Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2088
Peer-review started: August 4, 2022
First decision: September 30, 2022
Revised: October 1, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 15, 2022
Processing time: 103 Days and 6.9 Hours
Portal vein embolization (PVE) is currently considered the standard of care to improve the volume of an inadequate future remnant liver (FRL) and decrease the risk of post-hepatectomy liver failure (PHLF). PHLF remains a significant limitation in performing major liver surgery and is the main cause of mortality after resection. The degree of hypertrophy obtained after PVE is variable and depends on multiple factors. Up to 20% of patients fail to undergo the planned surgery because of either an inadequate FRL growth or tumor progression after the PVE procedure (usually 6-8 wk are needed before surgery). The management of PVE failure is still debated, with a lack of consensus regarding the best clinical strategy. Different additional techniques have been proposed, such as sequential transarterial chemoembolization followed by PVE, segment 4 PVE, intra-portal administration of stem cells, dietary supplementation, and hepatic vein emb
Core Tip: Portal vein embolization (PVE) is actually considered the standard of care for inducing volume augmentation of the future remnant liver. However, 20% of patients who have undergone PVE, reportedly never undergo curative resection, due to either insufficient future remnant liver (FRL) growth with an unacceptable risk of post-hepatectomy liver failure, or oncologic progression after PVE, while waiting for the adequate FRL hypertrophy (6-8 wk or more). The management of PVE failure is still highly debated, with different additional techniques that have been proposed, such as sequential transarterial chemoembolization followed by PVE, segment 4 PVE, intra-portal administration of stem cells, dietary supplementation, and hepatic vein embolization.
- Citation: Cassese G, Han HS, Lee B, Cho JY, Lee HW, Guiu B, Panaro F, Troisi RI. Portal vein embolization failure: Current strategies and future perspectives to improve liver hypertrophy before major oncological liver resection. World J Gastrointest Oncol 2022; 14(11): 2088-2096
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2088.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2088
The main goal of hepatic surgical oncology is to perform a R0 resection, by preserving a sufficient future remnant liver (FRL) to prevent post-hepatectomy liver failure (PHLF). Indeed, PHLF is still a major cause of mortality after major liver surgery[1]. To reduce the risk of PHLF it is necessary to preserve not only a sufficient amount of liver parenchyma, but also ensure adequate liver function[2]. Owing to advances in preoperative evaluation and optimization of the FRL, the postoperative mortality rate for major liver resections (≥ 3 segments) is currently showed to be less than 5%[3,4]. The FRL volume is the only factor that can be acted on, depending on the surgery and liver condition. An FRL ≥ 20% of the volume is considered safe in cases of healthy liver, ≥ 30% after chemotherapy, 40% in case of steatosis or cholestasis, and ≥ 50% in case of cirrhosis[5]. Prior to performing major hepatectomy, multiple patient factors should also be considered to optimize FRL growth, such as an age higher than 65 years, obesity or malnutrition, diabetes, chronic renal failure[4]. The degree of liver hypertrophy is also affected by many liver related factors, with the eventual presence of chronic liver disease or previous chemotherapy playing a fundamental role[6,7]. However, pooled data from a recent meta-analysis showed no difference in the degree of hypertrophy between patients receiving neo-adjuvant chemotherapy com
Portal vein embolization (PVE) is seen as the standard of care for inducing hypertrophy of the FRL. However, 20% of patients who have undergone PVE, reportedly never undergo curative resection, due to either insufficient FRL growth with an unacceptable risk of PHLF, or oncologic progression after the PVE procedure (6 wk or more before surgery)[11]. For patients with insufficient liver hypertrophy following PVE, adjunctive techniques such as hepatic vein embolization, segment 4 embolization, intra-portal administration of stem cells, dietary supplementation, and sequential transarterial embolization followed by PVE, have been proposed. However, evidence regarding the appropriate management of these patients after PVE failure is still lacking.
This review aims to summarize the up-to-date strategies available and future perspectives on the management of patients scheduled for major hepatic resection with insufficient FRL hypertrophy after PVE.
PVE was first described by Makuuchi et al[12] in 1984, in patients with cholangiocarcinoma (CCA) undergoing major hepatectomy[13]. However, the principle of contralateral liver lobe hypertrophy after hepatic vessel obliteration was first identified by James Cantlie 100 years before[14]. Currently, PVE is the standard of care procedure to obtain FRL hypertrophy in patients requiring major liver surgery, in case of marginal FRL. Reportedly, about 80% of patients are able to undergo the planned liver surgery after 6-8 wk[15].
PVE is a technique of interventional radiology, carried out under local anesthesia. Three approaches have been classically reported for this procedure: trans-hepatic, trans-splenic and trans-ileocolic. The trans-hepatic technique involves percutaneous access to the portal branches. The trans-ileocolic technique consists of a mini-laparotomy to isolate and cannulate the ileocolic vein, to access the portal vein. As it is a more invasive procedure, it is used when interventional radiology is not feasible. The trans-splenic technique is more recent, providing the advantage of eliminating the risk of tumor seeding. This access was initially thought to have a higher risk of bleeding complications; however, such concerns have been addressed and this approach is being increasingly used[16]. In contrast, a meta-analysis by Abulkhir et al[17] found that FRL hypertrophy was significantly higher using the trans-hepatic technique. Recently, Yamao et al[18] described for the first time the round ligament approach, suggesting its usefulness in elective cases for which it is difficult to safely perform trans-hepatic or trans-ileocecal approaches. In their study on 50 patients undergoing major hepatectomy, the authors observed no morbidity, neither mortality, related to the round ligament approach. The median functional hepatic remnant rate before and after the procedure was 55.6% and 63.2%, respectively.
Response to PVE has been found to be an important predictor of PHLF. Abdalla et al[19] proposed a degree of hypertrophy (DOH) cutoff of > 5% in case of healthy liver and > 10% in cirrhotic patients, to safely perform a major hepatectomy. Chapelle et al[20] investigated the hypertrophic response after PVE using hepatobiliary scintigraphy (HBS) and found a cut-off value of 1.72%/min/m2 of pre-PVE FRL-F for safe resection (81.3% sensitivity and 82.4% specificity). The increase in volume after PVE is not proportional to the increase in liver function (FRL-F), with a greater increase in FRL-F up to 3-4 wk after PVE procedure[21]. All previous studies agree that the smaller the FRL pre-PVE, the larger the FRL hypertrophy post-PVE[8,22,23].
PVE is contraindicated in cases of tumor invasion into the ipsilateral portal vein. A relative contraindication is portal hypertension since PVE may increase portal vein pressure and worsen the liver function and the clinical state[24].
Some previous studies suggested a negative impact of liver regeneration on long-term oncological outcomes, as regard to both disease-free survival (DFS) and overall survival (OS). Margonis et al[25] reported that a kinetic growth rate (KGR) higher than 1% could be related to an increased risk of recurrence. However, a meta-analysis focusing on the oncological outcomes of PVE showed that the procedure does not worsen the long term results of major liver surgery, without any higher risk in terms of hepatic recurrence, 3-year OS, and 5-year OS after PVE[26].
The true weight of most factors involved in PVE failure remains unclear, apart from the presence of the underlying liver disease. The main drawback of unresolved PVE: The 15%-25% rate of failure due to inadequate FRL hypertrophy or oncologic progression[11].
Several factors can influence the efficacy of PVE procedure. Regarding embolization materials that can be used for PVE, the combination of N-butil-cyanoacrylate (NBCA) with lipiodol is the most widely used, leading to reliable FRL hypertrophy with efficient embolization, and low rate of vascular recanalization[27]. Furthermore, recent reports showed similar results with resorbable materials, hypothesizing the advantage to prevent an accidental contralateral embolization[28]. A recent meta-analysis by Soykan et al[8] reported a significant difference in the degree of hypertrophy in favor of NBCA compared to the other agents. In the same study, other risk factors were investigated, and showed that sex and previous chemotherapy were not associated with a lower degree of hypertrophy, contrary to what has been previously reported. It is reported that five predictive factors for insufficient FRL growth: Age, FRL%, plasma indocyanine green detection rate (ICG-PDR), total bilirubin level, and a history of chemo
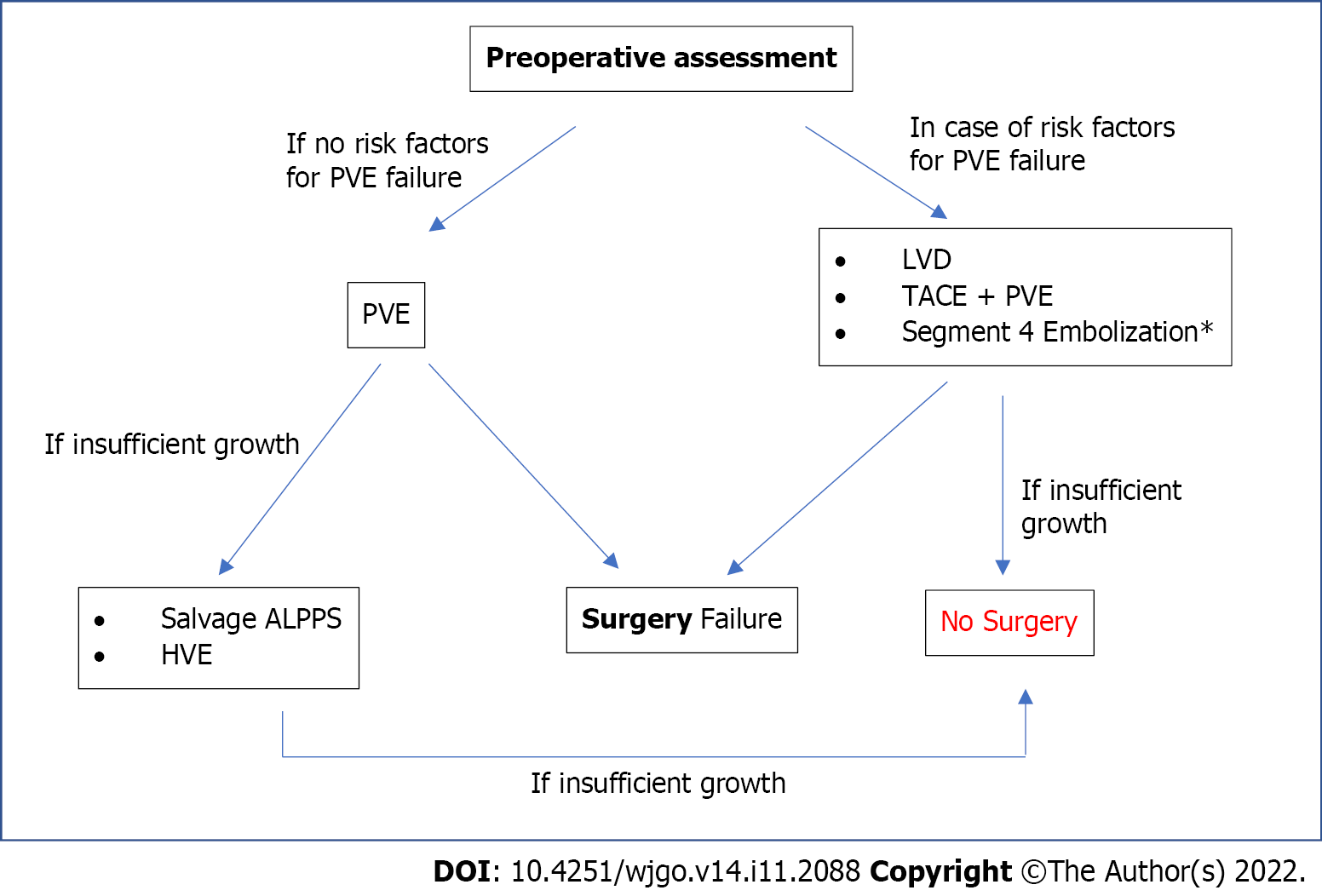
Insufficient FRL augmentation after PVE is a difficult issue to overcome because of two reasons: The need to act quickly to avoid tumor progression and the need to prevent PHLF. Different strategies have been suggested without consensus. In Figure 1, the authors propose their algorithm, which is discussed below.
When right trisectionectomy is planned, additional embolization of segment 4 (S4) can be performed. The first encouraging experience with this procedure was published by Kishi et al[29], which showed a higher FRL hypertrophy, resulting in a median volumetric increase of 54% vs 26% after PVE alone, without affecting post-procedural morbidity or perioperative outcomes. Recently, a larger Scandinavian study showed similar results (median increase of 47% vs 38%, respectively; P = 0.02), but with a heterogeneous cohort, including patient with cirrhosis, CCA, and colorectal liver metastases (CRLM)[30]. Furthermore, the pre-PVE FRL was smaller in the S4 group (333 mL vs 380 mL; P = 0.01), which is associated with a higher DOH. A Japanese, propensity score-matched study in patients with biliary carcinoma also reported an improved FRL after PVE with S4 embolization[31]. In contrast, three other studies showed no significant differences between PVE alone and PVE with S4 embolization[22,32,33]. Studies have always considered the time interval needed to obtain FRL increase after S4 portal embolization similar to that after PVE, without the advantage of faster hypertrophy. Furthermore, when the scheduled surgery is not a right trisectionectomy, this technique is useless[34].
Hepatic vein embolization (HVE) was introduced to obtain an additional increase in FRL after PVE failure. The first experience with sequential HVE after ipsilateral PVE was reported by Hwang et al[35] in 2004, in order to obtain an additional FRL hypertrophy in 42 patients. Another study reported an FRL augmentation rate of 28.9% after HVE (vs 13.3% after PVE alone), without significant complications[36]. The mechanism of action probably consists of a higher stress on the liver due to a major outflow obstruction, showing at the same time a protective effect of the residual arterial flow against any dangerous biliary ischemia. Similar outcomes were recently reported by Niekamp et al[37] in nine patients with CRLM who underwent salvage HVE following PVE failure. The standardized FRL increased from 16% to 26% after HVE and 22% after PVE (P = 0.0005). HVE was performed after a median of 40 d from PVE, and only four of the nine patients underwent hepatectomy. Thus, even though HVE is safer and more effective, the sequential association of PVE and HVE requires a long interval between them, without counteracting a possible progression of tumor disease. Hence, Guiu et al[38] published the first reports about the liver venous deprivation (LVD) technique, consisting in a simultaneous embolization of the hepatic vein(s) and ipsilateral portal vessels. LVD requires that both the ipsilateral portal and venous branch (+/− accessory veins) are occluded with an Amplatzer plug, placed approximately 1 cm from the ostium. NBCA is injected beyond the plug to close the intrahepatic part of the vein(s), as well as any collaterals. The extended LVD (e-LVD) is a variation of the technique in which the middle hepatic vein is also treated[39]. First data after 99 m-Tc mebrofenin hepatobiliary scintigraphy (HBS) reported a 66% improvement in FRL-F 7 d after e-LVD procedure. After 3 wk, the median volumetric gain was 63.3%, while the functional increase was 64.3%. Furthermore, subsequent studies have shown also safe perioperative and oncological results after the completion surgery[40-42]. Thus, preliminary studies have shown that LVD can induce a higher FRL hypertrophy than PVE, without adding additional periprocedural risks. However, to reach stronger conclusions, randomized studies comparing LVD and PVE are awaited (HyperLiv 01 and Dragon 1 are currently still ongoing).
In essence, HVE seems to be a safe salvage option after PVE failure, but carries the risk of tumor progression during the long waiting times. The LVD technique seems to be a better substitute for PVE, and aims to replace PVE owing to its higher and faster hypertrophic effects[43].
Associating liver partition and portal vein ligation (ALPPS) was first described by Schnitzbauer et al[44] in 2012 as a novel two-staged hepatectomy, with the main advantage of remarkably reducing the delay between the first and second procedure. During the first stage, the lesions in the FRL are treated, and an anticipated line of resection is transected with ligation of the contralateral first order portal branch. After only 1-2 wk, completion surgery is performed after a sufficient FRL is confirmed using CT-based volumetry[45]. The reported successful rate for the completion surgery was 99%, while the traditional two staged hepatectomy reached only about 75%[46,47]. The shorter interval needed for FRL augmentation could significantly decrease the risk of tumor progression. Furthermore, the two surgeries could possibly be performed during the same hospitalization, affecting the costs and the organization. However, there has been concern regarding the effective increase in FRL-F. Olthof et al[48] showed a median increase of 29% in the FRL-F 7 d after ALPPS stage 1, compared to a volumetric increase of 78%, in a study involving patients with perihilar cholangiocarcinoma (P < 0.01). Similar results have been reported in patients with CRLM[49]. To this end, the efficacy of HBS in predicting PHLF after ALPPS was proven by Tomassini et al[50]; patients presenting with a daily gain in FRL-F of ≤ 2.7%/min/m2 indicated a high risk of PHLF development, which requires re-discussion of the second stage. The ALPPS registry shows a mortality rate of 5% in a series which included only patients treated for colorectal liver metastasis aged < 60 years old[45]. The main disadvantage of this fast post-procedural hypertrophy is the risk for higher rates of perioperative morbidity and mortality[45].
ALPPS was proposed as a salvage procedure by Enne et al[51]. The study reported a mean FRL increase of 88% in 20 patients who underwent ALPPS after PVE failure, with an exceptional 100% success rate and no 90-d mortality. Similar results were reported by Sparrelid et al[49] in 11 patients with CRLM: A median FRL growth of 61.8%, with no 90-d mortality or high-grade complications (≥ 3b-complication according to Clavien-Dindo). Many variations of the original ALPPS procedure have been reported in the literature (mini ALPPS, partial ALPPS, radio-frequency-assisted liver partition with portal vein ligation, and Tourniquet modification), with the aim of reducing postoperative morbidity and bring some technical advantages. However, none of these ones have been proposed as salvage procedures. It may be beneficial to obtain data on this in the future. Additionally, Dondorf et al[52] reported the possibility of obtaining a significant further increase in FRL after additional ligation of the middle hepatic vein in combination with ALPPS (a sort of “surgical LVD”). Though higher morbidity and mortality were observed, they were most likely associated with the underlying liver conditions.
Although the actual role of salvage ALPPS is still debated, we believe that it can be considered a viable salvage option.
Herein, we present an option that can’t be performed after PVE failure, but in addition to PVE when there are risk factors of failure, as proposed in our flow-chart. Indeed, the presence of an underlying chronic liver disease is a risk factor for poor hypertrophy after PVE. One of the reasons could be the presence of arterio-portal tumoral shunts, typical of hepatocellular carcinoma (HCC), which could counteract the hemodynamic effect of PVE. Sequential trans arterial chemoembolization (TACE) followed by PVE has been shown to achieve a higher DOH than PVE alone[53]. Ipsilateral PVE is performed 7-10 d after the initial TACE, once the blood parameters have normalized. The benefits of this dual technique include improved FRL hypertrophy relative to PVE alone and induction of an anti-tumor effect in the embolized lobe[54,55]. Ogata et al[54] reported a mean FRL increase in the TACEPVE group of 12% vs 8% for the PVE alone group (P = 0.022), with a DOH of 10% vs 5%, respectively (P = 0.044). In the same study, the TACE + PVE group had a higher complete tumor necrosis incidence (83.00% vs 0.05%; P < 0.001) and 5-year DFS (37% vs 19%; P = 0.041), owing to better local control of the HCC nodule. A limitation of this strategy is the consequent inflammation of the hepatic pedicle, which makes subsequent surgery more challenging. Furthermore, areas of residual segmental infarction were found within the non-cancerous liver on histopathology; thus, TACE should be performed carefully, since many of these patients have pre-existing liver dysfunction[55].
Fürst et al[56] first reported caries in six patients undergoing PVE with CD133 (+) bone marrow stem cells (BMSC) administration to improve FRL hypertrophy following PVE. In their study, a significantly higher mean increase in FRL volume was reported (77.3% vs 39.1%, P = 0.039). The time to surgery was also shorter in patients who received stem cell infusion (27 d vs 45 d, P = 0.057). Similarly, am Esch et al[57] showed a median absolute gain of 138.66 in the PVE-BMSC group compared to 62.95 mL in the PVE-alone group (P = 0.004). Post hoc analysis revealed better survival in the PVE-BMSC group (P = 0.028) than in the PVE-alone group (P = 0.094) and controls.
Despite the encouraging results, further issues need to be investigated prior to their routine use. Stem cells have been reported to stimulate tumor growth in murine models of CRLM[58,59]. Furthermore, the effectiveness of this technique in patients with chronic liver disease and prolonged chemotherapy remains unknown[60].
Owing to tremendous technological advances, appropriate FRL optimization can reduce the risk of PHLF. Although PVE is considered the standard of care for FRL volume augmentation, up to 20% of patients fail to undergo the planned surgery. An in-depth knowledge of all the risk factors for PVE failure can help us to choose the most effective procedure. In our opinion, LVD could replace PVE in the future, particularly in cases with negative predictive factors for FRL hypertrophy, once its validity has been confirmed. Other strategies, such as the combination of PVE and TACE or segment 4 embolization, can be carefully considered when appropriate. To date, after PVE failure, ALPPS is reportedly the most effective salvage procedure to obtain a volumetric gain with only a short delay, thus preventing tumor progression. However, prospective and large-scale studies on this challenging scenario are still needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baba H, Japan; Jackson T, United States; Zhu X, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. J Surg Oncol. 2014;110:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Kishi Y, Vauthey JN. Issues to be considered to address the future liver remnant prior to major hepatectomy. Surg Today. 2021;51:472-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Xu HW, Liu F, Li HY, Wei YG, Li B. Outcomes following laparoscopic vs open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. 2018;32:712-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (61)] |
| 4. | Cassese G, Han HS, Al Farai A, Guiu B, Troisi RI, Panaro F. Future remnant liver optimization: preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg. 2022;77:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 6. | Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Beal IK, Anthony S, Papadopoulou A, Hutchins R, Fusai G, Begent R, Davies N, Tibballs J, Davidson B. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Soykan EA, Aarts BM, Lopez-Yurda M, Kuhlmann KFD, Erdmann JI, Kok N, van Lienden KP, Wilthagen EA, Beets-Tan RGH, van Delden OM, Gomez FM, Klompenhouwer EG. Predictive Factors for Hypertrophy of the Future Liver Remnant After Portal Vein Embolization: A Systematic Review. Cardiovasc Intervent Radiol. 2021;44:1355-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Tanaka K, Kumamoto T, Matsuyama R, Takeda K, Nagano Y, Endo I. Influence of chemotherapy on liver regeneration induced by portal vein embolization or first hepatectomy of a staged procedure for colorectal liver metastases. J Gastrointest Surg. 2010;14:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D'Angelica M, Getrajdman GI, DeMatteo R, Kemeny NE, Fong Y. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Alvarez FA, Castaing D, Figueroa R, Allard MA, Golse N, Pittau G, Ciacio O, Sa Cunha A, Cherqui D, Azoulay D, Adam R, Vibert E. Natural history of portal vein embolization before liver resection: a 23-year analysis of intention-to-treat results. Surgery. 2018;163:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Makuuchi M, Takayasu K, Takuma T. Preoperative Transcatheter Embolization of the Portal Venous Branch for Patients Receiving Extended Lobectomy Due to the Bile Duct Carcinoma. J Jpn Pract Surg Soc. 1984;45:1558-1564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 13. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 14. | Wellcome collection. “On a new arrangement of the right and left lobes of the liver”. Anatomical Society of Great Britain and Ireland. Wellcome Collection. [cited June 30, 2022]. Available from: https://wellcomecollection.org/works/jrwbtbys. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sugawara Y, Yamamoto J, Higashi H, Yamasaki S, Shimada K, Kosuge T, Takayama T, Makuuchi M. Preoperative portal embolization in patients with hepatocellular carcinoma. World J Surg. 2002;26:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Zurcher KS, Smith MV, Naidu SG, Saini G, Patel IJ, Knuttinen MG, Kriegshauser JS, Oklu R, Alzubaidi SJ. Transsplenic Portal System Catheterization: Review of Current Indications and Techniques. Radiographics. 2022;42:1562-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 18. | Yamao T, Tamura Y, Hayashi H, Takematsu T, Higashi T, Yamamura K, Imai K, Yamashita YI, Ikeda O, Baba H. Novel Approach via the Round Ligament in Portal Vein Embolization. World J Surg. 2021;45:2878-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Chapelle T, Op De Beeck B, Huyghe I, Francque S, Driessen A, Roeyen G, Ysebaert D, De Greef K. Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on (99m)Tc-mebrofenin hepatobiliary scintigraphy: can this tool predict post-hepatectomy liver failure? HPB (Oxford). 2016;18:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | de Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Zeile M, Bakal A, Volkmer JE, Stavrou GA, Dautel P, Hoeltje J, Stang A, Oldhafer KJ, Brüning R. Identification of cofactors influencing hypertrophy of the future liver remnant after portal vein embolization-the effect of collaterals on embolized liver volume. Br J Radiol. 2016;89:20160306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Kohno S, Isoda H, Ono A, Furuta A, Taura K, Shibata T, Togashi K. Portal Vein Embolization: Radiological Findings Predicting Future Liver Remnant Hypertrophy. AJR Am J Roentgenol. 2020;214:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB; American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Margonis GA, Sasaki K, Andreatos N, Pour MZ, Shao N, Ghasebeh MA, Buettner S, Antoniou E, Wolfgang CL, Weiss M, Kamel IR, Pawlik TM. Increased kinetic growth rate during late phase liver regeneration impacts the risk of tumor recurrence after colorectal liver metastases resection. HPB (Oxford). 2017;19:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Giglio MC, Giakoustidis A, Draz A, Jawad ZAR, Pai M, Habib NA, Tait P, Frampton AE, Jiao LR. Oncological Outcomes of Major Liver Resection Following Portal Vein Embolization: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23:3709-3717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | de Baere T, Roche A, Vavasseur D, Therasse E, Indushekar S, Elias D, Bognel C. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Tranchart H, Koffi GM, Gaillard M, Lainas P, Poüs C, Gonin P, Nguyen TH, Dubart-Kupperschmitt A, Dagher I. Liver regeneration following repeated reversible portal vein embolization in an experimental model. Br J Surg. 2016;103:1209-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Kishi Y, Madoff DC, Abdalla EK, Palavecino M, Ribero D, Chun YS, Vauthey JN. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Björnsson B, Hasselgren K, Røsok B, Larsen PN, Urdzik J, Schultz NA, Carling U, Fallentin E, Gilg S, Sandström P, Lindell G, Sparrelid E. Segment 4 occlusion in portal vein embolization increase future liver remnant hypertrophy - A Scandinavian cohort study. Int J Surg. 2020;75:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ito J, Komada T, Suzuki K, Matsushima M, Nakatochi M, Kobayashi Y, Ebata T, Naganawa S, Nagino M. Evaluation of segment 4 portal vein embolization added to right portal vein for right hepatic trisectionectomy: A retrospective propensity score-matched study. J Hepatobiliary Pancreat Sci. 2020;27:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | de Baere T, Teriitehau C, Deschamps F, Catherine L, Rao P, Hakime A, Auperin A, Goere D, Elias D, Hechelhammer L. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17:2081-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Massimino KP, Kolbeck KJ, Enestvedt CK, Orloff S, Billingsley KG. Safety and efficacy of preoperative right portal vein embolization in patients at risk for postoperative liver failure following major right hepatectomy. HPB (Oxford). 2012;14:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Nagino M, Kanai M, Morioka A, Yamamoto H, Kawabata Y, Hayakawa N, Nimura Y. Portal and arterial embolization before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Lee YJ, Sung KB, Moon DB, Ha TY, Cho SH, Oh KB, Han JM, Kim MH. Hepatic venous congestion in living donor liver transplantation: preoperative quantitative prediction and follow-up using computed tomography. Liver Transpl. 2004;10:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, Kim MH, Lee SK, Lee SG. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg. 2015;39:2990-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Niekamp AS, Huang SY, Mahvash A, Odisio BC, Ahrar K, Tzeng CD, Vauthey JN. Hepatic vein embolization after portal vein embolization to induce additional liver hypertrophy in patients with metastatic colorectal carcinoma. Eur Radiol. 2020;30:3862-3868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, Fabre JM, Quenet F, Herrero A, Panaro F, Baudin G, Ramos J. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26:4259-4267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Guiu B, Quenet F, Escal L, Bibeau F, Piron L, Rouanet P, Fabre JM, Jacquet E, Denys A, Kotzki PO, Verzilli D, Deshayes E. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol. 2017;27:3343-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Khayat S, Cassese G, Quenet F, Cassinotto C, Assenat E, Navarro F, Guiu B, Panaro F. Oncological Outcomes after Liver Venous Deprivation for Colorectal Liver Metastases: A Single Center Experience. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kobayashi K, Yamaguchi T, Denys A, Perron L, Halkic N, Demartines N, Melloul E. Liver venous deprivation compared to portal vein embolization to induce hypertrophy of the future liver remnant before major hepatectomy: A single center experience. Surgery. 2020;167:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 42. | Cassese G, Troisi RI, Khayat S, Quenet F, Tomassini F, Panaro F, Guiu B. Liver venous deprivation vs associating liver partition and portal vein ligation for staged hepatectomy for colo-rectal liver metastases: a comparison of early and late kinetic growth rates, and perioperative and oncological outcomes. Surg Oncol. 2022;43:101812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Laurent C, Fernandez B, Marichez A, Adam JP, Papadopoulos P, Lapuyade B, Chiche L. Radiological Simultaneous Portohepatic Vein Embolization (RASPE) Before Major Hepatectomy: A Better Way to Optimize Liver Hypertrophy Compared to Portal Vein Embolization. Ann Surg. 2020;272:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 934] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 45. | Lang H, Baumgart J, Mittler J. Associated Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Registry: What Have We Learned? Gut Liver. 2020;14:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016;103:1768-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15:483-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, Topp SA, Vivarelli M, Aldrighetti LA, Robles Campos R, Oldhafer KJ, Jarnagin WR, van Gulik TM. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017;19:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Sparrelid E, Jonas E, Tzortzakakis A, Dahlén U, Murquist G, Brismar T, Axelsson R, Isaksson B. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J Gastrointest Surg. 2017;21:967-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Tomassini F, D'Asseler Y, Linecker M, Giglio MC, Castro-Benitez C, Truant S, Axelsson R, Olthof PB, Montalti R, Serenari M, Chapelle T, Lucidi V, Sparrelid E, Adam R, Van Gulik T, Pruvot FR, Clavien PA, Bruzzese D, Geboes K, Troisi RI. Hepatobiliary scintigraphy and kinetic growth rate predict liver failure after ALPPS: a multi-institutional study. HPB (Oxford). 2020;22:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Enne M, Schadde E, Björnsson B, Hernandez Alejandro R, Steinbruck K, Viana E, Robles Campos R, Malago M, Clavien PA, De Santibanes E, Gayet B; ALPPS Registry Group. ALPPS as a salvage procedure after insufficient future liver remnant hypertrophy following portal vein occlusion. HPB (Oxford). 2017;19:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Dondorf F, Deeb AA, Bauschke A, Felgendreff P, Tautenhahn HM, Ardelt M, Settmacher U, Rauchfuss F. Ligation of the middle hepatic vein to increase hypertrophy induction during the ALPPS procedure. Langenbecks Arch Surg. 2021;406:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Esposito F, Lim C, Lahat E, Shwaartz C, Eshkenazy R, Salloum C, Azoulay D. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford). 2019;21:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 55. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Fürst G, Schulte am Esch J, Poll LW, Hosch SB, Fritz LB, Klein M, Godehardt E, Krieg A, Wecker B, Stoldt V, Stockschläder M, Eisenberger CF, Mödder U, Knoefel WT. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | am Esch JS, Schmelzle M, Fürst G, Robson SC, Krieg A, Duhme C, Tustas RY, Alexander A, Klein HM, Topp SA, Bode JG, Häussinger D, Eisenberger CF, Knoefel WT. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg. 2012;255:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 58. | Audollent R, Eveno C, Contreres JO, Hainaud P, Rampanou A, Dupuy E, Brouland JP, Pocard M. Bone marrow-derived endothelial and hematopoietic precursors cells enhance the metastasis of colon cancer in an orthotopic murine model. Int J Cancer. 2011;129:2304-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol. 2009;217:282-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 60. | Huang SY, Aloia TA. Portal Vein Embolization: State-of-the-Art Technique and Options to Improve Liver Hypertrophy. Visc Med. 2017;33:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |