Published online Apr 15, 2022. doi: 10.4251/wjgo.v14.i4.920
Peer-review started: October 9, 2021
First decision: December 12, 2021
Revised: January 4, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 15, 2022
Processing time: 187 Days and 19.3 Hours
The effectiveness of regorafenib plus programmed cell death-1 (PD-1) inhibitor in treating microsatellite stable (MSS) metastatic colorectal cancer (mCRC) remains controversial.
To investigate the benefits of regorafenib combined with PD-1 inhibitor in treating MSS mCRC and explore indicators predicting response.
This retrospective study included a total of 30 patients with microsatellite stable metastatic colorectal cancer treated with regorafenib combined with programmed cell death-1 inhibitor at Henan Provincial People’s Hospital between December 2018 and December 2020. During a 4-wk treatment cycle, regorafenib was performed for 3 continuous weeks. PD-1 inhibitor was intravenously injected starting on the first day of the oral intake of regorafenib. We reviewed tumor response, progression-free survival (PFS), overall survival, and treatment-related adverse events (TRAEs) and evaluated association between platelet-to-lymphocyte ratio (PLR) and outcomes in this retrospective study.
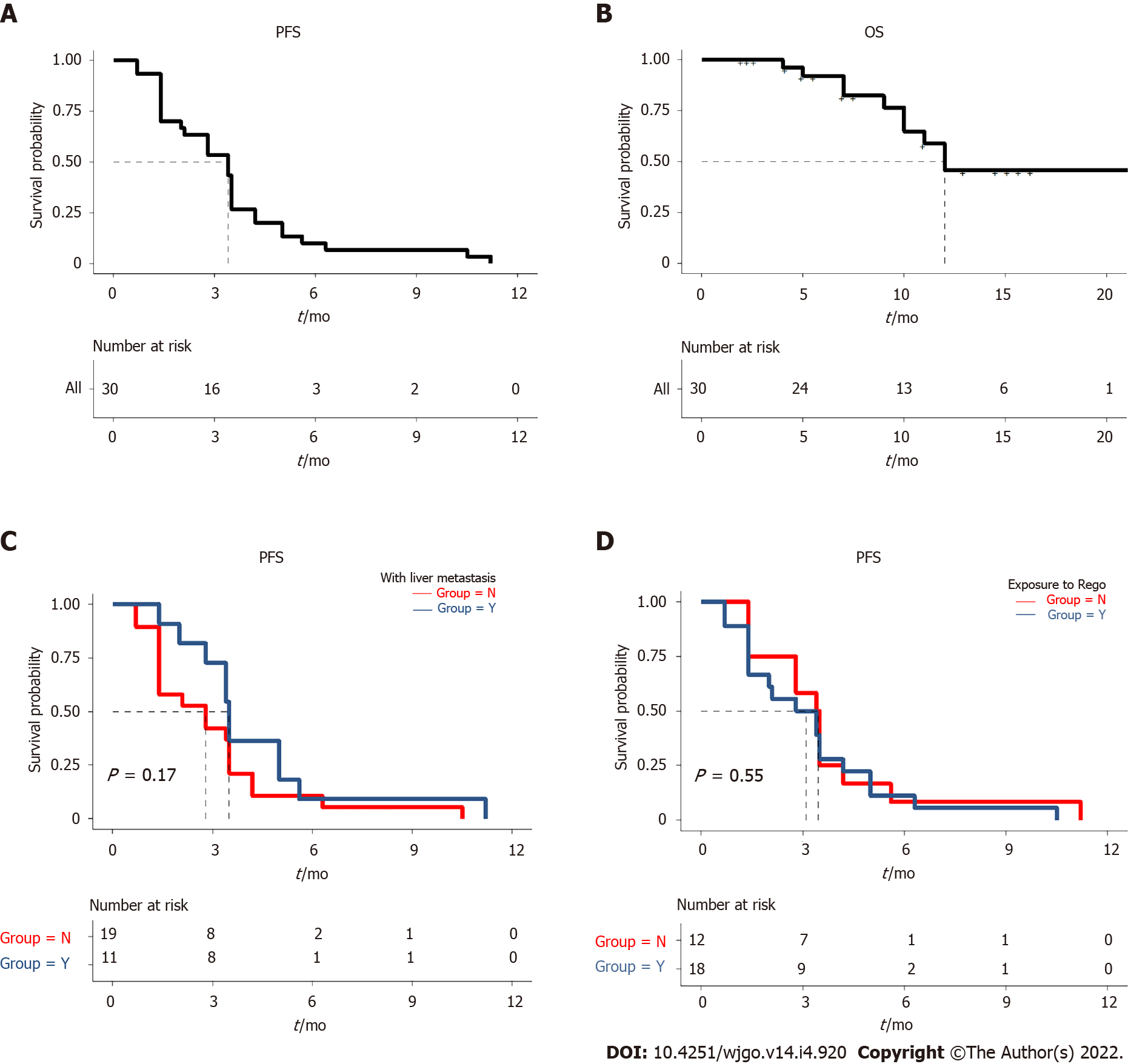
Stable disease and progressive disease were found in 18 (60.0%) and 12 (40.0%) patients, respectively. The disease control rate was 60.0%. The median follow-up time was 12.0 mo, and median PFS was 3.4 mo [95% confidence interval (CI): 2.2-4.6 mo]. Of the 12 patients with progressive disease, 10 (83.3%) had liver metastasis before starting the combined treatment. Among the 18 patients with SD, 10 (55.6%) did not have liver metastases. One patient without liver metastases at baseline was found with a substantially prolonged PFS of 11.2 mo. The liver metastasis, the choice of programmed cell death-1 inhibitor other than nivolumab or pembrolizumab and previous exposure to regorafenib was’t associated with treatment outcome. The median PFS in the low-PLR group was 4.2 mo (95%CI: 3.5-4.9 mo), compared with 2.8 mo (95%CI: 1.4-4.2 mo) in the high-PLR group (P = 0.005). The major TRAEs included hand-foot syndrome (33.3%), hypertension (23.3%), malaise (20.0%), and gastrointestinal reaction (16.7%). The incidence of grade 3 TRAEs was 13.3% (4/30), which comprised abnormal capillary proliferation (n = 1), transaminase elevation (n = 1), and hand-foot syndrome (n = 2). No grade 4 or higher toxicity was observed.
Regorafenib combined with PD-1 inhibitor could lead to a longer PFS in some patients with MSS mCRC. The PLR might be a prediction of the patient response to this therapy.
Core Tip: The use of regorafenib combined with programmed cell death-1 inhibitor in the treatment of refractory microsatellite stable colorectal cancer has contradictory results in some small-scale studies. The purpose of this paper is to analyze the real-world data of our center in the past 2 years so as to provide more treatment experience and reference for treatment selection. The progression-free survival and overall survival of patients with refractory microsatellite stable colorectal cancer treated with regorafenib combined with programmed cell death-1 inhibitor were analyzed retrospectively, and the safety and adverse reactions under different doses were reviewed. The platelet-to-lymphocyte ratio was found as a potential screening index for patients with prolonged progression-free survival.
- Citation: Xu YJ, Zhang P, Hu JL, Liang H, Zhu YY, Cui Y, Niu P, Xu M, Liu MY. Regorafenib combined with programmed cell death-1 inhibitor against refractory colorectal cancer and the platelet-to-lymphocyte ratio’s prediction on effectiveness. World J Gastrointest Oncol 2022; 14(4): 920-934
- URL: https://www.wjgnet.com/1948-5204/full/v14/i4/920.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i4.920
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide. More than 1.8 million new patients with CRC were reported in 2018, of which 881000 died of CRC[1,2]. In China, CRC is the fifth leading cause of cancer-related death. The number of CRC-related deaths was about 191000 in 2015[3]. A variety of patients are diagnosed with advanced CRC, accompanied by distant metastases in addition to the primary tumor[4]. Despite multidisciplinary management based on surgery, systemic therapy, and radiotherapy[5-7], the prognosis of patients with advanced CRC is still poor, with 5-year survival rates of 71% and 14% for regional and distant disease, respectively[8].
The Guidelines of the Chinese Society of Clinical Oncology (CSCO) recommend chemotherapy with or without targeted therapy (such as cetuximab and bevacizumab) for the first- and second-line therapies of advanced CRC[9]. Although the CSCO guidelines also recommended later-line therapy for CRC after progression, the treatment efficacy is generally limited[9]. Regorafenib is an oral, small-molecular multi-target kinase inhibitor that can exert anti-tumor effects through inhibiting several key processes, such as tumor cell proliferation, metastasis, angiogenesis, and immune escape[10,11]. The international CORRECT trial promoted regorafenib as the standard drug for treating metastatic CRC (mCRC)[12].
The emergence of immunotherapy in recent years has brought long-term survival benefits for many patients. Still, to date, only patients with microsatellite instablility-high mCRC could benefit from immunotherapy using a single drug[13]. Considering that the efficacy of single targeted drug was suboptimal, combination therapy could bring new hopes for patients. The preliminary findings from the REGONIVO study reported the efficacy of regorafenib combined with nivolumab. The objective response rate (ORR) was 33% in 24 Asian patients with proficient mismatch repair (MMR)/ microsatellite stable (MSS) refractory mCRC, and the median progression-free survival (PFS) was prolonged by more than 6 mo. Therefore, the findings possibly provided new chances for patients with repeated failures after third- or further-line therapies to prolong the survival time and improve the quality of life[14]. The combination of regorafenib with programmed cell death-1 (PD-1) for patients with refractory CRC after multi-line standard therapies is now used by many medical centers worldwide. A retrospective study in 18 patients with refractory MSS mCRC, including five Asian patients, performed by the National Cancer Institute in the United States recently failed to show the effectiveness of regorafenib combined with nivolumab or pembrolizumab but suggested that patients without liver metastases could benefit from the treatment[15]. A study conducted in 23 Chinese patients with MSS advanced CRC also did not demonstrate the effectiveness of this combination[16]. These two studies[15,16] were inconsistent with the REGONIVO study[14]. Hence, additional clinical studies are needed to investigate the benefits and adverse events of regorafenib combined with PD-1 inhibitors.
The effects of specific treatments among different patients and different studies vary greatly. Thus, identifying factors that could predict the prognosis of patients treated with regorafenib combined with PD-1 inhibitors is clinically relevant. Carcinoembryonic antigen (CEA) is commonly used in clinical practice to monitor treatment efficacy in patients with CRC[17,18]. Other indicators are also related to the prognosis of patients with cancer[19-23], such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR).
Despite the emergence of new drugs and the changes in the combination of targeted therapy, the effectiveness of mCRC treatment is still suboptimal, especially the treatments after third-line therapy, with a 5-year survival rate of only 11%[24]. Therefore, this retrospective study investigated patients with MSS mCRC treated with regorafenib combined with a PD-1 inhibitor in the last 2 years. This study may suggest a novel therapeutic approach that could be tried in a clinical trial.
This retrospective study included patients with MSS mCRC treated with regorafenib combined with PD-1 inhibitor at Henan Provincial People’s Hospital between December 2018 and December 2020. This study was approved by the ethics committee of People’s Hospital of Zhengzhou University (Henan Province, China) and performed in accordance with the Declaration of Helsinki. The requirement for informed consent was waived by the committee because of the retrospective nature of the study.
The inclusion criteria were: (1) Histologically or cytologically proven with MSS mCRC; (2) Treated with more than two lines of standard chemotherapy regimens (including fluorouracil, oxaliplatin, and irinotecan, with or without biological agents such as bevacizumab and cetuximab); (3) Treated with regorafenib combined with PD-1 inhibitor (due to the accessibility of drugs and financial burden of patients, other low-cost PD-1 inhibitors approved in China, such as camrelizumab, sintilimab, toripalimab, and tislelizumab, could also be used in addition to nivolumab and pembrolizumab); (4) With evaluable lesions and with detailed clinical data and follow-up results; and (5) Downregulation of the expression of four MMR enzymes (MutL homolog 1/MutS homolog 2/MutS homolog 6/PMS1 homolog 2) assessed by immunohistochemistry, or the MMR/MSI status was evaluated by the 2B3D method.
During a 4 wk treatment cycle, oral drug administration was performed for 3 continuous weeks. Regorafenib was orally administered at 80, 120, or 160 mg once per day. Dose reduction or temporary discontinuation of regorafenib was performed for the patients with treatment-related toxicities. PD-1 inhibitor was intravenously injected starting on the 1st day of the oral intake of regorafenib, including 240 mg every 3 wk for toripalimab, 200 mg every 2 or 3 wk for camrelizumab, 240 mg every 2 wk for nivolumab, 200 mg every 3 wk for pembrolizumab and sintilimab, or 200 mg every 3 wk for tislelizumab.
Collected data included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), site of the primary tumor, site of the metastases, MSI/MMR, gene status, lines of treatment, and previous treatments. The blood routine examination and CEA results before treatment and after three and five cycles of combination therapy were longitudinally analyzed. CEA was detected by microarray chemiluminescence immunoassay (Sunlant Biological SLXP-001, Wuxi, Jiangsu Province, China). The platelet and lymphocyte counts were measured using a Hessian-Meikang XN-9000 automatic modular blood and body fluid analyzer. The PLR was calculated as the absolute platelet count divided by the absolute lymphocyte count in full blood.
According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, tumor responses were evaluated every two or three cycles of immunotherapy. If signs of rapid disease progression were noted, the evaluation was performed at earlier time points. ORR included complete response (CR) and partial response (PR). Disease control rate (DCR) was defined as the sum of the ORR and stable disease (SD) rate. PFS was defined as the time from the start of treatment to disease progression or death. Overall survival (OS) referred to the time from the start of treatment to death. Toxicity was evaluated according to the Common Toxicity Criteria for Adverse Events Version 5.0 (CTCAE 5.0) and the 2019 CSCO immune checkpoint inhibitor-related toxicity management guideline[25]. Follow-up was censored on April 15, 2021.
SPSS 26.0 (IBM, Armonk, NY, United States) was used for statistical analysis. Continuous data with a normal distribution (according to the Shapiro-Wilk test) were described using means ± SD, while continuous data without a normal distribution were described as medians and interquartile range (Q1, Q3). The t-test was used for comparing continuous data with a normal distribution, and the rank-sum test was used for comparing continuous data without a normal distribution. Categorical data were presented as n (%) and tested using the χ2 test. The receiver operating characteristic (ROC) curve was used to estimate the best cutoff value of NLR and PLR before treatment. The patients were divided into the low-NLR, high-NLR, low-PLR, and high-PLR groups according to the cut-off value of ROC curve. The Kaplan-Meier method was used for univariable survival analysis. The log-rank test was used to analyze the differences in PFS and OS among different influencing factors. Two-sided P values < 0.05 were considered statistically significant.
Between December 2018 and December 2020, a total of 30 patients with MSS mCRC were included. Table 1 and Supplementary Table 1 present the characteristics of the patients. The patients were treated with regorafenib combined with PD-1 inhibitor as the third- (46.7%) or further-line therapy (53.3%). Of these patients, 12 (40.0%) were ≥ 60 years and 18 (60.0%) < 60 years. Fourteen (46.7%) and sixteen (53.3%) were males and females, respectively. Twenty-one (70.0%) and 9 (30.0%) patients had an ECOG PS of 0-1 and ≥ 2, respectively. The primary lesion was on the left side (including the left colon, sigmoid colon, and rectum) in 24 patients (80.0%) and on the right side (including the ascending colon and liver curvature of the transverse colon) in 6 patients (20.0%). Eighteen (60.0%) patients had liver metastases, 11 (36.7%) had lung metastases, 6 (20.0%) had peritoneal metastases, and 18 patients (60.0%) had multiple metastases in ≥ two sites (Table 1). All patients were proven with MSS (proficient MMR) type CRC using immunohistochemistry or polymerase chain reaction, and 1 (3.3%) patient had been treated with PD-1 inhibitor before. The genetic test showed that 7 (36.7%) and 12 (60.0%) patients had RAS mutation and wild-type RAS, respectively, while 11 patients did not have a genetic mutation test. No patient had BRAF mutation.
| No. | Age (yr) | Sex | ECOG PS | Primary tumor | Site of metastases | Liver metastases | RAS and BRAF mutation | Lines of treatment | Prior 5-Fu | Prior platinum | Prior irinotecan | Prior bevacizumab | Prior cetuximab | Prior regorafenib | Prior PD-1 |
| 1 | 56 | Female | 1 | Sigmoid colon | Abdominal metastases | No | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 2 | 62 | Female | 3 | Ascending colon | Multiple metastases | Yes | Not tested | 4 | Yes | Yes | Yes | Yes | No | Yes | No |
| 3 | 51 | Female | 0 | Rectum | Multiple metastases | Yes | Not tested | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 4 | 52 | Female | 1 | Sigmoid colon | Multiple metastases | No | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 5 | 66 | Female | 1 | Ascending colon | Multiple metastases | Yes | Not tested | 4 | Yes | Yes | Yes | Yes | No | Yes | No |
| 6 | 57 | Female | 2 | Sigmoid colon | Multiple metastases | No | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 7 | 48 | Female | 3 | Liver curvature of the transverse colon | Multiple metastases | Yes | Not tested | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 8 | 61 | Female | 2 | Rectum | Pelvic metastases | No | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 9 | 65 | Female | 1 | Rectum | Ovary metastases | No | Wild-type | 4 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 10 | 65 | Female | 0 | Ascending colon | Lung metastases | No | Not tested | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 11 | 56 | Female | 2 | Left half of the colon | Ovary metastases | No | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 12 | 27 | Male | 0 | Sigmoid colon | Pelvic metastases | No | Wild-type | 4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13 | 58 | Male | 1 | Sigmoid colon | Liver metastases | Yes | Not tested | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 14 | 70 | Female | 1 | Rectum | Lung metastases | No | Not tested | 4 | Yes | Yes | Yes | Yes | No | No | No |
| 15 | 54 | Male | 1 | Ascending colon | Multiple metastases | Yes | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 16 | 55 | Male | 1 | Rectum | Multiple metastases | Yes | NRAS mutation | 4 | Yes | Yes | Yes | Yes | No | Yes | No |
| 17 | 48 | Female | 0 | Rectum | Multiple metastases | Yes | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 18 | 55 | Male | 1 | Rectum | Multiple metastases | Yes | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 19 | 64 | Male | 2 | Rectum | Multiple metastases | Yes | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 20 | 58 | Female | 0 | Ascending colon | Multiple metastases | Yes | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 21 | 44 | Female | 2 | Sigmoid colon | Multiple metastases | Yes | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 22 | 51 | Male | 1 | Rectum | Multiple metastases | Yes | Not tested | 4 | Yes | Yes | Yes | Yes | No | Yes | No |
| 23 | 56 | Male | 1 | Rectum | Lung metastases | No | Not tested | 4 | Yes | Yes | Yes | Yes | No | No | No |
| 24 | 61 | Male | 2 | Rectum | Multiple metastases | Yes | Not tested | 4 | Yes | Yes | Yes | Yes | No | No | No |
| 25 | 73 | Male | 0 | Rectum | Multiple metastases | Yes (radiofrequency) | Wild-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
| 26 | 61 | Male | 1 | Rectum | Multiple metastases | Yes | Wild-type | 4 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 27 | 58 | Male | 2 | Sigmoid colon | Multiple metastases | No | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 28 | 57 | Female | 1 | Sigmoid colon | Liver metastases | Yes (hepatectomy) | KRAS mutation | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 29 | 67 | Male | 2 | Rectum | Liver metastases | Yes | Not tested | 3 | Yes | Yes | Yes | Yes | No | No | No |
| 30 | 61 | Male | 1 | Sigmoid colon | Peritoneal metastasis | No | Wide-type | 3 | Yes | Yes | Yes | Yes | Yes | No | No |
Nineteen (63.3%) patients were treated with regorafenib for a median treatment duration of 4.3 mo [95% confidence interval (CI): 2.0-6.7 mo] before the combination therapy in this study. During the combination therapy, the initial dose of regorafenib was 80, 120, and 160 mg in 18, 8, and 4 patients, respectively. Of the 30 patients receiving combination therapy, 9 were treated with sintilimab, 8 with toripalimab, 5 with camrelizumab, 4 with tislelizumab, 3 with nivolumab, and 1 with pembrolizumab (Table 1 and Supplementary Table 1).
According to RECIST 1.1, 18 (60.0%) of the 30 patients who received combination therapy achieved SD, and 12 (40.0%) had progressive disease (PD); no PR or CR was found. The DCR was 60.0% (Table 2 and Supplementary Table 1). Of the 12 patients with PD, 10 (83.3%) had liver metastases at baseline. Among the 18 patients with SD, 10 (55.6%) had no liver metastases.
| No. | Initial dose of regorafenib (mg) | Dose adjustment (mg) | PD-1 inhibitor used in combination | Number of treatment cycles | Best response | PFS (mo) | OS (mo) |
| 1 | 120 | Camrelizumab | 16 | SD | 11.2 | 12.0 | |
| 2 | 80 | Tislelizumab | 7 | SD | 5.0 | 7.0 | |
| 3 | 160 | 160→80 | Nivolumab | 4 | PD | 3.4 | Not reached |
| 4 | 80 | Camrelizumab | 4 | PD | 3.4 | 4.0 | |
| 5 | 80 | Tislelizumab | 3 | SD | 2.0 | 5.0 | |
| 6 | 80 | Toripalimab | 5 | SD | 3.5 | Not reached | |
| 7 | 80 | Tislelizumab | 7 | SD | 5.0 | 9.0 | |
| 8 | 80 | Pembrolizumab | 5 | PD | 3.5 | Not reached | |
| 9 | 120 | Sintilimab | 4 | SD | 2.8 | Not reached | |
| 10 | 160 | 160→80 | Toripalimab | 8 | SD | 5.6 | Not reached |
| 11 | 80 | Tislelizumab | 2 | SD | 1.4 | Not reached | |
| 12 | 160 | Toripalimab | 4 | SD | 2.8 | Not reached | |
| 13 | 120 | Nivolumab | 6 | SD | 4.2 | Not reached | |
| 14 | 80 | Toripalimab | 5 | SD | 3.5 | Not reached | |
| 15 | 120 | Sintilimab | 6 | SD | 3.5 | Not reached | |
| 16 | 80 | Nivolumab | 15 | SD | 10.5 | Not reached | |
| 17 | 160 | 160→80 | Sintilimab | 4 | PD | 2.8 | Not reached |
| 18 | 80 | Camrelizumab | 5 | PD | 3.5 | Not reached | |
| 19 | 80 | Toripalimab | 2 | SD | 1.4 | Not reached | |
| 20 | 120 | Camrelizumab | 4 | PD | 3.4 | 7.0 | |
| 21 | 80 | Toripalimab | 2 | PD | 1.4 | Not reached | |
| 22 | 120 | Camrelizumab | 1 | SD | 0.7 | 12.0 | |
| 23 | 80 | Sintilimab | 2 | PD | 1.4 | 10.0 | |
| 24 | 80 | Toripalimab | 9 | SD | 6.3 | 11.0 | |
| 25 | 120 | 120→80 | Sintilimab | 2 | PD | 1.4 | Not reached |
| 26 | 80 | Sintilimab | 3 | SD | 2.1 | 10.0 | |
| 27 | 80 | Toripalimab | 6 | SD | 4.2 | Not reached | |
| 28 | 120 | Sintilimab | 2 | PD | 1.4 | Not reached | |
| 29 | 80 | Sintilimab | 1 | PD | 0.7 | not reached | |
| 30 | 80 | Sintilimab | 2 | PD | 1.4 | Not reached |
Up to April 15, 2021, the median follow-up time was 12.0 mo. The median PFS of the patients was 3.4 mo (95%CI: 2.2-4.6 mo) (Figure 1A and B). One patient without liver metastases at baseline was found with a substantially prolonged PFS of 11.2 mo. Still, of the patients with liver metastases at baseline, no prolonged PFS was observed in 2 patients even after the combination therapy using local hepatectomy and radiofrequency ablation. The median PFS was 2.8 mo (95%CI: 0.8-4.7 mo) for the 18 patients with liver metastases and 3.5 mo (95%CI: 3.0-4.0 mo) for the 12 patients without liver metastases, with no significant difference (P = 0.17) (Figure 1C). The median PFS of the 19 patients treated with previous regorafenib treatment was 2.8 mo (95%CI: 1.0-4.6 mo), while the median PFS of the 11 patients naïve to regorafenib was 3.4 mo (95%CI: 2.8-4.0 mo); the difference was not statistically significant (P = 0.55) (Figure 1D). The median PFS was not significantly different between the patients with or without RAS mutation (P = 0.37). The 4 patients with imported PD-1 inhibitor (nivolumab or pembrolizumab) and 26 patients with domestic PD-1 inhibitor (sintilimab, toripalimab, camrelizumab, or tislelizumab) did not differ in median PFS (P = 0.31). At the time of April 15, 2021, the data for OS were still not mature. Detailed PFS and OS data can be found in Table 2.
Of the 30 patients, treatment-related adverse events (TRAEs) occurred in 17 patients (56.7%). The major TRAEs included hand-foot syndrome (33.3%), hypertension (23.3%), malaise (20.0%), and gastrointestinal reaction (16.7%). The other TRAEs included transaminase elevation (13.3%), diarrhea (10.0%), abnormal capillary proliferation (6.7%), thrombocytopenia (6.7%), hypothyroidism (6.7%), proteinuria (6.7%), rash (6.7%), anemia (3.3%), myocardial enzyme elevation (3.3%), and oral mucositis (3.3%). The incidence of grade 3 TRAEs was 13.3% (4/30), which comprised abnormal capillary proliferation (n = 1), transaminase elevation (n = 1), and hand-foot syndrome (n = 2). No grade 4 or higher toxicity was observed. For patients treated with different initial doses of regorafenib, more grade 3 TRAEs were observed in the 160 mg group. Specifically, 3 of the 4 patients treated with an initial dose of regorafenib of 160 mg had grade 3 TRAEs, including grade 3 hand-foot syndrome in 2 patients and grade 3 abnormal capillary proliferation in 1 patient. For all 3 patients, the dose of regorafenib was reduced to 80 mg. In the 8 patients treated with an initial dose of regorafenib of 120 mg, 1 patient had grade 3 transaminase elevation. For this patient, regorafenib and PD-1 inhibitor were discontinued until the transaminase levels returned to normal. Subsequently, the regorafenib dose was reduced to 80 mg to treat the patient combined with a PD-1 inhibitor. All 18 patients in the 80 mg group had good tolerance; they could tolerate the therapy after symptomatic treatment until PD. Table 3 lists the details of the TRAEs.
| Adverse event | Number of patients (n = 30), n (%) | ||
| Any grade | Grade 1-2 | Grade 3 | |
| Any event | 17 (56.7) | 13 (43.3) | 4 (13.3) |
| Hand-foot syndrome | 10 (33.3) | 8 (26.7) | 2 (6.7) |
| Hypertension | 7 (23.3) | 7 (23.3) | 0 |
| Malaise | 6 (20.0) | 6 (20.0) | 0 |
| Gastrointestinal reaction | 5 (16.7) | 5 (16.7) | 0 |
| Transaminase elevation | 4 (13.3) | 3 (10.0) | 1 (3.3) |
| Diarrhea | 3 (10.0) | 3 (10.0) | 0 |
| Abnormal capillary proliferation | 2 (6.7) | 1 (3.3) | 1 (3.3) |
| Hypothyroidism | 2 (6.7) | 2 (6.7) | 0 |
| Proteinuria | 2 (6.7) | 2 (6.7) | 0 |
| Rash | 2 (6.7) | 2 (6.7) | 0 |
| Thrombocytopenia | 2 (6.7) | 2 (6.7) | 0 |
| Anemia | 1 (3.3) | 1 (3.3) | 0 |
| Myocardial enzyme elevation | 1 (3.3) | 1 (3.3) | 0 |
| Oral mucositis | 1 (3.3) | 1 (3.3) | 0 |
| Leukopenia/neutropenia | 0 | 0 | 0 |
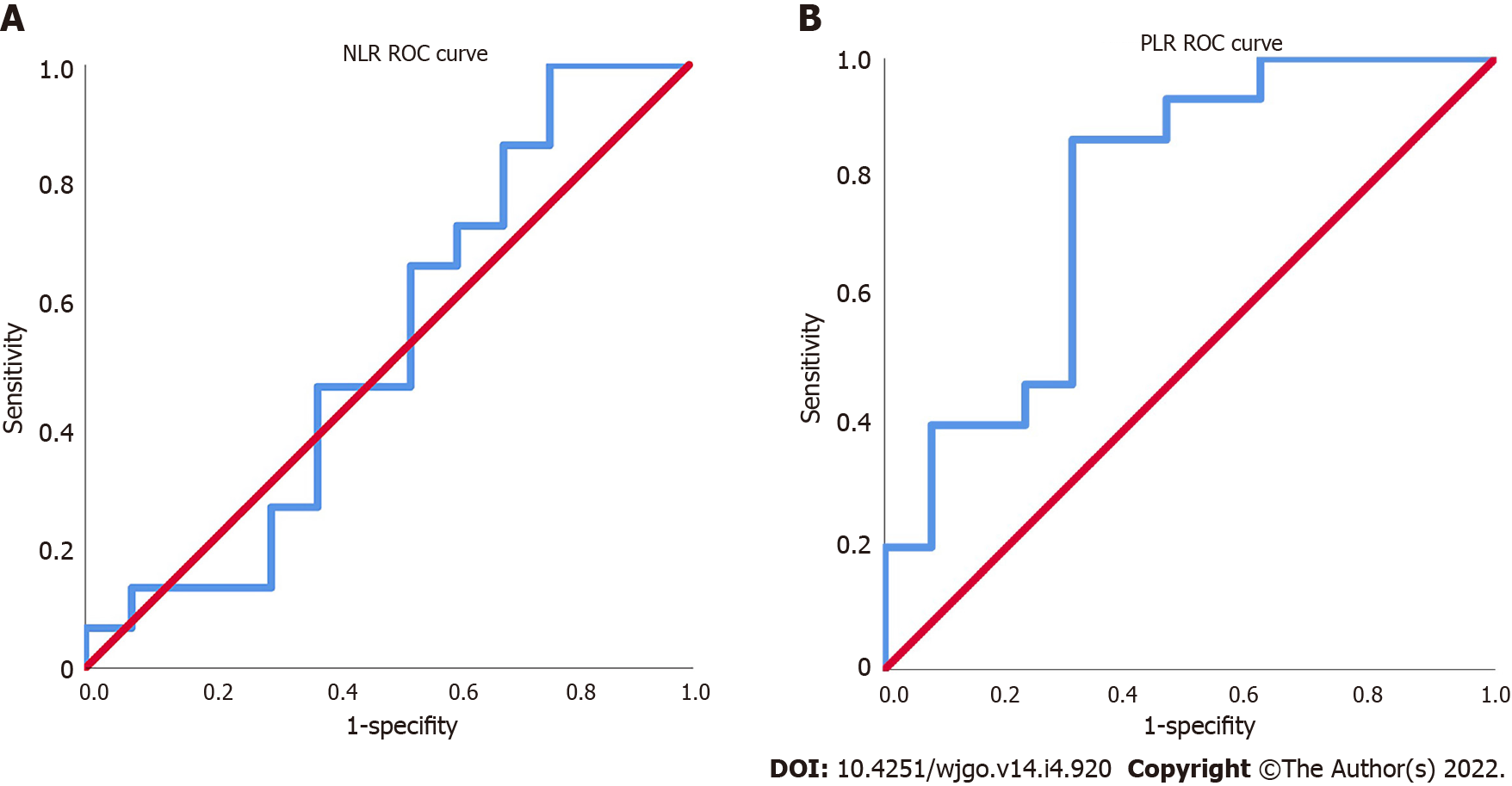
The NLR and PLR of the 30 patients were calculated, and the ROC curves were plotted. The area under the ROC curve (AUC) of NLR was 0.533, which could not effectively predict the treatment efficacy (Figure 2A). The AUC of PLR was 0.774, the maximum Youden index was 0.549, the corresponding cutoff value of PLR was 118, and the sensitivity and specificity were 85.7% and 69.2%, respectively (Figure 2B).
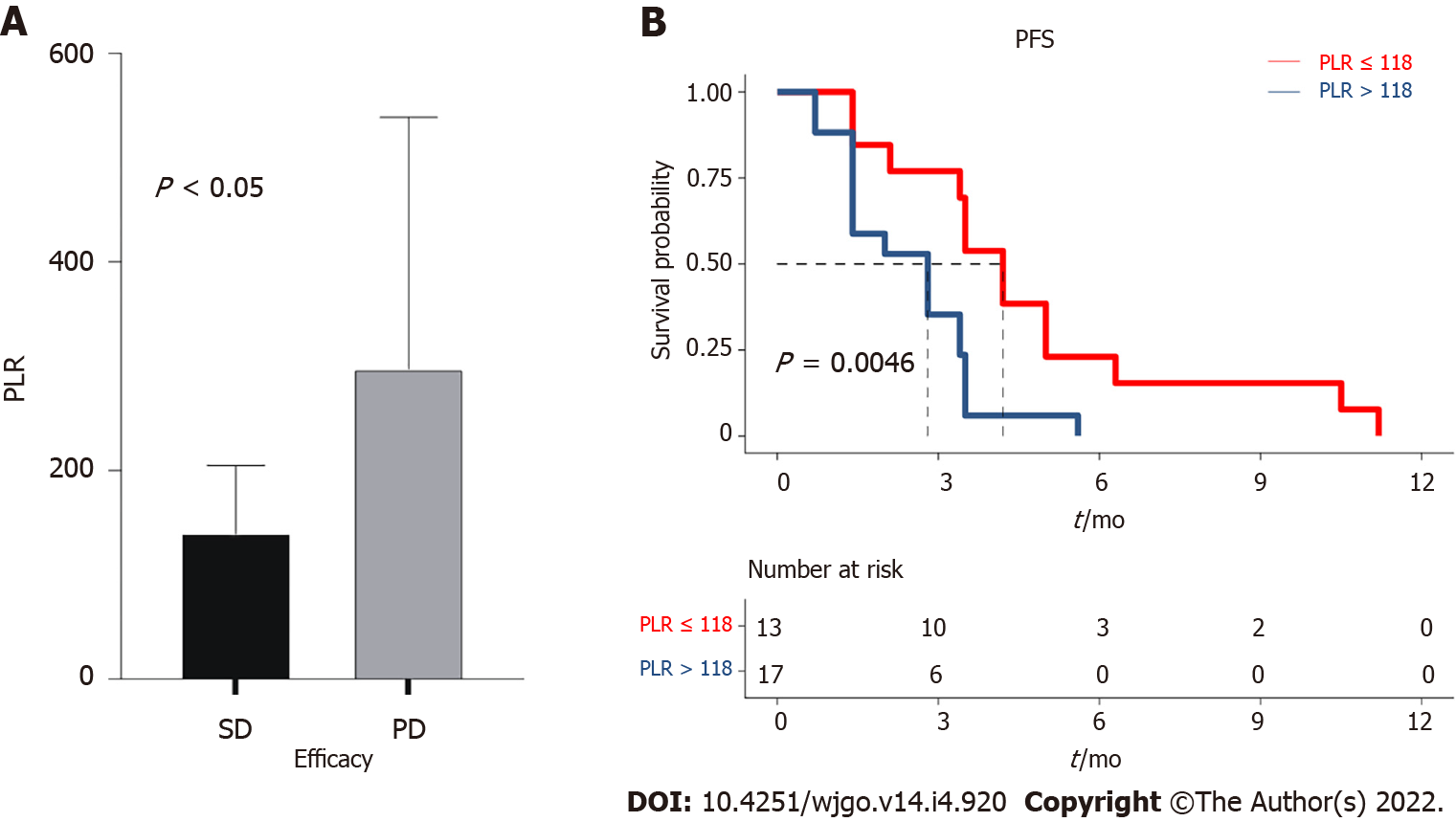
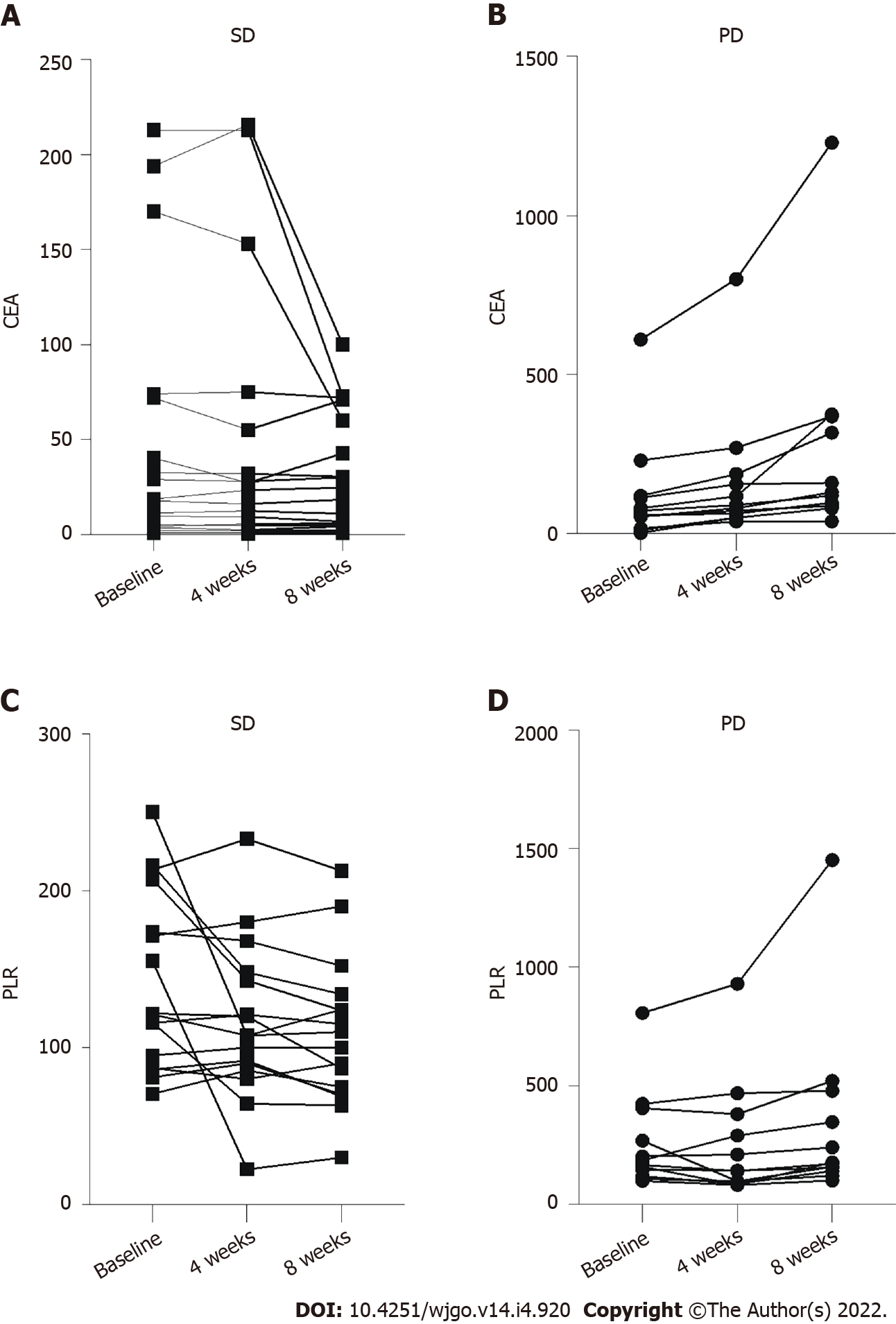
The median PLR was 151.1 (96.0-211.8). The PLR values differed significantly between the SD and PD groups (P = 0.047) (Figure 3A). Subsequently, the patients were divided into the PLR < 118 group (low-PLR group, n = 11) and PLR ≥ 118 group (high-PLR group, n = 19) according to the cutoff value. The median PFS in the low-PLR group was 4.2 mo (95%CI: 3.5-4.9 mo), compared with 2.8 mo (95%CI: 1.4-4.2 mo) in the high-PLR group (P = 0.005) (Figure 3B). The PLR may be an indicator to predict patient response and selected those who with longer PFS to regorafenib combined with PD-1 inhibitor. For the 12 patients with PD, the CEA increased after one and two cycles of treatment (Figure 4A). For the 18 patients in the SD group, the CEA in 1 patient increased transiently with a low amplitude after the first treatment cycle (higher than the normal range). In contrast, after the second treatment cycle, the CEA in all the patients was stable or decreased compared with before treatment (Figure 4B). The PLR in 3 patients decreased slightly after the first treatment cycle, while the PLR in 11 patients increased after two treatment cycles (Figure 4C). Still, the PLR level in 5 patients increased slightly after the first treatment cycle (higher than the cutoff value). The PLR in 1 patient still increased after the second treatment cycle (Figure 4D). The sensitivity of PLR was slightly lower than that of CEA for predicting treatment response.
This study aimed to investigate the benefits of regorafenib combined with a PD-1 inhibitor in treating MSS mCRC and explore indicators predicting treatment response and prognosis. Treatment using regorafenib combined with PD-1 inhibitor could lead to a longer PFS in some patients with MSS mCRC with failure to standard treatment. Our study also analyzed and compared the PLR of the patients in different treatment cycles with the corresponding CEA levels and explored the possible predictive value of PLR for predicting the response to treatment.
Recently, many studies on combination therapy with immunotherapy were performed to improve the immune responses and clinical efficacies on malignant tumors[26,27]. Still, studies on immunotherapy combined with vascular endothelial growth factor (VEGF) inhibitors have not shown significant improvements in PFS or OS[28,29]. Combination therapy using regorafenib and PD-1 inhibitors has already shown synergistic effects in mouse models[15,30]. In addition, the REGONIVO trial in Japan reported that the response rate was 36%, and the median PFS was 7.9 mo in 25 patients with mCRC (including one with microsatellite instablility-high mCRC) treated with regorafenib plus nivolumab[14]. On the other hand, a retrospective study in the United States did not replicate the findings of the REGONIVO study[15]. Specifically, the clinical responses of the patients were relatively poor; the rate of disease progression was as high as 69%, and the rate of SD was only 31%, without PR or CR[15]. A retrospective study in Shandong, China analyzed the data of 23 patients; SD was found in 18 patients, and the DCR was 78.3%; PD was found in 5 patients, and the PD rate was 21.7%[16]. In the present study, the data of 30 patients were retrospectively analyzed, making it the largest study to date. Of these patients, 18 had SD during the treatment, and the DCR was 60.0%, which was substantially higher than the 31% reported by the American study[15]. The median PFS was 3.4 mo in this study, which was not as outstanding as in the REGONIVO study (7.9 mo) but was longer than the 2.0 mo reported by the American study and comparable with the 3.1 mo reported by the Shandong study. The PFS in 2 patients was longer than 10 mo and was 11.2 mo for the patient with the longest PFS.
The differences between the findings of this study and those of the American study could be due to the following reasons. First, 77.8% of the patients included in the American study had baseline liver metastases. Second, only 4 Asian patients were included in the American study, and patients of different ethnicities could respond differently to the treatment. Liver metastasis was considered an important factor influencing the study results. As an immune-tolerant organ, the liver is related to high percentages of immune suppressor cells[31]. The immune tolerance of the liver is used by primary hepatocellular carcinoma and liver metastases to inhibit the anti-tumor immune responses and decrease the efficacy of treatments using immune checkpoint inhibitors[32]. In addition, several studies demonstrated that liver metastases could also exert systemic immunosuppression effects in patients with cancer, which consequently inhibited the intra- and extrahepatic immune responses[33,34]. A promising method to overcome the inherent immune escape of liver tumors is the combination therapy of liver cancer using anti-VEGF drugs and immune checkpoint inhibitors, as the anti-VEGF drugs could reverse the VEGF-mediated immune suppression, promote T-cell infiltration of tumor microenvironment, and consequently enhance the treatment effects of ICIs[35]. The multicenter study in Shandong[25] and the REGONIVO study[14] included 56.5% and 52.0% of patients with liver metastases, respectively, while this frequency was 77.8% in the American study. The present study included 60% of patients with liver metastases. The relatively encouraging findings of the REGONIVO study could be related to the good ECOG PS of the patients, while the failure of the American study could be directly related to the high percentage of patients with liver metastases. In the present study, the ECOG PS and the percentage of liver metastases were more evenly distributed. Moreover, the number of patients with each domestic PD-1 inhibitor was limited in our study. Due to the national conditions and patients’ financial burden, the number of patients who received nivolumab (n = 3) or pembrolizumab (n = 1) was also very low. Thus, we analyzed the difference in PFS between patients with imported PD-1 inhibitor (nivolumab or pembrolizumab) and those with domestic anti-PD-1 inhibitor (sintilimab, toripalimab, camrelizumab, or tislelizumab), which showed no statistical significance. This comforting result was also supported by previous clinical trials of domestic PD-1 inhibitors. Thus, the study could provide more objective evidence for evaluating the clinical efficacy of this treatment regimen.
In the present study, the doses of regorafenib included 160, 120, and 80 mg. The dose of regorafenib in all 4 patients treated with 160 mg was reduced to 80 mg due to grade 3 TRAE, and the regorafenib dose in some patients treated with 120 mg was also reduced to 80 mg due to abnormal transaminase levels. Regorafenib treatment could be continued at this dose for all patients, suggesting that 80 mg could be used as the best dose in the combination therapy for further investigation. Besides nivolumab and pembrolizumab used worldwide, four other PD-1 inhibitors common in China were included in this study because many patients cannot afford nivolumab and pembrolizumab.
Since the cost of the combination treatment is relatively high, discovering predictive markers for treatment efficacy is important to identify the patients who could best benefit from the treatment. The findings showed that the specificity of PLR was slightly lower than that of CEA (the PLR of one patient with SD still increased slightly after two treatment cycles), while the sensitivity was comparable (the trend of changes in most patients conformed to the treatment efficacy after the first treatment cycle). The PFS was significantly different between the PLR-low and -high groups, indicating that the PLR was negatively correlated to PFS. The findings suggested that the PLR could be used as a reference to predict the treatment efficacy and PFS of patients when selecting the combination therapy. An Italian study demonstrated that PLR was an independent factor influencing the outcomes of CRC[36]. Moreover, patients with high PLR also showed a high expression level of programmed cell death-ligand 1 in circulating tumor cells, suggesting that PLR may also be a predictive marker of change in tumor immune microenvironment[36]. This may explain why PLR can predict the effectiveness of PD-1 inhibitor combination therapy but NLR cannot.
This study has limitations. Although we retrospectively included all the patients, only 30 were included in this study. The treatment responses of this treatment regimen need to be verified through prospective studies with large sample sizes. No multivariable Cox regression model could be established due to the relatively small sample size of this study, and the predict value of PLR should be further validated.
In conclusion, treatment using regorafenib combined with PD-1 inhibitor could lead to a longer PFS in some patients with MSS mCRC with failure to standard treatment. The PLR should be examined further for its ability to predict response to regorafenib combined with a PD-1 inhibitor. These results could help the design of a prospective trial in patients with refractory MSS mCRC.
The effectiveness of the combination therapy using regorafenib and programmed cell death-1 (PD-1) inhibitors in treating metastatic colorectal cancer (mCRC) in the REGONIVO trial in Japan and a retrospective study in the United States are inconsistent.
As the effectiveness of the combination therapy remains controversial, we evaluated the situation and data of the combination therapy including the efficacy and safety in our medical centre in order to provide more clinical evidence for this treatment.
The objectives of this study were to investigate the tumor response, progression-free survival, overall survival, and treatment-related adverse events of the treatment and explore a potential indicators predicting response and prognosis.
We identified patients with microsatellite stable (MSS) mCRC treated with regorafenib combined with PD-1 inhibitor at Henan Provincial People’s Hospital between December 2018 and December 2020. Collected data included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), site of the primary tumor, site of the metastases, MSI/MMR, gene status, lines of treatment, and previous treatments. The blood routine examination and CEA results before treatment and after three and five cycles of combination therapy were longitudinally analyzed.
We included 30 patients with MSS mCRC treated with regorafenib combined with PD-1 inhibitor. The disease control rate was 60.0%. The median follow-up time was 12.0 mo, and median PFS was 3.4 mo [95% confidence interval (CI): 2.2-4.6 mo]. The median PFS in the low-PLR group was 4.2 mo (95%CI: 3.5-4.9 mo), compared with 2.8 mo (95%CI: 1.4-4.2 mo) in the high-PLR group (P = 0.005). Four (13.3%) patients experienced grade 3 TRAE.
We find that some patients can benefit from the combination therapy even after multi-line therapy and adverse events are generally tolerable. The PLR might be a potential indicator to predict patient response to this combination therapy.
This study provides experiences and could help to design a prospective trial for patients with MSS mCRC those who failure to standard therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho YS, South Korea; Shomura M, Japan S-Editor: Wang LL L-Editor: Filipodia P-Editor: Li X
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4887] [Article Influence: 698.1] [Reference Citation Analysis (1)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55765] [Article Influence: 7966.4] [Reference Citation Analysis (132)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13204] [Article Influence: 1467.1] [Reference Citation Analysis (3)] |
| 4. | Fu Q, Zhang Q, Lou Y, Yang J, Nie G, Chen Q, Chen Y, Zhang J, Wang J, Wei T, Qin H, Dang X, Bai X, Liang T. Correction: Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene. 2019;38:5740-5741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2423] [Article Influence: 269.2] [Reference Citation Analysis (31)] |
| 6. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 2.2021. Fort Washington: National Comprehensive Cancer Network, 2021. |
| 7. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Rectal Cancer. Version 1.2021. Fort Washington: National Comprehensive Cancer Network, 2021. |
| 8. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 9. | Diagnosis And Treatment Guidelines For Colorectal Cancer Working Group CSOCOC. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res. 2019;31:117-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Dhillon S. Regorafenib: A Review in Metastatic Colorectal Cancer. Drugs. 2018;78:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Grothey A, Blay JY, Pavlakis N, Yoshino T, Bruix J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev. 2020;86:101993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi K, Komatsu Y, Satoh T, Uetake H, Yoshino T, Nishida T, Yamazaki N, Takikawa H, Morimoto T, Chosa M, Sunaya T, Hamada Y, Muro K, Sugihara K. Large-Scale, Prospective Observational Study of Regorafenib in Japanese Patients with Metastatic Colorectal Cancer in a Real-World Clinical Setting. Oncologist. 2019;24:e450-e457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7222] [Article Influence: 722.2] [Reference Citation Analysis (0)] |
| 14. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 527] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 15. | Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and Nivolumab or Pembrolizumab Combination and Circulating Tumor DNA Response Assessment in Refractory Microsatellite Stable Colorectal Cancer. Oncologist. 2020;25:e1188-e1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Li J, Cong L, Liu J, Peng L, Wang J, Feng A, Yue J, Li L, Wang X. The Efficacy and Safety of Regorafenib in Combination With Anti-PD-1 Antibody in Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Retrospective Study. Front Oncol. 2020;10:594125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Cho WK, Choi DH, Park HC, Park W, Yu JI, Park YS, Park JO, Lim HY, Kang WK, Kim HC, Cho YB, Yun SH, Lee WY. Elevated CEA is associated with worse survival in recurrent rectal cancer. Oncotarget. 2017;8:105936-105941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Auclin E, Taieb J, Lepage C, Aparicio T, Faroux R, Mini E, Folprecht G, Salazar R, Benetkiewicz M, Banzi M, Louvet C, Van Laethem JL, Tabernero J, Hickish T, de Gramont A, André T, Vernerey D. Carcinoembryonic Antigen Levels and Survival in Stage III Colon Cancer: Post hoc Analysis of the MOSAIC and PETACC-8 Trials. Cancer Epidemiol Biomarkers Prev. 2019;28:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Tanoglu A, Karagoz E. Neutrophil and platelet-to-lymphocyte ratio: new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer? Transpl Int. 2014;27:e80-e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Huang S, Li D, Zhuang L, Sun L, Wu J. A meta-analysis of the efficacy and safety of adjuvant sorafenib for hepatocellular carcinoma after resection. World J Surg Oncol. 2021;19:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016;17:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore). 2018;97:e0144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Kim JY, Jung EJ, Kim JM, Lee HS, Kwag SJ, Park JH, Park T, Jeong SH, Jeong CY, Ju YT. Dynamic changes of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predicts breast cancer prognosis. BMC Cancer. 2020;20:1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 2019;17:599-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Wang J, Zhang B, Chu Q, Su C, Wu H, Chen X, Wang B, Yin Y, Zhu B, Sun J. Attitudes and Practices of Immune Checkpoint Inhibitors in Chinese Patients With Cancer: A National Cross-Sectional Survey. Front Pharmacol. 2021;12:583126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol. 2020;13:1756284820917527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 27. | Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 28. | Mettu NB, Twohy E, Ou FS, Halfdanarson TR, Lenz HJ, Breakstone R, Boland PM, Crysler O, Wu C, Grothey A, Nixon AB, Bolch E, Niedzwiecki D, Fruth B, Schweitzer B, Elsing A, Hurwitz H, Fakih MG, Bekaii-Saab T. BACCI: A phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): An ACCRU network study. Anna Oncol. 2019;30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Grothey A, Tabernero J, Arnold D, De Gramont A, Ducreux MP, O'Dwyer PJ, Van Cutsem E, Bosanac I, Srock S, Mancao C, Gilberg F, Winter J, Schmoll HJ. Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): Findings from Cohort 2 of MODUL – a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann Oncol. 2018;29:714-715. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Shigeta K, Matsui A, Kikuchi H, Klein S, Mamessier E, Chen IX, Aoki S, Kitahara S, Inoue K, Shigeta A, Hato T, Ramjiawan RR, Staiculescu D, Zopf D, Fiebig L, Hobbs GS, Quaas A, Dima S, Popescu I, Huang P, Munn LL, Cobbold M, Goyal L, Zhu AX, Jain RK, Duda DG. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 31. | Zheng M, Tian Z. Liver-Mediated Adaptive Immune Tolerance. Front Immunol. 2019;10:2525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 32. | Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, Chopra Z, El Naqa I, Zhou J, Bian Y, Jiang L, Tezel A, Skvarce J, Achar RK, Sitto M, Rosen BS, Su F, Narayanan SP, Cao X, Wei S, Szeliga W, Vatan L, Mayo C, Morgan MA, Schonewolf CA, Cuneo K, Kryczek I, Ma VT, Lao CD, Lawrence TS, Ramnath N, Wen F, Chinnaiyan AM, Cieslik M, Alva A, Zou W. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 648] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 33. | Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, Daud A, Bluestone JA. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 34. | Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, Khurana N, Nosrati A, Krummel MF, Tucker A, Sosa EV, Sanchez PJ, Banayan N, Osorio JC, Nguyen-Kim DL, Chang J, Shintaku IP, Boasberg PD, Taylor EJ, Munster PN, Algazi AP, Chmielowski B, Dummer R, Grogan TR, Elashoff D, Hwang J, Goldinger SM, Garon EB, Pierce RH, Daud A. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res. 2017;5:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 441] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 35. | Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 901] [Article Influence: 90.1] [Reference Citation Analysis (1)] |
| 36. | Raimondi L, Raimondi FM, Di Benedetto L, Cimino G, Spinelli GP. PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |