Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.980
Peer-review started: February 21, 2021
First decision: May 8, 2021
Revised: June 3, 2021
Accepted: August 11, 2021
Article in press: August 11, 2021
Published online: September 15, 2021
Processing time: 201 Days and 3.2 Hours
Tenascin-C (TNC) is an adhesion modulatory protein present in the extracellular matrix that is highly expressed in several malignancies, including colon cancer. Although TNC is considered a negative prognostic factor for cancer patients, the substantial role of the TNC molecule in colorectal carcinogenesis and its malignant progression is poorly understood. We previously found that TNC has a cryptic functional site and that a TNC peptide containing this site, termed TNIIIA2, can potently and persistently activate beta1-integrins. In contrast, the peptide FNIII14, which contains a cryptic bioactive site within the fibronectin molecule, can inactivate beta1-integrins. This review presents the role of TNC in the development of colitis-associated colorectal cancer and in the malignant progression of colon cancer, particularly the major involvement of its cryptic functional site TNIIIA2. We propose new possible prophylactic and therapeutic strategies based on inhibition of the TNIIIA2-induced beta1-integrin activation by peptide FNIII14.
Core Tip: Exposure of the cryptic functional site TNIIIA2 from the Tenascin-C (TNC) molecule and its potent and sustained activation of beta1-integrins appear to be associated with the development of colon cancer and its malignant progression. Inhibition of the biological function of TNIIIA2 derived from TNC molecule may be a promising strategy for the prevention and treatment of colon cancer.
- Citation: Fujita M, Suzuki H, Fukai F. Involvement of integrin-activating peptides derived from tenascin-C in colon cancer progression. World J Gastrointest Oncol 2021; 13(9): 980-994
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/980.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.980
Extracellular matrix (ECM) proteins such as fibronectin (FN), collagen, and laminin provide a scaffold for cell adhesion and subsequently influence various physiological cellular processes, including cell differentiation, survival/proliferation, and migration. As one of the major components of the tumor microenvironment, the ECM affects the behavior of cells in the cancer microenvironment, such as cancer-associated fibroblasts (CAFs) and immune cells, resulting in cancer development[1]. It therefore plays major roles in carcinogenesis and the malignant progression of cancer.
Integrins are a family of heterodimeric transmembrane glycoproteins composed of alpha- and beta-subunits that directly interact with components of the ECM. These integrins primarily mediate cell adhesion, migration, survival, proliferation, and differentiation. In contrast to membrane receptors for humoral factors such as cytokines and chemokines, integrins are unique in their ability to alter the binding affinity for ECM ligands. Integrins exist mainly in two different structural states, an inactive conformation lacking ligand-binding affinity and an active one with high affinity[2]. On the other hand, integrin signaling contributes to the malignant progression of many cancers. For example, integrin alpha5beta1, a major FN receptor, is highly expressed in glioma/glioblastoma, with its expression levels reported to be associated with poor survival in glioma/glioblastoma patients[3]. Alpha5-integrin promotes cell proliferation and the dissemination of glioblastoma cells[4], modulates angiogenesis[5], and contributes to temozolomide chemoresistance[6]. Thus, the integrin alpha5beta1-mediated adhesive interaction of glioma cells may be associated with the acquisition of a highly aggressive phenotype in glioma/glioblastoma. Therefore, inhibition of integrin functions might be a promising therapeutic approach for cancer.
Tenascin-C (TNC) is a hexameric, multimodular ECM glycoprotein. It is poorly expressed in normal adult tissues but highly expressed in both inflammatory lesions and the tumor microenvironment[3,7-10]. TNC is an endogenous activator of toll-like receptor 4, which triggers and amplifies inflammatory responses[11]. In addition, TNC binds to integrin alphavbeta3 and alpha9beta1 to drive inflammatory responses by inducing the synthesis of proinflammatory cytokines, including interleukin (IL)-6, IL-1beta, and tumor necrosis factor-alpha[12]. TNC is highly expressed and is thought to act as a major driving regulator of acute and chronic inflammatory diseases, including cardiac disease[13], arthritis[14], nephritis[15], sepsis[16], stroke[17], asthma[18], chronic obstructive pulmonary disease[19], and viral infections[20]. Therefore, TNC may be a promising biomarker of disease activity and a therapeutic target in these inflammatory diseases.
Furthermore, the expression levels of TNC are associated with poor prognosis in patients with malignant tumors, such as glioma and breast and colon cancers[3,8,10]. Accumulating evidence indicates a relationship between TNC and tumor progression. For example, TNC plays key roles in several processes of tumor progression related to proliferation[21,22], migration, invasion[23-25], angiogenesis[26,27], immunosuppression[28,29], cancer stemness[30,31], and apoptosis resistance[32], supporting the belief that TNC contributes to cancer progression and aggression. In addition, TNC has been linked to carcinogenesis[33-36]. Analysis of the Rip1-Tag2 model of pancreatic beta-cell carcinogenesis, which drives a multistage carcinogenesis process, revealed that TNC contributes to multiple steps linked to carcinogenesis[34,35]. Moreover, Li et al[36] revealed that the expression levels of TNC are higher in adenomatous colon polyps and colon carcinoma in situ than in non-neoplastic colonic mucosa and are also correlated with TMN stages of colon cancer, further indicating that TNC might contribute to carcinogenesis and progression[36].
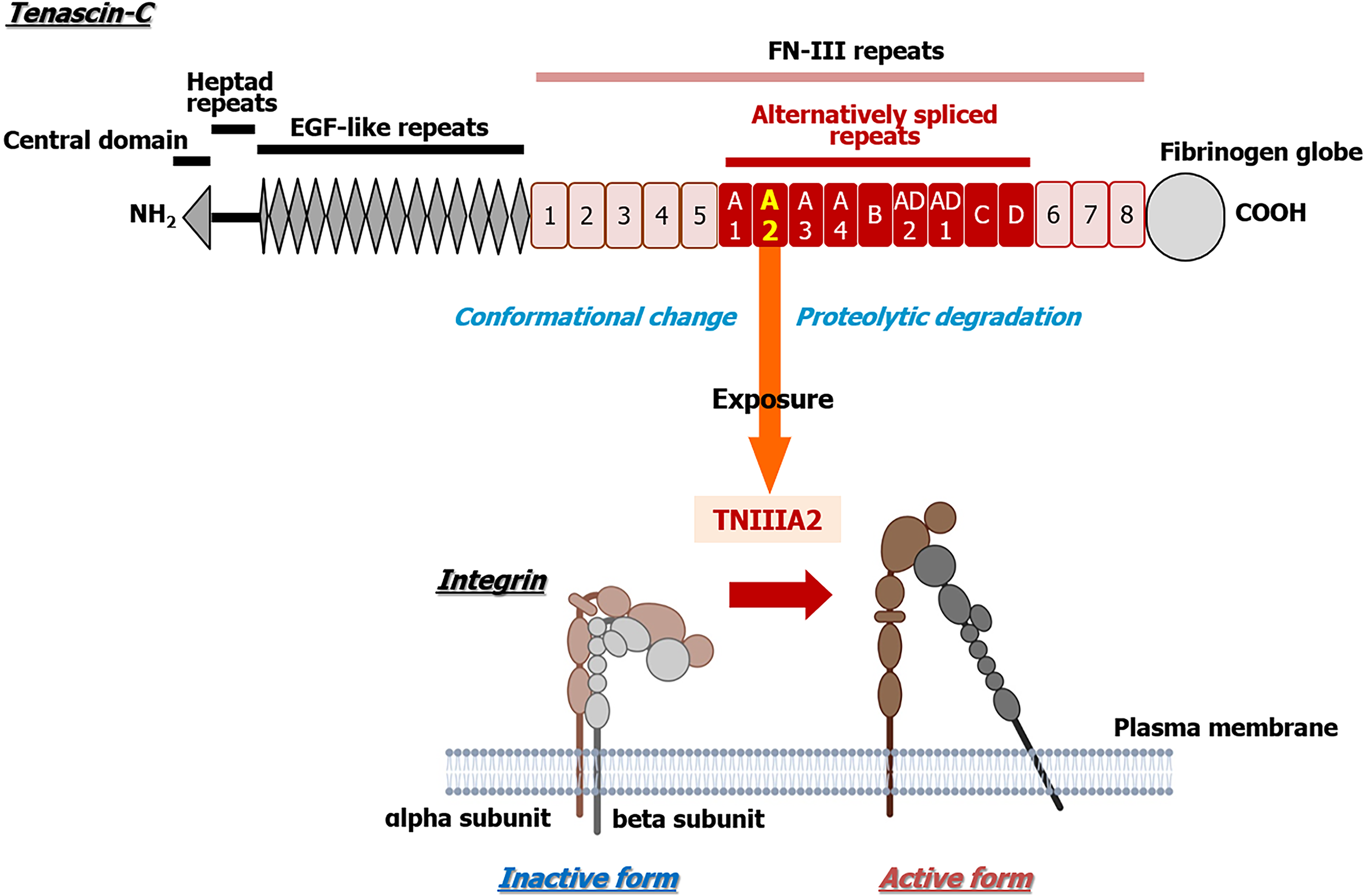
TNC contains several characteristic domains, such as a central domain, heptad repeats, epidermal growth factor (EGF)-like repeats, FN type III repeats (FN-III repeats), and a fibrinogen globe (Figure 1), which can interact with ECM proteins, soluble factors, and cell receptors and express various functions of TNC. In addition, human TNC contains nine alternative splicing sites in FN-III repeats, and 511 possible splice variants can theoretically be generated through alternative splicing[37]. This alternative splicing could control the versatile biological functions of TNC by modulating its interaction with specific binding partners, as well as by exposing post-translational sites and proteolytic cleavage sites[37]. However, the substantial role of the TNC molecule in colorectal carcinogenesis and its malignant progression has remained elusive.
This review presents the role of TNC in the malignant progression of colon cancer and the development of colitis-associated colorectal cancer (CAC), with a particular focus on the major involvement of TNIIIA2, the cryptic functional site of TNC. We propose new possibilities for prophylactic and therapeutic strategies based on peptide FNIII14-mediated inhibition of the TNIIIA2-induced beta1-integrin activation.
Most ECM proteins harbor functionally cryptic functional sites that are buried within their molecular structures. These cryptic sites, called matricryptic sites, are revealed via structural/conformational changes triggered by interactions with adjacent cells or other ECM components and by remodeling/processing by ECM-degrading protei
The mode of beta1-integrin activation induced by TNIIIA2 is entirely distinct from that induced by “inside-out” signaling, which is the commonly considered mode of integrin activation. Saito et al[47] have found that syndecan-4, one of the transmem
Colorectal cancer is the third most common type of gastrointestinal tract tumor worldwide and the third leading cause of death among men and women[54]. Because of recent substantial progress in diagnostic methods and advances in primary and adjuvant treatments, including standard chemotherapy and targeted treatments, the incidence and mortality of colorectal cancer has been improving[55,56], with a 5-year overall survival rate for colorectal cancer of about 60%[57,58]. However, patients with metastatic colorectal cancer, which comprise 20% of patients with new colorectal cancer diagnoses, show a high mortality rate, with a 5-year overall survival rate of approximately 20%[59-62]. Recently, systemic therapy involving molecular targeted drugs as well as cytotoxic drugs has been adopted for unresectable colorectal cancer. Combination with molecular targeted drugs such as bevacizumab, cetuximab, or panitumumab is recommended, depending on the RAS status[63]. However, although these drugs are effective, they have various problems, including certain adverse events, and eventually become ineffective. Therefore, further investigation is still necessary to develop novel strategies for colorectal cancer, and it is important to elucidate the molecular mechanisms that enable colorectal cancer to acquire malignant properties.
TNC is highly expressed in colon cancer, and high expression levels of TNC in tissue specimens are correlated with distant metastasis, tumor recurrence, advanced TNM stage, and poor prognosis[10,36]. Moreover, colon cancer cells highly expressing TNC show high metastatic potential and are associated with lymph nodes with metastasis[36]. In addition, serum TNC levels, particularly those of large-spliced variants, are higher in patients with colon cancer compared with controls[64]. Such levels are also correlated with tumor depth, lymph node metastasis, and disease progression[64]. Therefore, the levels of TNC in tissue and serum may be a diagnostic or prognostic biomarker in colon cancer. Furthermore, the Wnt/beta-catenin signaling pathway plays a central role in carcinogenesis, and its mutation and activation are found in almost all patients with colon cancers[65]. Because TNC is a Wnt/beta-catenin target gene in human colon tumors[66], the deregulation of Wnt/beta-catenin signaling might lead to the overexpression of TNC in colon cancer. Experimental observations indicated that TNC secreted by myofibroblasts might act as a proinvasive factor for colon cancer cells[67]. Furthermore, TNC promotes proliferation, migration, and invasion and also upregulates cancer stem cell markers via the Hedgehog signaling pathway[31]. However, the biochemical functions of TNC in the malignant progression of colon cancer have not yet been established.
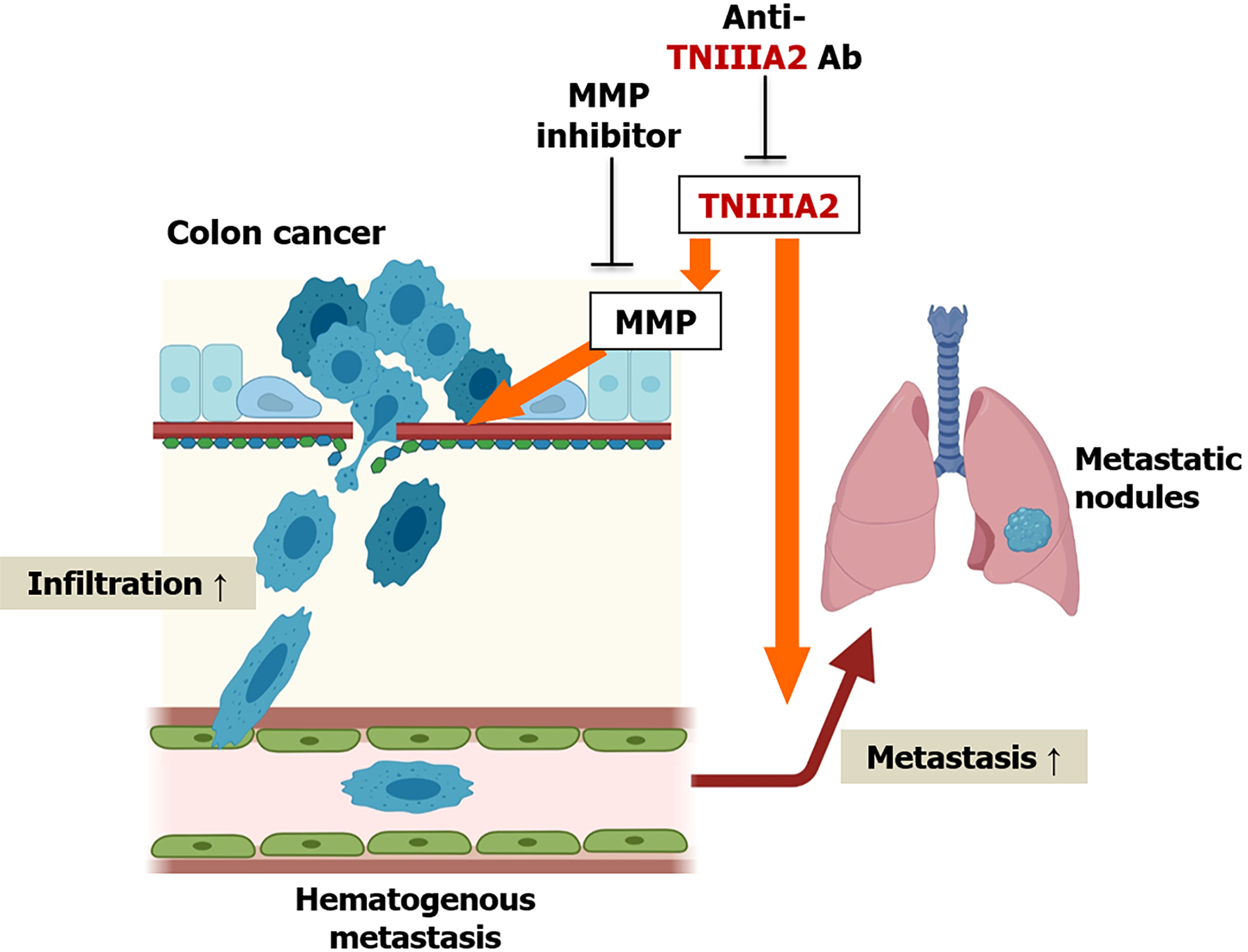
MMP-2 is highly expressed in colon cancer tissues and its expression levels increase with an increase in the tumor stage[68]. Furthermore, the expression levels of MMP-2 are correlated with lymph vessel invasion and disease progression in colon cancer[69]. MMP-7 is another Wnt/beta-catenin target gene[70] and both MMP-2 and MMP-7 can degrade TNC[71]. Furthermore, TNC variants containing the alternatively spliced domain types III-A1, -2, and -4 are highly expressed in colon cancer[49]. It is presumed that the functional cryptic site TNIIIA2 of TNC may be released into the tumor microenvironment of colon cancer and contribute to its pathogenesis. Supporting this hypothesis, peptide TNIIIA2 has been shown to act directly on colon cancer cells to enhance their in vitro invasive potential by inducing MMP secretion[72]; peptide TNIIIA2 or TNC promotes colon cancer cell invasion by upregulating MMPs[72]. The cell invasion induced by peptide TNIIIA2 or TNC is completely suppressed by anti-TNIIIA2 antibody or MMP-2 inhibitor[72]. Moreover, an in vivo observation involving a spontaneous metastasis mouse model mimicking hematogenous metastasis exhibited that peptide TNIIIA2 boosted the metastasis of colon cancer cells to the lung[72]. Taken together, the activation of beta1-integrin by peptide TNIIIA2 (one of the biochemical functions of TNC) may help to promote colon cancer cell metastasis via induction of MMP (Figure 2).
Alterations in the density, distribution, and composition of the ECM are common in malignancies. This process creates the tumor microenvironment that helps to confer cancer cells with malignant properties such as tumorigenesis and metastasis[1]. These alterations increase stiffness in the tumor microenvironment, which promotes pro-tumorigenic mechanosignaling. The increased ECM stiffness of colon cancer has been associated with cancer progression[73]. Through analysis of clinical specimens, a gradient of increasing ECM stiffness was observed from healthy to perilesional and colon cancer areas, which might predispose invasion[74]. Furthermore, the expression levels of lysyl oxidase (LOX), which catalyzes the covalent cross-linking of collagens and elastin, are closely correlated with the progression of colon cancer[75]. Compared with control cells or cells expressing a catalytically inactive LOX, colon cancer cells expressing LOX exhibit increased mechanosignaling, ECM stiffness, metastasis, and tumor burden in in vivo models via activation of beta1-integrin and the focal adhesion kinase-SRC signaling pathway[76], indicating that beta1-integrin activation might be associated with malignant progression via increased ECM stiffness in colon cancer. In a recent insightful study on the role of TNC in ECM stiffness in the tumor microenvironment, Barnes et al[77] demonstrated that the glycocalyx/ECM-integrin loop induces glioblastoma aggression in a tissue tension-dependent manner, with human recurrent glioblastomas showing an increase in TNC-enriched stiffened ECM and enhanced integrin mechanosignaling[77]. It has also been pointed out that glioblastoma cells expressing a V737N beta1-integrin autoclustering mutant exhibit increased mechanosignaling and ECM stiffness and facilitate tumor growth[77]. It is unlikely that at least the antiadhesive effect of TNC, which has been considered a major biochemical function of this protein, is responsible for the ECM stiffening and consequent enhanced integrin signaling. However, it remains unclear whether proadhesive activity (a biochemical function of TNC) is directly associated with ECM stiffness in the tumor microenvironment of colon cancer. Further investigations are required to determine whether activation of beta1-integrin by peptide TNIIIA2 could actually increase ECM stiffness in colon cancer.
Beta1-integrin is also highly expressed in colon cancer compared with normal mucosa. High expression levels of beta1-integrin have been associated with poor prognosis, and increased expression of beta1-integrin is independently correlated with decreased overall survival and disease-free survival in colon cancer patients[78]. In addition, alpha5-integrin, which is coupled with beta1-integrin, also shows upregulated expression in colon cancer and is expressed mainly in the tumor stroma of clinical samples[79]. Moreover, alpha5beta1-integrin expression is considered a significant independent prognostic factor. Experimental evidence indicates that overexpression of alpha5-integrin accelerates proliferation and suppresses apoptosis in colon cancer cells, with colon cancer cells overexpressing alpha5-integrin found to promote tumor growth in a murine xenograft tumor model[80]. In addition, blockade of alpha5-integrin inhibits cell attachment and induces apoptosis in colon cancer cells via Akt suppression[81]. Integrin alpha5beta1 also confers anoikis resistance in colon cancer cells via association with EGF receptor and the subsequent activation of ERK and Akt as well as suppression of the caspase signaling pathway[82]. Furthermore, depletion of alpha5-integrin expression in fibroblasts suppresses the tumorigenic activity of colon cancer in in vivo experiments, as determined by the co-injection of human colon cancer cells and human normal colonic fibroblast cells into immunocompromised mice[79]. This result indicated that CAFs expressing alpha5-integrin have a tumor-promoting effect in colon cancer. Pharmacological experiments have demonstrated that the non-peptidic alpha5beta1 integrin antagonist K34c suppresses the clonogenic survival of colon cancer cells[83]. In addition, ATN-161, a peptidic antagonist of integrin alpha5beta1 and alphavbeta3, reduced tumor vascularization, and combination therapy of ATN-161 and fluorouracil suppressed liver metastases in a murine model of colon cancer[84]. Therefore, integrin alpha5beta1 might be a promising target for cancer therapeutics.
A link between chronic inflammation and the pathogenesis of many malignancies has been well documented. Examples include Helicobacter pylori infection-associated gastric cancer and hepatitis virus infection-associated hepatocellular carcinoma[85,86]. In particular, inflammatory bowel disease (IBD) patients, including Crohn’s disease and ulcerative colitis, have an increased risk of developing CAC[87-89], which is a subtype of colorectal cancer[90]. Although the incidence of CAC seems to have decreased in recent years because of more frequent surveillance, improved surveillance techniques, and more effective IBD drugs for controlling inflammation, patients with IBD still have higher rates of death from colon cancer[91]. Indeed, a Scandinavian population-based study recently showed that patients with IBD and colorectal cancer had an increased risk of mortality compared with those with sporadic colorectal cancer[92,93]. Therefore, there is still an unmet medical need for the prevention and treatment of CAC. Unlike sporadic colorectal cancer, CAC onset does not show an adenoma-carcinoma sequence, but rather an inflammation-dysplasia-carcinoma sequence[94,95]. Nonetheless, the molecular basis of CAC onset is largely unknown. Thus, research into the molecular mechanisms underlying CAC onset is urgently needed for the development of novel therapeutics.
Several studies have reported that TNC is associated with ulcerative colitis and Crohn’s disease[96-99]. A genome-wide association study of African Americans found that single-nucleotide polymorphisms within the TNC gene are associated with IBD risk[100]. Ning et al[101] reported that TNC is highly expressed in the inflamed stromal area of the intestinal mucosa of IBD patients[101]. They also showed particularly high levels of serum TNC in patients with severe IBD compared with those with mild or moderate IBD[101]. Riedl et al[96] determined that the serum levels of TNC are correlated with clinical and histological parameters of disease activity in IBD patients[96]. Moreover, high levels of TNC mRNA in the mucosa of ulcerative colitis have been associated with a poor response to infliximab therapy, an effective treatment for moderate-to-severe IBD, indicating that TNC may contribute to therapeutic resistance against IBD. Therapy resistance may participate in the malignant progression of IBD due to a lack of inflammatory control, resulting in an increased risk of CAC onset. Indeed, TNC derived from intestinal myofibroblasts promotes the onset of CAC in an azoxymethane (AOM)/dextran sulfate sodium (DSS) model via angiogenesis[102]. Thus, TNC might contribute to the development and/or malignant progression of CAC. Identification of the biological functions of the TNC responsible for the development of CAC would enable the design of agents with prophylactic and therapeutic potential for these diseases. However, the biochemical functions of TNC in CAC onset have not yet been established.
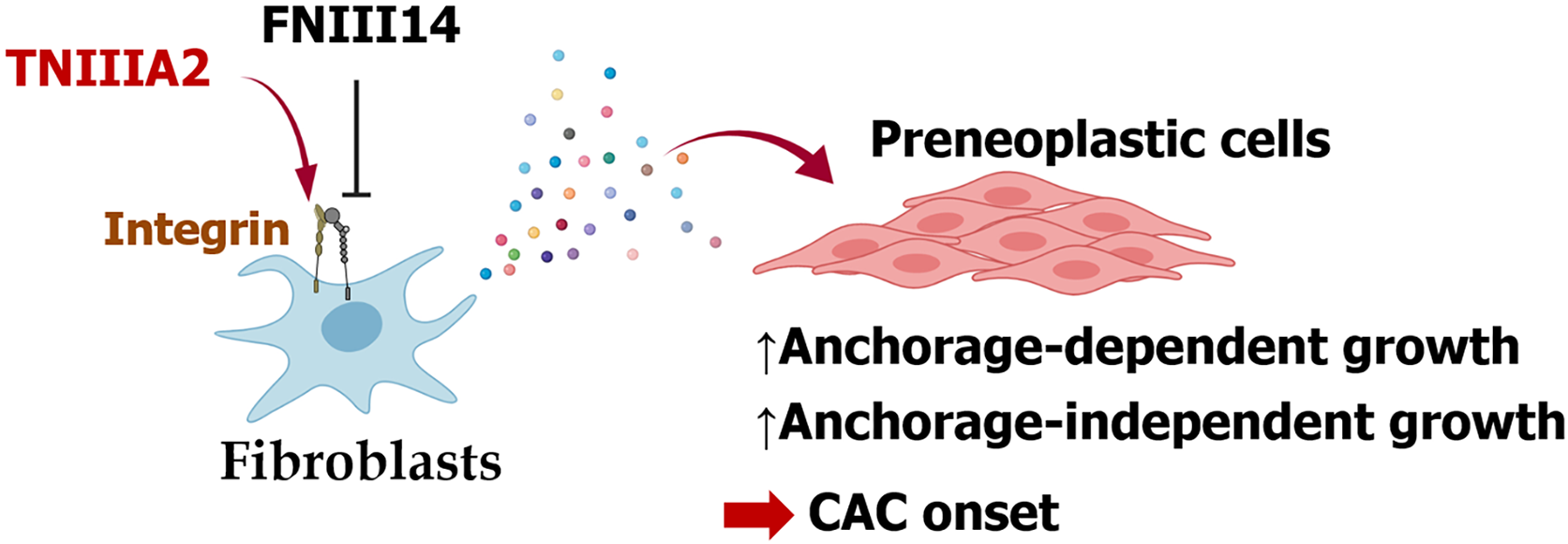
ECM remodeling is often augmented in these pathological lesions, and proteolytic cleavage of ECM proteins is performed by several inflammatory proteinases, including MMPs and cathepsins. Indeed, increased expression levels of several MMPs have been observed in IBD and are associated with disease activity in IBD, indicating that degradation of the ECM, including TNC, might occur at high levels in IBD and during CAC onset[103]. Therefore, it is conceivable that the functional cryptic site TNIIIA2 might be exposed by the high levels of TNC molecules in the lesion and act as a specific pathogenic factor in the development of CAC. Supporting this assumption, our recent work demonstrated the presence of TNC and peptide TNIIIA2 in the stromal area of dysplastic lesions in AOM/DSS mice[104]. Assuming that peptide TNIIIA2 acts mainly on preneoplastic epithelial cells and fibroblasts, which are abundant in the stromal area of dysplastic lesions, our in vitro experiments focused on the effects of beta1-integrin activation on both preneoplastic epithelial cells and fibroblasts. Interestingly, although beta1-integrin activation by peptide TNIIIA2 promoted cell adhesion, it had no direct effect on the growth of preneoplastic epithelial cells[104]. Similarly, peptide TNIIIA2 had no direct effect on the growth of fibroblasts, but fibroblasts stimulated by peptide TNIIIA2 released humoral factors, or possibly factors, that drove the malignant transformation of premalignant epithelial cells in a paracrine manner, as judged by anchorage-independent cell growth and focus formation[104]. These factors secreted from peptide TNIIIA2-activated fibroblasts are also able to promote the survival/proliferation of colon cancer cells[104]. Furthermore, peptide FNIII14, a peptidic factor that induces a conformational change in beta1-integrin from the active to the inactive state[105], suppressed not only the TNIIIA2-induced dysregulated survival/proliferation of preneoplastic epithelial cells in vitro, but also polyp development in an AOM/DSS mouse model[104]. These results suggest that beta1-integrin activation by peptide TNIIIA2 in fibroblasts may be an important target for the prevention of CAC (Figure 3).
Several studies have demonstrated that cells in the tumor microenvironment, such as CAFs and immune cells, influence tumor progression. Among them, CAFs are key determinants of cancer development and progression[106-108]. Sasaki et al[109] demonstrated that CAC incidence is abrogated in CC chemokine ligand 3- or CC chemokine receptor 5-knockout mice treated with AOM/DSS and coincides with lower accumulation of fibroblasts in dysplastic lesions compared with wild-type mice[109]. These fibroblasts express heparin-binding EGF-like growth factor to stimulate the proliferation of tumor cells in CAC in mice[109]. In addition, epiregulin derived from fibroblast promotes the proliferation of intestinal epithelial cells through activation of the ERK signaling pathway, augmenting CAC growth[110]. These studies indicate that CAFs might be responsible for CAC development and progression. However, there is increasing evidence that TNC is upregulated in CAFs and that a high TNC expression as a CAF marker in tumor stroma is correlated with worse prognosis in several malignancies, such as breast ductal carcinoma[7], esophageal squamous cell carcinoma[9], colorectal cancer[10], and prostate cancer[111]. Taken together with our results, the evidence indicates that fibroblasts produce TNC in the tumor microenvironment and that this TNC might activate CAFs to promote tumor onset and progression.
Risk factors for CAC development include pancolitis, a younger age of IBD onset, a long disease duration, chronic cholestatic liver disease, family history[112], and stricture formation[113]. Intestinal fibrosis is a common complication in IBD, particularly Crohn’s disease, and the resulting clinically relevant strictures have been observed in about one-third of patients[114]. Intestinal fibrosis is likely to involve increased ECM stiffness, and this stiffness could perpetuate fibrogenesis[114], leading to the development of fibrotic strictures. More recently, accumulating evidence has linked increased ECM stiffness to several malignancies, with recent studies showing that cancer progression and aggression are correlated with the stiffness of a TNC-enriched ECM[115] (please see the previous section). In IBD, increased ECM stiffness has been observed in strictures, and the increased ECM stiffness enhances adhesive properties, such as the formation of focal adhesion and actin stress fibers of colonic fibroblasts[116]. Moreover, increased expression levels of TNC have been reported in lesions of ulceration in ulcerative colitis[98]. Erdem et al[117] reported the possible involvement of increased expression levels of TNC in the development of ulcerative colitis-related strictures[117]. Given that peptide TNIIIA2 can induce potent and persistent activation of beta1-integrin as well as its clustering[47,48], peptide TNIIIA2 in stromal lesions might contribute to the development of colitis-related strictures through increased ECM stiffness, leading to increased risk of CAC onset. Although further research is required to determine whether beta1-integrin activation by peptide TNIIIA2 actually increases ECM stiffness, TNIIIA2-targeting agents such as an anti-TNIIIA2 antibody might be a promising strategy for the prophylaxis or treatment of CAC development and malignant progression.
Several studies have suggested that integrin inactivation could be a promising strategy for controlling CAC development and progression. ATN-161, a peptidic antagonist of integrin alpha5beta1 and alphavbeta3, suppressed disease activity by blocking angiogenesis in IL-10-deficient mice that develop spontaneous Crohn’s disease-like colitis[118] as well as in a CD4+CD45RBhigh T-cell transfer model that induced chronic pancolitis[119]. Furthermore, ATN-161 also inhibits CAC deve
Several antagonists of integrin alpha5beta1 and alphavbeta3 were well tolerated in clinical testing[121,122] but failed to show therapeutic benefits in patients with malignancies. Although inhibition of integrin alpha5beta1 and alphavbeta3 might be a safe therapeutic strategy, alternative approaches should be considered, including the application of integrin inhibitors as anti-cancer drugs (reviewed in Ref.[123]). Regarding other therapeutic modalities, OS2966, a humanized monoclonal antibody targeting human beta1-integrins, is undergoing testing in a phase I clinical trial for the treatment of recurrent/progressive glioma[124]. In addition, one possible strategy may be to develop drugs with modes of inhibition other than competitive inhibition of integrin. Unlike integrin antagonists, peptide FNIII14—which has the ability to induce a conformational change in beta1-integrin from the active to the inactive state[105]—has shown therapeutic efficacy against several malignancies in animal models, including CAC, glioblastoma, neuroblastoma, and acute myelogenous leukemia[105]. Although further research is needed regarding its effect on the malignant progression of colon cancer, peptide FNIII14 may possess promising therapeutic properties.
Although TNC is considered a negative prognostic factor in several malignancies, the substantial role of TNC molecule in the development of colorectal cancer and its malignant progression has remained elusive. We suggest that one of the pathological roles of TNC, which is highly expressed in colon cancer, may be in activating beta1-integrins through TNIIIA2 function. This hypothesis and the previous findings open the door to prophylactic and therapeutic strategies for colon cancer that involve inhibition of TNIIIA2-induced beta1-integrin activation by peptide FNIII14.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Shivaji UN S-Editor: Fan JR L-Editor: A P-Editor: Li X
| 1. | Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 1324] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 2. | Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 797] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 3. | Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci. 2011;68:3175-3199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Blandin AF, Noulet F, Renner G, Mercier MC, Choulier L, Vauchelles R, Ronde P, Carreiras F, Etienne-Selloum N, Vereb G, Lelong-Rebel I, Martin S, Dontenwill M, Lehmann M. Glioma cell dispersion is driven by α5 integrin-mediated cell-matrix and cell-cell interactions. Cancer Lett. 2016;376:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Dudvarski Stanković N, Bicker F, Keller S, Jones DT, Harter PN, Kienzle A, Gillmann C, Arnold P, Golebiewska A, Keunen O, Giese A, von Deimling A, Bäuerle T, Niclou SP, Mittelbronn M, Ye W, Pfister SM, Schmidt MHH. EGFL7 enhances surface expression of integrin α5β1 to promote angiogenesis in malignant brain tumors. EMBO Mol Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F, Entz-Werle N, Lehmann-Che J, Godet J, Martin S, Teisinger J, Dontenwill M. Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012;72:3463-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Yang Z, Ni W, Cui C, Fang L, Xuan Y. Tenascin C is a prognostic determinant and potential cancer-associated fibroblasts marker for breast ductal carcinoma. Exp Mol Pathol. 2017;102:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res. 1995;1:1035-1041. [PubMed] |
| 9. | Yang ZT, Yeo SY, Yin YX, Lin ZH, Lee HM, Xuan YH, Cui Y, Kim SH. Tenascin-C, a Prognostic Determinant of Esophageal Squamous Cell Carcinoma. PLoS One. 2016;11:e0145807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Yang Z, Zhang C, Qi W, Cui C, Cui Y, Xuan Y. Tenascin-C as a prognostic determinant of colorectal cancer through induction of epithelial-to-mesenchymal transition and proliferation. Exp Mol Pathol. 2018;105:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 567] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 12. | Marzeda AM, Midwood KS. Internal Affairs: Tenascin-C as a Clinically Relevant, Endogenous Driver of Innate Immunity. J Histochem Cytochem. 2018;66:289-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Imanaka-Yoshida K, Tawara I, Yoshida T. Tenascin-C in cardiac disease: a sophisticated controller of inflammation, repair, and fibrosis. Am J Physiol Cell Physiol. 2020;319:C781-C796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Hasegawa M, Yoshida T, Sudo A. Tenascin-C in Osteoarthritis and Rheumatoid Arthritis. Front Immunol. 2020;11:577015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Izumi K, Miyazaki N, Okada H, Tsujimoto A, Matsumoto-Miyazaki J, Naito J, Yoshida G, Murata I, Nagashima K, Ohno M, Imanaka-Yoshida K, Okura H, Ohashi H, Takemura G. Tenascin-C expression in renal biopsies from patients with tubulointerstitial nephritis and its relation to disease activity and prognosis. Int J Clin Exp Pathol. 2020;13:1842-1852. [PubMed] |
| 16. | Yuan W, Zhang W, Yang X, Zhou L, Hanghua Z, Xu K. Clinical significance and prognosis of serum tenascin-C in patients with sepsis. BMC Anesthesiol. 2018;18:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Okada T, Suzuki H. The Role of Tenascin-C in Tissue Injury and Repair After Stroke. Front Immunol. 2020;11:607587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Yasuda M, Harada N, Harada S, Ishimori A, Katsura Y, Itoigawa Y, Matsuno K, Makino F, Ito J, Ono J, Tobino K, Akiba H, Atsuta R, Izuhara K, Takahashi K. Characterization of tenascin-C as a novel biomarker for asthma: utility of tenascin-C in combination with periostin or immunoglobulin E. Allergy Asthma Clin Immunol. 2018;14:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Löfdahl M, Kaarteenaho R, Lappi-Blanco E, Tornling G, Sköld MC. Tenascin-C and alpha-smooth muscle actin positive cells are increased in the large airways in patients with COPD. Respir Res. 2011;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Mills JT, Schwenzer A, Marsh EK, Edwards MR, Sabroe I, Midwood KS, Parker LC. Airway Epithelial Cells Generate Pro-inflammatory Tenascin-C and Small Extracellular Vesicles in Response to TLR3 Stimuli and Rhinovirus Infection. Front Immunol. 2019;10:1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Cai J, Lu W, Du S, Guo Z, Wang H, Wei W, Shen X. Tenascin-C Modulates Cell Cycle Progression to Enhance Tumour Cell Proliferation through AKT/FOXO1 Signalling in Pancreatic Cancer. J Cancer. 2018;9:4449-4462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Sarkar S, Mirzaei R, Zemp FJ, Wei W, Senger DL, Robbins SM, Yong VW. Activation of NOTCH Signaling by Tenascin-C Promotes Growth of Human Brain Tumor-Initiating Cells. Cancer Res. 2017;77:3231-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Sun Z, Schwenzer A, Rupp T, Murdamoothoo D, Vegliante R, Lefebvre O, Klein A, Hussenet T, Orend G. Tenascin-C Promotes Tumor Cell Migration and Metastasis through Integrin α9β1-Mediated YAP Inhibition. Cancer Res. 2018;78:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Cai J, Du S, Wang H, Xin B, Wang J, Shen W, Wei W, Guo Z, Shen X. Tenascin-C induces migration and invasion through JNK/c-Jun signalling in pancreatic cancer. Oncotarget. 2017;8:74406-74422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sun Z, Velázquez-Quesada I, Murdamoothoo D, Ahowesso C, Yilmaz A, Spenlé C, Averous G, Erne W, Oberndorfer F, Oszwald A, Kain R, Bourdon C, Mangin P, Deligne C, Midwood K, Abou-Faycal C, Lefebvre O, Klein A, van der Heyden M, Chenard MP, Christofori G, Mathelin C, Loustau T, Hussenet T, Orend G. Tenascin-C increases lung metastasis by impacting blood vessel invasions. Matrix Biol. 2019;83:26-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Cai HP, Wang J, Xi SY, Ni XR, Chen YS, Yu YJ, Cen ZW, Yu ZH, Chen FR, Guo CC, Zhang J, Ke C, Chen ZP. Tenascin-cmediated vasculogenic mimicry formation via regulation of MMP2/MMP9 in glioma. Cell Death Dis. 2019;10:879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Rupp T, Langlois B, Koczorowska MM, Radwanska A, Sun Z, Hussenet T, Lefebvre O, Murdamoothoo D, Arnold C, Klein A, Biniossek ML, Hyenne V, Naudin E, Velazquez-Quesada I, Schilling O, Van Obberghen-Schilling E, Orend G. Tenascin-C Orchestrates Glioblastoma Angiogenesis by Modulation of Pro- and Anti-angiogenic Signaling. Cell Rep. 2016;17:2607-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A, Bose P, Yong VW. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology. 2018;7:e1478647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Jachetti E, Caputo S, Mazzoleni S, Brambillasca CS, Parigi SM, Grioni M, Piras IS, Restuccia U, Calcinotto A, Freschi M, Bachi A, Galli R, Bellone M. Tenascin-C Protects Cancer Stem-like Cells from Immune Surveillance by Arresting T-cell Activation. Cancer Res. 2015;75:2095-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Yang Z, Zhang C, Feng Y, Qi W, Cui Y, Xuan Y. Tenascin-C is involved in promotion of cancer stemness via the Akt/HIF1ɑ axis in esophageal squamous cell carcinoma. Exp Mol Pathol. 2019;109:104239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Yang Z, Zhang C, Feng Y, Quan M, Cui Y, Xuan Y. Tenascin-C predicts poor outcomes for patients with colorectal cancer and drives cancer stemness via Hedgehog signaling pathway. Cancer Cell Int. 2020;20:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Shi M, He X, Wei W, Wang J, Zhang T, Shen X. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-κB pathway. Apoptosis. 2015;20:843-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Saupe F, Schwenzer A, Jia Y, Gasser I, Spenlé C, Langlois B, Kammerer M, Lefebvre O, Hlushchuk R, Rupp T, Marko M, van der Heyden M, Cremel G, Arnold C, Klein A, Simon-Assmann P, Djonov V, Neuville-Méchine A, Esposito I, Slotta-Huspenina J, Janssen KP, de Wever O, Christofori G, Hussenet T, Orend G. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep. 2013;5:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Spenlé C, Gasser I, Saupe F, Janssen KP, Arnold C, Klein A, van der Heyden M, Mutterer J, Neuville-Méchine A, Chenard MP, Guenot D, Esposito I, Slotta-Huspenina J, Ambartsumian N, Simon-Assmann P, Orend G. Spatial organization of the tenascin-C microenvironment in experimental and human cancer. Cell Adh Migr. 2015;9:4-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Li M, Peng F, Li G, Fu Y, Huang Y, Chen Z, Chen Y. Proteomic analysis of stromal proteins in different stages of colorectal cancer establishes Tenascin-C as a stromal biomarker for colorectal cancer metastasis. Oncotarget. 2016;7:37226-37237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Giblin SP, Midwood KS. Tenascin-C: Form vs function. Cell Adh Migr. 2015;9:48-82. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 38. | Passlick B, Sienel W, Seen-Hibler R, Wöckel W, Thetter O, Mutschler W, Pantel K. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6:3944-3948. [PubMed] |
| 39. | Cai M, Onoda K, Takao M, Kyoko IY, Shimpo H, Yoshida T, Yada I. Degradation of tenascin-C and activity of matrix metalloproteinase-2 are associated with tumor recurrence in early stage non-small cell lung cancer. Clin Cancer Res. 2002;8:1152-1156. [PubMed] |
| 40. | Mai J, Sameni M, Mikkelsen T, Sloane BF. Degradation of extracellular matrix protein tenascin-C by cathepsin B: an interaction involved in the progression of gliomas. Biol Chem. 2002;383:1407-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 42. | Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 823] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 43. | Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? Gut. 2015;64:367-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Sakai T, Kawakatsu H, Hirota N, Yokoyama T, Sakakura T, Saito M. Specific expression of tenascin in human colonic neoplasms. Br J Cancer. 1993;67:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Kusagawa H, Onoda K, Namikawa S, Yada I, Okada A, Yoshida T, Sakakura T. Expression and degeneration of tenascin-C in human lung cancers. Br J Cancer. 1998;77:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, Kodama H, Handa H, Yoshida T, Fukai F. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J Biol Chem. 2007;282:34929-34937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, Iyoda T, Takai T, Owaki T, Yajima H, Taira J, Hayashi R, Kodama H, Matsunaga T, Fukai F. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin α5β1. J Biol Chem. 2014;289:17699-17708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Dueck M, Riedl S, Hinz U, Tandara A, Möller P, Herfarth C, Faissner A. Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int J Cancer. 1999;82:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Fujita M, Yamamoto T, Iyoda T, Fujisawa T, Nagai R, Kudo C, Sasada M, Kodama H, Fukai F. Autocrine Production of PDGF Stimulated by the Tenascin-C-Derived Peptide TNIIIA2 Induces Hyper-Proliferation in Glioblastoma Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Fujita M, Yamamoto T, Iyoda T, Fujisawa T, Sasada M, Nagai R, Kudo C, Otsuka K, Kamiya S, Kodama H, Fukai F. Aggressive Progression in Glioblastoma Cells through Potentiated Activation of Integrin α5β1 by the Tenascin-C-Derived Peptide TNIIIA2. Mol Cancer Ther. 2019;18:1649-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Fujita M, Sasada M, Iyoda T, Nagai R, Kudo C, Yamamoto T, Osada S, Kodama H, Fukai F. Anoikis resistance conferred by tenascin-C-derived peptide TNIIIA2 and its disruption by integrin inactivation. Biochem Biophys Res Commun. 2021;536:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Iyoda T, Fujita M, Fukai F. Biologically Active TNIIIA2 Region in Tenascin-C Molecule: A Major Contributor to Elicit Aggressive Malignant Phenotypes From Tumors/Tumor Stroma. Front Immunol. 2020;11:610096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11942] [Article Influence: 2985.5] [Reference Citation Analysis (4)] |
| 55. | Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N, Ghai NR, Lee AT, Quesenberry CP, Fireman BH, Doubeni CA. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology. 2018;155:1383-1391.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 56. | Chibaudel B, Tournigand C, Bonnetain F, Richa H, Benetkiewicz M, André T, de Gramont A. Therapeutic strategy in unresectable metastatic colorectal cancer: an updated review. Ther Adv Med Oncol. 2015;7:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Ji K, Zhang M, Chu Q, Gan Y, Ren H, Zhang L, Wang L, Li X, Wang W. The Role of p-STAT3 as a Prognostic and Clinicopathological Marker in Colorectal Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0160125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 147] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 59. | Maida M, Macaluso FS, Ianiro G, Mangiola F, Sinagra E, Hold G, Maida C, Cammarota G, Gasbarrini A, Scarpulla G. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther. 2017;17:1131-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 60. | Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 434] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (11)] |
| 61. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 797] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 62. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1441] [Article Influence: 360.3] [Reference Citation Analysis (0)] |
| 63. | Taniguchi H, Yamazaki K, Yoshino T, Muro K, Yatabe Y, Watanabe T, Ebi H, Ochiai A, Baba E, Tsuchihara K; Japanese Society of Medical Oncology. Japanese Society of Medical Oncology Clinical Guidelines: RAS (KRAS/NRAS) mutation testing in colorectal cancer patients. Cancer Sci. 2015;106:324-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Takeda A, Otani Y, Iseki H, Takeuchi H, Aikawa K, Tabuchi S, Shinozuka N, Saeki T, Okazaki Y, Koyama I. Clinical significance of large tenascin-C spliced variant as a potential biomarker for colorectal cancer. World J Surg. 2007;31:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Wanitsuwan W, Kanngurn S, Boonpipattanapong T, Sangthong R, Sangkhathat S. Overall expression of beta-catenin outperforms its nuclear accumulation in predicting outcomes of colorectal cancers. World J Gastroenterol. 2008;14:6052-6059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knoll C, Kirchner T, Jung A. beta-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene. 2005;24:8200-8204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 68. | Li ZL, Wang ZJ, Wei GH, Yang Y, Wang XW. Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells. World J Gastrointest Oncol. 2020;12:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (3)] |
| 69. | Sis B, Sağol O, Küpelioğlu A, Sokmen S, Terzi C, Fuzun M, Ozer E, Bishop P. Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol Res Pract. 2004;200:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Buchert M, Rohde F, Eissmann M, Tebbutt N, Williams B, Tan CW, Owen A, Hirokawa Y, Gnann A, Orend G, Orner G, Dashwood RH, Heath JK, Ernst M, Janssen KP. A hypermorphic epithelial β-catenin mutation facilitates intestinal tumorigenesis in mice in response to compounding WNT-pathway mutations. Dis Model Mech. 2015;8:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Siri A, Knäuper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650-8654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Suzuki H, Sasada M, Kamiya S, Ito Y, Watanabe H, Okada Y, Ishibashi K, Iyoda T, Yanaka A, Fukai F. The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Liu C, Pei H, Tan F. Matrix Stiffness and Colorectal Cancer. Onco Targets Ther. 2020;13:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Nebuloni M, Albarello L, Andolfo A, Magagnotti C, Genovese L, Locatelli I, Tonon G, Longhi E, Zerbi P, Allevi R, Podestà A, Puricelli L, Milani P, Soldarini A, Salonia A, Alfano M. Insight On Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci Rep. 2016;6:22522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Wei B, Zhou X, Liang C, Zheng X, Lei P, Fang J, Han X, Wang L, Qi C, Wei H. Human colorectal cancer progression correlates with LOX-induced ECM stiffening. Int J Biol Sci. 2017;13:1450-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 76. | Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 77. | Barnes JM, Kaushik S, Bainer RO, Sa JK, Woods EC, Kai F, Przybyla L, Lee M, Lee HW, Tung JC, Maller O, Barrett AS, Lu KV, Lakins JN, Hansen KC, Obernier K, Alvarez-Buylla A, Bergers G, Phillips JJ, Nam DH, Bertozzi CR, Weaver VM. A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma. Nat Cell Biol. 2018;20:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 78. | Liu QZ, Gao XH, Chang WJ, Gong HF, Fu CG, Zhang W, Cao GW. Expression of ITGB1 predicts prognosis in colorectal cancer: a large prospective study based on tissue microarray. Int J Clin Exp Pathol. 2015;8:12802-12810. [PubMed] |
| 79. | Lu L, Xie R, Wei R, Cai C, Bi D, Yin D, Liu H, Zheng J, Zhang Y, Song F, Gao Y, Tan L, Wei Q, Qin H. Integrin α5 subunit is required for the tumor supportive role of fibroblasts in colorectal adenocarcinoma and serves as a potential stroma prognostic marker. Mol Oncol. 2019;13:2697-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Yu M, Chu S, Fei B, Fang X, Liu Z. O-GlcNAcylation of ITGA5 facilitates the occurrence and development of colorectal cancer. Exp Cell Res. 2019;382:111464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Murillo CA, Rychahou PG, Evers BM. Inhibition of alpha5 integrin decreases PI3K activation and cell adhesion of human colon cancers. Surgery. 2004;136:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Guha D, Saha T, Bose S, Chakraborty S, Dhar S, Khan P, Adhikary A, Das T, Sa G. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis. 2019;24:958-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 83. | Janouskova H, Ray AM, Noulet F, Lelong-Rebel I, Choulier L, Schaffner F, Lehmann M, Martin S, Teisinger J, Dontenwill M. Activation of p53 pathway by Nutlin-3a inhibits the expression of the therapeutic target α5 integrin in colon cancer cells. Cancer Lett. 2013;336:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, McCarty MF, Bucana CD, Mazar AP, Ellis LM. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 86. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 840] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 87. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1198] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 88. | Jess T, Loftus EV Jr, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ 3rd, Munkholm P, Sandborn WJ. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 89. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2079] [Article Influence: 86.6] [Reference Citation Analysis (1)] |
| 90. | Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 91. | Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19:599-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 92. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in Crohn's disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 93. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 94. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 95. | Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662-2669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Riedl S, Tandara A, Reinshagen M, Hinz U, Faissner A, Bodenmüller H, Buhr HJ, Herfarth C, Möller P. Serum tenascin-C is an indicator of inflammatory bowel disease activity. Int J Colorectal Dis. 2001;16:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Spenlé C, Lefebvre O, Lacroute J, Méchine-Neuville A, Barreau F, Blottière HM, Duclos B, Arnold C, Hussenet T, Hemmerlé J, Gullberg D, Kedinger M, Sorokin L, Orend G, Simon-Assmann P. The laminin response in inflammatory bowel disease: protection or malignancy? PLoS One. 2014;9:e111336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Geboes K, El-Zine MY, Dalle I, El-Haddad S, Rutgeerts P, Van Eyken P. Tenascin and strictures in inflammatory bowel disease: an immunohistochemical study. Int J Surg Pathol. 2001;9:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Riedl S, Kadmon M, Tandara A, Hinz U, Möller P, Herfarth C, Faissner A. Mucosal tenascin C content in inflammatory and neoplastic diseases of the large bowel. Dis Colon Rectum. 1998;41:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Brant SR, Okou DT, Simpson CL, Cutler DJ, Haritunians T, Bradfield JP, Chopra P, Prince J, Begum F, Kumar A, Huang C, Venkateswaran S, Datta LW, Wei Z, Thomas K, Herrinton LJ, Klapproth JA, Quiros AJ, Seminerio J, Liu Z, Alexander JS, Baldassano RN, Dudley-Brown S, Cross RK, Dassopoulos T, Denson LA, Dhere TA, Dryden GW, Hanson JS, Hou JK, Hussain SZ, Hyams JS, Isaacs KL, Kader H, Kappelman MD, Katz J, Kellermayer R, Kirschner BS, Kuemmerle JF, Kwon JH, Lazarev M, Li E, Mack D, Mannon P, Moulton DE, Newberry RD, Osuntokun BO, Patel AS, Saeed SA, Targan SR, Valentine JF, Wang MH, Zonca M, Rioux JD, Duerr RH, Silverberg MS, Cho JH, Hakonarson H, Zwick ME, McGovern DP, Kugathasan S. Genome-Wide Association Study Identifies African-Specific Susceptibility Loci in African Americans With Inflammatory Bowel Disease. Gastroenterology. 2017;152:206-217.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 101. | Ning L, Li S, Gao J, Ding L, Wang C, Chen W, Shan G, Zhang F, Yu J, Xu G. Tenascin-C Is Increased in Inflammatory Bowel Disease and Is Associated with response to Infliximab Therapy. Biomed Res Int. 2019;2019:1475705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Kawamura T, Yamamoto M, Suzuki K, Suzuki Y, Kamishima M, Sakata M, Kurachi K, Setoh M, Konno H, Takeuchi H. Tenascin-C Produced by Intestinal Myofibroblasts Promotes Colitis-associated Cancer Development Through Angiogenesis. Inflamm Bowel Dis. 2019;25:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Kofla-Dlubacz A, Matusiewicz M, Krzystek-Korpacka M, Iwanczak B. Correlation of MMP-3 and MMP-9 with Crohn's disease activity in children. Dig Dis Sci. 2012;57:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Fujita M, Ito-Fujita Y, Iyoda T, Sasada M, Okada Y, Ishibashi K, Osawa T, Kodama H, Fukai F, Suzuki H. Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Fujita M, Sasada M, Iyoda T, Fukai F. Involvement of Integrin-Activating Peptides Derived from Tenascin-C in Cancer Aggression and New Anticancer Strategy Using the Fibronectin-Derived Integrin-Inactivating Peptide. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1228] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 107. | Mukaida N, Sasaki S. Fibroblasts, an inconspicuous but essential player in colon cancer development and progression. World J Gastroenterol. 2016;22:5301-5316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 2450] [Article Influence: 490.0] [Reference Citation Analysis (0)] |
| 109. | Sasaki S, Baba T, Shinagawa K, Matsushima K, Mukaida N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int J Cancer. 2014;135:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Neufert C, Becker C, Türeci Ö, Waldner MJ, Backert I, Floh K, Atreya I, Leppkes M, Jefremow A, Vieth M, Schneider-Stock R, Klinger P, Greten FR, Threadgill DW, Sahin U, Neurath MF. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J Clin Invest. 2013;123:1428-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 111. | Ni WD, Yang ZT, Cui CA, Cui Y, Fang LY, Xuan YH. Tenascin-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochem Biophys Res Commun. 2017;486:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 112. | Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389-16397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 113. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 114. | Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:340-350.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 115. | Miroshnikova YA, Mouw JK, Barnes JM, Pickup MW, Lakins JN, Kim Y, Lobo K, Persson AI, Reis GF, McKnight TR, Holland EC, Phillips JJ, Weaver VM. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol. 2016;18:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 116. | Johnson LA, Rodansky ES, Sauder KL, Horowitz JC, Mih JD, Tschumperlin DJ, Higgins PD. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013;19:891-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 117. | Erdem E, Kochan K, Paker N, Gokden Y, Degirmenci AS, Kocak F, Gonen C. The correlation between tenascin-C expression, and formation of intestinal stricture. North Clin Istanb. 2014;1:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Danese S, Sans M, Spencer DM, Beck I, Doñate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 120. | Terasaki M, Ikuta M, Kojima H, Tanaka T, Maeda H, Miyashita K, Mutoh M. Dietary Fucoxanthin Induces Anoikis in Colorectal Adenocarcinoma by Suppressing Integrin Signaling in a Murine Colorectal Cancer Model. J Clin Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 121. | Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, Lewis N, Weiner L, Lam GN, Brown SC, Shaw DE, Mazar AP, Cohen RB. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 122. | Malric L, Monferran S, Gilhodes J, Boyrie S, Dahan P, Skuli N, Sesen J, Filleron T, Kowalski-Chauvel A, Cohen-Jonathan Moyal E, Toulas C, Lemarié A. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: an update. Oncotarget. 2017;8:86947-86968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 123. | Alday-Parejo B, Stupp R, Rüegg C. Are Integrins Still Practicable Targets for Anti-Cancer Therapy? Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 124. | Nwagwu CD, Immidisetti AV, Bukanowska G, Vogelbaum MA, Carbonell AM. Convection-Enhanced Delivery of a First-in-Class Anti-β1 Integrin Antibody for the Treatment of High-Grade Glioma Utilizing Real-Time Imaging. Pharmaceutics. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |