takenaka M, Shiomi H, Ueshima K, Ueno S, Okuda A, Matsuno J, Minaga K, Omoto S, Nakai A, Ikegawa T, Hakoda A, Higuchi K. Prospective multicenter evaluation of moving cell metallic stents in endoscopic multiple stent deployment for hepatic hilar obstruction. J Hepatobiliary Pancreat Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Augustin AM, Steingrüber M, Fluck F, Goetze O, Bley TA, Kickuth R. Percutaneous endobiliary forceps biopsy of biliary strictures for histopathologic examination. Diagn Interv Radiol. 2020;26:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Takenaka M, Yamao K, Minaga K, Nakai A, Omoto S, Kamata K, Kudo M. Novel metallic stent designed for endoscopic bilateral stent-in-stent placement in patients with hilar malignant biliary obstruction. Endoscopy. 2019;51:E30-E31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Das M, van der Leij C, Katoh M, Benten D, Hendriks BMF, Hatzidakis A. CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography, Biliary Drainage and Stenting. Cardiovasc Intervent Radiol. 2021;44:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 558] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 23. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Bill JG, Darcy M, Fujii-Lau LL, Mullady DK, Gaddam S, Murad FM, Early DS, Edmundowicz SA, Kushnir VM. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc Int Open. 2016;4:E980-E985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kongkam P, Orprayoon T, Boonmee C, Sodarat P, Seabmuangsai O, Wachiramatharuch C, Auan-Klin Y, Pham KC, Tasneem AA, Kerr SJ, Romano R, Jangsirikul S, Ridtitid W, Angsuwatcharakon P, Ratanachu-Ek T, Rerknimitr R. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: a multicenter observational open-label study. Endoscopy. 2021;53:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Corvino F, Centore L, Soreca E, Corvino A, Farbo V, Bencivenga A. Percutaneous "Y" biliary stent placement in palliative treatment of type 4 malignant hilar stricture. J Gastrointest Oncol. 2016;7:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Moon JH, Rerknimitr R, Kogure H, Nakai Y, Isayama H. Topic controversies in the endoscopic management of malignant hilar strictures using metal stent: side-by-side versus stent-in-stent techniques. J Hepatobiliary Pancreat Sci. 2015;22:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Kim GH, Gwon DI, Ko GY, Kim JH, Kim JW, Chu HH, Yoon HK, Sung KB. Percutaneous stent-in-stent placement with large cell-type stents for malignant hilar biliary obstruction. Acta Radiol. 2021;62:1625-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Ahn SJ, Bae JI, Han TS, Won JH, Kim JD, Kwack KS, Lee JH, Kim YC. Percutaneous biliary drainage using open cell stents for malignant biliary hilar obstruction. Korean J Radiol. 2012;13:795-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Lee TH, Moon JH, Kim JH, Park DH, Lee SS, Choi HJ, Cho YD, Park SH, Kim SJ. Primary and revision efficacy of cross-wired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures. Endoscopy. 2013;45:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |