INTRODUCTION
Vasoactive intestinal peptide (VIP) secreting tumour (VIPoma) is a rare functional neuroendocrine tumour (NET) secreting VIP in an uncontrolled manner. It is a non-beta pancreatic islet cell tumour that comprises < 10% of all pancreatic NETs[1].
VIPomas were initially reported by Priest and Alexander in 1957. Verner and Morrison in 1958, described a syndrome called WDHA (watery diarrhoea, hypokalemia, achlorhydria) as a consequence of a pancreatic malignancy that caused death due to dehydration and shock[2]. These tumours are also called Verner-Morrison syndrome, pancreatic cholera and WDHA syndrome, in view of the most frequent symptoms[2]. Its estimated incidence is of 1/10000000 individuals per year[3], affecting more women (65%) than men (35%)[4] and it usually appears in the 4th decade[3].
These originate in amine precursor uptake and decarboxylation cells of the gastro-enteropancreatic endocrine system and in adrenal or extra-adrenal neurogenic locations[5]. VIPomas are sporadic in 90% of cases, generally presenting as solitary lesions greater than 3 cm[5], with only in 5% of the cases being multicentric[6]. Pancreatic VIPomas might also be part of multiple endocrine neoplasia (MEN) 1 syndrome[6], which is an autosomal dominant syndrome that combines malignant lesions in the parathyroid glands, pancreatic islet cells, and the pituitary[7]. As well as its participation in familial MEN 1[5] and related disorders, the MEN1 locus has been involved in the development of MEN 1-type sporadic endocrine malignancies.
Pancreatic VIPomas are extremely rare in children, where it has been shown that VIP originates mainly from ganglioneuromas, ganglioneuroblastomas, neurofibromas, or most commonly from other neoplasias in the adrenal area[1,8]. Only a small number of neuroblastomas and ganglioneuroblastomas produce VIP, but this characteristic indicates a more favourable prognosis[8]. Patients with neurogenic neoplasias, usually show normal levels of gastrin, insulin, pancreatic polypeptide and somatostatin, as opposed to those with pancreatic VIPomas[8]. Most VIPomas are intrapancreatic, the majority of them in the body and tail while 25% are located in the head of the pancreas[9]. However, there are cases of extrapancreatic origin, mostly in adrenal glands (35%), followed by paraspinal retroperitoneal ganglia (30-35%), posterior mediastinum (20%), head and neck in 1%-5% and pelvis in 2%-3%; rare locations include the thymus, lung, kidney or anterior mediastinum[10,11].
PATHOPHYSIOLOGY
VIPomas most relevant symptoms are caused by the exaggerated, uncontrolled secretion of VIP. However, other products such as prostaglandin E2, may be secreted as well by this neoplasia[12].
VIP is generated as a precursor substance with a signal peptide of 22 amino acids, then cleaved to the active form of 28 amino acids[12,13]. This product encoding gene is in chromosome 6[14]. It works by attachment to receptors on intestinal epithelial cells and inducing activation of adenylate cyclase and adenosine 3’, 5’- cyclic phosphate (cAMP) production[11]. As such, it will control smooth muscle activity, blood flow in the gastrointestinal tract[15-17] and epithelial cell secretion. This will result in profuse refractory watery diarrhoea leading to water and electrolyte depletion, mainly hypokalemia[18-20]. Some studies have shown that there is a local control of VIP gene expression, suggesting that there is a post transcriptional regulation which may be crucial for normal VIP secretion[21].
VIP also shows a vasodilator effect (with flushing), glucogenolytic effect on the liver (with subsequent hyperglycaemia) and diminish gastric acid secretion[22] leading to hypochlorhydria/achlorhydria[23]. Hyperglycemia and impaired glucose tolerance affect ≤ 50% of patients[24]. The reasons behind these issues are in relation to direct glycogenolytic activity of the VIP on the liver and the inhibitory effect of hypokalemia on pancreatic islets cells insulin release. Hypercalcemia can also be seen without MEN1 syndrome or raised parathyroid hormone levels. It is believed that elevated bone resorption can be involved, however, it is not clear. In addition, the dehydration, as a consequence of severe diarrhoea, might also aggravate the hypercalcemia. This is seen in 50% of patients[25]. Hypochlorhydria or achlorhydria is described in 75% of patients with VIPoma as a result of the inhibition of histamine and pentagastrin-stimulated gastric acid secretion[26].
DIAGNOSIS
Diagnosis requires demonstration of secretory diarrhoea. Then the laboratory assessment (elevated VIP serum levels are required) and imaging studies will complete the diagnostic tools. Typically, the diagnosis is often delayed and diarrhoea may persist for years before a VIPoma is confirmed[27].
Laboratory
VIPomas are diagnosed when watery diarrhoea, hypokalemia, and achlorhydria are observed in the context of raised serum VIP[1]. Stool volumes of less than 700 mL/d virtually rule out a VIPoma. Generally in this context, stool volumes exceed 3 L/d. Stool osmolality must be compatible with a secretory diarrhoea, that is stool osmotic gap of < 50[8]. VIP levels are determined by radioimmunoassay and these are generally 2-10 times the normal range (20-30 pmol/L) in patients with VIPoma[9]. Figures > 75 pg/mL are consistent with a VIPoma[10], although levels usually reach 160-250 pmol/L or higher.
These levels should be drawn after fasting and a protease inhibitor (such as aprotinin) must be added to the blood sample, otherwise, VIP is degraded rapidly. Moreover, the sample must be kept frozen at -70 °C until it is processed. These levels should be determined when the patient is symptomatic (as VIP release from the tumour fluctuates and it is normal in between the episodes of diarrhoea) and should be repeated to verify the diagnosis. Hence, a normal figure may be a false negative[28].
Hypokalemia and non-anion gap acidosis are also frequently seen in VIPomas[1], same as hypochlorhydria or achlorhydria that may be assessed by checking gastric pH or basal gastric acid output l[1]. Measuring the stool weight and levels of potassium, it could be confirmed the high gastrointestinal potassium losses. Renal function must be assessed by blood urea nitrogen and serum creatinine levels. Magnesium should be determined as well[11]. Stool weight with potassium measurements will confirm the high gastrointestinal potassium losses.
VIPomas may release also other hormones, such as neurotensin, calcitonin or pancreatic polypeptide. Moreover, 66% of the cases with VIPoma will also show raised levels of gastrin and insulin[29]. It has been published one case in the literature, where a patient with VIPoma had increased dopamine levels suggesting that neuroendocrine cells are able to secrete both catecholamines, as well as pancreatic peptides[30].
Imaging
Imaging studies will initially check the pancreas, as it is known that 90% of VIPomas will be found there. These techniques are crucial not only to localize the neoplasia, but also to determine its size and the stage at diagnosis to help establish the treatment pathway[31]. In most cases, finding the VIPoma is easy as most will be larger than 3cm at diagnosis. Computed tomography (CT), magnetic resonance imaging (MRI) and somatostatin receptor scintigraphy (SRS) are three imaging techniques that may be utilised to find the tumour. Some published articles have used 99mTc sestamibi[32] to locate the neoplasia. Unfortunately there are no staging criteria for VIPomas.
CT: Multiphasic CT is crucial in determining the size, location, as well as involvement of adjacent structures, vessels, lymph nodes and presence of calcification[9,15]. CT will search for neck, mediastinal, or retroperitoneal masses and will identify the primary neoplasia in the majority of the cases. It will also help detecting or excluding liver metastases[10]. Peng et al[33] examined 31 patients and reported that CT was able to identify correctly all VIPomas in the body and tail of the pancreas. However, only 71% of the tumours in pancreatic head were identified. This neoplasia shows as a hyperattenuating lesion on arterial phase followed by an obscure mass on venous depiction. Calcifications may be detected as well. These are hypervascularized tumours rich in cells and fibrous tissue which is poorly supplied and therefore the contrast agent is held within the lesion[11].
MRI: MRI is useful for assessment of spinal tumours[34] or if CT is contraindicated (e.g., patient is allergic to iodine contrast or in renal failure). VIPomas are best seen on T1-weighted, fat-suppressed images as these are low signal-intensity tumours. MRI has better sensitivity for liver metastases detection. These may be observed as intensive peripheral ring enhancement on immediate post-gadolinium spoiled gradient-echo images[35]. This technique can detect tumours as small as 1 cm[36] and MRI should be performed in those cases with indeterminate lesions.
SRS: 90% of pancreatic NETs have a high number of somatostatin receptors[8]. Therefore, using radionuclide-labelled octreotide or lanreotide may be useful for studying abnormalities found on a CT or for identification of hidden or distant metastases[6]. It might help after surgery as well, if postoperative changes diminish the accuracy of a CT. This technique’s sensitivity for all pancreatic NETs is 80-90%; 92% for neoplasias > 1 cm[6].
Endoscopic ultrasound: This will help determine the accurate extent of the disease and it allows a biopsy of the pancreatic lesion.
Single-photon emission CT: Research has suggested that the use of single-photon emission CT (SPECT) might improve the value of somatostatin receptor scintigraphy for the localization of NETs, including VIPomas[37]. Several different radiotracers can be bound to octreotide, and applied together with SPECT or positron emission tomography (PET) imaging to localize areas of enhanced uptake.
PET: 18F-deoxyglucose-PET imaging has also been used to diagnose NETs. However, it may not be as sensitive as somatostatin receptor scintigraphy[38]. The FDA approved the newer functional PET technique with 68-Ga DOTATATE injection as the radioactive diagnostic product for the identification of somatostatin receptor-positive NETs in adult and paediatric patients. PET-CT Gallium-68 dotatate is 97% sensitive for the identification of VIPomas. Contrast enhanced CT and MRI sensitivities are 80 and 85%, respectively[8,11]. Moreover, a recent publication has suggested a role of the high sensitivity Ga-PET/CT not only in the identification of NETs but also in VIPomas prognostication and risk stratification[30].
Immunohistochemically: VIPomas stain positively for VIP, somatostatin, neuronspecific enolase, chromogranin A, synaptophysin and cytokeratin[39,40].
Other techniques: Chest radiography may help with identification/Location of paravertebral masses[41]. Endoscopic retrograde cholangiopancreatography might demonstrate blockage of the major pancreatic duct and perhaps some calcifications in the pancreatic body. Transabdominal ultrasonography help exclude liver metastases, which might show as hepatic calcifications. Electrocardiography can demonstrate QRS widening and T-wave flattening in cases with a very significant hypokalemia. Colonoscopy is another technique that might help with diferential diagnosis, to rule out a villous adenoma as another cause of potassium-losing diarrhoea.
CLINICAL MANIFESTATIONS
The onset of VIPomas is subtle and its symptoms can bevague. In most case, these neoplasias have already metastasized at diagnosis. A Chinese study with 41 patients showed that the average time from the appearance of symptoms to the final diagnosis was > 15 mo, although patients experience a range of distinguishing signs[43].
The major symptom of VIPomas is long-standing profuse watery diarrhoea of approximately 10 watery stools per day. This diarrhoea persists even after 72 h of fasting[44] as opposed as osmotic diarrhoea[44]. In actual fact, the majority of cases develop diarrhea (89%), weight loss (72%), and hypokalemia (67%)[29], as a consequence of VIP binding to intestinal epithelial cells, upregulating cAMP and leading to secretion of electrolytes into the bowel, causing profuse watery diarrhea[29]. These issues occur as a result of VIP binding to intestinal epithelial cells, thereby upregulating cAMP and leading to secretion of electrolytes into the bowel lumen, causing profuse watery diarrhea[2]. Stool volumes are during fasting at least 20 mL/kg/d but exceed 50 mL/kg/d in most cases. Non-fasting volumes exceed > 3000 mL/d. Faecal osmolality is accounted for by twice the sum of the sodium and potassium levels, evidencing the electrolyte loss. The stools are generally tea coloured and odourless without blood or mucus[1,2]. Initially diarrhoea may evolve in episodes, whereas it becomes constant as the neoplasm progresses. Unfortunately, the diarrhoea may be present for years before the diagnosis is made. As such, in around 33% of patients, the diarrhoea has been present for less than one year before the diagnosis, but 1/4 of patients have diarrhoea for a minimum of 5 years before the diagnosis is established. Faecal excretion of large amounts of potassium and bicarbonate will produce hypokalemia and non–anion gap acidosis. VIPomas will then lead to significant dehydration (45%-95%) and electrolytes imbalance, most frequently hypokalemia (70%-100%), achlorhydria (35%-76%), hypomagnesemia, hypophosphatemia and metabolic acidosis, all with linked symptoms[45].
Other frequent symptoms include muscular weakness, sickness (nausea, vomiting), lethargy and abdominal painful cramps or bloating. In some cases, skin rash has been shown as well[10] . Flushing similar to that seen with carcinoid syndrome appears in around 33% of patients during the episode of diarrhoea[33]. This flushing is secondary to prostaglandin production by the neoplasm. Patients will be exhausted and suffer from noticeable weight losses and renal failure, unless able to replace the lost fluids and electrolytes and flushing (15%-30%)[24,27]. Finally, ischemic stroke attributed to high haematocrit due to diarrhoea has been mentioned in an extremely rare case report[46].
TREATMENT/MANAGEMENT
General management
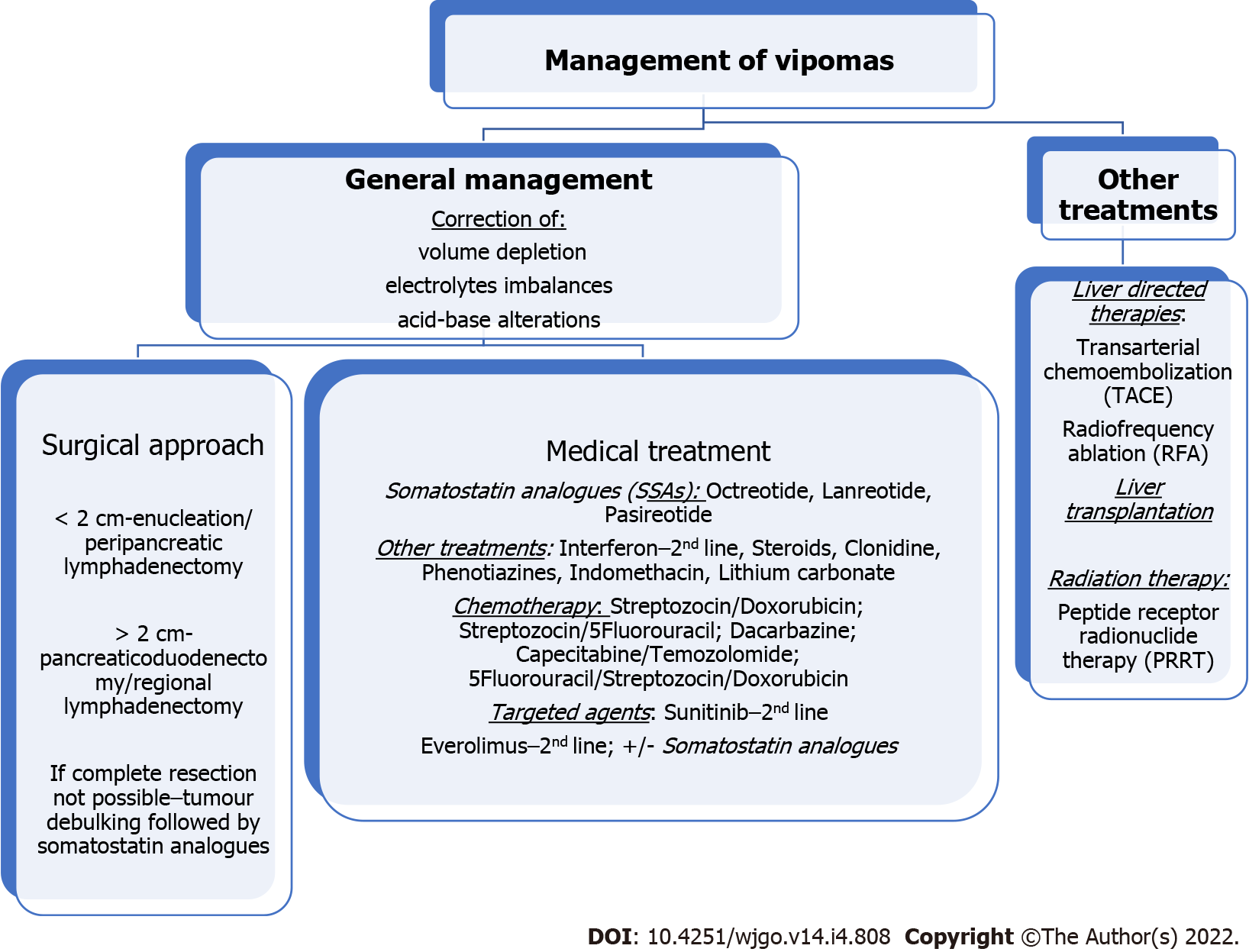
The management of VIPomas includes medical and surgical treatment. Its mortality rate is closely linked to uncontrolled WDHA syndrome. This will lead to a sustained dehydration with significant electrolyte and acid-base disturbances, and consequently renal failure, cardiac arrhythmias, neuromuscular deficits, shock, cardiac arrest and eventually death[49]. Therefore, the initial therapy for a VIPoma aims at controlling the symptoms and correcting any volume depletion, electrolytes and acid-base alterations. This entails a rapid replacement of fluids and electrolytes. The ideal fluid replacement should be with an isotonic electrolyte solution with adequate sodium, potassium and base if needed. In many cases, patients will need intravenous replacements and hospital admission[48]. In the absence of finding a neoplasia, symptomatic therapy is advised. This treatment in conjunction with octreotide, will improve preoperative electrolyte balance[30] if surgery is considered (Figure 1).
Figure 1 Management of vasoactive intestinal peptide secreting tumour.
Somatostatin analogues
Somatostatin analogues (SSAs) inhibit VIP secretion and are used to control symptoms[15]. Somatostatin is a peptide that reduces the secretion of an ample set of hormones[50]. Various studies on functional NETs have shown that controlling hormone levels is crucial to reduce patients’ morbidity and mortality[51]. SSAs (octreotide, lanreotide and pasireotide) replicate the effect of somatostatin on G-coupled receptors of cell membrane and will reduce VIP secretion. This will impact positively on diarrhoea control. SSAs will also inhibitit tumour growth[31] in more than 90% of patients[33]. The CLARINET trial (Controlled study of Lanreotide Antiproliferative Response in NETs) reported antiproliferative effects of lanreotide in NETs[52]. SSAs showed rates of tumour stabilization in 50%-60% of the pNETs[22]. Some authors have even suggested that that SSAs may produce a reduction in tumour size. Although this is still under debate, neoadjuvant therapy with octreotide has been applied in 69.2% of cases[45,53]. Overall, several studies have reported rates of diarrhoea control in more than 50% of the patients, while significant improvements are seen in 25%.
Unfortunately, there are some issues in relation to SSAs. One is the clearly documented resistance with long-term octreotide use, leading to the requirement of significantly high doses to achieve the wanted effect[54]. Another problem is the fact that the diarrhoea reappears when the SSAs is stopped. Thus, octreotide should carry on unless the tumour can be surgically completely removed. Octreotide is a synthetic longacting SSA that stops VIP secretion and is approved for treatment of VIPomas[55]. SSAs are generally well tolerated, although adverse events might occur. Indigestion, bloating, vomiting, bloating, diarrhoea with steatorrhea due to fat malabsorption, and mild glucose intolerance; However, these side effects tend to fade over time[52] (Figure 1).
Interferon
Interferon alfa is added to the treatment management when the highest tolerable dose of octreotide do not control symptoms. It may also cause a modest reduction in tumour size[56]. Interferon alpha (IFN-α) is approved for symptom control (3-5 million IU sc three times weekly) with similar results to SSAs. Due to its less favourable toxicity profile (fatigue, weight loss and, more rarely, depression), this is used in second-line as a supplemental therapy joined to SSA in cases with refractory syndrome[57] (Figure 1).
Steroids
Steroids may diminish symptoms in 50% of patients. This treatment may be used for those patients who did not show a good response to SSAs[58] (Figure 1).
Other agents
Before the availability of octreotide, some patients had shown responses with high dose of prednisone, clonidine, phenotiazine, indomethacin, lithium carbonate, etc[58]. Additional loperamide and opiates may be used as well[59] (Figure 1).
Chemotherapy
The activity of cytotoxic chemotherapy in metastatic VIPomas is difficult to assess as many series have published the results for all histologic subtypes of pNETs together. Overall response rate (ORR) is disappointing and as such, chemotherapy is not curative. Moertel et al[60] use a combination of streptozotocin and doxorubicin as the standard therapy for progressive or symptomatic unresectable NETs, including VIPomas. This combination reduced diarrhoea and diminished tumour size in 69% (in 14% it showed a complete regression). Further studies have reported that patients with advanced disease may respond to streptozocin-based chemotherapy, being doxorubicin/streptozotocin combination the gold standard with 5-fluorouracil replacing doxorubicin when the latter is contraindicated[10]. When the standard chemotherapy and SSAs lose effectiveness, 5-FU may be used in combination with interferon alfa.
Other chemotherapy drugs are dacarbazine and more recently capecitabine and temozolomide (CAPTEM). This last combination has shown efficacy from retrospective studies in pancreatic NETs where the number of VIPomas was very small. The study by Strosberg et al[61] showed that CAPTEM was able to normalise serum VIP level in one patient with VIPoma. They have only included two patients with thisdisease, the others had different NETs. Kouvaraki et al[62] reported that patients with locally advanced or metastatic pancreatic NETs that received 5-fluorouracil, doxorubicin, streptozozin showed a 40% of ORR, and showed longer progression free survival (PFS) and overall survival (OS). The extension of liver metastases seems to be the most important predictor of result. The median time to response was 4 mo. This study included only 2 cases of VIPoma and the authors’ data suggest that chromogranin A level after two to four cycles of this combination is a useful surrogate marker for the prediction of response. Temozolomide alone can also be recommended as an alternative chemotherapy in pancreatic NETs. There are no established second-line treatment, but regimens that can be used are 5-FU/Leucovorin/irinotecan (FOLFIRI), 5-FU/Leucovorin/oxaliplatin (FOLFOX), CAPTEM bevacizumab[63].
Chemotherapy may be considered as an alternative to hepatic-directed therapies such as resection, ablation or hepatic artery embolization, but it fails to control the hormonal syndrome[8]. It should be maintained at least for one or two cycles and it may show significant benefits at the end of the first mo. There is little experience with adjuvant chemotherapy after surgical resection in NET G1/G2. However, in aggressive NENs (NEC G3), adjuvant platinum-based chemotherapy can be used, although prospective clinical trials are advised[63]. Schizas et al[45] in their systematic review had reported that 6.8% of patients received systemic adjuvant therapy. The number of cases is very small as to be able to draw general conclusions. A multicenter trial evaluated 80 cases with metastatic NETs who were randomised to receive lanreotide, interferon alpha, or both. The authors did not find any significant differences between the arms of the study. Partial response was reported in 4% to 7.1%, stable disease in 17.9%-28% and progressive disease in 50%-56%[56]. Another study of 14 patients with metastatic NETs who received indium in-111 octreotide showed stable disease in 50 % of cases, partial response in 14%, and disease progression in 36%[64]. A quality-of-life study in 13 of these patients found a significant benefit in this[65,66].
European Neuroendocrine Tumour Society (ENETS) have published a position statement on peptide receptor radionuclide therapy (PRRT) for pancreatic neuroendocrine tumours (pNETS)[67]. The early findings with radioembolization with resin 90 Y-microspheres in liver metastases from a variety of NETs have been encouraging. Findings reported complete response in 2.7%, partial response in 60.5%, stabilisation in 22.7% and progressive disease in 4.9%. The median OS documented was 70 mo and no cases of radiation liver failure were reported[68]. Second-line therapy for VIPomas includes IFN-α as mentioned earlier in this article, everolimus and sunitinib[69-72]. ENETS 2016 guidelines approved everolimus and sunitinib as antiproliferative therapies in cases of progressive pNETs, after failure of SSA or chemotherapy.
Sunitinib
A randomized multicentric trial evaluating sunitinib (a tyrosine kinase inhibitor), included 171 advanced well-differentiated NETs, including patients with VIPomas. The results showed an ORR of 9.3% vs 0%, PFS 11.4 mo vs 5.5 mo in the sunitinib and placebo groups respectively. Nine deaths were reported with sunitinib (10%) vs 21 in the placebo group (25%). These results seem to be similar to those obtained with chemotherapy, but with a more favourable toxicity[69,70]. Sunitinib inhibits several receptor tyrosine kinase key to tumour growth, neoangiogenesis and dissemination[73].
Everolimus
mTOR is a serine-threonine kinase involved in cell growth control and cell apoptosis. Its effects are mediated through phosphoinositide 3-kinase/Akt pathway and stimulates downstream protein kinases crucial to cell cycle progression. mTOR inhibitors, alone or combined with octreotide have been studied in patients in pancreatic NETs. Everolimus is a selective mTOR inhibitor with antiangiogenic activity as well.
The RADIANT 1 is a multicentric single arm phase II trial that evaluates everolimus alone or in combination with octreotide in 160 cases of metastatic pNETs after chemotherapy failure[71]. The ORR was 9.6% in those patients not receiving octreotide, with a median PFS of 9.7 mo and OS 24.9 mo. A smaller group of 45 cases received everolimus and continued to receive octreotide. In these cases, the ORR was 4.4% with PFS of 16.7 mo and OS was not reached at the time of data cutoff.
The RADIANT 2 was a phase III trial randomising patients with NETs to everolimus[72] and SSA or to placebo and SSA. 429 patients were included, 6% pNETs. A PFS of 16.4 mo vs 11.3 favoured the combination.
RADIANT -3 trial is a phase III study that has been recently reported[74]. It included 410 cases with radiologic disease progression. Patients were randomized to receive everolimus or usual treatment which could include SSA. Findings showed a median PFS of 11 vs 4.6 mo and 34% vs 9% were reported alive and free of progression at 18 mo with everolimus or usual treatment respectively. 24% of patients in this trial had somatostatinomas, gastrinomas, insulinomas, glucagonomas or VIPomas. This means that everolimus may be used across the spectrum of pNET subtypes. In addition, authors reported that the benefit of everolimus was found in different subgroups of sex, age, geographic regions, race, performance status and previous therapy applied (chemotherapy, radiation or octreotide). As there is a risk of pneumonitis with this therapy, perhaps sunitinib would be a better option in those patients with underlying severe lung disease (Figure 1).
The future
Well-designed randomized clinical trials have significantly improved our treatment options for patients with these tumours. However, we are still far away from an ideal situation and as such, further research is crucial, although difficult, specially taking into account that VIPomas comprise only < 10% of pancreatic NETs.
Several trials have been carried out testing different agents. Some of these trials have finished recruitment and are still awaiting results such as NCT01466036, a phase II study of cabozantinib in advanced pancreatic NETs and carcinoids[73]. It recruited 62 cases and the primary end point is ORR. The final data collection date was expected in March 2021. Another one is NCT02893930, a phase II with sapanisertib[75] in patients with metastatic or refractory pancreatic NETs that cannot be surgically removed. It has been last updated in May 2021 but not results posted yet. NCT00075439 is a phase II study evaluating gefitinib[76] in patients with progressive metastatic NETs which has finished recruitment as well but awaiting results. EPO906 has been assessed in phase II trial in metastatic carcinoids and other NETs, including VIPomas. It has completed recruitment in 2007 but not available results. Other trials have been withdrawn such as the phase 1 trial with Veliparib (ABT-888) in combination with capecitabine and temozolomide in advanced well-differentiated NETs (NCT02831179)[77]. Another study with cabozantinib (a phase III) is still recruiting patients (NCT03375320). It will assess cabozantinib vs placebo in cases with advanced NETs or carcinoids[78].
Bevacizumab has also been investigated in a phase II study of everolimus and octreotide with or without bevacizumab in cases with advanced or metastatic pancreatic NETs that are not amenable for surgery. This has finished recruitment. PFS is the primary end point, being secondary end points ORR and OS. One hundred and fifty patients were recruited. At the most recent update (May 2021), the PFS results showed 14 m vs 16.7 m without and with bevacizumab respectively. OS is favouring as well the arm with bevacizumab with 34 m vs 37.6 m respectively. Although the study is not finished yet, results seem to favour the arm with bevacizumab. What we do not know yet is how many patients with VIPoma were included (NCT01229943)[79].
Data from other two clinical trials, SANET-p (NCT02589821) (NCT02588170)[80,81] have shown PFS benefit with surufatinib, with a tolerable safety[80] and SANET-ep pattern. Surufatinib is a new oral angio-immuno kinase inhibitor. It inhibits selectively the tyrosine kinase activity related to the vascular endothelial and fibroblast growth factor receptors, both inhibiting angiogenesis, and colony stimulating factor-1 receptor, which controls tumour-associated macrophages, stimulating an immune response against tumour cells. The FDA has conceded to surufatinib, an Orphan drug designation for pancreatic NET in 2019 and two Fast Track Designations for development in pancreatic and extra-pancreatic NETs in 2020.
In the SANET-p trial , 172 patients with pNETs were randomised to surufatinib or placebo. At a median follow-up of 19.3 mo in the experimental arm and 11.1 mo in the placebo arm, the investigator-assessed PFS was 10.9 mo (95%CI, 7.5-13.8) vs 3.7 mo (95%CI, 2.8-5.6) for surufatinib and placebo respectively (HR, 0.49; 95%CI, 0.32-0.76; P = 0.0011), being the most frequent adverse event of grade 3 or higher with surufatinib vs placebo, hypertension (38% vs 7%), proteinuria (10% vs 2%), and hypertriglyceridemia (7% vs 0%). Serious AE occurred in 22% of surufatinib arm vs 7% with placebo. Three patients died surufatinib, two of them due to AE and one due to disease progression. One died in the placebo arm due to disease progression.
We should continue to research further to identify actionable mutations or predictive factors for targeted therapy response to better select patients’ treatment. Also further efforts are needed to increase knowledge about the optimal sequential therapy that could impact positively in survival and also in quality of life.
CONCLUSION
VIPoma is a rare functional NET that typically presents as sporadic, solitary pancreatic neoplasia with only 5% of cases associated with MEN type I syndrome. It is characterised by a special clinical syndrome of refractory watery diarrhoea, electrolyte and acid-base imbalances related to the excessive VIP secretion.
The only curative option of treatment would be a complete surgical removal. Unfortunately, the majority of VIPomas have have already metastasized at the time of diagnosis leaving only palliative options for these patients. However, surgical debulking for these patients could be considered as it will help control symptoms and prolong survival. Other options include SSA and the newer chemotherapy regimens such as temozolomide, or drugs such as sunitinib or everolimus. Moreover, recent incorporation of treatment with PRRT has shown significant benefits and it is a safe addition to surgery or as a palliative treatment for those cases of widespread metastatic disease or unresectable primary tumour. As a priority, and regardless of the treatment to follow, all patients should have the water depletion, electrolyte imbalance and acid-base profile corrected.
With all these facts in mind, the prognosis may improve but hopefully further multinational clinical trials enrolling more patients with VIPoma can be carried out to get further insight in this rare but challenging disease.