Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1689
Peer-review started: April 7, 2022
First decision: June 2, 2022
Revised: June 16, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 15, 2022
Processing time: 154 Days and 23.5 Hours
Helicobacter pylori (H. pylori) is a Gram-negative bacterium found in the upper digestive tract. Although H. pylori infection is an identified risk factor for gastric cancer, its role in esophageal squamous cell carcinoma (ESCC) remains a topic of much debate.
To evaluate the association between H. pylori infection and the risk of precancerous lesions of ESCC, and further explore the association between dietary factors and the risk of H. pylori infection.
Two hundred patients with esophageal precancerous lesions (EPL) aged 63.01 ± 6.08 years and 200 healthy controls aged 62.85 ± 6.03 years were included in this case-control study. Epidemiological data and qualitative food frequency data were investigated. Enzyme-linked immunosorbent assay measuring serum immunoglobulin G antibodies was used to determine H. pylori seropositivity. An unconditional logistic regression model was used to assess the association between H. pylori infection and EPL risk dichotomized by gender, age, and the use of tobacco and alcohol, as well as the association between dietary factors and the risk of H. pylori infection.
A total of 47 (23.5%) EPL cases and 58 (29.0%) healthy controls had positive H. pylori infection. An inverse relation between H. pylori infection and the risk of EPL was found in the group of drinkers after adjustment for covariates [odds ratio (OR) = 0.32, 95% confidence interval (95%CI): 0.11-0.95]. Additionally, peanut intake was significantly associated with a decreased risk of H. pylori infection (OR = 0.39, 95%CI: 0.20-0.74).
Our study suggested that H. pylori infection may decrease the risk of EPL for drinkers in a rural adult Chinese population, and the consumption of peanut may reduce the risk of H. pylori infection. These findings should be framed as preliminary evidence, and further studies are required to address whether the mechanisms are related to the localization of lesions and alcohol consumption.
Core Tip: The association between Helicobacter pylori (H. pylori) infection and esophageal squamous cell carcinoma (ESCC) remains a topic of much debate. This study aimed to evaluate the association between H. pylori infection and the risk of precancerous lesions of ESCC, and further explore the association between dietary intake and the risk of H. pylori infection. Our findings suggested an inverse association between H. pylori infection and the risk of esophageal precancerous lesions in the group of drinkers [odds ratio (OR) = 0.32, 95% confidence interval (95%CI): 0.11-0.95]. Additionally, peanut consumption was significantly associated with a reduced risk of H. pylori infection (OR = 0.39, 95%CI: 0.20-0.74).
- Citation: Pan D, Sun GJ, Su M, Wang X, Yan QY, Song G, Wang YY, Xu DF, Wang NN, Wang SK. Inverse relations between Helicobacter pylori infection and risk of esophageal precancerous lesions in drinkers and peanut consumption. World J Gastrointest Oncol 2022; 14(9): 1689-1698
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1689.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1689
Esophageal cancer and gastric cancer are upper gastrointestinal cancers that share many risk factors[1-3]. However, their associations with Helicobacter pylori (H. pylori) infection can be completely different. It has been determined that H. pylori infection is an identified risk factor for gastric cancer[4], whereas the role of H. pylori in the risk of esophageal cancer remains controversial. Previous meta-analyses summarized that H. pylori infection is likely to be related to a reduced risk of esophageal adenocarcinoma (EAC)[5-9]. One of the reliable assumptions related to this phenomenon is that H. pylori infection causes gastric atrophy and parietal cell loss, thus leading to alleviated reflux and consequently, a decreased incidence of reflux esophagitis and Barrett’s esophagus (precursor for EAC)[10-12]. However, the impact of H. pylori infection on esophageal squamous cell carcinoma (ESCC) is not well understood, and research is inconclusive as to what population may be significantly influenced[9,13-15]. Previous meta-analyses also reported that in the general population, no significant association was found between H. pylori infection and ESCC risk[6-8], whereas an inverse association was observed in the Middle East[9]. In the other populations, the inverse relationship was found to be highly associated with age, smoking status, and drinking status[15].
H. pylori is a Gram-negative bacterium found in the upper digestive tract. In spite of the fact that H. pylori infection may reduce the risk of EAC, it may also cause an adverse effect on human health. Apart from the elevated risk of gastric cancer, H. pylori infection is also etiologically related to peptic ulcers, atrophic and non-atrophic gastritis, and lymphoma associated with gastric mucosa, and is able to induce reduced bioavailability and malabsorption of nutrients including iron and vitamin B12[16-18]. This case-control study aimed to investigate the association between H. pylori infection and the risk of precancerous lesions of ESCC, which is an identified early stage of carcinogenesis, and further examine the association between dietary factors and the risk of H. pylori infection.
