Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.2077
Peer-review started: May 21, 2022
First decision: June 23, 2022
Revised: July 8, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: October 15, 2022
Processing time: 146 Days and 8.3 Hours
Disseminated carcinomatosis of the bone marrow (DCBM) is a widespread metastasis with a hematologic disorder that is mainly caused by gastric cancer. Although it commonly occurs as a manifestation of recurrence long after curative treatment, the precise mechanism of relapse from dormant status remains unclear. Granulocyte colony-stimulating factor (G-CSF) can promote cancer progression and invasion in various cancers. However, the potential of G-CSF to trigger recurrence from a cured malignancy has not been reported.
A 55-year-old Japanese woman was diagnosed with Ewing sarcoma localized on the fifth lumbar vertebrae 6 years after curative gastrectomy for T1 gastric cancer. After palliative surgery to release nerve compression, pathological diagnosis of the resected specimen was followed by curative radiation and chemotherapy. During treatment, G-CSF was administered 32 times for severe neutropenia prophylaxis. Eight months after completing definitive treatment, she complained of severe back pain and was diagnosed as multiple bone metastases with DCBM from gastric cancer. Despite palliative chemotherapy, she died of disseminated intravascular coagulation 13 d after the diagnosis. Immunohistochemical examination of the autopsied bone marrow confirmed a diffuse positive staining for the G-CSF receptor (G-CSFR) in the relapsed gastric cancer cell cytoplasm, whereas the primary lesion cancer cells showed negative staining for G-CSFR. In this case, G-CSF administration may have been the key trigger for the disseminated relapse of a dormant gastric cancer.
When administering G-CSF to cancer survivors, recurrence of a preceding cancer should be monitored even after curative treatment.
Core Tip: Disseminated carcinomatosis of the bone marrow (DCBM) is a rare manifestation of recurrence of a treated cancer, mainly gastric cancer. We reported a case of DCBM 8 years after curative surgery for T1 gastric cancer. Immunostaining for granulocyte colony-stimulating factor (G-CSF) receptor was diffusely positive in the relapsed lesions, but it was negative in the primary lesion. The administration of G-CSF during treatment for Ewing sarcoma within 2 years before the relapse could have been the trigger for the gastric cancer recurrence. G-CSF administration in patients with history of cancer could be a risk factor for recurrence.
- Citation: Fujita K, Okubo A, Nakamura T, Takeuchi N. Disseminated carcinomatosis of the bone marrow caused by granulocyte colony-stimulating factor: A case report and review of literature. World J Gastrointest Oncol 2022; 14(10): 2077-2084
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/2077.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.2077
Disseminated carcinomatosis of the bone marrow (DCBM) is a rare metastatic disorder that originates from gastric cancer in about 90% of cases[1-4]. Although the reported incidence of bone recurrence from curatively resected gastric cancer was 0.7%-2.1%, 13.4%-17.6% of autopsied gastric cancer cases had bone metastasis[5-9]. The duration between primary surgery and DCBM diagnosis was reportedly longer than 5 years in 66.7% of cases[10]. Therefore, disseminated tumor cells (DTCs) could stay in a prolonged subclinically dormant status. However, the precise mechanisms of this metachronous relapse are not well-known[11].
We reported a case of DCBM 8 years after curative surgery of T1 gastric cancer. Within 2 years prior to the relapse, definitive treatment with multiple granulocyte colony-stimulating factor (G-CSF) infusions for Ewing sarcoma was administered. We focused on the relationships between G-CSF administration and gastric cancer relapse.
A 55-year-old woman followed up for cured Ewing sarcoma at the outpatient oncology department of our hospital complained of pain all over the body, especially in the lumbar area.
The patient’s pain started 8 mo after completing chemotherapy for Ewing sarcoma. The lumbar pain extended to the upper back and right shoulder for several weeks.
