Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.472
Peer-review started: January 8, 2021
First decision: February 24, 2021
Revised: March 22, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: June 15, 2021
Processing time: 150 Days and 6.3 Hours
Pancreatic adenocarcinoma is a lethal disease with a mortality rate that has not significantly improved over decades. This is likely due to several challenges unique to pancreatic cancer. Most patients with pancreatic cancer are diagnosed at a late stage of disease due to the lack of specific symptoms prompting an early investigation. A small subset of patients who are diagnosed at an early stage have a better chance at survival with curative surgical resection, but most patients still succumb to the disease in a few years. The dismal overall prognosis is due to suspected micro-metastasis at an early stage. Due to this reason, there is a recent interest in treating all patients with pancreatic cancers with systemic therapy upfront (including the ones that are surgically resectable). This approach is still not the standard of care due to the lack of robust prospective data available. Recent advancements in treatment regimens of chemotherapy, radiation and immunotherapy have improved the overall short-term survival but the long-term survival still remains poor. Novel approaches in diagnosis and treatment have shown promise in clinical studies but long-term clinical data is lacking. The following manuscript presents an overview of the epidemiology, diagnosis, staging, recent advances, novel approaches and controversies in the management of pancreatic adenocarcinoma.
Core Tip: Early diagnosis and treatment of pancreatic adenocarcinoma has remained a challenge over the last several decades. Despite best efforts, the long-term survival rate has not significantly improved. The following manuscript highlights the current advances and controversies in the management of pancreatic cancer. The standard systemic therapies have been presented in a format that is easy to read and follow. Novel approaches in diagnosis and management have been discussed in light of evidence-based medicine.
- Citation: Zeeshan MS, Ramzan Z. Current controversies and advances in the management of pancreatic adenocarcinoma. World J Gastrointest Oncol 2021; 13(6): 472-494
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/472.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.472
Pancreatic cancer ranks as the fourth leading cause of cancer related death in the United States and seventh leading cause of cancer deaths worldwide[1]. Approximately 57600 patients are diagnosed with pancreatic cancer annually[2], with a vast majority of these patients dying within the first year of diagnosis. The incidence of pancreatic cancer in the United States is 1.3 times higher in males than females[3]. There is a slightly increased risk in blacks than in whites[4]. Several other risk factors for pancreatic cancer have been identified[5], such as cigarette smoking[6,7], physical inactivity and obesity[8], high intake of saturated fat and/or processed or smoked meats[9], family history and genetic predisposition syndromes[10-12], nonhereditary chronic pancreatitis[13,14], and presence of pancreatic cysts such as intraductal papillary mucinous neoplasm of pancreas[15]. Due to high mortality of pancreatic cancer, many studies have looked at the prognostic indicators and predictors of mortality[16-19]. Several new modalities have been introduced in diagnosis and management of pancreatic cancer over the past few decades[20] but overall prognosis still remains poor[2].
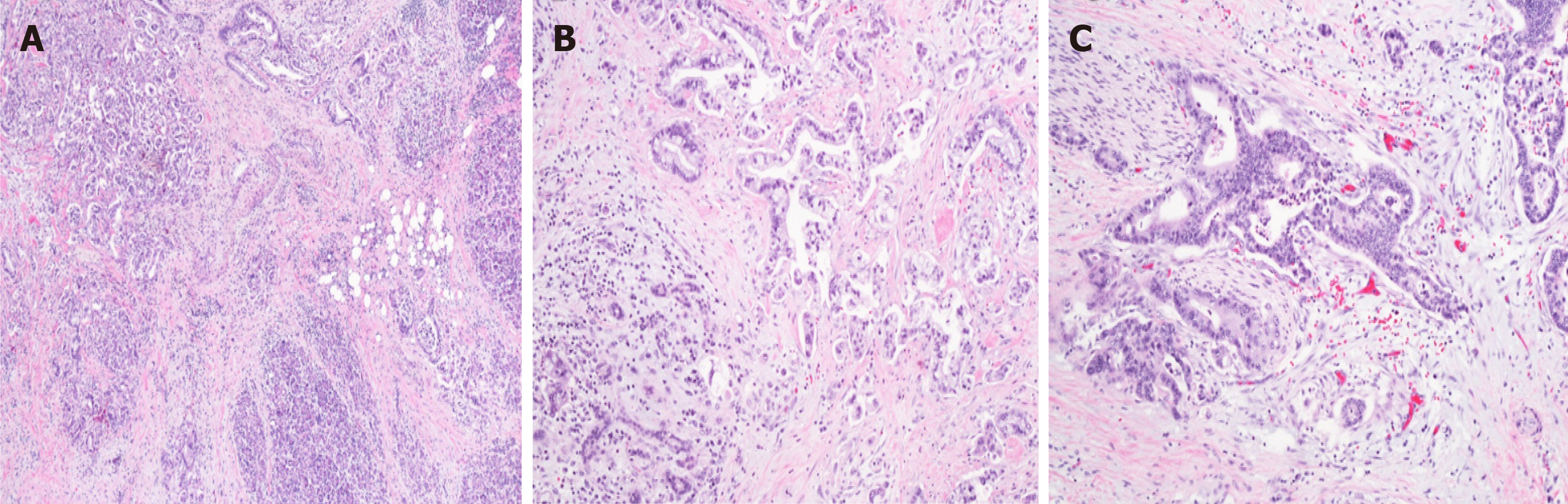
The most common type of pancreatic cancer is pancreatic adenocarcinoma, representing approximately 85% of all pancreatic neoplasms. Unfortunately, this accounts for the most aggressive type of pancreatic cancer with the poorest prognosis overall (Figure 1). Neoplasms arising from the endocrine pancreas (such as pancreatic neuroendocrine tumors) account for approximately 5% of all pancreatic tumors. Other rare tumors include acinar carcinoma, cystic neoplasms (mucinous cystadenoma, intraductal papillary mucinous tumors, solid pseudopapillary tumors, serous cystadenoma), metastatic tumors, etc.
The presentation of pancreatic cancer varies by the location of the tumor. Tumors located in the head of pancreas (approximately 60%-70%) usually present with painless jaundice[21] due to obstruction of the intra-pancreatic portion of distal common bile duct. Other symptoms include steatorrhea and weight loss. On the other hand, tumors in the body of pancreas (20%-25%) present somewhat late in the disease course with severe abdominal/back pain, anorexia and weight loss.
Rarely, a pancreatic mass is found as an incidental finding on computed tomography (CT) scan performed for another reason. Overall incidence of an incidental pancreatic mass over an eight year period in one study was reported as 7%, with one half of these were adenocarcinoma[22].
Symptoms alone are not sensitive to diagnose pancreatic cancer as many other diseases can present with similar symptoms[23]. Initial workup starts with simple blood tests and cross-sectional imaging, followed by additional testing based upon clinical presentation.
For patients suspected to have a mass in the head of pancreas causing biliary obstruction, initial blood work should include liver function tests (such as serum aminotransferases, alkaline phosphatase, and bilirubin). Evidence of cholestasis (elevation of alkaline phosphatase and bilirubin) could suggest obstruction of distal common bile duct in the right clinical scenario. Serum amylase and lipase can be checked to rule out acute pancreatitis in patients with severe epigastric pain but is not useful to diagnose pancreatic cancer.
The most important test in the diagnosis of pancreatic cancer is diagnostic imaging. Abdominal ultrasound is inexpensive, readily available and useful in certain clinical situations. It helps determine the presence of bile duct dilation, large masses in the head of pancreas (> 3 cm), any liver metastasis, ascites, etc. However, it has several limitations. It is not useful in the evaluation of the entire pancreas, as the retroperitoneal location of pancreas and overlying gas in the stomach and small intestine can obscure visualization of the entire pancreas.
The most useful test in diagnosing a pancreatic mass is abdominal CT scan. A special dedicated pancreas protocol CT scan [triple phase, helical, contrast enhanced, multidetector row CT with three dimensional reconstruction; multidetector computed tomography (MDCT)] has a sensitivity of 89%-97%[24-26]. The classic appearance of an exocrine pancreatic cancer is a poorly defined hypoattenuating mass within the pancreas, although isoattenuation can be seen in smaller tumors[27]. Other abnormalities may include abrupt cut off of pancreatic duct with proximal (upstream) pancreatic duct dilation, pancreatic atrophy, etc. Masses in the head of pancreas and ampulla can cause dilation of the bile duct and pancreatic duct (double duct sign)[28]. A good quality (pancreas protocol) CT also helps in the staging of tumors, which can range from delineating vascular anatomy in early-stage disease to evaluating distant metastasis in stage IV disease[29-31].
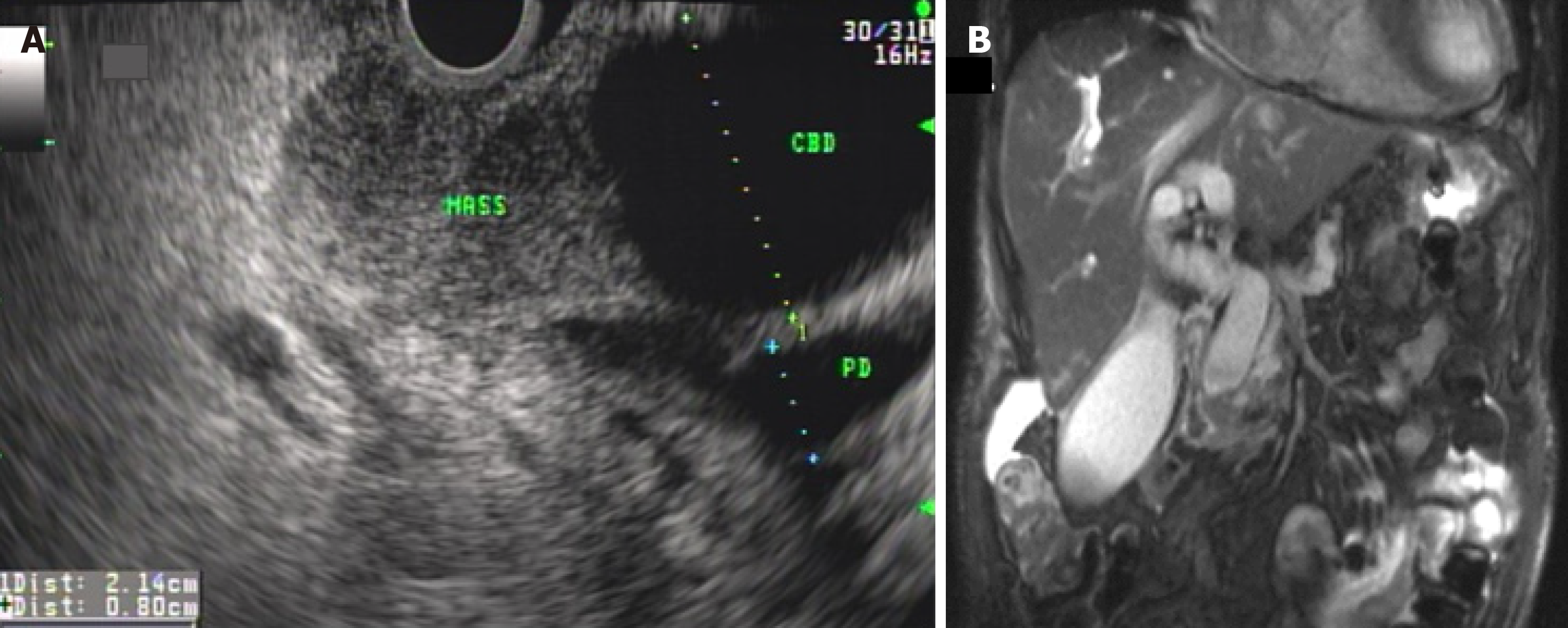
Magnetic resonance cholangiopancreatography (MRCP) creates a three-dimensional image of the pancreaticobiliary tree, liver and adjacent vascular structures. It is especially useful in outlining the pancreatic duct and biliary duct, obviating the need for having to inject dye during endoscopic retrograde cholangiopancreatography (ERCP) to obtain that information[32] (Figure 2). Moreover, subtle strictures, partly cystic masses and intrahepatic masses can be delineated with addition of contrast enhanced (gadolinium) injection during magnetic resonance imaging (MRI) of the abdomen. MRCP provides a road map in difficult situations such as patients with altered surgical anatomy (e.g., Bilroth II, Roux-en-Y gastric bypass, etc.), gastric or duodenal stenosis, bile duct obstruction in setting of chronic pancreatitis, etc.[33]. Despite the value of MRI/MRCP in certain clinical situations, MRI does not offer significant advantage over MDCT in routine workup of pancreatic cancer[34-36], except probable increased sensitivity for detecting small liver metastasis[37-40].
The role of tumor markers in diagnosing pancreatic cancer is controversial. The most useful and widely used tumor marker is cancer associated antigen 19-9 (CA 19-9). The reported sensitivity ranges from 70%-92% and specificity ranges from 68%-92%[41]. There are several caveats of using CA 19-9 in diagnosis of pancreatic cancer. The sensitivity is lower for smaller tumors[41,42]. In patients with a Lewis negative phenotype (approximately 5%-10% of the population), CA 19-9 is not a useful tumor marker[43,44]. The specificity is low as it is frequently elevated in patients with other cancers and various benign pancreaticobiliary tumors[44-46]. Due to low positive predictive value of CA 19-9, it is not used as a screening test for pancreatic cancer[47]. Nevertheless, there are two distinct advantages of using CA 19-9 in patients with pancreatic cancer. Firstly, it has some value as a prognostic marker, i.e., a markedly elevated CA 19-9 Likely signifies occult metastasis and hence, poor overall prognosis[48-51]. Secondly, it is useful in monitoring disease activity during treatment. For example, elevation in CA 19-9 Levels after curative surgical resection may indicate early cancer recurrence even before appearance of radiographic abnormality on surveillance imaging[52-54].
The diagnosis of pancreatic cancer is made on histological confirmation of biopsy specimens. The best modality to obtain tissue diagnosis is Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) of the pancreatic mass (Figure 2). The entire pancreas can be evaluated through the stomach and duodenum with the help of EUS. A special needle can be advanced through the wall of the upper gastrointestinal tract into the pancreatic mass without risking spread of cancer cells into the peritoneum as is seen with US or CT guided aspiration. The sensitivity and specificity of EUS-FNA has been reported at 89%-92% and 96% respectively[55,56]. Several advantages of EUS-FNA over US or CT guided approach (other than superior accuracy) include less risk of needle tract seeding[57], less risk of peritoneal seeding[58], ability to perform local staging, and cost[59]. Limitations of EUS are that it is operator dependent and it is suboptimal for evaluation of distant metastasis.
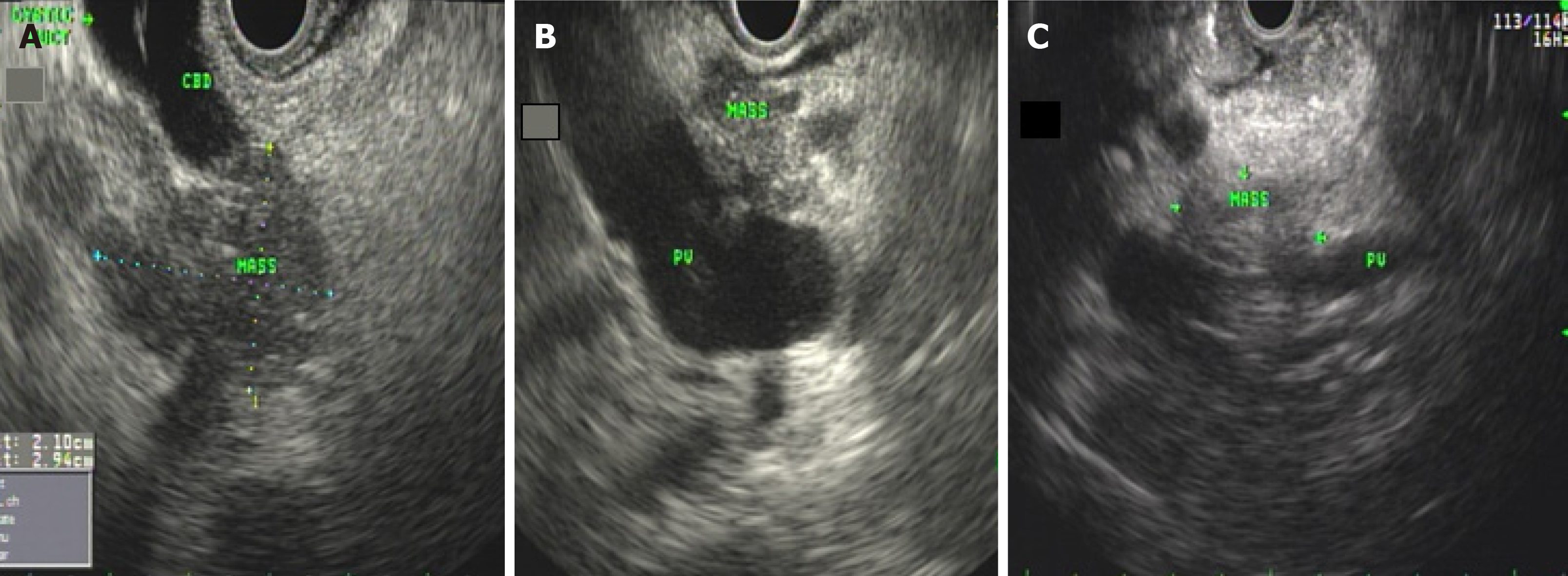
As an imaging tool, EUS is very sensitive and is commonly used in screening patients-with familial pancreatic cancer or other hereditary syndromes[60]. EUS not only helps biopsy the tumor but also provides simultaneous access to sampling of regional nodes, -ascites, liver lesions and malignant cyst fluid. Moreover, it helps assess resectability of tumor duringdiagnostic evaluation (Figure 3). Additionally, recent studies have explored the utility of EUS in many other sophisticated ways, such as injection of cytotoxic agents, application of radiofrequency ablation to ablate neoplastic lesions, introducing instruments directly into the lesions for diagnostic purposes, etc.
Despite overwhelming evidence to support the utility of EUS in evaluation of pancreatic cancer, there are certain limitations and challenges in its use. In certain situations, masses in setting of focal chronic pancreatitis (with focus of ductal adenocarcinoma) and/or autoimmune pancreatitis can be indistinguishable from pancreatic cancer and hence, pose a clinical challenge in diagnosis and management of these lesions. In these difficult situations, a multimodality approach involving clinical history (i.e., lack of alarm symptoms), radiological interpretation and short term follow up may be necessary. Additionally, certain technical limitations of EUS include difficulty in accessing tumors in the uncinate process of pancreas due to acute angulation in the second portion of duodenum, as well as the inherent limitations in obtaining rich aspirate with small FNA needles. The introduction of new and better needles such as fine needle biopsy needles with different designs and compositions have largely solved these problems. Despite these rare challenges and limitations, in the hands of experts, EUS imaging and sampling is very accurate and considered the gold standard in detecting and diagnosing pancreatic cancer.
Centers without personnel experienced with EUS-FNA rely on percutaneous biopsy of pancreatic masses to establish a diagnosis. Potential disadvantages of CT guided percutaneous biopsy approach include malignant seeding of the needle tract, though this theory has not been proven convincingly.
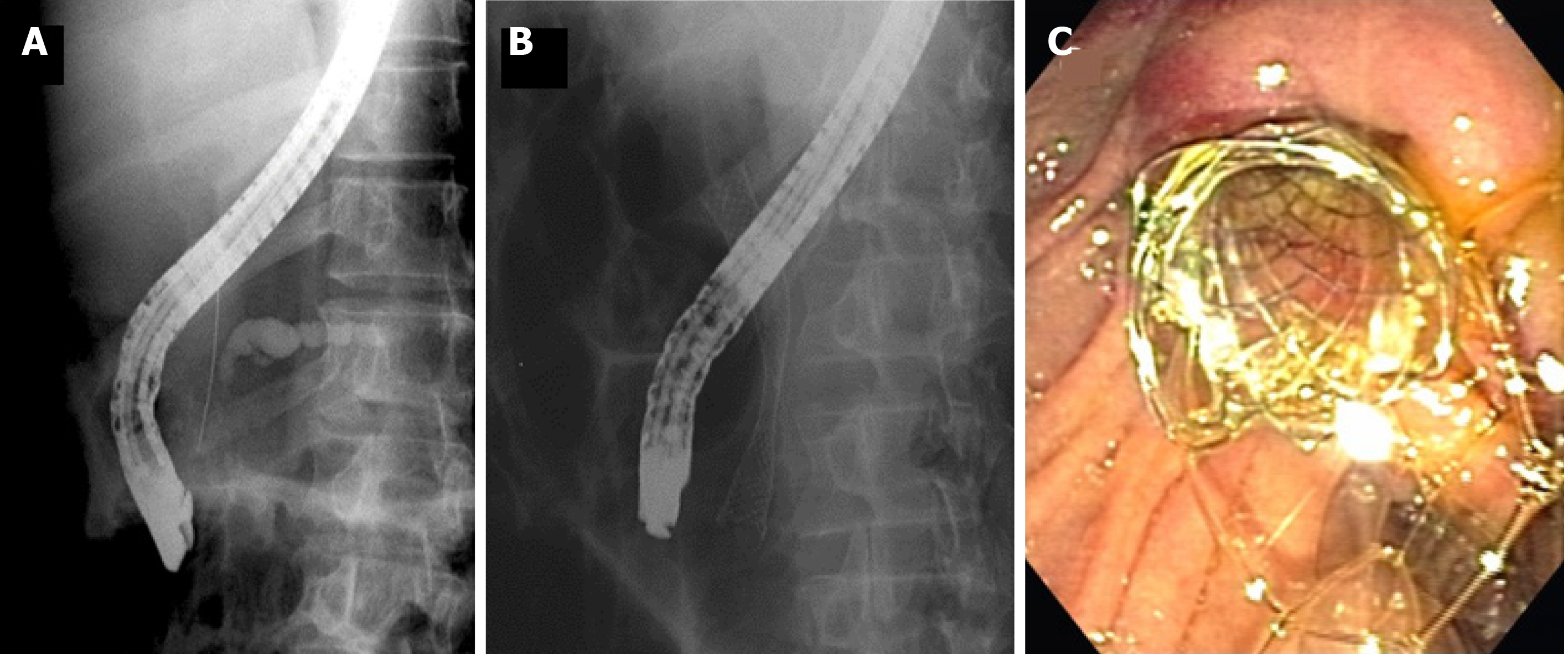
ERCP is a useful tool in evaluating the duodenum, ampulla, biliary and pancreatic system. In addition to direct visualization, ERCP can help obtain tissue samples for diagnosis, such as brush samples of indeterminate strictures for cytology, as well as intraductal biopsies. As ERCP has potential risks such as bleeding, perforation and pancreatitis, it is generally not considered the initial test for the diagnosis of suspected pancreatic cancer. EUS is still considered the gold standard in obtaining samples for tissue diagnosis. However, ERCP has great clinical utility in relieving malignant biliary obstruction by stent placement (Figure 4).
The role of positron emission tomography (PET) scan in routine staging of pancreatic cancer is controversial. Studies have shown data supporting the utility of PET scan in staging of pancreatic cancer[61-63], whereas other studies have shown conflicting results[64,65]. Use of 18F-flurodeoxyglucose (FDG) PET combined with CT (PET/CT) and MRI (PET/MRI) has generated interest in diagnosis, staging (lymph node involvement and metastasis)[66], assessment of pathological grade[67], assessment of treatment response, planning of radiation treatment, etc.[68-70]. There are certain advantages of PET/MRI over PET/CT such as lower radiation dose and superior soft tissue contrast[68]. However, the subgroup of patients with pancreatic cancer who will benefit from PET/CT or PET/MRI is not clearly understood. Hence, PET scan is not used routinely but only in certain select situations as illustrated in National Comprehensive Cancer Network and European Society for Medical Oncology guidelines[71].
The role of staging laparoscopy has evolved over time. The utility of staging laparoscopy relies on the pretext that small occult metastatic lesions can be missed by the available diagnostic imaging modalities and can be picked up by diagnostic laparoscopy. Hence, in certain clinical situations where the pre-test clinical probability of occult metastatic disease is high, staging laparoscopy can detect small sub-cm metastatic lesions on the peritoneum and surface of the liver and upstage the disease from resectable to stage IV metastatic disease. This also helps in re-directing the focus to palliative chemotherapy rather than neoadjuvant treatment in preparation for eventual needless surgical resection. Ideal candidates who may benefit from diagnostic laparoscopy include large tumors (> 3 cm), tumors in the body and tail of pancreas, elevated CA 19-9 > 1000, locally advanced but resectable disease, imaging suspicious for occult metastatic disease, etc.[72-74].
Routine use of laparoscopic ultrasound during staging laparoscopy has the potential of finding small metastatic lesions that can be missed by routine cross-sectional imaging or visual inspection during laparoscopy. When used in conjunction with laparoscopy, laparoscopic ultrasound can help in evaluation of primary tumors, peripancreatic vascular anatomy, detect small occult metastatic lesions and hence, change the surgical approach and prevent unnecessary radical surgery[75-79].
Several studies in the last decade have sparked an interest in novel mucosal imaging, but none has yet been accepted as a routine investigation in the evaluation of suspected pancreatic mass. Narrow band imaging technology uses light of specific blue and green wavelengths to augment certain mucosal features while visualizing the wall of pancreatic duct (with a small catheter inserted into the pancreatic duct) (‘pancreatoscopy’)[80].
Optical endomicroscopy permits imaging of the lining of pancreatic duct and wall of pancreatic cyst with the help of a small diameter probe introduced into the pancreatic duct at the time of EUS or ERCP. Such sophisticated imaging has a potential to increase diagnostic yield of sampling by targeted biopsies in the high yield area. Two imaging technologies used in this manner include confocal laser endomicroscopy[81,82] and high resolution microendoscopy[83]. Other imaging modalities such as optical coherence tomography have even lower clinical applicability as it employs infrared light to scan a few millimeters beneath the lining of the duct making it a time consuming and a low yield test[84-86].
Intraductal ultrasound (IDUS), a mini-ultrasound probe, can be used to evaluate indeterminate strictures. It is introduced within the pancreatic duct which makes it more invasive. In some studies it has been found to be useful in evaluation of early pancreatic cancers and determine margins of malignant cystic lesions such as intraductal papillary mucinous neoplasms before surgical resection[87]. IDUS is not commonly used in the United States due to limited clinical application and risk of pancreatitis associated with the procedure[88]. On the other hand, contrast enhanced EUS, which utilizes intravenous contrast to enhance a pancreatic lesion has been received with more interest in recent times[89]. In a meta-analysis, the pooled sensitivity of contrast-enhanced EUS for the differential diagnosis of pancreatic adenocarcinomas was 94% (95%CI: 0.91-0.95), and the specificity was 89% (95%CI: 0.85-0.92)[90].
Another modality using EUS, EUS elastography, helps distinguish between benign focal mass in chronic pancreatitis from pancreatic cancer by performing quantitative analysis of tissue stiffness. In one study, the sensitivity and specificity for detecting pancreatic malignancies were 100% and 92.9% respectively[91]. Three-dimensional reconstruction and spectrum analysis using EUS has shown -good results, and has the potential to be used more often in the future[92].
As pancreatic cancer has the potential to cause micro metastasis even in early stage of disease, research has been carried out to determine the molecular profiling of these tumors. Imaging agents such as peptides that bind to specific factors on the surface of pancreatic tumors have been developed and include: cathepsin E, integrin αvβ6, plectin 1, claudin-4 and oncolytic adenovarirus mutant[92-97]. Similarly, engineered biological agents such as oncolytic adenovirus have shown efficacy and tumor selectivity in preclinical pancreatic cancer models[98,99]. More robust clinical studies are needed before it can be used in routine evaluation of early pancreatic cancer.
A different approach focused on investigating normal pancreatic parenchyma has been developed. Unlike pancreatic tumor, normal pancreatic tissue expresses receptor for bombesin. Hence, a bombesin peptide-coupled nanoparticle (BN-CLIO[Cy5.5]) can be used to image normal pancreas and hence, differentiate it from pancreatic tumors[100]. Similarly, in other studies, microbubbles (small gas-filled microspheres) have been used to image the peri-tumoral vasculature with the help of ultrasound. This technology can be potentially used to deliver anti-cancer therapies in future studies[101].
A controversial subject is the need for pre-operative biopsy in patients with classic clinical and radiographic presentation of pancreatic adenocarcinoma. The advantage of performing biopsy is to confirm the diagnosis and minimize the risk of needless surgery for unsuspected benign disease. Disadvantages include the changes of false negative biopsy and risk of delaying definite and curative surgical resection in early pancreatic cancer. Another potential downside is the risk of rare iatrogenic complications, such as post-procedure pancreatitis or theoretical dissemination of tumor cells along the needle tract (and beyond) during CT guided biopsy. In light of these controversies, the decision to perform pre-operative biopsy rests on the discussion between the surgeon and the patient. Most centers in the US favor pre-operative biopsy as a routine. However, several experts, especially from non-US centers, favor proceeding to surgery directly (without pre-operative biopsy) in clearly resectable pancreatic head cancers[102], with an understanding that the presence of unsuspected benign diseases have been reported in 5%-11% of all resected tumors on final pathology results[103-105]. On the flip side, patients who definitely require tissue diagnosis include high risk surgical candidates, non-surgical candidates, patients due to undergo neoadjuvant or palliative chemotherapy. EUS/FNA is the ideal modality for tissue diagnosis in these patients.
Not all patients presenting with pancreatic masses have the classic presentation and supporting radiographic imaging for pancreatic cancer. Two important examples include chronic pancreatitis and autoimmune pancreatitis, where clinical presentation (lack of alarm symptoms) and imaging characteristics favor a non-malignant etiology. In these situations, a pre-operative biopsy is essential to rule out malignancy so that unnecessary surgery can be avoided.
A detailed discussion on all available treatment options is beyond the scope of this article. A brief overview of the treatment options with an emphasis on controversies and recent advancements will be discussed. Patients with pancreatic cancer should ideally be evaluated and treated in a high volume center in a multidisciplinary environment. The actual treatment algorithm depends upon the stage of disease (Tables 1-4) and is generally divided into four subgroups (resectable, borderline resectable, locally advanced and unresectable, and metastatic) (Table 5).
| T | Primary tumor |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ. This includes high-grade pancreatic intraepithelial neoplasia (PanIn-3), intraductal papillary mucinous neoplasm with high-grade dysplasia, intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia |
| T1 | Tumor ≤ 2 cm in greatest dimension |
| T1a | Tumor ≤ 0.5 cm in greatest dimension |
| T1b | Tumor > 0.5 cm and < 1 cm in greatest dimension |
| T1c | Tumor 1–2 cm in greatest dimension |
| T2 | Tumor > 2 cm and ≤ 4 cm in greatest dimension |
| T3 | Tumor > 4 cm in greatest dimension |
| T4 | Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size |
| N | Regional lymph nodes |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastasis in one to three regional lymph nodes |
| N2 | Metastasis in four or more regional lymph nodes |
| M | Distant metastasis |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stages | T | N | M |
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T1, T2, T3 | N1 | M0 |
| Stage III | T1, T2, T3 | N2 | M0 |
| T4 | Any N | M0 | |
| Stage IV | Any T | Any N | M1 |
| Resectability status | Arterial | Venous |
| Resectable | No arterial tumor contact (CA, SMA, or CHA) | No tumor contact with the SMV or PV or ≤ 180° contact without vein contour irregularity |
| Borderline resectable | Pancreatic head/uncinate process: Solid tumor contact with CHA without extension to CA or hepatic artery bifurcation. Solid tumor contact with the SMA of ≤ 180°; Solid tumor contact with variant arterial anatomy (ex: Accessory right hepatic artery, replaced right hepatic artery, replaced CHA, and the origin of replaced or accessory artery). Pancreatic body/tail: Solid tumor contact with the CA of ≤ 180°; Solid tumor contact with the CA of > 180° without involvement of the aorta and with intact and uninvolved gastroduodenal artery thereby permitting a modified Appleby procedure (controversial) | Solid tumor contact with the SMV or PV of > 180°, contact of ≤ 180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction. Solid tumor contact with the IVC |
| Locally advanced | Head/uncinate process: Solid tumor contact with SMA > 180°; Solid tumor contact with the CA > 180°. Pancreatic body/tail: Solid tumor contact of > 180° with the SMA or CA; Solid tumor contact with the CA and aortic involvement | Unreconstructible SMV/PV due to tumor involvement or occlusion (can be due to tumor or bland thrombus) |
Early curative resection of pancreatic cancer offers the best meaningful overall survival. However, only 15%-20% of pancreatic cancers are potentially resectable at presentation. Resectable tumors are the ones in which tumors have no contact with major surrounding arteries (such as celiac artery, superior mesenteric artery, or common hepatic artery) and surrounding veins (superior mesenteric vein or portal vein) (Table 5). Surgery of choice for tumors in the head of pancreas include Whipple surgery (pancreaticoduodenectomy) and distal pancreatectomy for tumors in the body and tail of pancreas. As per all major guidelines, these patients should undergo surgical resection if they are appropriate surgical candidates.
Pancreatic surgery carries a high morbidity and mortality but if done by experienced surgeons in high volume centers, the outcomes are superior[106,107]. Surgery also provides useful diagnostic and prognostic information[108]. Several factors such as tumor stage, status of surgical margins[17,109], lymph node status[110], tumor differentiation[111], pre and post- resection serum CA 19-9[109] and cigarette smoking[112,113] help predict overall prognosis. Five-year survival after pancreaticoduodenectomy is 10% in node positive disease[114] and 30% in node negative disease[115]. More importantly, about two thirds of patients undergoing surgical resection with curative intent will find positive lymph nodes, which correlates with a poor prognosis. Hence, this justification is used by some experts to support the use of neoadjuvant therapy upfront in all resectable tumors.
In contrast to the traditional practice of early resection in resectable tumors, the use of upfront neo-adjuvant therapy in clearly “resectable” pancreatic cancers has increased recently[116]. Some studies have supported its use[105,117-122] and others have largely debunked the idea[123,124]. The proponents of this approach highlight the fact that many such patients may already have micro-metastasis at the time of diagnosis. By providing chemo-radiation upfront, the tumors can be restaged after treatment and surgery can be offered only to the group of patients who still have localized disease. This approach will help decrease the incidence of patients presenting with grossly visible metastasis soon after surgery. Moreover, this approach helps systemic chemotherapy to be started as soon as possible, in contrast to the delay in starting chemotherapy up to 4 wk after surgery (as is routinely advised by the Oncologists). The decision to start upfront neoadjuvant therapy should ideally be made in a multidisciplinary environment in the setting of a clinical trial. There is no consensus on the best therapy for this purpose. In most centers, neoadjuvant therapy is not yet a standard treatment modality in resectable tumors outside of the context of a clinical trial[125]. For most patients with good functional status, the preferred treatment is a multiagent modified FOLFIRINOX regimen (oxaliplatin plus irinotecan with leucovorin and short term infusional flurouracil regimen), followed by chemoradiotherapy.
Another controversial subject in the management of patients with potentially resectable tumors in the head of pancreas presenting with biliary obstruction revolves around the need to perform pre-operative biliary drainage. Several studies have been done to address this question with the results revealing benefit[126], no clear benefit[127-129] or harm with this approach[130-134]. Despite overwhelming data showing potential lack of benefit or even harm in routine preoperative biliary drainage in patients with malignant biliary obstruction, many surgeons in the US routinely request biliary drainage due to perceived better post-operative outcome with this approach. The decision to choose the modality for biliary drainage (percutaneous transhepatic vs endoscopic) rests on the availability of expertise at the respective institution, location of the obstruction etc. Both techniques have advantages and disadvantages and should be used in the right clinical scenario after due consultation in a multidisciplinary environment[135]. Transhepatic biliary drainage is generally performed for more proximal intrahepatic biliary obstruction and endoscopic biliary drainage is performed for extrahepatic biliary obstruction. The type of stent used (plastic vs metal stent) depends upon the endoscopist and the surgeon’s preference. A permanent metal stent is more commonly used as it does not need to be replaced if the tumor is deemed unresectable at the time of surgery[136].
All patients who undergo resection of tumor (without neoadjuvant therapy) should undergo repeat staging of the disease with CT scan and tumor markers before starting adjuvant chemotherapy. Adjuvant chemotherapy should be started within 2 mo of the surgery and should be continued for six months. As in neoadjuvant therapy, for patients with good functional status, the preferred treatment is a multiagent modified FOLFIRINOX regimen (oxaliplatin plus irinotecan with leucovorin and short term infusional flurouracil regimen). For patients with poor functional status, gemcitabine alone or gemcitabine plus capecitabine are reasonable options (Table 6). Addition of radiation therapy in the adjuvant setting is somewhat controversial and is usually reserved in a subgroup of patients with excellent performance status[137].
| Drug | Dose and route | Administration | Toxicity |
| Adjuvant gemcitabine (cycle length: 4 wk)[156] | |||
| Gemcitabine | 1000 mg/m2 IV | Weekly (× 3 wk) followed by one week of rest | Myelotoxicity; Hepatotoxicity; Pulmonary toxicity; Thrombotic microangiopathy |
| Adjuvant gemcitabine plus capecitabine (GemCap; cycle length: 28 d)[157] | |||
| Gemcitabine | 1000 mg/m2 IV | Given on days 1, 8, and 15 | Myelotoxicity; Nonhematologic toxicity (including hepatoxicity); Pulmonary toxicity; Thrombotic microangiopathy |
| Capecitabine | 830 mg/m2 per dose by mouth | Given on days 1 through 21 | |
| Modified FOLFIRINOX (cycle length: 14 d)[158-162] | |||
| Oxaliplatin | 85 mg/m2 IV | Given on day 1 | Myelotoxicity; Diarrhea; Mucositis or hand-foot syndrome; Pulmonary toxicity; Neurotoxicity; Cardiotoxicity |
| Leucovorin | 400 mg/m2 IV | Given on day 1 | |
| Irinotecan | 150 mg/m2 IV | Given on day 1 | |
| Fluorouracil | 2400 mg/m2 IV | Given on day 1 | |
Pancreatic tumors are considered borderline resectable if there is suspected solid tumor contact with major surrounding vasculature (but less than 180 degrees of vascular involvement) on pre-operative imaging. If an obvious direct vascular invasion of > 180 degrees is noted such that a resection is not possible, then it is called locally advanced and unresectable disease (which accounts for approximately 40% of all pancreatic tumors) (Table 5).
There is no universal consensus on how to approach the treatment of these tumors. These patients are discussed in a multidisciplinary tumor board where appropriate treatment strategy is discussed in light of the patient’s functional status, tumor biology (status of genetic mutations), pre-treatment imaging and many other factors.
A reasonable approach in patients with a borderline resectable disease is to attempt at downstaging with chemotherapy/chemoradiation followed by surgical exploration (if no metastatic disease is found on restaging)[137]. For patients with unresectable disease, enrollment in clinical trials using new treatment strategies should be encouraged. Prompt initiation of chemotherapy is warranted. Patients with homologous recombination repair (HRR) mutations and good performance status could benefit from aggressive medical therapy with FOLFIRINOX (short term fluorouracil, plus leucovorin, irinotecan, and oxaliplatin). A detailed discussion on the various treatment regimens and available clinical trials is beyond the scope of this article but a brief summary of the most common treatment regimens is summarized in Table 7.
| Drug | Dose and route | Administration | Toxicity |
| Gemcitabine monotherapy (cycle length: 8 wk for first cycle, then 4 wk)[163-166] | |||
| Gemcitabine | 1000 mg/m2 IV | Weekly (× 7 wk) followed by one week of rest in the first cycle, then weekly (× 3 wk) followed by one week of rest in all subsequent cycles | Myelotoxicity; Hepatoxicity; Pulmonary toxicity; Thrombotic microangiopathy |
| Gemcitabine plus nanoparticle albumin-bound paclitaxel (nabpaclitaxel) (cycle length: 4 wk)[167,168] | |||
| Nabpaclitaxel | 125 mg/m2 IV | Given on days 1, 8, and 15 | Myelotoxicity; Sepsis; Thrombotic microangiopathy; Peripheral neuropathy; Hepatotoxicity; Pulmonary toxicity |
| Gemcitabine | 1000 mg/m2 IV | Given on days 1, 8, and 15 | |
| Gemcitabine plus capecitabine (cycle length: 21 d)[157,169] | |||
| Gemcitabine | 1000 mg/m2 IV | Given on days 1 and 8 | Myelotoxicity; Nonhematologic toxicity (including hepatoxicity); Pulmonary toxicity; Thrombotic microangiopathy |
| Capecitabine | 650 mg/m2 per dose by mouth | Given on days 1 through 14 | |
| Gemcitabine plus cisplatin (cycle length: 21 d)[170] | |||
| Cisplatin | 25 mg/m2 IV daily | Given on days 1 and 8 | Myelotoxicity; Thrombotic microangiopathy; Pulmonary toxicity; Hepatotoxicity; Neurotoxicity; Nephrotoxicity |
| Gemcitabine | 1000 mg/m2 IV daily | Given on days 1 and 8 | |
| FOLFIRINOX (fluorouracil plus leucovorin, irinotecan, and oxaliplatin) (cycle length: 14 d)[160,161] | |||
| Oxaliplatin | 85 mg/m2 IV | Given on day 1 | Myelotoxicity; Diarrhea; Mucositis or hand-foot syndrome; Pulmonary toxicity; Neurotoxicity; Cardiotoxicity |
| Leucovorin | 400 mg/m2 IV | Given on day 1 | |
| Irinotecan | 180 mg/m2 IV | Given on day 1 | |
| Fluorouracil | 400 mg/m2 IV bolus | Given on day 1 | |
| FU | 2400 mg/m2 IV | Given on day 1 | |
| Modified FOLFIRINOX (cycle length: 14 d)[158,159,161] | |||
| Oxaliplatin | 85 mg/m2 IV | Given on day 1 | Myelotoxicity; Diarrhea; Mucositis or hand-foot syndrome; Pulmonary toxicity; Neurotoxicity; Cardiotoxicity |
| Leucovorin | 400 mg/m2 IV | Given on day 1 | |
| Irinotecan | 150 mg/m2 IV | Given on day 1 | |
| Fluorouracil | 2400 mg/m2 IV | Given on day 1 | |
| Modified FOLFOX6 (fluorouracil plus leucovorin and oxaliplatin) (cycle length: 14 d)[160,171,172] | |||
| Oxaliplatin | 85 mg/m2 IV | Given on day 1 | Myelotoxicity; Neurotoxicity; Diarrhea; Cardiopulmonary toxicity |
| Leucovorin | 400 mg/m2 IV | Given on day 1 | |
| Fluorouracil | 400 mg/m2 IV bolus | Given on day 1 | |
| FU | 2400 mg/m2 IV | Given on day 1 | |
| Liposomal irinotecan and fluorouracil (cycle length: 14 d)[173] | |||
| Liposomal irinotecan | 70 mg/m2 IV | Given on day 1 | Myelotoxicity; Diarrhea; Neurotoxicity; Cardiotoxicity |
| Leucovorin | 400 mg/m2 IV | Given on day 1 | |
| Fluorouracil | 2400 mg/m2 IV | Given on day 1 | |
| Pembrolizumab monotherapy for microsatellite-unstable (mismatch repair-deficient) advanced cancer (cycle length: q3 weeks or q6 weeks)[174,175] | |||
| Pembrolizumab | 200 mg IV | Given on day 1, every 3 wk | Pulmonary toxicity; Hepatotoxicity; Neurotoxicity; Dermatologic toxicityCardiotoxicity |
| OR | |||
| Pembrolizumab | 400 mg IV | Given on day 1, every 6 wk | |
Prognosis of metastatic pancreatic cancer is poor with an expected 5-year mortality to be greater than 97%. Hence, it is important to discuss the patient’s preference and goals of care before initiation of treatment. Early involvement of the palliative care team is beneficial. Genetic testing should be performed to determine the presence of HRR deficiency. Genes associated with HRR deficiency include BRCA1/2, PALB2, ATM, BAP1, BARD1, BLM, BRIP1, CHEK2, FAM175A, FANCA, FANCC, NBN, RAD50, RAD51, RAD51C, and RTEL1.
For metastatic disease in the setting of known HRR mutation, a platinum based chemotherapy regimen is preferred[138]. For patients with excellent functional status (ECOG PS 0 or 1) and serum bilirubin < 1.5x upper limit of normal, an aggressive medical therapy with FOLFIRINOX or modified FOLFIRINOX should be considered. Other alternatives included FOLFOX (leucovorin plus infusional fluorouracil plus oxaliplatin, if serum bilirubin is > 1.5), and a combination of gemcitabine plus cisplatin (Table 7).
After 16 wk of chemotherapy, maintenance treatment is considered based upon the results of genetic mutation analysis. For patients with certain genetic mutations such as germline BRCA mutation and PALB2 mutation, maintenance therapy with poly(ADP-ribose) polymerase inhibitor Olaparib is initiated[138,139].
If no HRR mutation is detected in patients with good functional status and low serum bilirubin (< 1.5 × ULN), an aggressive regimen such as FOLFIRINOX should be considered. Other alternatives include modified FOLFIRINOX and gemcitabine plus nonparticle albumin-bound paclitaxel (nabpaclitaxel). However, for patients with higher bilirubin (> 1.5 × ULN), a gemcitabine-based regimen can prove to be toxic and should be avoided; instead, a FOLFOX based regimen should be considered in this setting) (Table 7).
For patients with suboptimal functional status (ECOG PS of 2), monotherapy with gemcitabine or gemcitabine plus capecitabine can be considered. Gemcitabine plus nabpaclitaxel can be toxic and should be reserved for highly selected patients with high tumor burden (Table 7).
For patients with very poor functional status or severe existing co-morbidities, systemic chemotherapy should be considered cautiously. Palliative care should be involved early on with an emphasis on the control of symptoms (such as severe pain).
Symptom palliation in patients with advanced pancreatic cancer is very important and is an integral part of the overall treatment plan[140]. The most common symptoms that require palliation include relief of obstructive jaundice (in tumors of the head of pancreas), duodenal obstruction (from tumor invasion) and severe debilitating pain. Other symptoms include risk of thromboembolism, anxiety/depression, anorexia and weight loss.
Palliative options in patients with malignant obstructive jaundice include biliary stenting and surgical biliary bypass. Randomized trials between the two approaches have shown no difference in survival; patients with stents have less procedure related morbidity and mortality but a higher rate of hospital readmissions from stent occlusion[141-143]. Since the advent of self-expandable metal biliary stents, however, stent occlusion has become less common compared to the traditional plastic biliary stents[143,144]. Biliary stenting can be performed endoscopically or percutaneously (by Interventional Radiology). Endoscopic biliary stenting is preferable as it is associated with much lower complication rates and shorter hospital stays[145-147]. A permanent expandable metal biliary stent can be placed right after obtaining samples for tissue diagnosis (during EUS-FNA), allowing for one-step, efficient and effective care to these patients[148]. As there is no surgery involved, patients can be started on chemotherapy soon afterwards (without waiting for the post-op recovery as is seen in patients undergoing surgical bypass procedure). If endoscopic management is not feasible, external biliary drainage can be attempted. Percutaneous transhepatic biliary access (by Interventional Radiology) results in the placement of a percutaneous internal-external drain which can be replaced by percutaneous metal biliary stent placement in a few weeks[146]. In rare situations, surgical biliary bypass (such as hepaticojejunostomy, choledochojejunostomy or cholecystojejunostomy) may be needed.
Locally advanced pancreatic cancer can infiltrate the wall of duodenum resulting in malignant duodenal obstruction in in approximately 15%-20% of patients[149]. This can be treated by surgical gastrojejunostomy or endoscopic enteral stent placement. Recent data on the utility of endoscopic stent placement has revealed good short-term efficacy, improved cost-effectiveness and shorter recovery time[150]. There are few studies comparing surgical bypass (gastrojejunostomy) to endoscopic stent placement in patients with malignant gastric outlet obstruction[151]. The decision on proceeding with one option vs. the other should be made in light of the patient’s preference, performance status, disease stage, overall health condition and expected life expectancy. Overall, if the life expectancy is short (say 2-3 mo), an endoscopic stent is favored due to prompt relief of symptoms and short duration of recovery. If the expected life expectancy is longer, then surgical bypass is a more reasonable and durable approach due to better long-term results[151].
Cancer-related pain is a very common symptom in patients with advanced pancreatic cancer[152], resulting in decreased performance status and dismal quality of life. Opioid analgesics are most commonly used in managing severe pain associated with pancreatic cancer. Other adjunctive medications include gabapentin, pregabalin, nortriptyline, or duloxetine. In select situations, celiac plexus neurolysis (CPN) can be more effective for immediate and long-term pain relief[152,153]. CPN is preferred over radiation as the onset of action is quicker and long lasting[154,155]. In patients with pain associated with underlying depression and anxiety, antidepressant medications may be beneficial.
The risk of venous thromboembolism is 4-7 folds higher in pancreatic cancer as compared to other common adenocarcinomas. Patient education is key to recognize early signs of thromboembolism. Prophylaxis is recommended with low molecular weight heparin, low dose unfractionated heparin or fondaparinux in high-risk patients such as hospitalized patients with known pancreatic cancer. Lifelong treatment is generally required in patients who develop thromboembolism. Common recommended agents include low molecular weight heparin or a direct oral anticoagulant (e.g., rivaroxaban, apixaban, edoxaban).
Poor appetite and weight loss in patients with advanced pancreatic cancer is common. Unfortunately, this correlates with disease activity and in many situations is a direct biological sequelae of tumor progression. Other factors such as severe depression and severe debilitating pain may contribute to these symptoms as well. Early referral to Nutritionist and/or dietician, dietary supplements and appetite stimulants (such as megestrol acetate) may help in these difficult situations. Patients who exhibit signs of pancreatic insufficiency (such as diarrhea and weight loss) may benefit from oral pancreatic enzyme replacement therapy.
Despite recent advances in the diagnosis and treatment of pancreatic cancer, the survival of pancreatic cancer has not significantly improved. This poor prognosis is mainly due to the aggressive tumor biology of pancreatic adenocarcinoma and its potential for micro metastasis at an early stage of the disease. Early diagnosis and curative resection, when possible, correlates with improved survival but surgery in itself carries a definite morbidity and mortality, even in specialized centers. Neoadjuvant therapy (instead of surgery upfront) in these patients is being offered in some centers but whether this approach consistently translates to better survival is not known. On the other hand, controversies such as the need for routine pre-operative biliary drainage and histological diagnosis before surgery have been addressed by good quality studies, but the results have not translated into clinical practice universally. Nevertheless, there is an overall consensus that while we continue to find the best treatment options, patients with pancreatic cancer should be managed in light of published guidelines at high volume centers in a multi-disciplinary setting.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mu PY S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 2. | Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Dhakal I, Ning B, Kesteloot H. Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969-2002: a comparison with the US population. Eur J Cancer Prev. 2008;17:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI, Abrams RA, Laheru D, Jaffee EM, Hidalgo M, Yeo CJ. Pancreatic cancer. Curr Probl Cancer. 2002;26:176-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (2)] |
| 6. | Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham E, Bamlet WR, Holly EA, Bertuccio P, Gao YT, Hassan M, Yu H, Kurtz RC, Cotterchio M, Su J, Maisonneuve P, Duell EJ, Boffetta P, La Vecchia C. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (2)] |
| 7. | Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, Zheng W, Albanes D, Amundadottir L, Bingham SA, Boffetta P, Boutron-Ruault MC, Chanock SJ, Clipp S, Hoover RN, Jacobs K, Johnson KC, Kooperberg C, Luo J, Messina C, Palli D, Patel AV, Riboli E, Shu XO, Rodriguez Suarez L, Thomas G, Tjønneland A, Tobias GS, Tong E, Trichopoulos D, Virtamo J, Ye W, Yu K, Zeleniuch-Jacquette A, Bueno-de-Mesquita HB, Stolzenberg-Solomon RZ. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 421] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Arem H, Reedy J, Sampson J, Jiao L, Hollenbeck AR, Risch H, Mayne ST, Stolzenberg-Solomon RZ. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst. 2013;105:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Benzel J, Fendrich V. Familial Pancreatic Cancer. Oncol Res Treat. 2018;41:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol. 2013;107:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Pilarski R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am Soc Clin Oncol Educ Book. 2019;39:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, Fontham EH, Maisonneuve P, Bueno-de-Mesquita HB, Ghadirian P, Kurtz RC, Ludwig E, Yu H, Lowenfels AB, Seminara D, Petersen GM, La Vecchia C, Boffetta P. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:2964-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017; 153: 1284-1294. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Singh G, Nassri A, Kim D, Zhu H, Ramzan Z. Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma. World J Gastrointest Pharmacol Ther. 2017;8:60-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, Nguyen NQ, Leong RW, Cosman PH, Kelly MI, Sutherland RL, Henshall SM, Kench JG, Biankin AV. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1122] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 19. | Kim D, Zhu H, Nassri A, Mokdad A, Kukreja S, Polanco P, Huerta S, Ramzan Z. Survival analysis of veteran patients with pancreatic cancer. J Dig Dis. 2016;17:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S, Alguacil J, Corominas JM, Solà R, Salas A, Real FX. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Goodman M, Willmann JK, Jeffrey RB. Incidentally discovered solid pancreatic masses: imaging and clinical observations. Abdom Imaging. 2012;37:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | DiMagno EP, Malagelada JR, Taylor WF, Go VL. A prospective comparison of current diagnostic tests for pancreatic cancer. N Engl J Med. 1977;297:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 114] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Valls C, Andía E, Sanchez A, Fabregat J, Pozuelo O, Quintero JC, Serrano T, Garcia-Borobia F, Jorba R. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, Broemeling LD, Cleary KR, Charnsangavej C. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, Kim SJ, Baek JH, Kim SH, Lee JY, Han JK, Choi BI. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Nino-Murcia M, Jeffrey RB Jr, Beaulieu CF, Li KC, Rubin GD. Multidetector CT of the pancreas and bile duct system: value of curved planar reformations. AJR Am J Roentgenol. 2001;176:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1710] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 30. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology 2014; 146: 291-304. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 32. | Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Lopez Hänninen E, Amthauer H, Hosten N, Ricke J, Böhmig M, Langrehr J, Hintze R, Neuhaus P, Wiedenmann B, Rosewicz S, Felix R. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Megibow AJ, Zhou XH, Rotterdam H, Francis IR, Zerhouni EA, Balfe DM, Weinreb JC, Aisen A, Kuhlman J, Heiken JP. Pancreatic adenocarcinoma: CT vs MR imaging in the evaluation of resectability--report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Irie H, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, Masuda K. Comparison of helical CT and MR imaging in detecting and staging small pancreatic adenocarcinoma. Abdom Imaging. 1997;22:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Sheridan MB, Ward J, Guthrie JA, Spencer JA, Craven CM, Wilson D, Guillou PJ, Robinson PJ. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol. 1999;173:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Holzapfel K, Reiser-Erkan C, Fingerle AA, Erkan M, Eiber MJ, Rummeny EJ, Friess H, Kleeff J, Gaa J. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging. 2011;36:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, Sano K, Araki T. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011;260:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Trede M, Rumstadt B, Wendl K, Gaa J, Tesdal K, Lehmann KJ, Meier-Willersen HJ, Pescatore P, Schmoll J. Ultrafast magnetic resonance imaging improves the staging of pancreatic tumors. Ann Surg. 1997;226:393-405; discussion 405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Balci NC, Semelka RC. Radiologic diagnosis and staging of pancreatic ductal adenocarcinoma. Eur J Radiol. 2001;38:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 352] [Reference Citation Analysis (3)] |
| 42. | Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350-355. [PubMed] |
| 43. | Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501-5503. [PubMed] |
| 45. | Molina V, Visa L, Conill C, Navarro S, Escudero JM, Auge JM, Filella X, Lopez-Boado MA, Ferrer J, Fernandez-Cruz L, Molina R. CA 19-9 in pancreatic cancer: retrospective evaluation of patients with suspicion of pancreatic cancer. Tumour Biol. 2012;33:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 260] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 48. | Maithel SK, Maloney S, Winston C, Gönen M, D'Angelica MI, Dematteo RP, Jarnagin WR, Brennan MF, Allen PJ. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2008;15:3512-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Maisey NR, Norman AR, Hill A, Massey A, Oates J, Cunningham D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer. 2005;93:740-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y, Ohge H, Sueda T. Prognostic impact of perioperative serum CA 19-9 Levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA, Samra JS, Gill AJ, Kench JG, Merrett ND, Das A, Musgrove EA, Sutherland RL, Biankin AV; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Koom WS, Seong J, Kim YB, Pyun HO, Song SY. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Abdel-Misih SR, Hatzaras I, Schmidt C, Saab TB, Klemanski D, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Failure of normalization of CA19-9 following resection for pancreatic cancer is tantamount to metastatic disease. Ann Surg Oncol. 2011;18:1116-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, Konski A, Benson AB 3rd, MacDonald J, Willett CG. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918-5922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 55. | Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 57. | Fornari F, Civardi G, Cavanna L, Di Stasi M, Rossi S, Sbolli G, Buscarini L. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol. 1989;24:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 59. | Chen VK, Arguedas MR, Kilgore ML, Eloubeidi MA. A cost-minimization analysis of alternative strategies in diagnosing pancreatic cancer. Am J Gastroenterol. 2004;99:2223-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 61. | Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, Eikman EA, Malafa M. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA, Nuutila P, Ovaska JT. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 63. | Nishiyama Y, Yamamoto Y, Yokoe K, Monden T, Sasakawa Y, Tsutsui K, Satoh K, Ohkawa M. Contribution of whole body FDG-PET to the detection of distant metastasis in pancreatic cancer. Ann Nucl Med. 2005;19:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Izuishi K, Yamamoto Y, Sano T, Takebayashi R, Masaki T, Suzuki Y. Impact of 18-fluorodeoxyglucose positron emission tomography on the management of pancreatic cancer. J Gastrointest Surg. 2010;14:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, Beger HG, Reske SN. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Wartski M, Sauvanet A. 18F-FDG PET/CT in pancreatic adenocarcinoma: A role at initial imaging staging? Diagn Interv Imaging. 2019;100:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Xing H, Hao Z, Zhu W, Sun D, Ding J, Zhang H, Liu Y, Huo L. Preoperative prediction of pathological grade in pancreatic ductal adenocarcinoma based on 18F-FDG PET/CT radiomics. EJNMMI Res. 2021;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Yeh R, Dercle L, Garg I, Wang ZJ, Hough DM, Goenka AH. The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma. Abdom Radiol (NY). 2018;43:415-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Arnone A, Laudicella R, Caobelli F, Guglielmo P, Spallino M, Abenavoli E, Martini AL, Filice R, Comis AD, Cuzzocrea M, Linguanti F, Evangelista L, Alongi P. Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Lee JW, O JH, Choi M, Choi JY. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Seufferlein T, Bachet JB, Van Cutsem E, Rougier P; ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii33-vii40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 72. | Liu RC, Traverso LW. Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc. 2005;19:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016;7:CD009323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Piccolboni P, Settembre A, Angelini P, Esposito F, Palladino S, Corcione F. Laparoscopic ultrasound: a surgical "must" for second line intra-operative evaluation of pancreatic cancer resectability. G Chir. 2015;36:5-8. [PubMed] |
| 76. | de Werra C, Quarto G, Aloia S, Perrotta S, Del Giudice R, Di Filippo G, Furino E, Amato B, Benassai G. The use of intraoperative ultrasound for diagnosis and stadiation in pancreatic head neoformations. Int J Surg. 2015;21 Suppl 1:S55-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Cirimbei S, Puşcu C, Lucenco L, Brătucu E. The role of intraoperative ultrasound in establishing the surgical strategy regarding hepato-bilio-pancreatic pathology. Chirurgia (Bucur). 2013;108:643-651. [PubMed] |
| 78. | Doran HE, Bosonnet L, Connor S, Jones L, Garvey C, Hughes M, Campbell F, Hartley M, Ghaneh P, Neoptolemos JP, Sutton R. Laparoscopy and laparoscopic ultrasound in the evaluation of pancreatic and periampullary tumours. Dig Surg. 2004;21:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Minnard EA, Conlon KC, Hoos A, Dougherty EC, Hann LE, Brennan MF. Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg. 1998;228:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Yelamali A, Mansard MJ, Dama R, Rebela P, Rao GV, Reddy DN. Intraoperative pancreatoscopy with narrow band imaging: a novel method for assessment of resection margins in case of intraductal papillary mucinous neoplasm. Surg Endosc. 2012;26:3682-3685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Giovannini M, Bories E, Monges G, Pesenti C, Caillol F, Delpero JR. Results of a phase I-II study on intraductal confocal microscopy (IDCM) in patients with common bile duct (CBD) stenosis. Surg Endosc. 2011;25:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 83. | Regunathan R, Woo J, Pierce MC, Polydorides AD, Raoufi M, Roayaie S, Schwartz M, Labow D, Shin D, Suzuki R, Bhutani MS, Coghlan LG, Richards-Kortum R, Anandasabapathy S, Kim MK. Feasibility and preliminary accuracy of high-resolution imaging of the liver and pancreas using FNA compatible microendoscopy (with video). Gastrointest Endosc. 2012;76:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Testoni PA, Mariani A, Mangiavillano B, Arcidiacono PG, Di Pietro S, Masci E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am J Gastroenterol. 2007;102:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Hwang JH, Cobb MJ, Kimmey MB, Li X. Optical coherence tomography imaging of the pancreas: a needle-based approach. Clin Gastroenterol Hepatol. 2005;3:S49-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Testoni PA, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J Gastroenterol. 2008;14:6444-6452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Cheon YK, Cho YD, Jeon SR, Moon JH, Jeong SW, Hur KY, Jin SY, Lee JS. Pancreatic resection guided by preoperative intraductal ultrasonography for intraductal papillary mucinous neoplasm. Am J Gastroenterol. 2010;105:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Varadarajulu S, Eloubeidi MA, Wilcox CM. Prospective evaluation of indeterminate ERCP findings by intraductal ultrasound. J Gastroenterol Hepatol. 2007;22:2086-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 90. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 91. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 92. | Kumon RE, Repaka A, Atkinson M, Faulx AL, Wong RC, Isenberg GA, Hsiao YS, Gudur MS, Deng CX, Chak A. Characterization of the pancreas in vivo using EUS spectrum analysis with electronic array echoendoscopes. Gastrointest Endosc. 2012;75:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Cruz-Monserrate Z, Abd-Elgaliel WR, Grote T, Deng D, Ji B, Arumugam T, Wang H, Tung CH, Logsdon CD. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut. 2012;61:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Hausner SH, Abbey CK, Bold RJ, Gagnon MK, Marik J, Marshall JF, Stanecki CE, Sutcliffe JL. Targeted in vivo imaging of integrin alphavbeta6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer Res. 2009;69:5843-5850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 95. | Neesse A, Hahnenkamp A, Griesmann H, Buchholz M, Hahn SA, Maghnouj A, Fendrich V, Ring J, Sipos B, Tuveson DA, Bremer C, Gress TM, Michl P. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut. 2013;62:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | Kojima T, Kyuno D, Sawada N. Targeting claudin-4 in human pancreatic cancer. Expert Opin Ther Targets. 2012;16:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Stella Man YK, Foster J, Carapuça E, Davies JA, Parker AL, Sosabowski J, Halldén G. Systemic delivery and SPECT/CT in vivo imaging of 125I-labelled oncolytic adenoviral mutants in models of pancreatic cancer. Sci Rep. 2019;9:12840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Nattress CB, Halldén G. Advances in oncolytic adenovirus therapy for pancreatic cancer. Cancer Lett. 2018;434:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Man YKS, Davies JA, Coughlan L, Pantelidou C, Blázquez-Moreno A, Marshall JF, Parker AL, Halldén G. The Novel Oncolytic Adenoviral Mutant Ad5-3Δ-A20T Retargeted to αvβ6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol Cancer Ther. 2018;17:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjug Chem. 2006;17:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 102. | Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 103. | van Heerde MJ, Biermann K, Zondervan PE, Kazemier G, van Eijck CH, Pek C, Kuipers EJ, van Buuren HR. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig Dis Sci. 2012;57:2458-2465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | de la Fuente SG, Ceppa EP, Reddy SK, Clary BM, Tyler DS, Pappas TN. Incidence of benign disease in patients that underwent resection for presumed pancreatic cancer diagnosed by endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA). J Gastrointest Surg. 2010;14:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Gerritsen A, Molenaar IQ, Bollen TL, Nio CY, Dijkgraaf MG, van Santvoort HC, Offerhaus GJ, Brosens LA, Biermann K, Sieders E, de Jong KP, van Dam RM, van der Harst E, van Goor H, van Ramshorst B, Bonsing BA, de Hingh IH, Gerhards MF, van Eijck CH, Gouma DJ, Borel Rinkes IH, Busch OR, Besselink MG; Dutch Pancreatic Cancer Group. Preoperative characteristics of patients with presumed pancreatic cancer but ultimately benign disease: a multicenter series of 344 pancreatoduodenectomies. Ann Surg Oncol. 2014;21:3999-4006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-57; discussion 257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1388] [Article Influence: 49.6] [Reference Citation Analysis (34)] |
| 107. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (34)] |
| 108. | Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, Hirose K, Schulick RD, Choti MA, Wolfgang CL, Pawlik TM. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 109. | Kinsella TJ, Seo Y, Willis J, Stellato TA, Siegel CT, Harpp D, Willson JK, Gibbons J, Sanabria JR, Hardacre JM, Schulak JP. The impact of resection margin status and postoperative CA19-9 Levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008;31:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Helm J, Centeno BA, Coppola D, Melis M, Lloyd M, Park JY, Chen DT, Malafa MP. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115:4080-4089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Meyer W, Jurowich C, Reichel M, Steinhäuser B, Wünsch PH, Gebhardt C. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today. 2000;30:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Yuan C, Morales-Oyarvide V, Babic A, Clish CB, Kraft P, Bao Y, Qian ZR, Rubinson DA, Ng K, Giovannucci EL, Ogino S, Stampfer MJ, Gaziano JM, Sesso HD, Cochrane BB, Manson JE, Fuchs CS, Wolpin BM. Cigarette Smoking and Pancreatic Cancer Survival. J Clin Oncol. 2017;35:1822-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 113. | Pelucchi C, Galeone C, Polesel J, Manzari M, Zucchetto A, Talamini R, Franceschi S, Negri E, La Vecchia C. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 114. | Kang MJ, Jang JY, Chang YR, Kwon W, Jung W, Kim SW. Revisiting the concept of lymph node metastases of pancreatic head cancer: number of metastatic lymph nodes and lymph node ratio according to N stage. Ann Surg Oncol. 2014;21:1545-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 115. | Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, Weiss MJ, Hruban RH, Gönen M, Klimstra DS, Mino-Kenudson M. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 2017;265:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 116. | Ellis RJ, Ho JW, Schlick CJR, Merkow RP, Bentrem DJ, Bilimoria KY, Yang AD. National Use of Chemotherapy in Initial Management of Stage I Pancreatic Cancer and Failure to Perform Subsequent Resection. Ann Surg Oncol. 2020;27:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Hu Q, Wang D, Chen Y, Li X, Cao P, Cao D. Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma. Radiat Oncol. 2019;14:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 119. | de Geus SW, Eskander MF, Bliss LA, Kasumova GG, Ng SC, Callery MP, Tseng JF. Neoadjuvant therapy vs upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery. 2017;161:592-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 120. | Fathi A, Christians KK, George B, Ritch PS, Erickson BA, Tolat P, Johnston FM, Evans DB, Tsai S. Neoadjuvant therapy for localized pancreatic cancer: guiding principles. J Gastrointest Oncol. 2015;6:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 121. | Lee AJ, Simoneau E, Chiang YJ, Lee JE, Kim MP, Aloia TA, Vauthey JN, Katz MH, Tzeng CD. Is early-stage pancreatic adenocarcinoma truly early: stage migration on final pathology with surgery-first vs neoadjuvant therapy sequencing. HPB (Oxford). 2019;21:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Rangarajan K, Pucher PH, Armstrong T, Bateman A, Hamady Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis. Ann R Coll Surg Engl. 2019;101:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 123. | Zhan HX, Xu JW, Wu D, Wu ZY, Wang L, Hu SY, Zhang GY. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 124. | Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MH. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 125. | Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant Treatment in Pancreatic Cancer. Front Oncol. 2020;10:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 126. | Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985;97:641-648. [PubMed] |
| 127. | Eshuis WJ, van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, Kuipers EJ, Coene PP, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 128. | Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries-Jones R, Kernoff L, Marks IN. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 263] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 129. | Mumtaz K, Hamid S, Jafri W. Endoscopic retrograde cholangiopancreaticography with or without stenting in patients with pancreaticobiliary malignancy, prior to surgery. Cochrane Database Syst Rev. 2007;CD006001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 697] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 131. | Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, Wang C. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012;CD005444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, Wang C. Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br J Surg. 2013;100:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 134. | Scheufele F, Schorn S, Demir IE, Sargut M, Tieftrunk E, Calavrezos L, Jäger C, Friess H, Ceyhan GO. Preoperative biliary stenting vs operation first in jaundiced patients due to malignant lesions in the pancreatic head: A meta-analysis of current literature. Surgery. 2017;161:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 135. | Rizzo A, Ricci AD, Frega G, Palloni A, DE Lorenzo S, Abbati F, Mollica V, Tavolari S, DI Marco M, Brandi G. How to Choose Between Percutaneous Transhepatic and Endoscopic Biliary Drainage in Malignant Obstructive Jaundice: An Updated Systematic Review and Meta-analysis. In Vivo. 2020;34:1701-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 136. | Singal AK, Ross WA, Guturu P, Varadhachary GR, Javle M, Jaganmohan SR, Raju RP, Fleming JB, Raju GS, Kuo YF, Lee JH. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: single-center experience with 79 cases. Dig Dis Sci. 2011;56:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 137. | Cellini F, Arcelli A, Simoni N, Caravatta L, Buwenge M, Calabrese A, Brunetti O, Genovesi D, Mazzarotto R, Deodato F, Mattiucci GC, Silvestris N, Valentini V, Morganti AG. Basics and Frontiers on Pancreatic Cancer for Radiation Oncology: Target Delineation, SBRT, SIB technique, MRgRT, Particle Therapy, Immunotherapy and Clinical Guidelines. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 138. | Hammel P, Kindler HL, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Joo S, Yoo HK, Patel N, Golan T; POLO Investigators. Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Ann Oncol. 2019;30:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1626] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 140. | House MG, Choti MA. Palliative therapy for pancreatic/biliary cancer. Surg Clin North Am. 2005;85:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 141. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting vs surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 554] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 142. | Andersen JR, Sørensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis vs operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 393] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 143. | Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 144. | Levy MJ, Baron TH, Gostout CJ, Petersen BT, Farnell MB. Palliation of malignant extrahepatic biliary obstruction with plastic vs expandable metal stents: An evidence-based approach. Clin Gastroenterol Hepatol. 2004;2:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 145. | Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006;CD004200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic vs percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 451] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 147. | Inamdar S, Slattery E, Bhalla R, Sejpal DV, Trindade AJ. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 148. | Chen VK, Arguedas MR, Baron TH. Expandable metal biliary stents before pancreaticoduodenectomy for pancreatic cancer: a Monte-Carlo decision analysis. Clin Gastroenterol Hepatol. 2005;3:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Singh SM, Longmire WP Jr, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg. 1990;212:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 155] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 150. | Yim HB, Jacobson BC, Saltzman JR, Johannes RS, Bounds BC, Lee JH, Shields SJ, Ruymann FW, Van Dam J, Carr-Locke DL. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc. 2001;53:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (2)] |
| 152. | Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 153. | Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cancer pain-time effect: a prospective, randomized multicenter study. J Pain Symptom Manage 2014; 48: 944-56. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 154. | Ceha HM, van Tienhoven G, Gouma DJ, Veenhof CH, Schneider CJ, Rauws EA, Phoa SS, González González D. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer. 2000;89:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 155. | Morganti AG, Trodella L, Valentini V, Barbi S, Macchia G, Mantini G, Turriziani A, Cellini N. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care. 2003;19:258-262. [PubMed] |
| 156. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 157. | Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 158. | Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, Sata N, Miyashita K, Mizuno N, Tsuji K, Okusaka T, Furuse J. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 159. | Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Pant S, Shah MA, Sahai V, Uronis HE, Zaidi N, Laheru D. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020;JCO2001364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 160. | Cercek A, Park V, Yaeger R, Reidy-Lagunes D, Kemeny NE, Stadler ZK, Segal NH, Varghese A, Saltz LB. Faster FOLFOX: Oxaliplatin Can Be Safely Infused at a Rate of 1 mg/m2/min. J Oncol Pract. 2016;12:e548-e553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 161. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 162. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1943] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 163. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4177] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 164. | Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M, Kindler HL. A phase III trial of pemetrexed plus gemcitabine vs gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 165. | Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J, Androulakis N, Kotsakis A, Samonis G, Georgoulias V. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 166. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Köhne CH, Mingrone W, Stemmer SM, Tàmas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W; Swiss Group for Clinical Cancer Research; Central European Cooperative Oncology Group. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 167. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4883] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 168. | Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, Munshi HG, Benson AB 3rd, O'Dwyer PJ. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 169. | Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 170. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3166] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 171. | Cheeseman SL, Joel SP, Chester JD, Wilson G, Dent JT, Richards FJ, Seymour MT. A 'modified de Gramont' regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87:393-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 172. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2200] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 173. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 829] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 174. | Pantuck M, McDermott D, Drakaki A. To treat or not to treat: Patient exclusion in immune oncology clinical trials due to preexisting autoimmune disease. Cancer. 2019;125:3506-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 175. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2587] [Article Influence: 369.6] [Reference Citation Analysis (0)] |