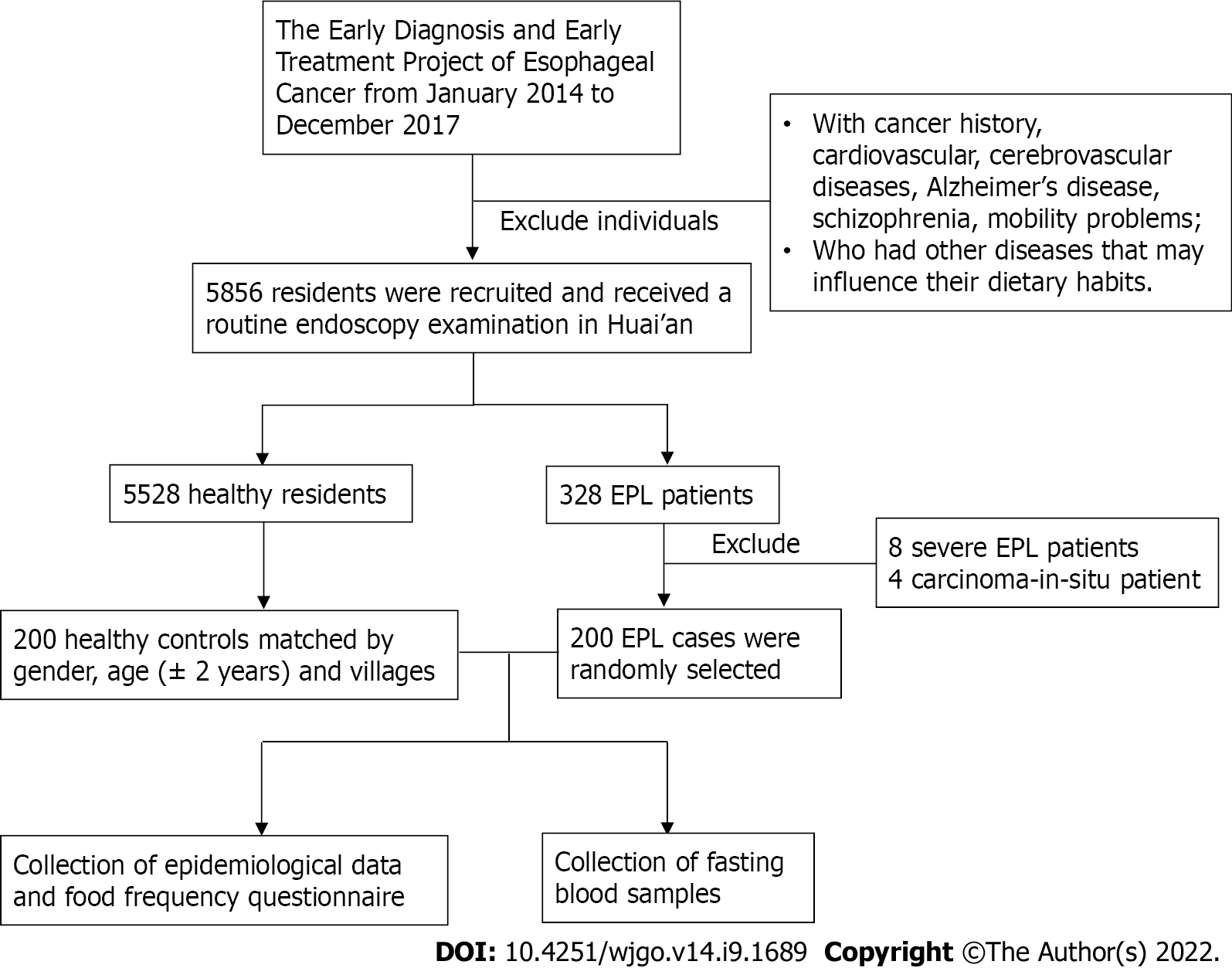
This study was carried out in a high-incidence area for ESCC located in Huai’an District, Huai’an City, Jiangsu Province, China, where the crude incidence rate from 1998 to 2016 was 91.85/100000[19]. As described in our previous studies[20-22], the Early Diagnosis and Early Treatment Project of Esophageal Cancer (EDETPEC) supported by the government and Cancer Foundation of China has been carried out in the endemic regions including Huai’an District since 2010. Local residents were required to undergo routine endoscopies. A detailed introduction to esophageal precancerous lesions (EPL) based on histological criteria for dysplasia and methods for EPL diagnosis has already been given in a previous study[21]. The localization of EPL was based on the definition of upper thoracic esophagus (from thoracic inlet to level of tracheal bifurcation; 18-23 cm from incisors), mid thoracic esophagus (from tracheal bifurcation midway to gastroesophageal junction; 24-32 cm from incisors), and lower thoracic esophagus (from midway between tracheal bifurcation and gastroesophageal junction to gastroesophageal junction, including abdominal esophagus; 32-40 cm from incisors)[23]. Figure 1 shows the flowchart of the study population and data collection process. This study included 200 EPL cases aged 62.85 ± 6.03 years and 200 healthy controls aged 63.01 ± 6.08 years matched by gender, age (± 2 years), and villages. The collection of epidemiological data and dietary intake data based on questionnaire method has been introduced in detail previously[21]. Subjects were required to provide the amount of beer/wine/liquor/any other alcoholic drinks consumed per day, which meant that the average alcohol units consumed per day could be estimated. Separated serum samples were obtained by centrifuging collected fasting blood samples at 3000 rpm for 5 min. Enzyme-linked immunosorbent assay (ELISA, KingMed Diagnostics Group Co., Ltd. Guangzhou, China) measuring serum immunoglobulin G (IgG) antibodies was used to determine H. pylori seropositivity. Sensitivity of the ELISA test was 97.9% [95% confidence interval (95%CI): 88.9%-99.9%] and specificity was 100% (95%CI: 86.8%-100%).
Epidata version 3.1 (EpiData Association, Odense, Denmark) was used for inputting and validating the epidemiological data and dietary intake data. Then, SPSS version 22.0 (SPSS, Chicago, IL, United States) was used to establish a database and perform statistical analyses. Two independent samples t-test and conditional logistic regression model were used to evaluate the differences in general characteristics and potential factors between healthy controls and EPL cases, wherever appropriate. The Fisher’s exact test was used to analyze the difference in localization of EPL and H. pylori infection. An unconditional logistic regression model was used to assess the association between H. pylori infection and EPL risk dichotomized by gender, age, and tobacco and alcohol use, as well as the association between dietary factors and H. pylori infection. Covariates including gender, age, body mass index (BMI), education level, annual income, number of cigarettes per day, and alcohol units consumed per day were adjusted in the logistic regression model. Meanwhile, odds ratio (OR) and 95%CI were calculated accordingly. Statistical significance was defined as P < 0.05 (two-tailed).
The study protocol was approved by the Institutional Review Board of Southeast University Zhongda Hospital (Approval No. 2016ZDKYSB017), and the written informed consent was obtained.
Two hundred EPL cases aged 63.01 ± 6.08 years and 200 healthy controls aged 62.85 ± 6.03 years were enrolled. Among the pairs, 100 were males and 100 were females. Table 1 shows that 47 (23.5%) and 58 (29.0%) out of 200 cases and 200 controls, respectively, had H. pylori infection. Two independent samples t-test and conditional logistic regression analysis indicated that there were no statistically significant differences in age, BMI, education level, annual income per person, current drinking status, or H. pylori infection between the two groups after adjustment for covariates (P > 0.05). Compared with non-smokers, a smoking habit of more than 20 cigarettes a day was significantly associated with an elevated risk of EPL (P < 0.05).
| Category | Cases, n = 200 | Controls, n = 200 | Adjusted OR (95%CI)1 | P value |
| Age (yr), mean ± SD | 63.01 ± 6.08 | 62.85 ± 6.03 | 0.792a | |
| BMI (kg/m2), mean ± SD | 24.52 ± 3.33 | 24.36 ± 3.37 | 0.631a | |
| Normal (18.5-23.9) | 82 (41.0%) | 84 (42.0%) | 1.00 (reference) | |
| Underweight (< 18.5) | 4 (2.0%) | 5 (2.5%) | 0.61 (0.12-3.02) | 0.545 |
