INTRODUCTION
Gastric cancer (GC) is a major global problem, ranking fifth in incidence and third in cancer-related mortality worldwide, and it was responsible for over 1000000 new cases in 2018 and an estimated 783000 deaths[1]. The incidence of GC varies by sex and region. The incidence is twice as high in men as in women. Incidence rates are markedly elevated in Eastern Asia, whereas the rates in Northern America and Northern Europe are generally low and are equivalent to those seen across African regions[1]. Risk factors include Helicobacter pylori ( H. pylori) infection, family history (hereditary diffuse GC due to germline mutations in CDH1), dietary intake of foods preserved by salting and low fruit intake, Epstein–Barr virus infection, alcohol consumption, active tobacco smoking, consumption of foods contaminated with aflatoxin, nitrates and fungi, and pernicious anemia[2]. The GC incidence has decreased in a growing number of countries partly due to decreasing risk factors, improving standards of living and increasing use of screening programs in high-risk populations[3,4]. GC screening is recommended in regions of high prevalence and patients at risk. In Japan and South Korea, national population-based screening programs for early GC are performed most commonly using barium radiography or endoscopic screening aimed at patients over 40 or 50 years old. These strategies have achieved a 5-year survival rate of more than 50% in Japan (60.3%) and South Korea (55.7%), which is higher than that in China (35.9%) from 2010 to 2014[5], and endoscopy is with superiority over other screening techniques such as barium radiography and is more cost-effective[6-8]. In Asian guidelines, high-risk individuals, including male patients, Chinese patients, patients with first-degree relatives who have GC, patients with persistent H. pylori infection and patients with age 40–45 years or older, should be considered for targeted invasive screening in regions with moderate or low incidence of GC[9]. In contrast with Asian guidelines, European guidelines recommend that patients with advanced stages of atrophic gastritis (including severe atrophic changes or intestinal metaplasia (IM) in both the antrum and corpus, OLGA/OLGIM III/IV) should be followed up with high-quality endoscopy every 3 years. Patients with advanced stages of atrophic gastritis and with a family history of GC may benefit from a more intensive follow-up (every 1–2 years after diagnosis; every 3–5 years for those with autoimmune gastritis)[10]. Patients with IM specifically at higher risk of GC (those with incomplete IM or extensive IM and a family history) and/or who are at overall increased risk for GC (racial/ethnic minorities, immigrants from regions with high GC incidence, or individuals with a first-degree relative with GC) may reasonably elect to undergo repeat endoscopy within 1 year for risk stratification[11]. H. pylori gastritis accounts for more than 80% of gastritis, and persistent infection may eventually cause chronic atrophic gastritis (CAG) through the canonical process originating from acute-on-chronic inflammation. H. pylori eradication abolishes the inflammatory response[12]. A noninvasive test-and-treat strategy is appropriate for uninvestigated dyspepsia[13]. An endoscopy-based strategy combined with Sydney system biopsies is supposed to be considered in patients who have dyspeptic symptoms and/or alarm symptoms or older patients, particularly in low-prevalence H. pylori populations (< 10%)[14]. A screen-and-treat strategy is recommended in communities at high risk of GC[15]. GC still carries a poor prognosis, in part due to the late stage of diagnosis. Recognition of precancerous conditions is indispensable for the management of early GC. In this review, we have focused on precancerous gastric conditions, including CAG, metaplasia, foveolar hyperplasia and gastric hyperplastic polyps derived from gastric epithelium, and provide an overview of gastric epithelium organization and renewal.
HISTOLOGICAL CONSTRUCTURE OF GASTRIC EPITHELIUM AND REGENERATION PROCESS
General histological structure of gastric epithelium
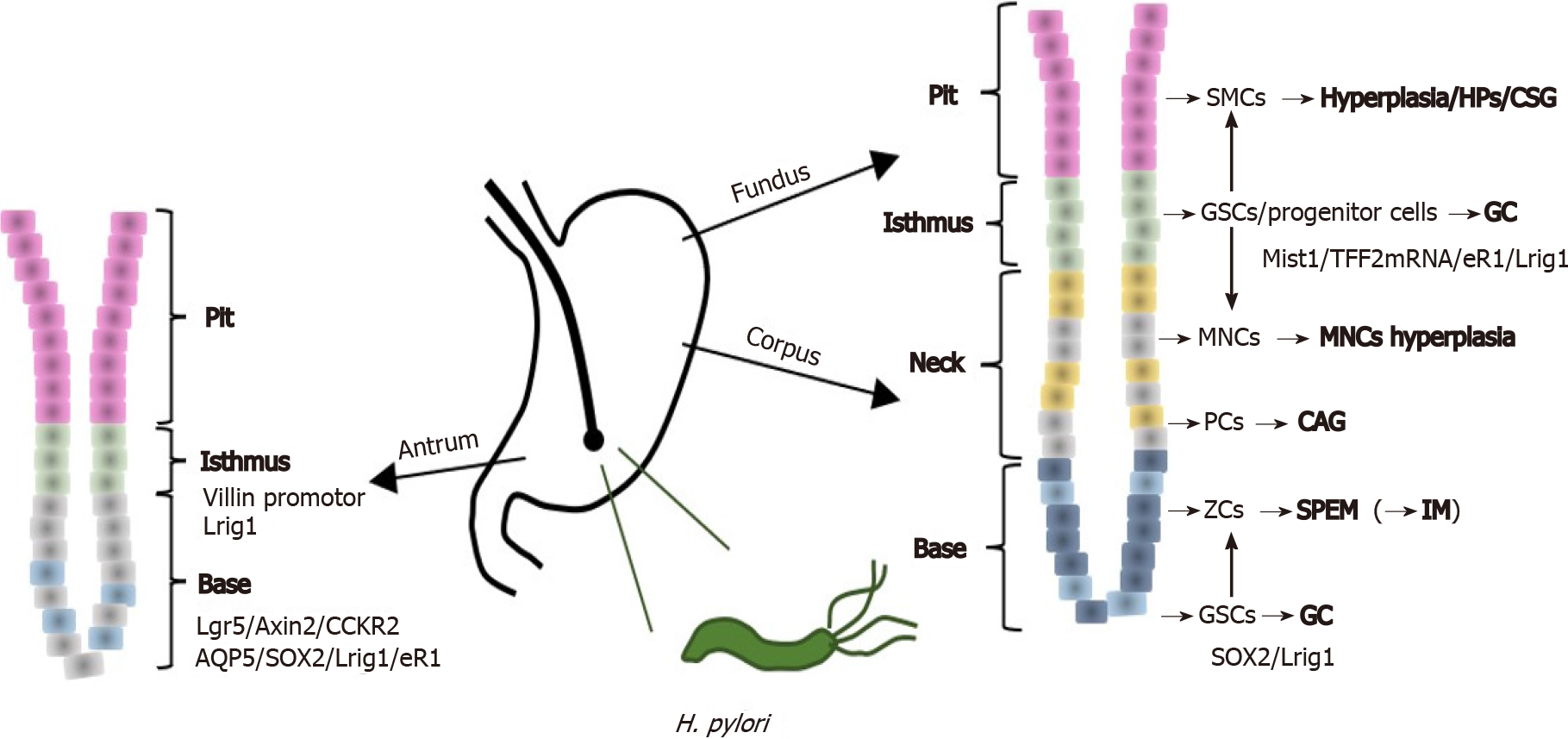
Histologically, the self-renewing gastric epithelium is covered by surface mucous cells (SMCs) which also line the approximately 3 million funnel-shaped gastric pits. Gastric glands in the isthmus, neck and base open into the bottom of these pits. The combination of a pit and a gland is called a gastric unit[16]. The gastric corpus and body are characterized by oxyntic glands with parietal cells (PCs) and chief cells/zymogenic cells (ZCs). The gastric units of the antrum consist predominantly of mucous cells and are largely devoid of PCs and ZCs[17]. The epithelium of fundic units contains mesenchymal stem cells (MSCs), PCs, mucous neck cells (MNCs), ZCs and several types of enteroendocrine cells[16]. The antral unit contains SMCs, antral gland cells (AGCs) and endocrine cells (gastrin-producing G cells, D and EC cells). The genes used to identify cell clusters include ATP4a (PCs)[18], trefoil factor family 2 (TFF2) and MUC6 (MNCs)[19], MUC5AC and TFF1 (SMCs)[20], and gastric intrinsic factor (GIF) (ZCs)[21]. Based on their expression patterns, mucinous AGCs resemble MNCs. The human antral mucosa (particularly the SMCs in it) is known to have a much higher turnover rate than the fundic mucosa[22]. The different proteins expressed by SMCs of the fundic and antral units, such as gastric lipase, TFF3, FCGBP and lysozyme, may be the basis of the different self-renewal rates and may further influence the spatial organization of the bacterial microbiota[23]. These cells originate by stepwise maturation from multipotent gastric stem cells (GSCs) and progenitor cells derived from GSCs such as pre-SMCs, pre-MNCs, pre-PCs and pre-AGCs residing in the isthmus of the gastric gland[16,24]. ZCs are generated by transdifferentiation of MNCs during their downward migration toward the base of the glands[25].
GASTRIC EPITHELIAL RENEWAL AND GSCs
Subjected to countless daily injuries, the stomach still functions as a remarkably efficient digestive organ and microbial filter mainly because of its regeneration and repair. Rapid repair of superficial lesions occurs via a cell migration process called restitution, which starts within minutes. Continuous regeneration via differentiation and proliferation of stem cells and progenitor cells leads to self-renewal within days to months[22]. Pit cells are rapidly turned over after cell death, phagocytosed by a neighboring cell or extruded to the surface, and their half-life, which is regulated by Indian hedgehog and epidermal growth factor (EGF), is approximately 3 d[26,27]. The half-life of PCs and ZCs is several months. PCs are generated from GSC-derived pre-PCs, a process regulated by sonic hedgehog (SHH), gastrin and bone morphogenetic protein (BMP)[27]. In the past several years, strong evidence that ZCs differentiate from MNCs (a process that takes 14 d) has been reported, and the process is regulated by Mist1 and retinoic acid[16,28,29]. The degree of maturation correlates with the distance migrated from the GSCs. MNCs are located in the neck region, ZCs are located at the base, and intermediate pre-ZCs with characteristics of both MNCs and ZCs are located between the neck and the base region[25,30]. Transitional cells with coexpression of MUC6 and GIF are found in the normal gastric mucosa of mice[31]. Proper maturation of the MNC-zymogenic lineage is strictly dependent on PCs, perhaps via their functional secretion of SHH, BMP and amphiregulin[27]. Genetic ablation of PCs blocked the pattern of the entire zymogenic lineage: Progenitors showed premature expression of differentiated cell markers, and fully differentiated ZCs failed to develop, and these aberrancies may also correlate with a notable change in gastric unit architecture[32]. GSCs are characterized as undifferentiated granule-free and mitotic cells, and progenitor cells have a few granules or few microvilli[33]. The mesenchymal compartment surrounding the glands is less studied and poorly understood. It can be significantly affected by H. pylori infection and infiltrated by immune cells, including macrophages, neutrophils, regulatory T cells, natural killer cells and inflammatory mediators such as cytokines, chemokines and metalloproteinases, which may further contribute to epithelial–mesenchymal transition and carcinogenesis[34,35]. MSCs have multipotent potential to differentiate into various cell types and woundhealing ability, as well as tumorpromoting potential[36]. MSCs have immunoregulatory effects on macrophages and polarize them toward M2-like tumor-associated macrophages (TAMs). In turn, M2-TAMs modulate the transition of “naive” MSCs into tumor-derived MSCs, contributing to carcinogenesis by the induction of EMT process, metastasis, immune invasion and immunotherapy resistance of tumor cells[37,38].
GSCs can bidirectionally migrate and differentiate into various cell types after the apoptosis of mature cells[16]. The lateral expansion of GSC clones is impeded by the physical barrier of long-lived PCs. Only when PCs are lost can GSC clones expand laterally around the gland circumference, which may indicate the reversibility of atrophy in the appropriate setting[39,40]. GSCs or progenitor cells reside in the isthmus region within a “niche”, an area providing an optimal microenvironment to regulate physiological tissue renewal and injury repair. The ability of stromal niche cells to control and modify GSC dynamics constitutes a sophisticated mechanism that orchestrates epithelial regeneration and the maintenance of tissue integrity[41]. These cell populations include the villin+, Lgr5+, Axin2+ and CCKR2+ stem cells in the antrum, TFF2 mRNA+, Mist1+ cells and Troy+ or Lgr5+ mature ZCs in the corpus, and Sox2+, eR1+, Lrig1+, Bmi1+ cells in both the antrum and the corpus. These cells are the main suppliers of regenerated glands in response to injury and inflammation regulated by Wnt5a[42]. Villin promotor stem cells are normally quiescent, but multiply in response to IFN-γ, locate in the isthmus and can differentiate into all types of cells in the antral glands. Disruption of Klf4 in villin+ gastric progenitor cells promotes the formation and progression of GC[43]. Mouse gastric corpus gland epithelium is maintained by two stem cell populations: A rapidly cycling population in the isthmus region with a broad expression signature and a quiescent reserve population in the base, marked by expression of Troy or Lgr5[39]. Single adult Lgr5+ stem cells predominantly restricted to the base of mature pyloric glands build gastric organoids closely resembling pyloric gastric units in vitro. Neonate Lgr5+ stem cells located at the base of the prospective corpus and pyloric glands contribute to the development of mature gastric epithelium in both the pylorus and corpus regions[44]. Activation of Wnt signaling in these Lgr5+ cells can induce GC[44]. Lgr5 or Troy is also expressed at the gland base by mature ZCs, and these ZCs can be cultured to generate long-lived gastric organoids, displaying plasticity and an ability to replenish entire gastric units, essentially serving as quiescent “reserve” stem cells for gland regeneration after injury[45,46]. In this same zone, Axin2+/Lgr5− stem cells adjacent to Axin2+/Lgr5+ stem cells are highly proliferative and able to repopulate entire glands, including those of the base, upon the depletion of the Lgr5+ population, which can be regulated by stroma-derived R-spondin 3 produced by gastric myofibroblasts after H. pylori infection[41]. CCK2R+/Lgr5− stem cells in the antrum and are stimulated by gastrin through CCK2R to enhance antral gland fission and carcinogenesis[47]. The TFF2 mRNA-expressing cells above the neck region are the progenitors of the oxyntic mucosa but not of the pit or enterochromaffin-like (ECL) cell lineages in the corpus. SOX2-positive cells are found in the pylorus and corpus of the mice stomach and participate in normal regeneration[48]. Runx1 enhancer element (eR1) expression is present in the stomach corpus and pyloric gland, and eR1+ stem cells can generate organoids via Wnt signaling pathway-dependent WENR. Activation of eR1 induces foveolar hyperplasia and antralization. Lrig1+ cells give rise to gastric lineage epithelial cells in both the gastric corpus and antrum. The replacement of damaged gastric oxyntic glands occurs via the differentiation of Lrig1+ cells into normal gastric lineage cells after acute oxyntic atrophy in the gastric corpus rather than via transdifferentiation of chief cell lineages[49].
GASTRIC PRECANCEROUS CONDITIONS
Different gastric lesions, such as chronic superficial/atrophic gastritis and gastric hyperplastic polyps, can be derived from their corresponding cells after injury caused by H. pylori infection, autoimmune gastritis, reactive or chemical stimulation, liver cirrhosis and portal hypertension[50,51]. Pathophysiologically, the stomach follows two patterns of adaptation after injury. The superficial response is a pattern whereby SMCs migrate and rapidly proliferate to repair erosions induced by acid or other irritants. Pit cells come from pre-pit cells arising in the isthmus region from undifferentiated granule-free cells through a pre-pit cell precursor stage and subsequently differentiate during their upward migration to the luminal surface, which takes approximately 3 days[52]. Metaplastic response [spasmolytic polypeptide-expressing metaplasia (SPEM) and IM] can follow gastric atrophy which is histologically characterized by the loss (atrophy) of acid-producing (oxyntic) PCs accompanied by invariable loss of mature ZCs when the portion of the gastric gland from the isthmus down to the base is damaged[53].
Chronic gastritis: CAG
Chronic gastritis (CG) is an inflammatory condition of the gastric mucosa. H. pylori infection is the most common cause, especially of CAG[54], compared with other relatively common causes, including bile reflux, autoimmune disease, long-term use of nonsteroidal anti-inflammatory drugs and other drugs and ethanol intake. Globally, on average, more than half of people may have CG, with approximately 70%–90% having H. pylori infection[55,56]. According to the updated Sydney system classification of CG, CG can be divided into atrophic and non-atrophic gastritis (chronic superficial gastritis)[57]. H. pylori-induced chronic non-atrophic gastritis can progress to atrophic gastritis[58,59]. Atrophy of the gastric mucosa is defined as the decrease or disappearance of the original gastric glands, which may be replaced by SPEM/pseudopyloric metaplasia (PPM), pyloric metaplasia, IM or fibrosis[60]. Corpus gland atrophy is characterized by the loss of PCs leading to impaired gastric acid and intrinsic factor secretion (malabsorption of iron and vitamin B12 resulting in anemia)[61,62]. The diagnosis of corpus gland atrophy is mainly based on endoscopy findings, such as H. pylori gastritis and open- and closed-type gastritis, the findings of pathology assessment with histological staging systems (OLGA and OLGIM), and the findings of noninvasive methods, such as the combination of pepsinogens, gastrin-17 and anti-H. pylori antibody serum assays, serum ghrelin assays, volatile organic compound assays and gastric juice pH assays based on hypochlorhydric conditions)[63-65]. Under endoscopy, IM is the most reliable marker of atrophy. Patients with advanced stages of gastritis, that is, atrophy and/or IM affecting both the antral and corpus mucosa, should be considered at a high risk for GC[10]. High-definition endoscopy with chromoendoscopy (CE) and image-enhanced endoscopy (IEE) with ME, such as NBI with ME, are better than high-definition and conventional white-light endoscopy[10,66,67]. Artificial intelligence (AI) has also been applied to diagnose CAG based on different images. For example, convolutional neural network (CNN) models based on conventional white light images to diagnose CAG achieved more than 90% (93%-94.2%) higher accuracy than experts, as well as being able to distinguish H. pylori gastritis from autoimmune gastritis[68,69]. When differentiating CAG from GC, the accuracy, sensitivity and specificity of a CNN model based on ME-NBI images were 85.3%, 95.4% and 71.0%, respectively (0.02 s/image)[70]. CG can be found in the gastric antrum, predominantly in the gastric body, and the involvement of the entire stomach is seen on endoscopy. Mild, moderate or severe CAG can be diagnosed according to histopathological results. The OLGA system stages gastritis by combining the extent of atrophy scored histologically with the topography of atrophy identified through biopsy mapping. It is recommended that patients with advanced stages of CAG (including severe atrophic change, IM in both the antrum and corpus or OLGA/OLGIM III/IV disease) should be followed up with a high-quality endoscopy every 3 years, and those with autoimmune CAG should be followed up every three-five years[10,64,71]. For patients with mild to moderate atrophy restricted to the antrum, there is no evidence to recommend surveillance.
In patients with CAG, the annual incidence of GC is 0.1%. Within 1, 5 and 10 years of follow-up after initial diagnosis, GC is diagnosed in 0.3%, 0.6% and 0.8% of patients, respectively. The median interval between initial diagnosis and GC is 1.6 years[72]. In terms of reversibility, H. pylori eradication may contribute to the regression of CAG, although this is controversial[73]. In clinical research, significant differences in CAG between H. pylori-eradicated and H. pylori-negative groups disappeared at the 1-year follow-up[74]. In patients > 59 years old, atrophy was alleviated in both the 5th and 10th years, and patients ≤ 59 years old showed alleviation of atrophy in the 10th year[75]. Moreover, it has been reported that CAG can be improved after some drug treatments[76-78]. These results indicate the reversibility of CAG. Another clinical study showed that severe gastric atrophy persisted in the mucosa adjacent to the GC for a long time following H. pylori eradication, indicating the irreversibility of H. pylori-induced gastric atrophy[79]. CAG may continue for a long time and may even become severe, even after eradication[80]. In addition, the longer the follow-up, the greater the risk of developing diffuse-type GC became in patients who have mild-to-moderate gastric atrophy[80]. It is likely that slight atrophy is reversible after stimulus removal, whereas severe atrophy caused by long-term infection may not be readily reversed. Molecular alterations related to CAG are not common, especially mutations, and this is further evidence for reversibility. For instance, single-nucleotide polymorphisms (SNPs) have been studied, such as the C allele of TLR1 rs4833095 T/C and the IL10 gene promoter -819C/T (rs1800871) polymorphism related to the immune response, which might increase the risk of CAG and GC[81-83]. rs7521584 minor allele homozygosity and p53 gene mutation might be associated with the degree of gastric mucosal atrophy induced by H. pylori infection[84,85]. Gastric microbiota alteration is also a potential factor. Significant mucosal microbial dysbiosis was observed in gastric mucosal samples from patients with CAG, IM and dysplasia, including alterations of Fusobacterium, Neisseria, Prevotella, Veillonella, and Rothia, compared with the microbial environment in superficial gastritis, indicating the potential correlation of these alterations with disease progression[86].
SPEM
Gland cells loss or dropout is followed by replacement with metaplastic cells coexpressing proteins, including TFF2 and MUC6 expressed by MNCs and pepsinogen expressed by mature ZCs, and is not affected by GSCs[87]. This metaplasia occurs because of dysregulated transdifferentiation of the mucous neck-ZC lineage and is termed as SPEM; it is inherent to the gastric corpus and has an unclear initiation mechanism (transdifferentiation is a process in which one differentiated cell type converts into a completely different cell type present in the tissue.)[88]. SPEM is often considered synonymous with PPM. PPM is a histological name, and SPEM is a molecular name[89]. However, pyloric and PPMs are not always coincident with SPEM in H. pylori gastritis and autoimmune gastritis, which may be because SPEM resembles MNC hyperplasia on histochemical staining and can occur with MNC hyperplasia[90,91]. SPEM can be identified in mice with chronic infection of H. pylori or autoimmune gastritis, and it also develops in mice immediately after acute epithelial injury, such as in response to drugs including DMP777, L-635 and high-dose tamoxifen. SPEM is characterized by abundant mucins in cells that are found below the isthmus region, which often expands to the gland base[92]. Tamoxifen/DMP777 can cause > 90% PCs loss, increased proliferation of stem/progenitor cells and reduced mutation and morphology change of ZCs, which is the characteristic of SPEM, without neoplastic transformation over 3–6 mo, and gastric histology returns to nearly normal status within 3 mo after withdrawing the drug[53,93]. Furthermore, in mice treated with PCs alone responding to apoptosis-inducing diphtheria toxin (DT)[94], > 90% of PCs were killed, and increased proliferation occurred in the isthmus and neck but not in the base, while ZCs maintained largely normal morphology with apical GIF granules[95]. In contrast with tamoxifen and DMP777, DT targeted at PCs has never caused substantial SPEM. SPEM develops in response to both acute injury and chronic inflammation. The emergence of SPEM can also be altered by endocrine or paracrine regulation. For example, mice with gastrin or histidine deficiency had accelerated development of SPEM after DMP777 administration compared with wild-type mice as well as attenuated EGF receptor signaling[96]. Thus, the emergence of SPEM is regulated by a complex coordinated mechanism including PC loss due to inflammation (not apoptosis), which may be accompanied by ZC loss, damage to other mucosal cell lineages and their cytokines and immune reactions. No factor has been found to be solely responsible for the emergence of SPEM.
SPEM is a regenerative lesion in gastric mucosa, and SPEM cells can redifferentiate into ZCs following recovery from injury. As a mucous-secreting reparative lineage, SPEM cells arise in the setting of acute injury or ulceration. SPEM around ulcers contributes to the healing of these lesions[89]. During chronic inflammation, such as that induced by H. pylori infection, SPEM glands can connect to allow deeper penetration of H. pylori and expansion of its intragastric niche[97]. Heterogeneity in mouse SPEM cell lineages has been identified, and chronic inflammation can lead to the further evolution of goblet-cell IM, which shows varying levels of intestinalization-related transcripts, including TFF3, MAL2, CFTR and MUC4[98]. In addition, inflammation is necessary for SPEM to progress directly to dysplasia induced by H. pylori infection without the development of phenotypic IM[99,100]. Inflammation is also considered a potential precursor to IM and GC in a chronic inflammatory setting[99]. Both SPEM and IM are associated with GC.
GASTRIC IM
IM, an adaptive response to chronic injury, generates epithelium in the stomach that resembles small intestinal epithelium with or without a brush border characterized by the presence of intestinal goblet cells labeled by MUC2 and TFF3. The expression of MUC2 in IM was found to be higher in GC of earlier stages and was correlated with lower tumor depth of invasion and a lower rate of lymph node metastasis, and these features are considered reliable markers of IM[101-103]. Foveolar hyperplasia, SPEM and IM are histological changes observed in atrophic gastritis, and the lesions express TFF1, TFF2, and TFF3, respectively. TFF3 can easily form a disulfide-linked heterodimer with FCGBP (TFF3–FCGBP), as both TFF3 and FCGBP are secreted from intestinal goblet cells. TFF3 expression seems to be dependent on the STAT1/3 signaling induced by IL-6 during inflammation. The TFF3–FCGBP interaction is likely part of the innate immune defense of gastric mucous, defensing microorganisms and promoting mucosal wound healing in mice and humans[104-106]. Serum level of TFF3 was found to be a potential noninvasive marker of GC, and the use of TFF3 serum levels combined with pepsinogen could improve GC screening[107,108]. In addition, only the serum TFF3 Level was stable and not significantly affected by H. pylori eradication[109]. GC patients with negative TFF3 expression exhibited longer survival than the positive TFF3 expression group. TFF3 was also demonstrated to be an independent prognostic indicator of recurrence and overall survival in GC[110]. Caudal-related homeobox 1 (CDX1) and CDX2 are intestine-specific transcription factors strictly confined to the gut, expressed from the duodenum to the rectum. Expression of nuclear CDX1 and CDX2 has been noted in human IM and is involved in the progression to dysplasia and GC[111]. CDX1 is predominantly expressed in the undifferentiated cells of the intestinal crypts, whereas CDX2 is mostly present in the villi or differentiated cell compartment of the small intestine[112]. Differences in differentiation, structure, and proliferation between IM induced by CDX1 vs CDX2 have also been found. CDX1 may play a role in the transdifferentiation of the mucosa into an intestinal type. The maintenance of intestinal differentiation may depend on CDX2[113]. CDX1 expression can be transcriptionally activated by nuclear factor-kappaB (NF-κB) signaling after direct binding to the unmethylated promoter. In normal gastric mucosa, CDX1 is not expressed due to high levels of CDX1 promoter methylation and the absence of inflammation. The methylation level of the CDX1 promoter decreases during H. pylori infection and IM and reaches the lowest level in low-grade intraepithelial neoplasia[114]. In H. pylori infection, the contribution of CDX2 to IM is regulated by the NOD1-mediated innate immune response to bacterial infection. In vivo, prolonged infection of NOD1-deficient mice with H. pylori led to increased CDX2 expression and IM with increased nuclear expression of the NF-κB p65 subunit and decreased expression of TRAF3[115]. Eradication of H. pylori did not show beneficial effects on aberrant CDX1/CDX2 expression. Reversal of IM may be associated with a decrease in CDX2 mRNA expression[116]. CDX2 activation in IM can be driven by the reflux of bile acids via the FXR/NF-κB signaling pathway and can be increased by microRNA (microRNA-92a-1–5p) targeting FOXD1[117]. SOX2 is a member of the high-mobility group domain proteins and has been identified as an adult stem cell marker. SOX2 can negatively regulate the CDX2 promoter. It was associated with gastric differentiation in incomplete IM and lost in the progression to dysplasia, whereas CDX2 was acquired de novo in IM and maintained in dysplasia[118].
Factors affecting gene expression, including mutations, DNA methylation and SNPs, as well as epigenetic factors, including microRNAs, long noncoding RNAs and mRNAs, have been associated with IM and have a potential role in screening, treatment and prognostic purposes[119]. IM patients with shortened telomeres and chromosomal alterations were likely to develop subsequent dysplasia or GC, whereas patients exhibiting normal epigenetic patterns were likely to experience regression. IM in patients with intermediate levels of DNA methylation changes tended to regress, while IM in patients with high levels of DNA methylation changes tended to persist/progress, indicating that levels of aberrant DNA methylation might influence the “point of no return”[120]. Further heterogeneous levels of DNA methylation were found in isolated metaplastic gland samples and isolated nonmetaplastic gland samples from the same IM mucosa. DNA methylation of mir34-b/c was found to be common in isolated cancer and IM glands. The mir34-b/c gene methylation status among isolated samples of IMs and isolated non-IM glands had an impact on IM development[121]. SNP analysis showed a negative association between the minor allele C of TLR4 rs11536889 and the progression of IM[122].
IM may arise from SPEM, mentioned above, or perhaps directly from ZCs[123] or gastric isthmus GSCs[124]. Elucidating the origin of gastric IM is difficult because H. pylori infection in mice does not result in IM. It has been demonstrated that IM can be mostly surrounded by SPEM foci and developed subsequently to SPEM with the SPEM- and MUC2-positive goblet cells during the course of H. pylori infection in Mongolian gerbils[125]. Dual TFF2 and TFF3 positivity was seen in about 50% of the foci of IM and in about 33% of SPEM samples adjacent to GC. The expression patterns of TFFs and MUCs may indicate that IM evolves from SPEM[126]. SPEM was found to originate from ZCs in mice, and cells redifferentiated into ZCs upon injury resolution; in this process, activation of Ras may promote metaplasia and inhibition of Ras signaling may reverse metaplasia. The acquisition of genetic/epigenetic mutations in long-lived stem cells may leads to GC and precancerous lesions such as metaplasia and dysplasia[42]. For example, it was demonstrated that short-term SPEM might arise from neck progenitors rather than transdifferentiated mature ZCs, and long-term SPEM might originate from isthmus progenitors[127]. A downward migration of the stem cell zone toward the base to form SPEM was shown in one study, and depleting Lgr5+ ZCs did not impair metaplasia development, indicating that Mist1+ isthmus cells, rather than Mist1+ ZCs, were the origin[124,127]. Therefore, the origin of gastric IM is presently under debate.
Gastric IM can be classified into complete and incomplete types on H&E-stained sections. Types I, II and III are classified based on the type of secretory mucins identified by the special stains Alcian blue pH 2.5 (AB)/periodic acid-Schiff (PAS) and high iron diamine/AB. Type I corresponds to complete IM, and types II and III are subclassifications of incomplete IM[128,129]. The pooled prevalence of IM, a precancerous condition, was 4.8%; the pooled incidence rate for the progression of IM to GC in the absence of dysplasia was 12.4 per 10000 person-years; the median time to progression to GC from IM was estimated to be 6.1 years[130]; and the pooled global regression rate of IM to normal mucosa or non-atrophic gastritis at 1 year, 3 years, 5 years, and 10 years was 29.7%, 19.4%, 25.9% and 19.4%, respectively[129]. However, IM reversibility is still controversial. IM in the corpus was most likely to progress to more than one location[131]. Incomplete type, extensive IM and a first-degree relative with GC have been shown to be higher risk factors (carrying a more than 2-fold increased risk for GC) than complete type, limited IM and no family history. Therefore, the AGA recommends patients with IM specifically at higher risk of GC (those with incomplete IM, extensive IM or a family history) and/or who are at overall increased risk for GC (racial/ethnic minorities, immigrants from regions with high GC incidence, or individuals with a first-degree relative with GC) may reasonably choose repeat endoscopy within 1 year for the risk stratification[11]. European guidelines recommend that endoscopic surveillance with CE and guided biopsies within 3 years may be considered in patients with IM at a single location with a family history of GC, incomplete IM or persistent H. pylori gastritis[10]. British guidelines recommend that patients with IM should undergo endoscopy for no less than 7 min with photographic documentation of gastric regions, including location and extent and pathology, and recommend endoscopic surveillance every 3 years for patients with extensive IM, a strong family history or persistent H. pylori infection. IEE is recommended to accurately detect and risk-stratify IM. Endoscopic grading is documented as distal gastric (antrum or incisura—low risk) or proximal gastric (corpus—high risk). Biopsies are directed at mucosa within Sydney protocol areas where enhanced imaging discloses IM[132]. In addition, AI, especially the deep CNN system, has been introduced into research to aid real-time diagnosis of IM based on IEE images and has achieved acceptable results[133,134]. For example, the accuracy of an AI model for diagnosing IM was between 0.86 and 0.91, similar to expert's accuracy for detecting IM (0.89 vs 0.82), and was superior to that of nonexperts for detecting IM (0.74 vs 0.82)[133]. Although IM has been considered a point of no return, H. pylori eradication can achieve the most benefit before the development of precancerous lesions, including IM and CAG. There are also some studies indicating that IM may be reversible, and the most common treatment is eradication of H. pylori to reverse IM. For example, in one study, IM in the antrum and corpus improved significantly in the H. pylori-eradicated group. A significant difference in IM between the H. pylori-eradicated and H. pylori-negative groups disappeared at ≥ 5 years of follow-up in the antrum and at 3 years of follow-up in the corpus[74]. Chronic celecoxib users had a higher regression rate of IM after eradication of H. pylori[135]. Resveratrol can reduce bile acid-induced IM through the PI3K/AKT/p-FoxO4 signaling pathway and has a potential reversing effect on IM, especially that caused by bile acid reflux[136].
FOVEOLAR HYPERPLASIA AND GASTRIC HYPERPLASTIC POLYPS
Both foveolar hyperplasia and gastric hyperplastic polyps are the expansion of normal foveolar/pit cells. A hyperproliferative response to tissue injury such as erosions and ulcers accompanied by increased cellular exfoliation results in the histopathological appearance of foveolar hyperplasia. They commonly arise from the background mucosa containing chronic active or reactive inflammation caused by H. pylori infection, liver cirrhosis and portal hypertension, autoimmune gastritis and reactive or chemical stimulation (e.g., bile reflux or NSAIDs and gastric stump), etc.[137]. The hyperplasia originated from pre-pit/progenitor cells in isthmus[138]. Hyperplastic polyps consist of elongated, grossly distorted, branching and dilated hyperplastic foveola lying in an edematous stroma rich in vasculature. Smooth muscle fibers may also be apparent in the lamina propria[139]. Although glands are usually not involved in the formation of polyps, they may contain pyloric glands, ZCs and PCs[140]. Both the epithelium and mesenchymal stromal cells can show marked regenerative changes and contain varying degrees of chronic and active inflammation[139]. Hyperplastic polyps represent 30%–93% of all gastric epithelial polyps and rarely undergo neoplastic progression, but are associated with an increased risk of synchronous cancer occurring elsewhere in gastric mucosa[141]. The rate of dysplasia arising in hyperplastic polyps was reported from 1.9% to 19% and the rate of GC was from 0.6% to 2.1%[142]. Molecular alterations have also been studied including expression of p53, K-ras, microsatellite instability, p21WAF1/CIP1 and cyclin D1 Levels, involving in the process of neoplastic transformation, which still remain controversial[143].
Endoscopically, they are found most frequently in the antrum and are often multiple between 0.5 and 1.5 cm in diameter. Size larger than 1 cm has been considered as a risk factor of malignant transformation. The larger a GHP is, the more likely it is to become cancerous, as its continuously increasing volume indicates the persistence of hyperplasia and inflammatory stimulating factors. One carcinogenesis process is intestinal type: Persistent inflammation can increase the damage of the proper gastric glands followed by atrophy, IM, dysplasia and adenocarcinoma. The other is gastric type: The metaplasia of foveal epithelium occurs and resists the repeated inflammatory stimulation. It is tall columnar cells resembling foveolar cells, further developing to dysplasia and gastric type adenocarcinoma[143]. On the management, hyperplastic polyps should be biopsied and an examination of the whole stomach should be made for mucosal abnormalities and biopsied abnormalities[144]. Test for H. pylori and eradicate it when present, as it is the most common cause. One meta-analysis showed that after eradicating H. pylori, most GHPs were eliminated, and the GHP elimination rate was increased by more than 20 times than that in the control group[145]. In view of the potential neoplastic transformation risk, hyperplastic polyps larger than 10 mm should be resected completely[144]. However, there is a controversial issue on the recurrence and neoplastic transformation after endoscopic resection, even R0 resection[137]. When it does not harbor foci of dysplasia and/or carcinoma, whether R0 resection has little impact on recurrence, as inflammatory hyperplasia foveolar cells are not able to divide and proliferate themselves[137]. Similarly, either ESD or EMR resection of a GHP may have little effect on recurrence. How about the residual inflammatory hyperplasia foveolar cells? As the resection deep is at least mucosa, it means proper gastric glands in the lamina propria will be removed together, further losing the regeneration of pit and gland epithelium and being followed by scar formation/fibrosis. The residual cells can initially derive from the removed glands or adjacent glands. If they are from the removed glands, there is no chance of recurrence. However, if they are from adjacent glands, endoscopic resection will increase the rate of recurrence when the background mucosa contains chronic active or reactive inflammation. If there is free of chronic active/reactive inflammation, the residual cells from adjacent glands will die from necrosis/apoptosis, or they continue the inflammatory hyperplasia and recover after the recession of the acute inflammation induced by resection. It can be explained by ESD removing early GC after eradication of H. pylori, in which rare GHP can be found during surveillance, as the background mucosa is commonly chronic atrophy instead of active inflammation and IM instead of foveolar cells[146,147]. However, if the background mucosa contains atrophy and IM with chronic active/reactive inflammation, the possibility of neoplastic transformation will increase derived from the residual cells. When a GHP harbors foci of dysplasia and/or carcinoma, R0 resection will be pivotal to prevent recurrence, as these cells have already contained the potential for malignant proliferation themselves, no matter whether there is a new origin of neoplasms. We can see the “recurrence” is actually from adjacent glands in the background mucosa with chronic active/reactive inflammation. The background mucosa where GHPs derive is one of determinants. Eliminating the common causes should be applied at the same time as resection when a GHP is more than 10 mm which will reduce the recurrence rate and bring benefits for patients to a large extent better than being in the dilemma (whether resection or not)[148,149] (Figure 1).
Figure 1 An illustration of gastric unit and precancerous conditions shows the histological structure of gastric epithelium, possible gastric stem cells types and potential origin of different gastric precancerous conditions.
CAG: Chronic atrophic gastritis; CSG: Chronic superficial gastritis; GC: Gastric cancer; GSCs: Gastric stem cells; HPs: Hyperplastic polyps; IM: Intestinal metaplasia; MNCs: Mucous neck cells; PCs: Parietal cells; SMCs: Surface mucous cells; SPEM: Spasmolytic Polypeptide-Expressing Metaplasia; ZCs: Chief cells/zymogenic cells; H. pylori: Helicobacter pylori.
CONCLUSION
Reducing the rate of GC and increasing endoscopic eradication therapy have always been core goals of GC management. Timely and effective detection and surveillance of precancerous conditions can facilitate early detection and treatment of GC in the early stage and even prevent the occurrence of GC. We provided a detailed overview of gastric epithelium organization and its renewal and reviewed precancerous conditions, including CAG, SPEM, IM, foveolar hyperplasia and GHPs derived from gastric epithelium, based on a histological perspective, covering their epidemiology, clinical management and advances, histological structures, causes, and potential origins and reversibility. Regarding the origin, process and reversibility, they are the mainly controversial parts, such as whether CAG and IM are reversible and what the point of no return is, whether IM derives from SPEM or which cells are the origin of IM and what the association is between R0 resection and recurrence and neoplastic transformation of gastric hyperplastic polyps. We discussed these issues in detail based on a review of almost all related articles. More clinical and basic research on the molecular alterations of these gastric lesions may shed light on the controversies in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batyrbekov K, Mohamed SY S-Editor: Fan JR L-Editor: A P-Editor: Fan JR