Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1478
Peer-review started: March 21, 2022
First decision: April 25, 2022
Revised: May 7, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 15, 2022
Processing time: 142 Days and 11.9 Hours
Intrahepatic cholangiocarcinoma (iCCA) is an aggressive malignancy with an increasing incidence worldwide and poor prognosis, despite several advances and continuous efforts to develop effective treatments. Complete surgical resection is the mainstay of treatment and offers a potentially curative option, but is only possible in less than a third of patients, owing to advanced disease. Chemo
Core Tip: Intrahepatic cholangiocarcinoma (iCCA) maintains a dismal prognosis despite best available therapy. Complete surgical resection offers a potentially curative option but is feasible in a limited number of cases. This review explores the evolving role of stereotactic ablative radiotherapy (SABR) in the management of iCCA either as an adjuvant to surgical resection, or in cases or recurrent or unresectable disease. Data on the use of SABR as a neoadjuvant/downstaging modality are scarce. Notably, published studies are limited to predominantly retrospective case series. High quality prospective trials evaluating SABR are urgently needed.
- Citation: Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2022; 14(8): 1478-1489
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1478.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1478
Cholangiocarcinoma (CCA) is a rare, aggressive malignancy arising from the biliary epithelium. The overall incidence worldwide is less than 6 cases per 100000, however, this varies significantly from country to country and is significantly more common in East Asia[1,2], with incidences of up to 90 per 100000 reported in Thailand[3].
Prognosis in CCA is dismal with fewer than 10% surviving 5 years after diagnosis. Overall survival (OS) is significantly higher with extrahepatic vs intrahepatic tumours (15% vs < 5%, respectively)[4]. The reasons for the poor survival are predominantly related to the insidious growth of the tumours, with limited clinical symptoms until the disease is disseminated, by which point surgical resection which is the sole curative option is precluded.
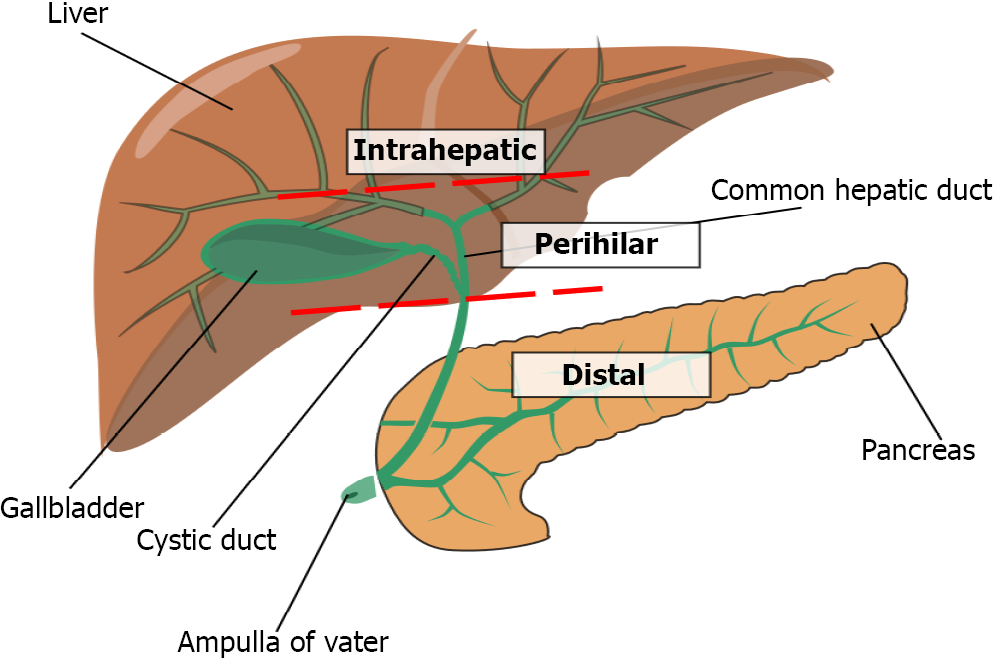
CCA can be further subdivided by the site of origin in the biliary tract (Figure 1): Intrahepatic CCAs (iCCA) arise from sites proximal to the second order branches of the right or left hepatic duct up to the canals of Hering, while perihilar CCAs (phCCA), also known as Klatskin tumours, arise between the second order branches of the right and/or left hepatic duct and the cystic duct confluence. Distal CCAs (dCCA) arise between the cystic duct confluence and the ampulla of Vater[5-7]. phCCA and dCCAs are collectively termed extrahepatic CCAs (eCCAs) and account for approximately 80% of all diagnoses of CCAs overall, while the remainder are intrahepatic[6,8]. Morphologically, depending on their pattern of growth and appearance, they are categorised in three different types. The mass-forming type, which is the most frequent, accounts for presentation with a mass, the periductal-infiltrating type is characterised by growth along the wall of the bile duct, and the intraductal-growing type by intraluminal growth[7].
Histologically, CCAs can be broadly subdivided into papillary and mucinous carcinomas[9]. iCCAs show greater variability with further subdivision into small and large bile duct cancer. Small bile ducts are lined by cuboidal epithelium and hepatic stem cells, which may be associated with more aggressive tumours and rarely, mixed hepatocellular CCAs. Large bile duct iCCAs are broadly similar to phCCA and dCCA[10].
CCAs are typically asymptomatic in their early stages and manifest clinically only at an advanced stage. Non-specific symptoms such as abdominal pain, night sweats and weight loss may be present in the early stage[11].
Jaundice is a hallmark feature of eCCA as obstruction of large distal bile ducts is needed to obstruct the biliary outflow significantly. Given that iCCAs affect the smaller proximal bile ducts, jaundice is much less frequent, and presentation is more likely to be incidental finding on imaging or after work-up for deranged liver function tests[12].
iCCAs further differ clinically from extrahepatic tumours in that they are more likely to arise on a background of diseased liver parenchyma, much like hepatocellular carcinoma. eCCAs, in contrast, are associated with chronic bile duct inflammation, such as with primary sclerosing cholangitis, chole
Complete surgical resection is the only prospect for cure in CCA, but this is only possible in < 30% of patients due to advanced disease at presentation[14,15]. Surgery ranges from hepatectomy in iCCA, hepatectomy and/or hilar resection in phCCA, or pancreatoduodenectomy in extrahepatic tumours, to liver transplantation in selected cases of CCA[7,16].
Adjuvant gemcitabine-based chemotherapy is now recommended in most international guidelines[17-20], with evidence of increased disease-free survival (DFS)[21]; overall 5-year survival can reach from 44% in dCCA to 20%-40% in phCCA and iCCA[8,16].
In the palliative setting, data is more robust in supporting chemotherapy with several randomised studies confirming the survival benefit of gemcitabine and platinum-based therapies, with a median progression free survival (PFS) of 8.0 mo[22,23]. Second line chemotherapy with FOLFOX regimens has also been shown to be of limited benefit, with an improvement in OS by 1 mo, although PFS was poor at 8.6% at 1 year[24].
Despite institution of surgery or chemotherapy where appropriate, recurrence rates remain high and, consequently, patient survival is still poor in CCA. Locally advanced disease, oligometastases and medical comorbidities may also preclude surgical intervention. Locoregional therapies such as radiofrequency ablation (RFA)[25] and trans-arterial chemo- or radio-embolization (TACE or TARE, the latter also known as selective internal radiotherapy)[26] have been developed for locally advanced and oligometastatic disease. These therapies have also reduced cancer recurrence as adjuvant therapies along with surgery[27].
Radiotherapy is another alternative treatment modality encompassing standard external beam, brachytherapy and stereotactic forms studied. This has several advantages to RFA and TACE/TARE, in particular being non-invasive and, not requiring the target to be near blood vessels as in TARE/TACE.
Although radiotherapy is not included in guidelines for the treatment of CCA, it has been shown to improve survival vs chemotherapy alone for unresectable iCCA in large propensity matched population studies, with reduced hazards of mortality [hazard ratio (HR): 0.80 (95%CI: 0.71-0.91, P = 0.001)][28,29].
Targeted radiotherapy is challenging due to the radiosensitivity of the liver parenchyma and surrounding gastrointestinal tract, which may result in radiation hepatitis, vomiting, diarrhoea and bowel obstruction resulting from stricturing[30,31]. Stereotactic ablative radiotherapy (SABR) allows for high energy beams of radiation focused on target sites avoiding damage to surrounding tissues.
This review gives an overview of the technology of SABR and its application to intrahepatic CCA, which possesses unique characteristics in comparison to other sites.
SABR uses multiple beams of radiation focused to a single point in three-dimensional space using a collimation system, as opposed to a single unfocused beam used in conventional radiotherapy. This allows a much larger dose of radiation in a single fraction, whilst avoiding exposure to surrounding tissues[32]. In some cases, the course may be completed in a single fraction. This concept was developed initially by Phillips et al[33] at the Karolinska Institute in Sweden in the 1960s to treat intracranial lesions. Their technology would eventually become known as the Gamma Knife (Elekta Instruments Inc., Tucker GA, United States)[33]. It was not until the early 1990s until similar technology was applied outside the brain. Immobilisation of the patient or tracking of viscera is necessary when targeting the thorax and abdomen to avoid off-target viscera and mitigate against motion such as during respiration[34].
Uematsu et al[35] were one of the first to realise the clinical benefits of SABR, in 1998, in patients with locally advanced non-small cell lung cancer who were technically operable but unfit for surgery[35]. Successive studies demonstrated that SABR allowed progression-free survival in 80%-90% of these patients, nearly double that of conventional radiotherapy, with significantly lower toxicity[36].
Following the above reports Herfarth et al[37] applied this technology to the liver for unresectable, predominantly metastatic tumours of varying origin. They again showed impressive local control (LC) rates of 81% at 18 mo[37]. Larger, contemporary series of SABR mirror Herfarth’s early results in both hepatocellular carcinoma[38] and oligometastatic disease in the liver[39,40]. These series are predominantly observational, and no large-scale interventional trial has been published in this population.
Modern approaches to applying SABR in the liver involve immobilising the abdomen using body moulds or vacuum cushions. Movement from respiration is controlled by using controlled breath holding techniques or respiratory gating or tumour tracking with image guidance. Stereotactic frames and/or implanted fiducial markers may be used to provide a reference for anatomical delineation. The above methods are combined with 4D computed tomography scanning to apply SABR, and accuracy to between 2 and 3 mm is achievable[41,42].
Patients suitable for SABR to the liver, typically have fewer than 3 tumours at no larger than 6cm each, situated greater than 5 mm from adjacent viscera so that ablative doses may be more easily achieved, although these criteria will vary depending on institutional experience[41,42].
The side effect profile of SABR in relation to the liver most commonly consists of nausea and fever, which can be seen within a few hours of treatment. These may be prevented with prophylactic antiemetics[43].
Late side effects include radiation induced liver disease (RILD), which may occur between 2 wk and 8 mo after completion of treatment. This includes clinical symptoms of fatigue, tender anicteric hepatomegaly and ascites. Biochemically, there is elevated alkaline phosphatase, whilst transaminases and bilirubin remain normal[44].
Non-classical RILD (typically in patients with underlying liver disease) occurs within 3 mo of radiotherapy and consists of liver enzymes more than five times the upper limit of normal or a decline in liver function as measured by a worsening Child-Pugh score of 2 or more in the absence of classical RILD.
These occur in less than 5% of patients and are associated with cumulative doses (in conventionally fractionated radiotherapy) higher than 30-32 Gy and 28 Gy in patients with underlying liver disease.
Other specific toxicities are related to off-target effects on the gastrointestinal tract, with nausea, vomiting and diarrhoea being common. Other effects are common to all radiation therapies, and these include skin necrosis (much less common in the era of volumetric modulated arc therapy) and systemic effects such as fatigue and fever. It should be reiterated that these side effects, when they do occur, are typically milder and less frequent than with equivalent conventional radiotherapy[45].
As mentioned above, the standard of care for curative treatment of iCCA is surgical resection followed by adjuvant chemotherapy. For palliative treatment, chemotherapy with gemcitabine and platinum regimes are recommended[17,18]. We therefore focus on five scenarios where SABR may be useful in the treatment algorithm: (1) Primary therapy in patients with technically resectable disease but precluded from resection due to medical comorbidities; (2) Primary therapy in technically unresectable disease; this may be due to diffuse or metastatic disease; (3) Recurrent disease after surgical resection; (4) Following surgical resection to prevent local recurrence (adjuvant therapy); and (5) As a down
| Ref. | Country | Design | Patient characteristics (reason for inoperability) | Total patients | No. iCCA (%) | Median follow-up/months (range) | Outcomes (1 yr)1 | Major side effects (CTC > 3) | ||
| Local control (%) | Progression free survival (%) | Overall survival (%) | ||||||||
| Shen et al[46], 2017 | China | Retrospective | Unresectable: (1) 7/28 Medical; (2) 16/28 Technical; and (3) 5/28 Advanced age | 28 | 28 (100) | 16 (3-42) | 89.3 | 50.0 | 57.1 | 0 |
| Liu et al[47], 2017 | Taiwan | Retrospective | Unresectable: (1) Medical 3/15; and (2) Surgical 12/15 | 15 | 12 (80) | 29.0 | 48.5 | - | 50.3 | 0 |
| Thuehøj et al[48], 2022 | Denmark | Retrospective | Unresectable, locally advanced | 41 | 15 (37) | 9.5 (0-66.5) | 85.4 | 31.7 | 48.8 | - |
| Tao et al[49], 2016 | United States | Retrospective | Unresectable, locally advanced | 79 | 79 (100) | 24 (4-33) | 81.0 | 91.0 | 87.0 | 0 |
| Tse et al[51], 2008 | Canada | Prospective, phase I | Unresectable, locally advanced (includes HCC) | 41 | 10 (24) | 17.6 (range 10.8-39.2) | 65.0 (all patients) | - | 58.0 | 0 |
| Mahadevan et al[52], 2015 | United States | Retrospective | Unresectable: (1) Medical 3/34; and (2) Surgical 29/34. R1 Resection: 2/34 | 34 | 31 (91) | 38 (8-71) | 88.0 | - | 58.0 | 0 |
| Barney et al[53], 2012 | United States | Retrospective | Unresectable: 6/12 lesions. Recurrent: 6/12 lesions | 10 | 6 (60) | 14 (2-26) | 100% | - | KM 73.0% | 0 |
| Brunner et al[54], 2019 | Germany and Switzerland | Retrospective, multicentre | Unresectable, unclear reasons | 64 | 41/82 lesions (50%) | 35 (7-91) for survivors | 89 | - | 81 | 0 |
| Weiner et al[55], 2016 | United States | Prospective, phase I | Unresectable, locally advanced (includes HCC) | 26 | 14 (54) including 2 biphenotypic ICCA and HCC | 8.8 (0.3-33) | 91 (all patients) | 68 | 51 | Grade IV lymphopenia-1 patient; Grade V hepatic failure-2 patients |
| Kozak et al[56], 2020 | United States | Retrospective | Unresectable disease | 40 | 26 (63) | 18 (1-100) | 70 (all patients) | - | 66 (all patients) | 0 |
| Sebastian et al[59], 2019 | United States | Retrospective, population database study, comparative study between SABR, TARE and CRT | Unresected, locally advanced disease | 27-SABR; 52-CRT; TARE-60 | 141 (100%) | 17 | - | - | Propensity matched hazard ratio of overall survival for SABR vs CRT-0.22; vs TARE 0.58 | Not reported |
| Jung et al[60], 2014 | South Korea | Retrospective | Unresectable and recurrent disease after surgery | 28-Unresectable; 30-Recurrent | 33 (57) | 10 (1-97) | Unresectable-76; Recurrent-91 | Overall-26 | Unresectable-29; Recurrent-53 | 2-Cholangitis; 1-Gastric perforation |
| Franzese et al[61], 2020 | Italy | Retrospective | 49/51 (96%) Recurrent metastatic disease after surgical resection | 51 (includes GB adenoCa) | 34 (66)-iCCA and eCCA grouped together | 14 (3-95) | 74.7 | 32.8 | 63.2 | 0 |
| Ibarra et al[62], 2012 | United States | Retrospective | Unresectable disease | 21-HCC; 11-iCCA | 11 (34) | 7.8 (1.4-17.9) | 55.5 | - | 45 | 0 |
Shen et al[46] reported data on SABR in inoperable iCCA. In this series 12/28 (42.8%) were inoperable due to medical co-morbidities or advanced age whilst the remainder were technically inoperable. Data was not stratified by the reason for inoperability, although on multivariable analysis, there was no difference in response based on this. The overall disease control rate with SABR was 89.3%, of which 42.9% had stable disease, 35.7% a partial response and 10.7% a complete response at first follow-up (median 16 mo). Predictors of successful response were median biologically effective doses (BED) of > 100 Gy and having solitary lesions. Median OS was 15.0 mo and median PFS was 11.0 mo. OS and PFS were 32.1% and 21.4% at 2 years, respectively[46].
A Taiwanese study included patients with solely medically inoperable tumours (14/15 iCCA). 1- and 2-year OS were 50.3 and 14.4%, while LC was achieved in only 48.5% at 1 year. The reason is likely the lower BED used at 45 Gy and the authors reported significantly higher survival with doses at > 75 Gy, with 1-year OS at 58.3%[47]. A Danish study with predominantly patients with eCCA but who were also medically inoperable showed similar OS and LC rates[48].
The largest study of SABR in iCCA (79 patients) showed 1-year OS of 87% and 3-year OS of 44%. LC rates were 81% and 31%, respectively, for the same time period with a PFS of 88% and 39%. Patients in this study were excluded if treatment was directed with palliative intent, which may explain the higher survival rates, although the authors’ definition of this is unclear. All patients had favourable performance status: 94% scored at 0 or 1, 6% scored 2 and no patients had performance status > 2. 20% of patients had extrahepatic metastatic disease and 58% had nodal disease, implying a poor prognosis pre-treatment[49]. Nevertheless, the survival figures in this study are similar to curative resection, which according to a recent review confers an overall 3-year survival ranging from 32% to 47% and a similar 3-year recurrence free survival which is between 6 to 47%[50]. Survival also correlated with the radiation dose, with a BED greater than 80.5 Gy associated with 3-year OS of 73% vs 38% for patients receiving lower doses.
These results may suggest that SABR could be a suitable alternative to surgical resection in patients unfit for surgery, however comparative studies, in particular, randomized trials are needed to confirm this.
Tse et al[51] provided one of the first reports of SABR in iCCA. Their phase I study included 10 patients with iCCA who were unresectable due to metastatic disease, pre-dominantly confined to the liver or with locoregional lymphadenopathy. The median OS was 15.0 mo with 58% 1-year OS[51].
In Mahadevan et al’s retrospective study of locally advanced 31 iCCAs (11 further phCCAs or dCCAs), 1-year OS was 58% and 4-year OS was 19%. LC was achieved in 88% at 1 year and 79% at 4 years for the overall cohort. Median PFS was 11 mo after SABR[52].
Barney et al[53] performed a retrospective study consisting predominantly of patients with either primary or recurrent oligometastatic disease. OS was 73% at 1 year and LC was achieved in 100% of patients (of whom 25% had a complete response and 42% a partial response). 40% of patients had PFS[53].
A large multicenter German and Swiss study with 64 patients (41 iCCA) showed 1-year OS of 63% and LC at 89%. After multivariable analysis, as above, improved survival and LC were achieved with higher radiation doses, without a significant increase in toxicity[54].
Weiner et al[55] performed a phase II study of SABR in unresectable primary liver lesions of which 14/26 (54%) were iCCA or biphenotypic with HCC. 1-year OS was 51% and PFS was 68% with only 2 of 26 (4%) patients in the study having local progression at the SABR site[55].
Kozak et al[56] performed a retrospective study of SABR in 40 patients with unresectable CCA (23 patients iCCA and the remainder phCCA) assessing the location of failure with respect to the radiation field. Median OS for patients with iCCA was 10 mo, 1-year OS for the entire cohort was 66%, and median follow-up was 18 mo. 12 patients (30%) had in-field local failure, whilst seventeen (42.5%) had out of field hepatic failure. Seven patients (17%) experienced regional failure predominantly in perihilar and para-aortic nodes, whilst 15 patients (37.5%) had distant failure of which the lungs were the most common site of progression (7 patients, 46.7%)[56]. Given the high rates of out of field recurrence, the authors proposed elective nodal irradiation in the perihilar space to prevent regional recurrence, however there are no trials on this.
Bisello et al[57] proposed a series of guidelines on clinical target volumes for biliary tract cancers, including iCCA, to incorporate sites of potential regional progression. They proposed a margin of 9.8mm from the primary tumour boundary to incorporate all microscopic spread[57]. This is at the cost of potential for increased toxicity, in particular around the central biliary tree with suggested dosing limited to for example 42 Gy in 15 fractions or 35 Gy in 5 fractions[58].
One study compared SABR to TARE and conventional chemoradiotherapy in unresectable iCCA using the United States National Cancer Database. Median OS was 20 mo with SABR and significantly greater than TARE and chemoradiotherapy after adjusting for confounders with propensity weighting and multivariable regression [HR: 0.44 (95%CI: 0.21-0.91)][59].
Of note, Jackson et al[28] performed a propensity matched study of patients with inoperable iCCA identified from the United States National Cancer Database comparing patients who received any form of radiotherapy (not specifically SABR). After propensity score matching, they showed that the addition of radiotherapy to the standard chemotherapy regimen significantly reduced the hazards of death [HR: 0.83 (95%CI: 0.71-0.97, P = 0.018)][28].
Jung et al[60] studied patients with unresectable and recurrent disease, of which 57% were iCCAs. 1- and 2-year OS in the recurrent disease group were 53% and 28%, respectively, LC rates were 91% and 81%, respectively, at the same time periods. Overall PFS for all patients were 26% and 23% at 1 and 2 years. Of note, 2 patients developed transient liver failure following SABR in this study[60].
Franzese et al[61] performed a retrospective study of SABR in recurrent biliary tract cancer after surgical resection, of which 18/51 (35%) had iCCA. 1-year OS and PFS were 63.2% and 32.8%, respectively, whilst LC rates were 74.7% at 1 year[61].
Ibarra et al[62] performed a small multi-centre study of 11 patients undergoing SABR for iCCA, with 50% reported as undergoing this following surgical resection and recurrence (the remainder were for unresectable disease, of whom 45% had distant disease). 1-year survival was 45% and LC was estimated to be 55.5% in this study[62].
Hammad et al[63] performed a study using the United States National Cancer Database of patients with iCCA who underwent surgical resection. Of the 525 out of 2897 patients who underwent postoperative conventional radiotherapy, 230 (43.8%) had positive resection margins, compared to 704 (24.3%) in the non-radiotherapy group. There was no significant OS benefit [0.99 (95%CI: 0.84–1.16) P = 0.931] for patients who underwent radiotherapy, after propensity score matching and multivariable Cox regression. LC and PFS were not reported[63].
Kim et al[64] published a small case series of 18 patients with incompletely resected iCCA (R1) of whom 7 underwent adjuvant chemoradiotherapy. They found significant increases in OS, LC and PFS with chemoradiotherapy: (LC: 5.6 mo vs not reached, P < 0.001, PFS: 5.6 mo vs 8.3 mo, P = 0.047, OS: 15.0 mo vs 26.6 mo, P = 0.064)[64].
While there are no large studies of SABR specifically, given its advantages over conventional radiotherapy, the above studies could be regarded as showing some promise in its potential use for incomplete resection.
Studies on SABR as standard adjuvant therapy following resection of iCCA are limited, however there is a limited number of studies evaluating conventional radiotherapy following resection.
Jiang et al[65] assessed adjuvant conventional radiotherapy where macroscopic regional lymph nodes were identified following surgical resection on imaging. Out of 100 patients, 24 received radiotherapy, whilst 76 did not, but it was not specified whether the latter patients received any further treatment. Median OS was significantly superior at 68.8% in the radiotherapy group and 12.1% in the non-radiotherapy group (P = 0.01). After multivariable analysis, radiotherapy was independently associated with survival [HR: 0.482 (95%CI: 0.27-0.86)][65]. A further meta-analysis of studies assessing adjuvant radiotherapy in iCCA did not show a significantly improved patient survival[66].
Studies assessing SABR for downstaging of iCCA (neoadjuvant therapy) have mainly focused on doing this to allow liver transplantation. Wong et al[67] and Sandler et al[68] both reported impressive OS of 80 and 75% at 1 year in the few (4 in each study) patients who underwent liver transplantation following successful SABR. However, 18/22 (82%) in Wong’s study and 27/31 (87%) patients in Sandler’s failed to proceed to transplant, predominantly due to tumour progression.
Conventional chemoradiotherapy has been attempted with promising results in a small case series. Of 7 patients with locally advanced, unresectable iCCA, five (71.4%) became resectable following chemoradiotherapy and one patient remained disease free after resection at 18 mo. 5-year OS was 23.6%[69].
Rayar et al[70] reported their experience of using TARE as a downstaging modality for unresectable iCCA. Of 45 patients who underwent downstaging TARE and chemotherapy, eight (17.7%) ultimately underwent surgical resection with curative intent. With a median follow-up of 15.6 mo, only two patients died perioperatively and only one died from unrelated disease. Of the remainder, two were found to have recurrence at follow-up[70]. Similarly, Edeline and colleagues reported a similar proportion of patients with iCCA downstaged to resectability (9/41, 22%) with TARE, a further two patients remained unresectable, but underwent liver transplantation. For the resected patients, 1-year OS was 88.9% and DFS was 66.8%. For both of the patients undergoing liver transplantation, solitary lung recurrence occurred at 15 and 16 mo and both were alive at 19 and 18 mo of follow-up[71].
Side effects were shown to be transient and mild in the majority of patients in these studies of SABR. Those studies which reported liver function tests, showed mildly deranged values of all parameters (alkaline phosphatase, alanine transaminase, aspartate transaminase and bilirubin) in most patients following SABR. Very few studies reported greater than 40% of patients having grade II symptoms. Of these, the majority are gastrointestinal side effects with nausea and diarrhoea being common.
Although bowel obstruction and perforation may be complications of radiotherapy, only one case of gastric perforation requiring surgery was found in the studies included in the review. Radiation hepatitis was rare and liver failure was reported in only 2 patients in all the studies included in this review.
One study evaluated the quality of life in patients undergoing SABR in the liver and showed a reduction in quality of life in terms of appetite and fatigue within 1 mo of treatment but returning to baseline after 3 mo. These features demonstrate overall that SABR is tolerated well, relative to other therapies[72].
A search of the clinicaltrials.gov registry (search terms “cholangiocarcinoma” and “stereotactic”) showed 2 actively recruiting trials evaluating stereotactic radiotherapy. Of these two, the CORRECT trial (NCT03898895) is a multicentre randomized trial evaluating a programmed cell death ligand 1 checkpoint inhibitor (Camrelizumab) with either SABR or conventional radiotherapy vs standard gemcitabine chemotherapy in unresectable iCCA[73]. The second is a phase II trial of nivolumab with SABR in unresectable iCCA and dCCA[74].
Of the remainder, 4 studies assess all types of liver tumours, 2 assess phCCA only, and the rest assess a mix of extrahepatic and intrahepatic tumours. These are all phase I and II trials.
In addition, the ABC-07 trial is actively recruiting and is a multicentre randomized controlled trial comparing chemotherapy vs chemotherapy and SABR in unresectable CCA (of all types) and gallbladder carcinoma[75].
Furthermore, the ACCTICA-1 trial is primarily assessing the superiority of gemcitabine and cisplatin vs capecitabine in patients with resected CCA and gallbladder adenocarcinoma. However, within this trial there is a sub-study evaluating conventional radiotherapy in patients with R1 resections[76,77].
Thus far, there have been no published randomized trials of SABR in any subgroup of iCCA, and the majority are retrospective single institution studies. Few studies have compared SABR to a control group or other locoregional therapies. There is limited literature on SABR as a downstaging modality prior to standard surgical resection of iCCA, despite evidence of excellent LC in patients who are inoperable.
High quality prospective clinical trials of SABR are urgently needed in homogeneous groups of iCCA, to explore its role as an adjuvant and neoadjuvant therapy either prior to resection or liver transplantation, and as a treatment modality in recurrent and unresectable disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: General Medical Council (UK), No. 7451513; Royal College of Surgeons of England, No. 9092145; International College of Surgeons, No. M21313; Faculty of Surgical Trainers of Edinburgh, Royal College of Surgeons of Edinburgh, No. 188646; American College of Surgeons, No. 03340060.
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y, China; Ryckman JM, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2055] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 3. | Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488-96; discussion 1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 6. | Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Saffioti F, Mavroeidis VK. Review of incidence and outcomes of treatment of cholangiocarcinoma in patients with primary sclerosing cholangitis. World J Gastrointest Oncol. 2021;13:1336-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-73; discussion 473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 865] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 9. | Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract Res Clin Gastroenterol. 2015;29:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1551] [Article Influence: 310.2] [Reference Citation Analysis (0)] |
| 11. | Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 13. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 693] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 14. | Ustundag Y, Bayraktar Y. Cholangiocarcinoma: a compact review of the literature. World J Gastroenterol. 2008;14:6458-6466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 15. | Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 16. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 17. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 18. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 577] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 19. | Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 20. | Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 21. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 823] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 22. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3169] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 23. | Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, Gollins S, Lofts F, Evans L, Meyer T, Anthoney A, Iveson T, Highley M, Osborne R, Bridgewater J. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101:621-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 25. | Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, Cucchetti A, Golfieri R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma-a Systemic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2021;44:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Labib PL, Davidson BR, Sharma RA, Pereira SP. Locoregional therapies in cholangiocarcinoma. Hepat Oncol. 2017;4:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Jackson MW, Amini A, Jones BL, Rusthoven CG, Schefter TE, Goodman KA. Treatment Selection and Survival Outcomes With and Without Radiation for Unresectable, Localized Intrahepatic Cholangiocarcinoma. Cancer J. 2016;22:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Shao F, Qi W, Meng FT, Qiu L, Huang Q. Role of palliative radiotherapy in unresectable intrahepatic cholangiocarcinoma: population-based analysis with propensity score matching. Cancer Manag Res. 2018;10:1497-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. 2017;49:e359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Olcina MM, Giaccia AJ. Reducing radiation-induced gastrointestinal toxicity - the role of the PHD/HIF axis. J Clin Invest. 2016;126:3708-3715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Phillips MH, Stelzer KJ, Griffin TW, Mayberg MR, Winn HR. Stereotactic radiosurgery: a review and comparison of methods. J Clin Oncol. 1994;12:1085-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 35. | Uematsu M, Shioda A, Tahara K, Fukui T, Yamamoto F, Tsumatori G, Ozeki Y, Aoki T, Watanabe M, Kusano S. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998;82:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 37. | Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Höss A, Schlegel W, Wannenmacher MF. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 350] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Thomas HR, Feng M. Stereotactic Body Radiation Therapy (SBRT) in Hepatocellular Carcinoma. Curr Hepatology Reports. 2021;20:12-22. [DOI] [Full Text] |
| 39. | Scorsetti M, Comito T, Clerici E, Franzese C, Tozzi A, Iftode C, Di Brina L, Navarria P, Mancosu P, Reggiori G, Fogliata A, Tomatis S, Torzilli G, Cozzi L. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. 2018;13:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D'Ambrosio D, Sharma S, Perry D, Kolker J, Davis J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat Oncol. 2018;13:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 41. | Paravati AJ, Healy E, Murphy JD, Song W, Hattangadi-Gluth J. Stereotactic body radiation therapy for primary hepatic malignancies and liver metastases. Transl Cancer Res. 2013;2:507-520. [DOI] [Full Text] |
| 42. | Koay EJ, Hanania AN, Hall WA, Taniguchi CM, Rebueno N, Myrehaug S, Aitken KL, Dawson LA, Crane CH, Herman JM, Erickson B. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract Radiat Oncol. 2020;10:e495-e507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 564] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 44. | Koay EJ, Owen D, Das P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin Radiat Oncol. 2018;28:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 45. | Sawrie SM, Fiveash JB, Caudell JJ. Stereotactic body radiation therapy for liver metastases and primary hepatocellular carcinoma: normal tissue tolerances and toxicity. Cancer Control. 2010;17:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Shen ZT, Zhou H, Li AM, Li B, Shen JS, Zhu XX. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:93541-93550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Liu MY, Lo CH, Lin CS, Chao HL, Yang JF, Lin KT, Fan CY, Su YF, Huang WY. Stereotactic ablative radiotherapy for patients with unresectable or medically inoperable cholangiocarcinoma. Tumori. 2017;103:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Thuehøj AU, Andersen NC, Worm ES, Høyer M, Tabaksblat EM, Weber B, Mortensen HR. Clinical outcomes after stereotactic ablative radiotherapy in locally advanced cholangiocarcinoma. Acta Oncol. 2022;61:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, Kaseb AO, Bishop AJ, Swanick CW, Koay EJ, Thames HD, Hong TS, Das P, Crane CH. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol. 2016;34:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 50. | Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 51. | Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Mahadevan A, Dagoglu N, Mancias J, Raven K, Khwaja K, Tseng JF, Ng K, Enzinger P, Miksad R, Bullock A, Evenson A. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J Cancer. 2015;6:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Brunner TB, Blanck O, Lewitzki V, Abbasi-Senger N, Momm F, Riesterer O, Duma MN, Wachter S, Baus W, Gerum S, Guckenberger M, Gkika E. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol. 2019;132:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Weiner AA, Olsen J, Ma D, Dyk P, DeWees T, Myerson RJ, Parikh P. Stereotactic body radiotherapy for primary hepatic malignancies - Report of a phase I/II institutional study. Radiother Oncol. 2016;121:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Kozak MM, Toesca DAS, von Eyben R, Pollom EL, Chang DT. Stereotactic Body Radiation Therapy for Cholangiocarcinoma: Optimizing Locoregional Control With Elective Nodal Irradiation. Adv Radiat Oncol. 2020;5:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Bisello S, Renzulli M, Buwenge M, Calculli L, Sallustio G, Macchia G, Deodato F, Mattiucci G, Cammelli S, Arcelli A, Giaccherini L, Cellini F, Brandi G, Guerri S, Cilla S, Golfieri R, Fuccio L, Morganti AG, Guido A. An atlas for clinical target volume definition, including elective nodal irradiation in definitive radiotherapy of biliary cancer. Oncol Lett. 2019;17:1784-1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Osmundson EC, Wu Y, Luxton G, Bazan JG, Koong AC, Chang DT. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Sebastian NT, Tan Y, Miller ED, Williams TM, Alexandra Diaz D. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin Transl Radiat Oncol. 2019;19:66-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Jung DH, Kim MS, Cho CK, Yoo HJ, Jang WI, Seo YS, Paik EK, Kim KB, Han CJ, Kim SB. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J. 2014;32:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Franzese C, Bonu ML, Comito T, Clerici E, Loi M, Navarria P, Franceschini D, Pressiani T, Rimassa L, Scorsetti M. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: single-institution analysis of outcome and toxicity. J Cancer Res Clin Oncol. 2020;146:2289-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Ibarra RA, Rojas D, Snyder L, Yao M, Fabien J, Milano M, Katz A, Goodman K, Stephans K, El-Gazzaz G, Aucejo F, Miller C, Fung J, Lo S, Machtay M, Sanabria JR. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Hammad AY, Berger NG, Eastwood D, Tsai S, Turaga KK, Christian KK, Johnston FM, Pawlik TM, Gamblin TC. Is Radiotherapy Warranted Following Intrahepatic Cholangiocarcinoma Resection? Ann Surg Oncol. 2016;23:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Kim KS, Kim HY, Kim K, Yi NJ, Suh KS, Chie EK. Postoperative Chemoradiotherapy for R1 Resected Intrahepatic Cholangiocarcinoma. J Liver Dis. 2018;18:115-120. [DOI] [Full Text] |
| 65. | Jiang W, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, Zhang JY, Chen YX, Tan YS. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol. 2010;136:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PLoS One. 2020;15:e0229292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Wong M, Kim J, George B, Eriksen C, Pearson T, Robbins J, Zimmerman MA, Hong JC. Downstaging Locally Advanced Cholangiocarcinoma Pre-Liver Transplantation: A Prospective Pilot Study. J Surg Res. 2019;242:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Sandler KA, Veruttipong D, Agopian VG, Finn RS, Hong JC, Kaldas FM, Sadeghi S, Busuttil RW, Lee P. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol. 2016;1:237-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, Matsumoto M, Hata Y, Noda Y, Sui K, Sueda T. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J Surg. 2018;42:2910-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, Boucher E, Boudjema K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 71. | Edeline J, Du FL, Rayar M, Rolland Y, Beuzit L, Boudjema K, Rohou T, Latournerie M, Campillo-Gimenez B, Garin E, Boucher E. Glass Microspheres 90Y Selective Internal Radiation Therapy and Chemotherapy as First-Line Treatment of Intrahepatic Cholangiocarcinoma. Clin Nucl Med. 2015;40:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Klein J, Dawson LA, Jiang H, Kim J, Dinniwell R, Brierley J, Wong R, Lockwood G, Ringash J. Prospective Longitudinal Assessment of Quality of Life for Liver Cancer Patients Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;93:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Kuang M. COmbination of Radiotherapy With Anti-PD-1 Antibody for unREseCtable inTrahepatic Cholangiocarcinoma. [cited 10 March 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03898895. |
| 74. | Shamseddine A. A Study of BMS-936558 With SBRT After Induction Chemotherapy in Cholangiocarcinoma. [cited 10 March 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT04648319?term=Stereotactic&cond=Cholangiocarcinoma&draw=2&rank=4. |
| 75. | ISRCTN registry. A trial looking at whether stereotactic radiotherapy together with chemotherapy is a useful treatment for people with locally advanced bile duct cancer (ABC-07). (e-pub ahead of print. [cited 10 March 2022]. Available from: https://www.isrctn.com/ISRCTN10639376. |
| 76. | Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J, Schrum S, Shannon J, Vettorazzi E, Wege H. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 77. | NIH. Adjuvant Chemotherapy With Gemcitabine and Cisplatin Compared to Standard of Care After Curative Intent Resection of Biliary Tract Cancer-Full Text View-ClinicalTrials.gov. [cited 10 March 2022]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02170090. |