| Overweight (24.0-28.0) | 84 (42.0%) | 89 (44.5%) | 0.95 (0.61-1.49) | 0.836 |
| Obese (> 28.0) | 30 (15.0%) | 22 (11.0%) | 1.61 (0.83-3.12) | 0.164 |
| Education level | ||||
| Illiterate | 100 (50.0%) | 96 (48.0%) | 1.00 (reference) | |
| Primary school education | 74 (37.0%) | 77 (38.5%) | 0.79 (0.45-1.39) | 0.413 |
| Middle school education and higher | 26 (13.0%) | 27 (13.5%) | 0.81 (0.36-1.79) | 0.599 |
| Annual income/person (RMB) | ||||
| 1-5000 | 53 (26.5%) | 42 (21.0%) | 1.00 (reference) | |
| 5001-10000 | 88 (44.0%) | 86 (43.0%) | 0.75 (0.45-1.25) | 0.267 |
| > 10000 | 59 (29.5%) | 72 (36.0%) | 0.67 (0.37-1.21) | 0.183 |
| Current smoking status (number of cigarettes/d) | ||||
| Non-smoker | 126 (63.0%) | 134 (67.0%) | 1.00 (reference) | |
| 1-10 | 20 (10.0%) | 17 (8.5%) | 1.39 (0.67-2.87) | 0.381 |
| 11-20 | 38 (19.0%) | 41 (20.5%) | 1.10 (0.61-1.97) | 0.755 |
| > 20 | 16 (8.0%) | 8 (4.0%) | 3.11 (1.00-9.63) | 0.049 |
| Current drinking status (alcohol units consumed/d, 1 unit is 8 g or 10 mL of pure alcohol) | ||||
| Non-drinker | 147 (73.5%) | 151 (75.5%) | 1.00 (reference) | |
| < 4 | 10 (5.0%) | 10 (5.0%) | 1.03 (0.41-2.59) | 0.954 |
| 4- | 26 (13.0%) | 23 (11.5%) | 1.02 (0.52-2.02) | 0.946 |
| 8- | 17 (8.5%) | 16 (8.0%) | 1.06 (0.47-2.42) | 0.885 |
| H. pylori infection | ||||
| Negative | 153 (76.5%) | 142 (71.0%) | 1.00 (reference) | |
| Positive | 47 (23.5%) | 58 (29.0%) | 0.75 (0.46-1.24) | 0.265 |
Based on routine endoscopy examination, the study found that the number of cases whose EPL developed in upper, mid, and lower thoracic esophagus was 3, 130, and 67, respectively. Table 2 shows that the control group had the highest positive rate of H. pylori infection (29.0%), followed by EPL cases of upper and mid thoracic esophagus (24.8%) and EPL cases of lower thoracic esophagus (20.9%), but there was no statistically significant differences.
| Group | H. pylori infection | P valuea | ||
| Negative | Positive | Positive rate | ||
| Controls | 142 | 58 | 29.0% | |
| EPL cases (upper and mid thoracic esophagus) | 100 | 33 | 24.8% | 0.384 |
| EPL cases (lower thoracic esophagus) | 53 | 14 | 20.9% | |
As shown in Table 3, when subjects were dichotomized according to gender, age, and the use of tobacco and alcohol, the protective effect of H. pylori infection against the risk of EPL was found in the group of drinkers after adjustment for covariates (OR = 0.32, 95%CI: 0.11-0.95). Supplementary Tables 1 and 2 shows that there may be a nonsignificant decreasing trend of H. pylori infection rate when alcohol consumption is increasing.
| Cases | Controls | Crude OR (95%CI) | P value | Adjusted OR (95%CI)1 | P value | |
| Male | n = 100 | n = 100 | ||||
| H. pylori (-) | 78 (78.0%) | 72 (72.0%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 22 (22.0%) | 28 (28.0%) | 0.73 (0.38-2.38) | 0.328 | 0.64 (0.32-1.27) | 0.200 |
| Female | n = 100 | n = 100 | ||||
| H. pylori (-) | 75 (75.0%) | 70 (70.0%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 25 (25.0%) | 30 (30.0%) | 0.78 (0.42-1.45) | 0.429 | 0.82 (0.42-1.58) | 0.548 |
| Age < 65 years | n = 107 | n = 107 | ||||
| H. pylori (-) | 76 (71.0%) | 73 (68.2%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 31 (29.0%) | 34 (31.8%) | 0.88 (0.49-1.57) | 0.656 | 0.89 (0.47-1.67) | 0.708 |
| Age ≥ 65 years | n = 93 | n = 93 | ||||
| H. pylori (-) | 77 (82.8%) | 69 (74.2%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 16 (17.2%) | 24 (25.8%) | 0.60 (0.29-1.22) | 0.156 | 0.59 (0.27-1.28) | 0.183 |
| Cigarette smoking (-) | n = 126 | n = 134 | ||||
| H. pylori (-) | 97 (77.0%) | 93 (69.4%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 29 (23.0%) | 41 (30.6%) | 0.68 (0.39-1.18) | 0.170 | 0.74 (0.42-1.32) | 0.310 |
| Cigarette smoking (+) | n = 74 | n = 66 | ||||
| H. pylori (-) | 56 (75.7%) | 49 (74.2%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 18 (24.3%) | 17 (25.8%) | 0.93 (0.43-1.99) | 0.845 | 0.80 (0.34-1.86) | 0.601 |
| Alcohol drinking (-) | n = 147 | n = 151 | ||||
| H. pylori (-) | 109 (74.1%) | 109 (72.2%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 38 (25.9%) | 42 (27.8%) | 0.91 (0.54-1.51) | 0.702 | 0.94 (0.55-1.61) | 0.831 |
| Alcohol drinking (+) | n = 53 | n = 49 | ||||
| H. pylori (-) | 44 (83.0%) | 33 (67.3%) | 1.00 (reference) | - | 1.00 (reference) | - |
| H. pylori (+) | 9 (17.0%) | 16 (32.7%) | 0.42 (0.17-1.07) | 0.070 | 0.32 (0.11-0.95) | 0.040 |
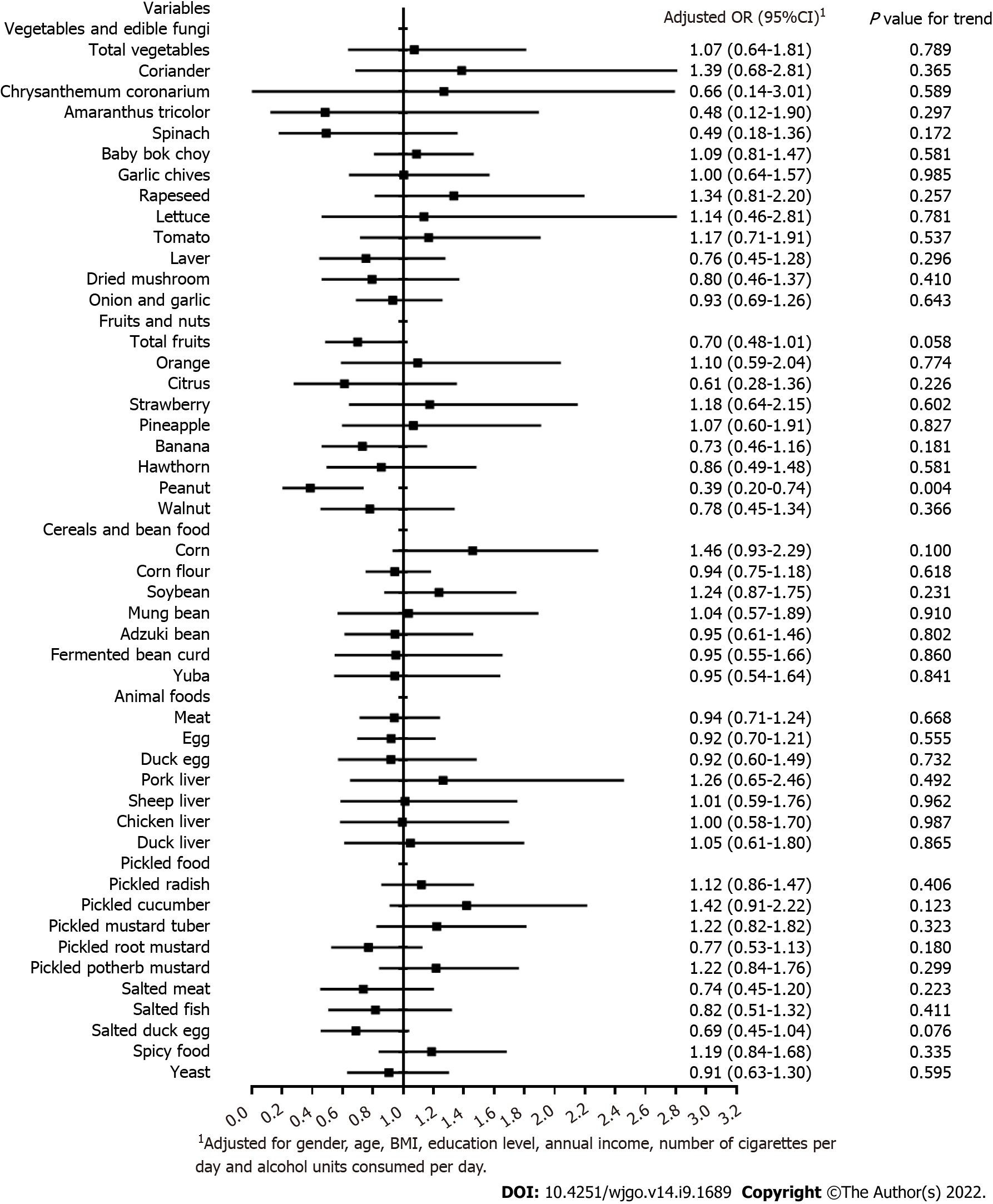
Figure 2 illustrates the association between dietary factors and the risk of H. pylori infection after the adjustment for covariates via the unconditional logistic regression model. The result indicated that peanut intake was significantly associated with a reduced risk of H. pylori infection (OR = 0.39, 95%CI 0.20-0.74). Supplementary Table 3 shows that there may be a significant positive association between peanut consumption and alcohol drinking (P for trend < 0.05).
This study revealed that in drinkers, there was an association between H. pylori infection and a reduced risk of EPL, which is an identified early stage of esophageal carcinogenesis. However, the relationship between H. pylori infection and ESCC is still subject to much discussion. Some researchers believed that infection with H. pylori can increase the risk of ESCC by causing gastric atrophy that promotes excessive bacterial growth and causes endogenous nitrosamine production[24-26]. However, other studies which held that H. pylori infection probably plays a protective role in ESCC postulated that the protection is mediated via gastric atrophy, whereas the mechanism is related to a reduced load of esophageal acid[27,28]. Therefore, it is likely that ESCC might be affected in a double-edged manner by H. pylori infection, which is dependent on population and other possible external factors. For example, previous studies have indicated that acid regurgitation may be facilitated by the reduction in lower esophageal sphincter’s pressure and the retard of both esophageal motility and gastric emptying due to large consumption of alcoholic beverages[29-33]. Therefore, the current hypothesis is that H. pylori infection just alleviates esophageal reflux caused by alcohol to some extent, thus reducing the risk of esophageal carcinogenesis caused by acid reflux. Our results also reported that EPL cases of lower thoracic esophagus had the lowest positive rate of H. pylori infection, which may support the hypotheses to some extent, although the difference was not statistically significant. In addition, there is more data indicating the positive role of this bacterium for humans. For example, a recent review considered the data on H. pylori and suggested that H. pylori may be a latent or opportunistic pathogen rather than a true pathogen of some diseases, and is possibly part of the normal human microbiome as a commensal or even a symbiont organism[34]. However, it was reported that a regular but moderate alcohol intake could possibly facilitate elimination of H. pylori infection[35]. Supplementary material also shows a nonsignificant decreasing trend of H. pylori infection rate when alcohol consumption is increasing. This partly supports the hypothesis that there is a possibility that drinkers without H. pylori infection could have more alcohol consumption. In other words, the decreased EPL risk in drinkers with H. pylori infection is possibly related to a lower alcohol consumption. However, because the result was not statistically significant, and there was no significant association between alcohol consumption and EPL risk in Huai’an in both this study and the previous epidemiological investigation[21], it is hard to address whether the reduced risk of EPL in drinkers with H. pylori infection was related to a reduced alcohol intake.
Additionally, the present study reported that the consumption of peanuts may provide protection from H. pylori infection. Since peanuts are high in fat, the duodenal mucosa secretes the hormone enterogastrone when fatty food is present in the stomach or small intestine[36]. Enterogastrone inhibits gastric movements and secretion of gastric acid, possibly by blocking the production or activity of gastrin, the hormone that initially leads to these functions[37]. Therefore, the reduced amount of acid produced may influence the growth of H. pylori, as H. pylori is dependent on acidity to survive for a long time[38]. In addition, in China, people are likely to drink and eat peanuts at the same time, and Supplementary material shows that there was a positive association between peanut consumption and alcohol drinking. Therefore, the inverse association between the consumption of peanut and the risk of H. pylori infection may be mediated by alcohol drinking. However, there is still a lack of evidence to verify the above hypotheses, thus further researches are required to evaluate the relationship between peanut consumption and H. pylori infection.
At present, about 50% of the global population and more than 70% of the population in some developing countries are infected by H. pylori[39]. However, this study reported that the positive rates of H. pylori infection were only 23.5% and 29.0% in EPL cases and healthy controls. In an early study conducted by Gao et al[40], Huai’an, Jiangsu Province was selected as a high incidence area of upper digestive tract cancers, and Pizhou, Jiangsu Province was selected as a low incidence area. They used ELISA and latex agglutinate test for the detection of H. pylori infection, and found that the prevalence of H. pylori infection among the gastric cancer group/upper digestive tract cancer group in the low incidence area of Pizhou (66.67%/63.46%) was significantly higher than that in the high incidence area of Huai’an (38.64%/39.33%). However, in the high incidence area of Huai’an, the prevalence of H. pylori infection in non-cancer controls and the healthy family members of the cancer cases was higher than that of cases. Therefore, the previous study and our study found that the prevalence of H. pylori infection in Huai’an may be much lower than that in other areas, and the prevalence in upper digestive tract cancers or EPL cases can be lower than that in non-cancer population in this region.
In summary, our study suggested that H. pylori infection is likely to decrease EPL risk in drinkers for a rural adult Chinese population, and the consumption of peanuts may be related to a reduced risk of H. pylori infection. However, the sample size used is a limitation of the study, which may bring difficulties to evaluate statistical significance in some statistical analyses, thus the findings should be framed as preliminary evidence. A case-control study might be difficult to determine causality, so the statement of “protective role” might be overestimated. Hence, it is necessary to design a large-scale prospective cohort study to address the impact of H. pylori infection on ESCC, the localization of lesions, and the association with dietary intake and the use of alcohol in the future. Additionally, the low prevalence of H. pylori infection in Huai’an is a peculiar finding, which implies that further investigations are recommended.
The role of Helicobacter pylori (H. pylori) infection in esophageal squamous cell carcinoma (ESCC) remains a topic of much debate.
To assess the relationship between H. pylori infection and the risk of precancerous lesions of ESCC, which is an identified early stage of carcinogenesis.
This study aimed to evaluate the association between H. pylori infection and the risk of esophageal precancerous lesions (EPL) in a high-incidence area in Huai’an, and further explore the association between dietary factors and the risk of H. pylori infection.
The study was based on a case-control design. Epidemiological data were collected and H. pylori seropositivity was tested. An unconditional logistic regression model was used to analyze the association between H. pylori infection and EPL risk with adjustment for confounders, as well as the association between dietary factors and risk of H. pylori infection.
The control group had the highest positive rate of H. pylori infection (29.0%), followed by EPL cases of upper and mid thoracic esophagus (24.8%) and EPL cases of lower thoracic esophagus (20.9%). The protective effect of H. pylori infection against the risk of EPL was observed in the group of drinkers after adjustment for covariates [odds ratio (OR) = 0.32, 95% confidence interval (95%CI): 0.11-0.95]. Peanut intake was significantly associated with a reduced risk of H. pylori infection (OR = 0.39, 95%CI: 0.20-0.74).
H. pylori infection may decrease the risk of EPL in drinkers for a rural adult Chinese population, and the consumption of peanuts may be related to a reduced risk of H. pylori infection.
A well-designed prospective cohort study is required to address the impact of H. pylori infection on ESCC, the localization of lesions, and the association with dietary intake and alcohol drinking. Additionally, the low prevalence of H. pylori infection in Huai’an is a peculiar finding, which implies that further investigations are recommended.
Thanks to all the subjects and researchers for their contributions and hard work. We really appreciate Mr Rob Unwin’s assistance in polishing the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Chinese Nutrition Society, No. M102201117M.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LW, Taiwan; Reshetnyak VI, Russia S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Yildirim M, Kaya V, Yildiz M, Demirpence O, Gunduz S, Dilli UD. Esophageal cancer, gastric cancer and the use of pesticides in the southwestern of Turkey. Asian Pac J Cancer Prev. 2014;15:2821-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Li M, Wan X, Wang Y, Sun Y, Yang G, Wang L. Time trends of esophageal and gastric cancer mortality in China, 1991-2009: an age-period-cohort analysis. Sci Rep. 2017;7:6797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Castro C, Peleteiro B, Bento MJ, Lunet N. Trends in gastric and esophageal cancer incidence in northern Portugal (1994-2009) by subsite and histology, and predictions for 2015. Tumori. 2017;103:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 5. | Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila). 2008;1:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 7. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413-1417, 1417.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Zhuo X, Zhang Y, Wang Y, Zhuo W, Zhu Y, Zhang X. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol). 2008;20:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gao H, Li L, Zhang C, Tu J, Geng X, Wang J, Zhou X, Jing J, Pan W. Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol Res Pract. 2019;2019:1953497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Shaheen NJ, Whiteman DC, Anderson LA, Vaughan TL, Corley DA, El-Serag HB, Rubenstein JH, Thrift AP. Helicobacter pylori Infection Is Associated With Reduced Risk of Barrett's Esophagus: An Analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Am J Gastroenterol. 2018;113:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Vohlonen IJ, Hakama M, Härkönen M, Malila N, Pukkala E, Koistinen V, Sipponen P. Oesophageal cancer incidence in 20-year follow-up in a population-based sample of 12 000 middle-age men with or without Helicobacter pylori infection in Finland. Gut. 2018;67:1201-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Poyrazoglu OB, Dulger AC, Gultepe BS. Helicobacter Pylory infection in patients with esophageal squamous cell carcinoma. Clinics (Sao Paulo). 2017;72:150-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Khoshbaten M, Zadimani A, Bonyadi MR, Mohammadzadeh M, Gachkar L, Pourhoseingholi MA. Helicobacter pylori infection reduces the risk of esophageal squamous cell carcinoma: a case-control study in iran. Asian Pac J Cancer Prev. 2011;12:149-151. [PubMed] |
| 15. | Wu DC, Wu IC, Lee JM, Hsu HK, Kao EL, Chou SH, Wu MT. Helicobacter pylori infection: a protective factor for esophageal squamous cell carcinoma in a Taiwanese population. Am J Gastroenterol. 2005;100:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Vitale G, Barbaro F, Ianiro G, Cesario V, Gasbarrini G, Franceschi F, Gasbarrini A. Nutritional aspects of Helicobacter pylori infection. Minerva Gastroenterol Dietol. 2011;57:369-377. [PubMed] |
| 17. | Aimasso U, D'onofrio V, D'eusebio C, Devecchi A, Pira C, Merlo FD, De Francesco A. Helicobacter pylori and nutrition: a bidirectional communication. Minerva Gastroenterol Dietol. 2019;65:116-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S, Viviana G, Valentina T, Riccardo LL, Antonio G. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014;20:12809-12817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 19. | Wang S, Pan D, Chen Z, Song G, Han R, Sun G, Su M. Trends in Incidence and Mortality of Esophageal Cancer in Huai'an District, a High-Risk Area in Northern Jiangsu Province, China. Cancer Control. 2022;29:10732748221076824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Pan D, Su M, Huang G, Luo P, Zhang T, Fu L, Wei J, Wang S, Sun G. MTHFR C677T genetic polymorphism in combination with serum vitamin B2, B12 and aberrant DNA methylation of P16 and P53 genes in esophageal squamous cell carcinoma and esophageal precancerous lesions: a case-control study. Cancer Cell Int. 2019;19:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Pan D, Su M, Zhang T, Miao C, Fu L, Yang L, Song G, Raine PJ, Wang S, Sun G. A Distinct Epidemiologic Pattern of Precancerous Lesions of Esophageal Squamous Cell Carcinoma in a High-risk Area of Huai'an, Jiangsu Province, China. Cancer Prev Res (Phila). 2019;12:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Pan D, Wang S, Su M, Sun G, Zhu X, Ghahvechi Chaeipeima M, Guo Z, Wang N, Zhang Z, Cui M. Vitamin B12 may play a preventive role in esophageal precancerous lesions: a case-control study based on markers in blood and 3-day duplicate diet samples. Eur J Nutr. 2021;60:3375-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Ashraf HH, Palmer J, Dalton HR, Waters C, Luff T, Strugnell M, Murray IA. Can patients determine the level of their dysphagia? World J Gastroenterol. 2017;23:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyrén O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 25. | Iijima K, Koike T, Abe Y, Yamagishi H, Ara N, Asanuma K, Uno K, Imatani A, Nakaya N, Ohara S, Shimosegawa T. Gastric hyposecretion in esophageal squamous-cell carcinomas. Dig Dis Sci. 2010;55:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Houben GM, Stockbrügger RW. Bacteria in the aetio-pathogenesis of gastric cancer: a review. Scand J Gastroenterol Suppl. 1995;212:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol. 1998;93:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Akiyama T, Inamori M, Iida H, Mawatari H, Endo H, Hosono K, Yoneda K, Fujita K, Yoneda M, Takahashi H, Goto A, Abe Y, Kobayashi N, Kubota K, Saito S, Nakajima A. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett's epithelium in Japanese men. BMC Gastroenterol. 2008;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Kaufman SE, Kaye MD. Induction of gastro-oesophageal reflux by alcohol. Gut. 1978;19:336-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 95] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Keshavarzian A, Polepalle C, Iber FL, Durkin M. Esophageal motor disorder in alcoholics: result of alcoholism or withdrawal? Alcohol Clin Exp Res. 1990;14:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Mincis M, Chebli JM, Khouri ST, Mincis R. [Ethanol and the gastrointestinal tract]. Arq Gastroenterol. 1995;32:131-139. [PubMed] |
| 33. | Wang F, Li G, Ning J, Chen L, Xu H, Kong X, Bu J, Zhao W, Li Z, Wang X, Li X, Ma J. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int J Biol Sci. 2018;14:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: Commensal, symbiont or pathogen? World J Gastroenterol. 2021;27:545-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (3)] |
| 35. | Kuepper-Nybelen J, Rothenbacher D, Brenner H. Relationship between lifetime alcohol consumption and Helicobacter pylori infection. Ann Epidemiol. 2005;15:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Gough AL, Rai VS, Mariano EC, Greco RS, Landor JH. Effect of an intravenous fat preparation on canine gastric secretion. Am J Surg. 1980;139:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 37. | Thulin L, Johansson C. Gastrointestinal hormones. Acta Chir Scand Suppl. 1978;482:69-72. [PubMed] |
| 38. | Waldum HL, Kleveland PM, Sørdal ØF. Helicobacter pylori and gastric acid: an intimate and reciprocal relationship. Therap Adv Gastroenterol. 2016;9:836-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Li J, Perez-Perez GI. Helicobacter pylori the Latent Human Pathogen or an Ancestral Commensal Organism. Front Microbiol. 2018;9:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Gao C, Li Z, Ding J, Hu X, Xu T, Liu T, Takezaki T, Tajima K. The Relationship between Helicobacter pylori Infection and Gastric Cancer in High and Low Incidence Areas for Upper Digestive Tract Cancers in Jiangsu Province. Zhongguo Aizheng Zazhi. 2000;9:395-396. |