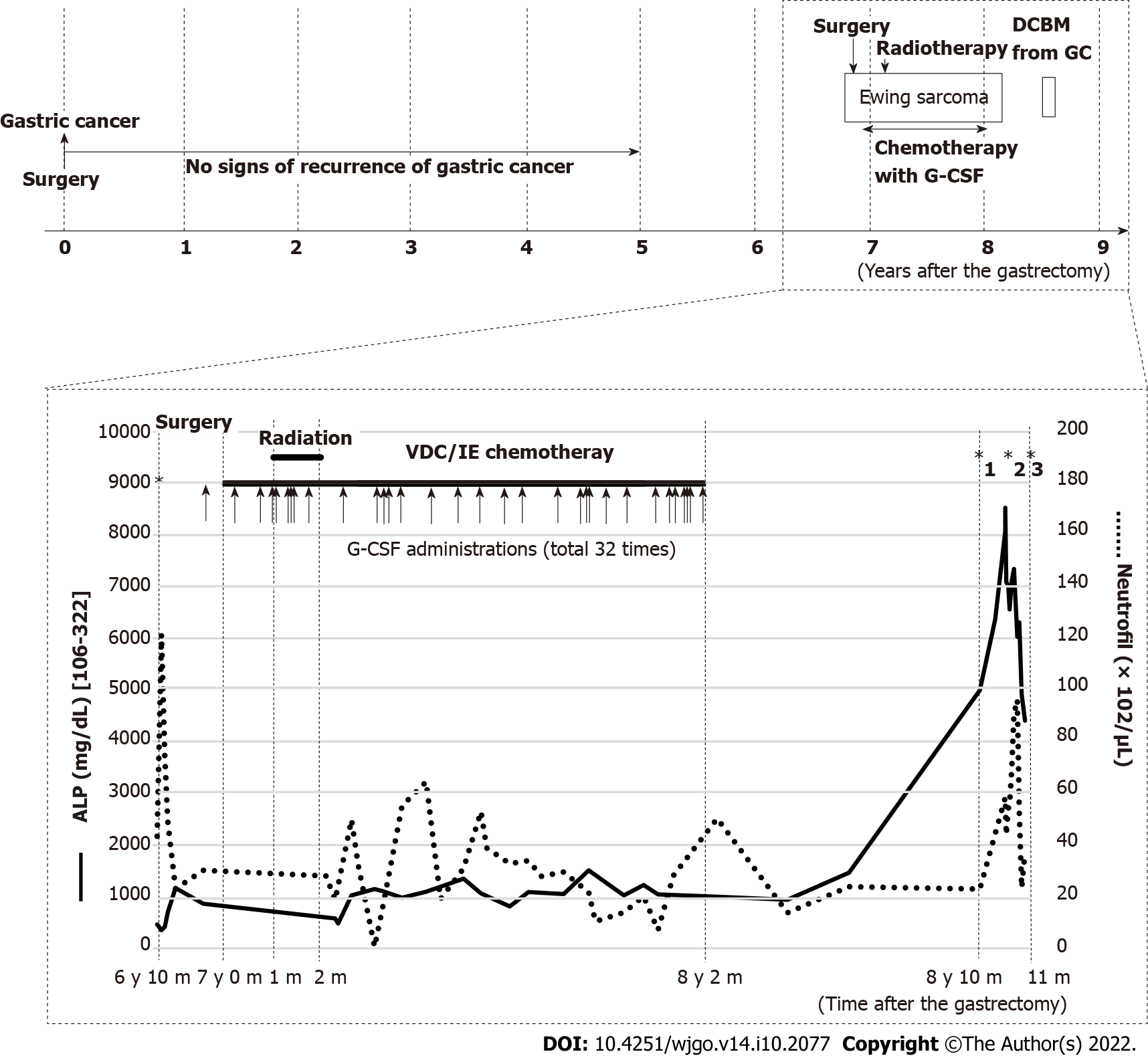
The patient had undergone curative distal gastrectomy with lymphadenectomy for early gastric cancer (T1aN1M0)[12], and completed a 5-year postoperative follow-up without any signs of recurrence based on tumor markers, gastroduodenoscopy, and computed tomography (CT) scans. Seven years after the gastrectomy, she had persistent pain on the right hip joint and right lumbar area, which was attributed to a soft tissue tumor on the right fifth lumbar vertebra seen on CT and magnetic resonance imaging (MRI). The pathologic diagnosis of the palliatively resected tumor was Ewing sarcoma, which was confirmed by chromosomal analysis of ESWR1 break apart. After induction radiotherapy (50.4 Gy/28 Fr), she received adjuvant chemotherapy with 8 courses of vincristine (16 mg in total), 4 courses of doxorubicin (344 mg in total), 8 courses of cyclophosphamide (13840 mg in total), 33 courses of ifosfamide (81.2 mg in total), and 32 courses of etoposide (2240 mg in total). Six times of red blood cell (RBC) transfusion were required for grade 4 anemia. Grade 4 neutropenia was treated with antibiotics and 18 doses of 2700 μg of filgrastim (filgrastimBS®, Nippon Kayaku, Tokyo, Japan). For severe neutropenia prophylaxis, 14 doses of 50.4 mg of pegfilgrastim (G-Lasta®, Kyowa Kirin, Tokyo, Japan) were given (Figure 1). After completion of chemotherapy, CT and MRI revealed no residual tumor.
The patient had no prior history of smoking or alcohol consumption. There was no relevant family history in relation to this case report.
On admission, the patient’s temperature was 36.3 °C, heart rate was 82 beats per minute, respiratory rate was 19 breaths per minute, blood pressure was 122/86 mmHg, and oxygen saturation at room air was 95%. Our primary clinical consideration was bone metastasis from recurrent Ewing sarcoma or gastric cancer.
Laboratory examinations showed evident increases in serum alkaline phosphatase (ALP) at 8081 IU/L (normal, 106-322 IU/L) and pancytopenia (RBC 2.23 × 1012/μL, hemoglobin 7.2 g/dL, white blood cell 8500/μL with 69% neutrophils, and platelet 2.9 × 104). The following tumor markers were elevated: Carcinoembryonic antigen (CEA) at 120.3 ng/mL (normal, < 5 ng/mL) and carbohydrate antigen 125 (CA125) at 45.5 U/mL (normal, < 35 U/mL). Notably, the ALP range was 1000-1500 IU/L during chemotherapy for Ewing sarcoma and remarkably increased when the patient complained of pain (Figure 1). Additionally, the CEA and CA125 were normal throughout the five-year follow-up of the resected gastric cancer but were not available during the treatment for Ewing sarcoma.
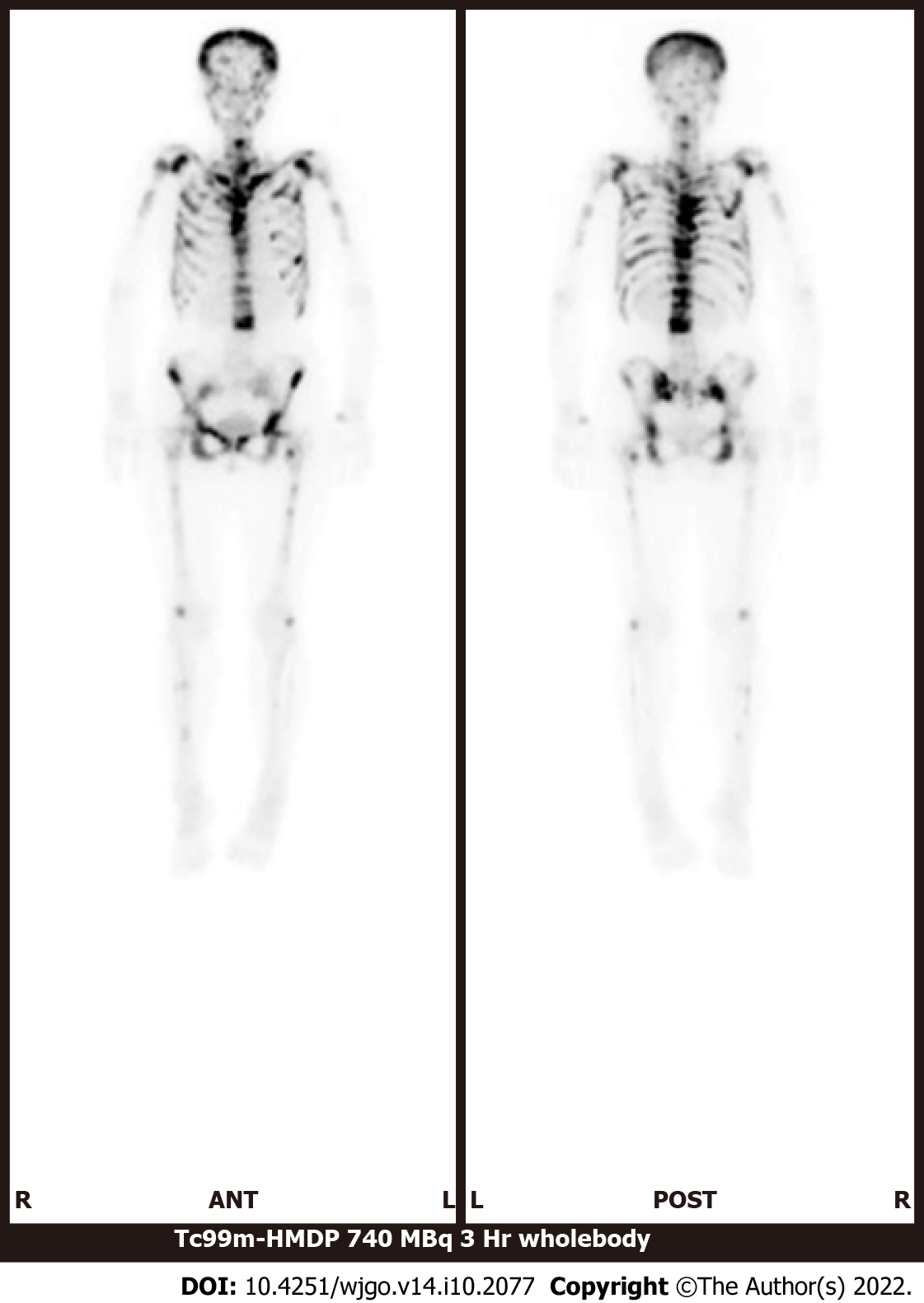
Bone scintigraphy, using technetium-99m hydroxymethylene diphosphonate, revealed an increased uptake in the spine, limbs, pelvis, and skull and decreased radioactivity in the kidneys (Figure 2). These characteristic image findings are called superscans (also termed super bone scans and super scan patterns) and can indicate bone marrow involvement[13,14].
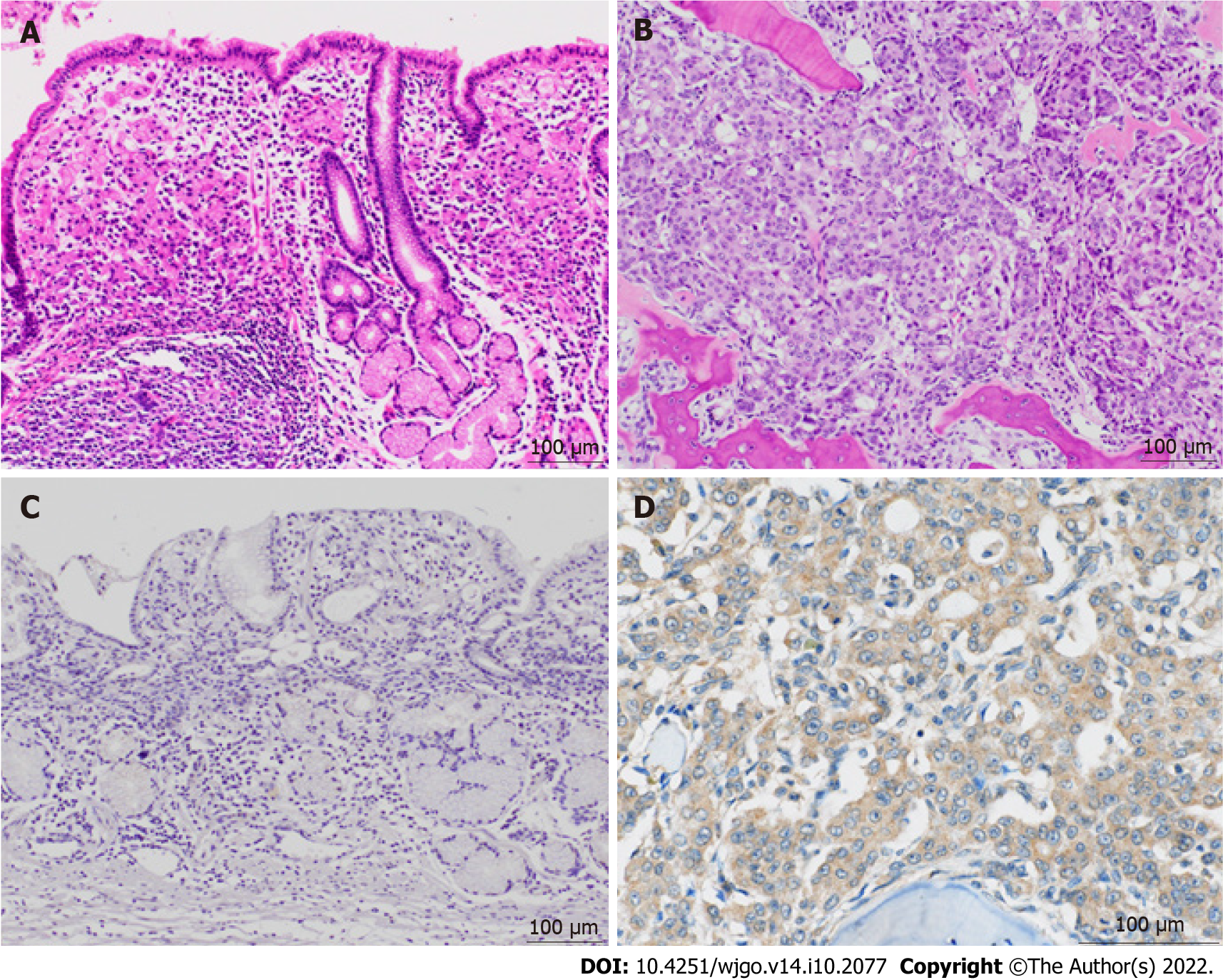
Bone marrow biopsy from the iliac crest revealed adenocarcinoma, which seemed to be a recurrence from gastric cancer. The immunohistochemical findings of the adenocarcinoma cells were as follows: CK7(+), CK20(+), MUC2(-), MUC5AC(+), MUC6(focal+), CDX2(−), and CA19-9(−). The results were identical to those of the primary lesion of the resected stomach 8 years prior, except for CDX2, which was focally positive in the primary lesion.
Postmortem autopsy revealed the following metastatic lesions from gastric cancer: (1) Bilateral bronchopulmonary lymph nodes; (2) Scattered minute tumor emboli in the lungs; and (3) Diffuse bone marrow infiltration in the vertebrae (cervical, thoracic, and lumbar), ribs, and iliac bone. There were no recurrences of gastric cancer in the peritoneal cavity and stomach and of Ewing sarcoma all over the body. The histological and immunohistochemical findings of the autopsied bone marrow were identical to those of the bone marrow biopsy. To further investigate the mechanism of relapse, additional immunostainings on the primary and relapsed bone marrow lesions were done using anti-G-CSF antibody (clone 5.24, 1:600, Sigma-Aldrich, St. Louis, Missouri, United States) and anti-G-CSF receptor (G-CSFR) antibody (1:300, Bioss antibodies, Woburn, Massachusetts, United States). Immunostaining for G-CSF was negative in both lesions. In contrast, G-CSFR was diffusely positive in the cytoplasm of the cancer cells in the relapsed lesions but was negative in the primary lesion (Figure 3).
The final diagnosis was DCBM from gastric cancer that was curatively resected 8 years prior.
Weekly intravenous chemotherapy that comprised methotrexate 140 mg, fluorouracil 840 mg, and calcium folinate 12 mg per course was started but needed to be stopped on day 7 because of deteriorating general condition of the patient[15-18].
Despite chemotherapy, disseminated intravascular coagulation progressed, and the patient died 13 d after the diagnosis of DCBM.
This case suggested the potential of G-CSF administration to cause recurrence presenting as DCBM from a curatively resected gastric cancer 8 years prior. Although the precise mechanism of DCBM as a manifestation of a metachronous recurrence of cured cancer is unclear, recent studies have indicated the reactivation of dormant DTCs by various factors, which are mainly related with angiogenesis and the immunologic antitumor surveillance system[19-23]. The administration of G-CSF has been reported to be one of the factors that can promote cancer progression and invasion in various cancers[24], and this interaction was confirmed in vivo using gastric cancer cells expressing G-CSFR[25]. However, previous clinical documentations have seldom documented that G-CSF could trigger recurrence of cured malignancies. In this report, we focus on the direct and indirect effects of G-CSF on the metachronous relapse of cured malignancies.
G-CSF can directly promote the proliferation and spread of gastric cancer cells, especially those with stem-like properties, such as CD44 and aldehyde dehydrogenase expression, by activating G-CSFR and the RERK1/2 and RSK1 phosphorylation pathways[26,27]. In the present case, G-CSFR staining was negative in the primary lesion but was diffusely positive in the relapsed lesion. This observation provided two possible explanations. First, a small amount of slow growing G-CSFR-positive gastric cancer cells could survive in a dormant state for a long period. Second, residual DTCs may develop and express G-CSFR throughout years of dormant state. G-CSF can promote the growth of solid tumors not only through G-CSFR on tumor cells but also by modulating immune cell activities or bone remodeling. G-CSF can activate myeloid derived suppressor cells and regulate T cells and macrophages, both of which can lead to the progression of solid tumors by suppressing CD8-positive T cells[28-31]. In addition, G-CSF can accelerate bone infiltration of tumor cells by activating osteoclasts and inhibiting osteoblasts[32,33]. These direct and indirect effects of G-CSF could be a positive trigger for the reactivation of dormant cancer cells.
About 90% of gastric cancer cases have positive G-CSFR staining, and some cancers have been reported to express G-CSFR[27,34]. G-CSF administration for the second primary cancers could be a risk factor for recurrence of a preceding G-CSFR-expressing primary cancer that was assumed to be cured for a long time. Therefore, G-CSF administration should be performed carefully in patients who have a preceding cancer. Considering the high incidence of G-CSFR-expressing gastric cancer, no other similar cases of gastric cancer recurrence caused by G-CSF have been reported. The possibility of G-CSF causing recurrence of a preceding cancer might have been overlooked. Because this one case is not enough to accurately evaluate the risk of G-CSF to cause recurrence, further research on the interaction between G-CSF and tumor proliferation and relapse are needed.
G-CSF administration in cancer survivors could be a risk factor for recurrence of a preceding cancer, even after curative treatment.
The authors thank Ms. Ayumi Karasawa, Mr. Yusuke Kohno, and Ms. Sayuri Hirashima for their excellent technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao L, China; Guo F, China; Liu T, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Iguchi H. Recent aspects for disseminated carcinomatosis of the bone marrow associated with gastric cancer: What has been done for the past, and what will be needed in future? World J Gastroenterol. 2015;21:12249-12260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Kikuchi Y, Matsuzaki M, Tokura N, Kanayama K, Kanayama M, Shiratori M, Shinohara M, Igarashi Y, Sumino Y, Nakano K. Disseminated carcinomatosis of the bone marrow due to gastric cancer: Analysis of cases at Toho University and in Japan. J Med Soc Toho. 2010;57:127-136. |
| 3. | Ubukata M, Seshimo A, Aratake K, Miyake K, Amano K, Ueda Y, Kameoka S. A case of disseminated carcinomatosa of the bone marrow from gastric cancer occurring 5 years after a curative resection. J Jap Surg. 2012;37:1120-1125. [DOI] [Full Text] |
| 4. | Hasuda N, Koshizuka K, Oyachi N, Takano K, Matsumoto M. A case report of disseminated carcinomatosis of the bone marrow from early gastric cancer 4 years after operation. J Jap Surg Assoc. 2008;69:355-359. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kobayashi M, Okabayashi T, Sano T, Araki K. Metastatic bone cancer as a recurrence of early gastric cancer -- characteristics and possible mechanisms. World J Gastroenterol. 2005;11:5587-5591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Mikami J, Kimura Y, Makari Y, Fujita J, Kishimoto T, Sawada G, Nakahira S, Nakata K, Tsujie M, Ohzato H. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J Surg Oncol. 2017;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, Seo Y, Fukuda T, Tomoda H. Diffuse bone metastasis with hematologic disorders from gastric cancer: clinicopathological features and prognosis. Oncol Rep. 1999;6:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, Yalcin S. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Okuno T, Yamaguchi H, Kitayama J, Ishigami H, Nishikawa T, Tanaka J, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Kawai K, Kazama S, Ishihara S, Sunami E, Watanabe T. A case of disseminated carcinomatosis of the bone marrow originating from gastric cancer 3 years after intraperitoneal chemotherapy against peritoneal carcinomatosis. World J Surg Oncol. 2016;14:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S. Overt bone metastasis and bone marrow micrometastasis of early gastric cancer. Surg Today. 2011;41:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4405] [Article Influence: 550.6] [Reference Citation Analysis (4)] |
| 13. | Pour MC, Simon-Corat Y, Horne T. Diffuse increased uptake on bone scan: super scan. Semin Nucl Med. 2004;34:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Lin CY, Chen YW, Chang CC, Yang WC, Huang CJ, Hou MF. Bone metastasis versus bone marrow metastasis? Kaohsiung J Med Sci. 2013;29:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Takashima A, Shirao K, Hirashima Y, Takahari D, Okita NT, Nakajima TE, Kato K, Hamaguchi T, Yamada Y, Shimada Y. Sequential chemotherapy with methotrexate and 5-fluorouracil for chemotherapy-naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136:243-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Hamaguchi T, Shirao K, Yamamichi N, Hyodo I, Koizumi W, Seki S, Imamura T, Honma H, Ohtsu A, Boku N, Mukai T, Yamamoto S, Fukuda H, Yoshida S; Gastrointestinal Oncology Study Group of Japan Clinical Oncology Group. A phase II study of sequential methotrexate and 5-fluorouracil chemotherapy in previously treated gastric cancer: a report from the Gastrointestinal Oncology Group of the Japan Clinical Oncology Group, JCOG 9207 trial. Jpn J Clin Oncol. 2008;38:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, Conroy T, Fickers M, Leyvraz S, Buyse M. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9:827-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Hen O, Barkan D. Dormant disseminated tumor cells and cancer stem/progenitor-like cells: Similarities and opportunities. Semin Cancer Biol. 2020;60:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Triana-Martínez F, Loza MI, Domínguez E. Beyond Tumor Suppression: Senescence in Cancer Stemness and Tumor Dormancy. Cells. 2020;9:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Saleh T, Bloukh S, Carpenter VJ, Alwohoush E, Bakeer J, Darwish S, Azab B, Gewirtz DA. Therapy-Induced Senescence: An "Old" Friend Becomes the Enemy. Cancers (Basel). 2020;12:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 22. | Jahanban-Esfahlan R, Seidi K, Manjili MH, Jahanban-Esfahlan A, Javaheri T, Zare P. Tumor Cell Dormancy: Threat or Opportunity in the Fight against Cancer. Cancers (Basel). 2019;11:1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1283] [Cited by in RCA: 1154] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 24. | Theron AJ, Steel HC, Rapoport BL, Anderson R. Contrasting Immunopathogenic and Therapeutic Roles of Granulocyte Colony-Stimulating Factor in Cancer. Pharmaceuticals (Basel). 2020;13:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Baba M, Hasegawa H, Nakayabu M, Shimizu N, Suzuki S, Kamada N, Tani K. Establishment and characteristics of a gastric cancer cell line (HuGC-OOHIRA) producing high levels of G-CSF, GM-CSF, and IL-6: the presence of autocrine growth control by G-CSF. Am J Hematol. 1995;49:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Fan Z, Li Y, Zhao Q, Fan L, Tan B, Zuo J, Hua K, Ji Q. Highly Expressed Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte Colony-Stimulating Factor Receptor (G-CSFR) in Human Gastric Cancer Leads to Poor Survival. Med Sci Monit. 2018;24:1701-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Pilatova K, Bencsikova B, Demlova R, Valik D, Zdrazilova-Dubska L. Myeloid-derived suppressor cells (MDSCs) in patients with solid tumors: considerations for granulocyte colony-stimulating factor treatment. Cancer Immunol Immunother. 2018;67:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Karagiannidis I, Jerman SJ, Jacenik D, Phinney BB, Yao R, Prossnitz ER, Beswick EJ. G-CSF and G-CSFR Modulate CD4 and CD8 T Cell Responses to Promote Colon Tumor Growth and Are Potential Therapeutic Targets. Front Immunol. 2020;11:1885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Karagiannidis I, de Santana Van Vilet E, Said Abu Egal E, Phinney B, Jacenik D, Prossnitz ER, Beswick EJ. G-CSF and G-CSFR Induce a Pro-Tumorigenic Macrophage Phenotype to Promote Colon and Pancreas Tumor Growth. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Motallebnezhad M, Jadidi-Niaragh F, Qamsari ES, Bagheri S, Gharibi T, Yousefi M. The immunobiology of myeloid-derived suppressor cells in cancer. Tumour Biol. 2016;37:1387-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Li S, Li T, Chen Y, Nie Y, Li C, Liu L, Li Q, Qiu L. Granulocyte Colony-Stimulating Factor Induces Osteoblast Inhibition by B Lymphocytes and Osteoclast Activation by T Lymphocytes during Hematopoietic Stem/Progenitor Cell Mobilization. Biol Blood Marrow Transplant. 2015;21:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Li S, Zhai Q, Zou D, Meng H, Xie Z, Li C, Wang Y, Qi J, Cheng T, Qiu L. A pivotal role of bone remodeling in granulocyte colony stimulating factor induced hematopoietic stem/progenitor cells mobilization. J Cell Physiol. 2013;228:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Yeo B, Redfern AD, Mouchemore KA, Hamilton JA, Anderson RL. The dark side of granulocyte-colony stimulating factor: a supportive therapy with potential to promote tumour progression. Clin Exp Metastasis. 2018;35:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |