Published online Feb 15, 2021. doi: 10.4251/wjgo.v13.i2.147
Peer-review started: November 6, 2020
First decision: November 30, 2020
Revised: December 11, 2020
Accepted: December 26, 2020
Article in press: December 26, 2020
Published online: February 15, 2021
Processing time: 86 Days and 20.9 Hours
Breast cancer is the most common tumor in women, and about one-third of cases develop metastatic disease. However, metastatic breast cancer rarely invades the common bile duct (CBD) directly without involving the liver, and involvement of the gastrointestinal tract is rare. Cases of such metastases pose a particular diagnostic challenge.
A 55-year-old female presented to the Department of Gastroenterology with complaint of a 2 mo history of right upper abdominal pain accompanied by pain in the right back, aggravated after eating greasy diet. The patient had received a diagnosis of breast cancer 3 years prior. Physical examination showed obvious superficial protuberant erythema on the left neck and chest skin, with slight tenderness and burning sensation. Endoscopic retrograde cholangiopancre-atography showed an obstruction at the end of the CBD. Histopathology of the CBD and symptomatic skin biopsies showed positivity for cytokeratin 7 and trans-acting T-cell-specific transcription factor breast cancer biomarkers. A cancer embolus was also found in the skin vasculature. Accordingly, the diagnosis of breast cancer metastases to the skin and biliary ducts was made. A plastic biliary sent was placed, which relieved the right upper abdominal pain and protected against unnecessary hepatectomy surgery.
Although rare, biliary metastasis should be considered in patients with bile duct stenosis and a history of breast cancer.
Core Tip: Breast cancer is the most common tumor in women, and about one-third of cases develop metastatic disease. However, metastatic breast cancer rarely invades the common bile duct (CBD) directly without involving the liver, and involvement of the gastrointestinal tract is rare. We report a case of CBD metastasis from breast cancer that was diagnosed according to findings from endoscopic retrograde cholangiopancre-atography imaging and histopathology of CBD and symptomatic skin biopsies. This case highlights the importance of making an accurate diagnosis before undertaking an operative approach. Although CBD metastasis of breast cancer is rare, it should not be completely ignored.
- Citation: Tang J, Zhao GX, Deng SS, Xu M. Rare common bile duct metastasis of breast cancer: A case report and literature review . World J Gastrointest Oncol 2021; 13(2): 147-156
- URL: https://www.wjgnet.com/1948-5204/full/v13/i2/147.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i2.147
Breast cancer is the most common tumor in women[1] and about one-third of cases will progress to metastatic disease[2], which is the leading cause of cancer-related death[3]. Only 5%-10% of newly diagnosed breast cancer patients present with distant metastasis; more troubling, the risk of developing metastatic disease is high among patients with localized primary disease following successful primary tumor resection and adjuvant therapy[4-6]. It is estimated that up to 30% of node-negative breast cancer patients will develop metastatic disease, despite receiving standard treatment[4-6].
Breast cancer metastasis includes contiguous, lymphatic, and hematogenous forms of spread[7]. While hematogenous spread of breast cancer can target any site in the human body, the most common sites are bone, lung, lymph nodes, liver, and brain[7]. Metastasis to the digestive system, kidney, and retroperitoneal organs is rare, and to the biliary system is remarkably rare[8-10]. Widespread liver metastases can compress or infiltrate the bile duct, which may lead to obstructive jaundice, while a direct metastatic involvement of the extrahepatic bile duct in the absence of hepatic lesions is exceptional[8-10].
We describe, herein, a special case of secondary tumor of the common bile duct (CBD) from a primary breast cancer tumor that had been treated by operation 3 years earlier. Our review of the literature highlighted that this diagnosis may be difficult and controversial in rare sites. For early diagnosis, endoscopy and detailed pathological analysis are necessary, which may help prevent unnecessary surgical intervention.
A 55-year-old female presented to the Department of Gastroenterology (Shanghai East Hospital, Shanghai, China) with complaint of right upper abdominal pain lasting over a 2 mo period that was accompanied by pain in the right back and aggravated after eating greasy diet.
The patient’s symptoms started 2 mo prior, with recurrent episodes of right upper abdominal pain.
The patient had been diagnosed with breast cancer 3 years prior but had an unremarkable medical history otherwise.
Physical examination revealed mild tenderness in the right upper quadrant of the abdomen. Superficial protuberant erythema with clear margins were observed on the left neck and chest; the patient indicated a burning sensation and slight tenderness associated with the erythema.
Laboratory tests upon admission showed elevated gamma-glutamyl transpeptidase (238 IU/L; normal range: 10-60 IU/L) but normal level of alkaline phosphatase (67 IU/L) and negative findings for inflammatory biomarkers. All blood tumor markers (alpha-fetoprotein: 2.01 ng/mL; carcinoembryonic antigen: 1.71 ng/mL; cancer antigen-125: 12.1 U/mL; cancer antigen-199: 9.26 U/mL; and cancer antigen-135: 14.3 U/mL) were within normal ranges.
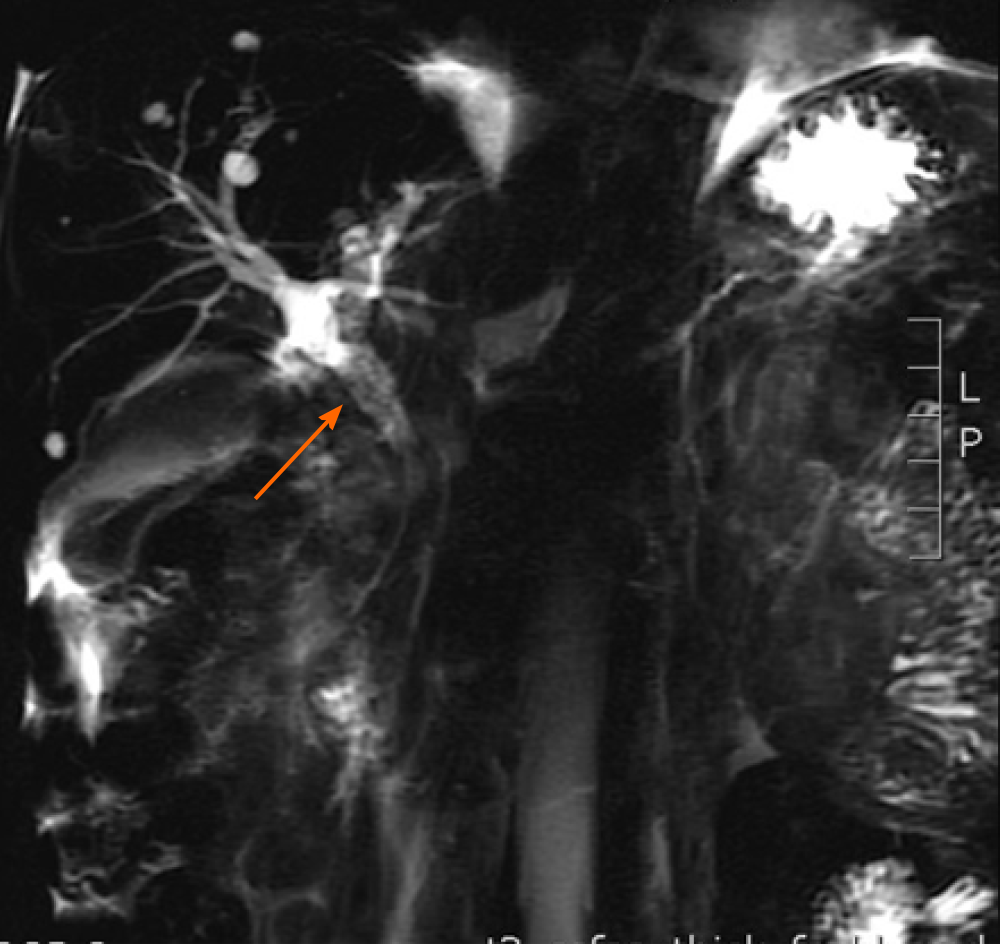
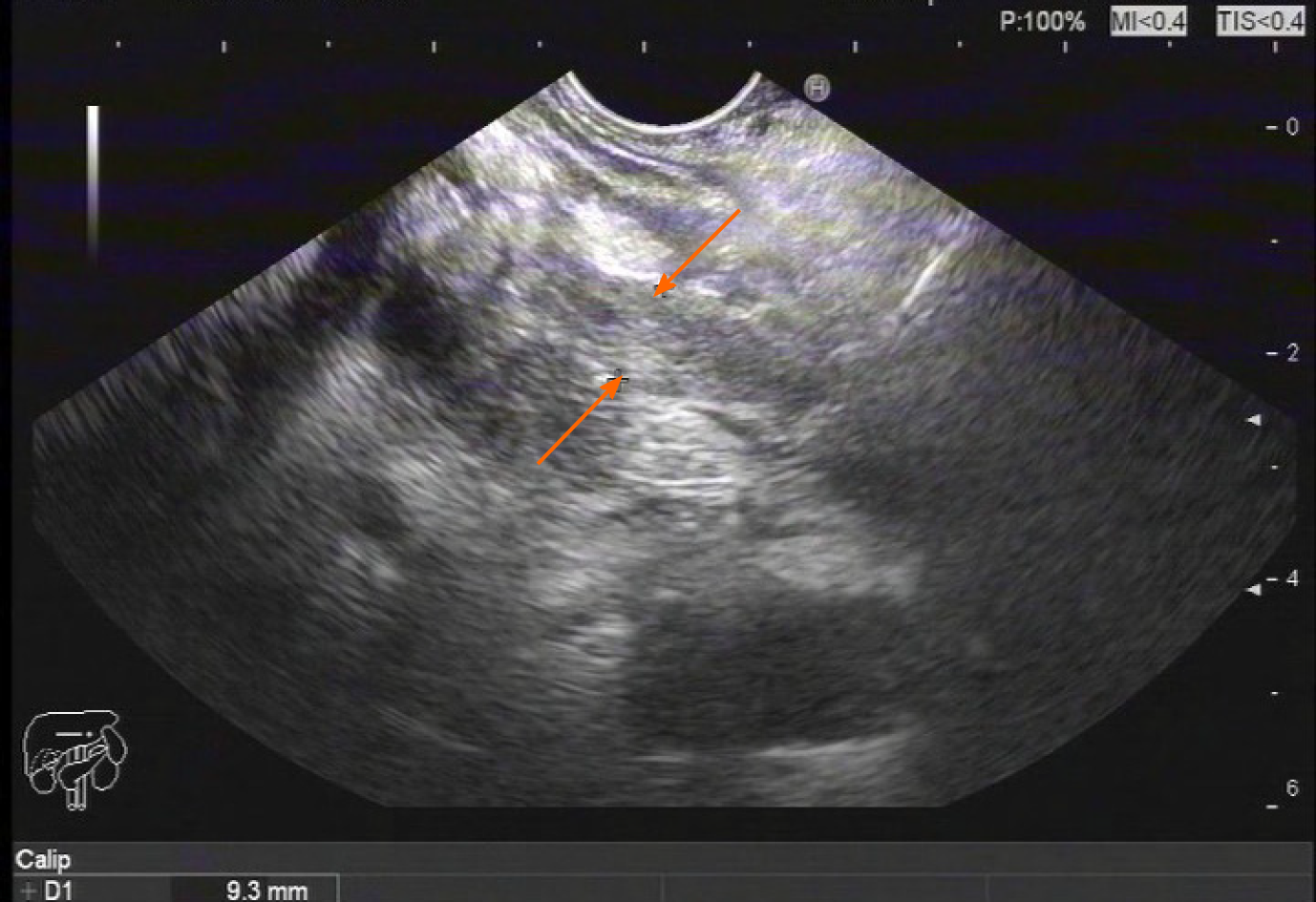
Magnetic resonance cholangiopancreatography revealed enlarged head of the pancreas, narrowed CBD within the pancreas head, and slightly dilated upper bile ducts (Figure 1). Ultrasound gastroscopy revealed changes indicative of chronic pancreatitis, widening of the bile duct wall, and immunoglobulin G (IgG)4-related cholangitis (Figure 2). Subsequent laboratory tests, however, showed the IgG-4 level to be within the normal range, excluding the possibility of IgG4-related cholangitis.
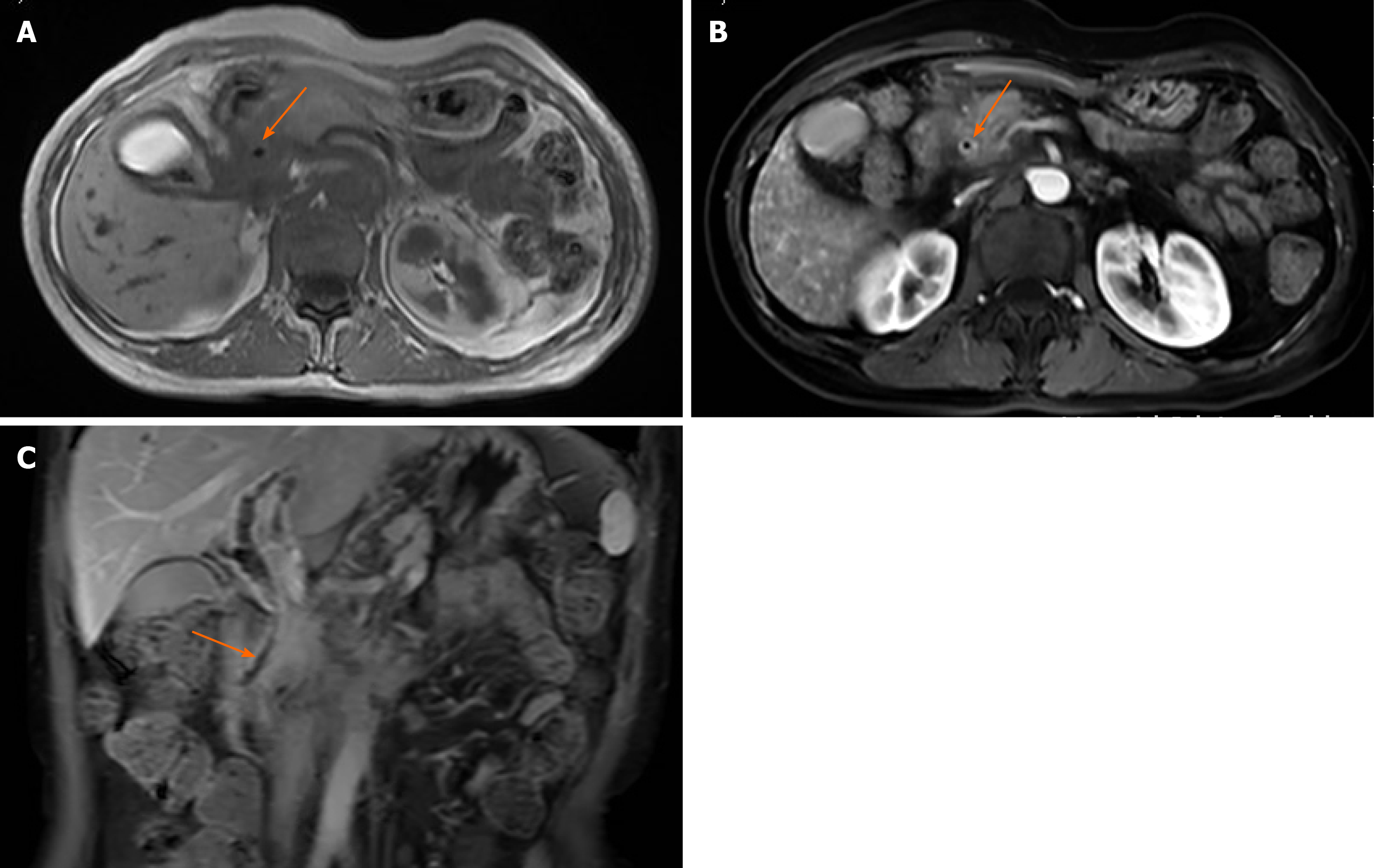
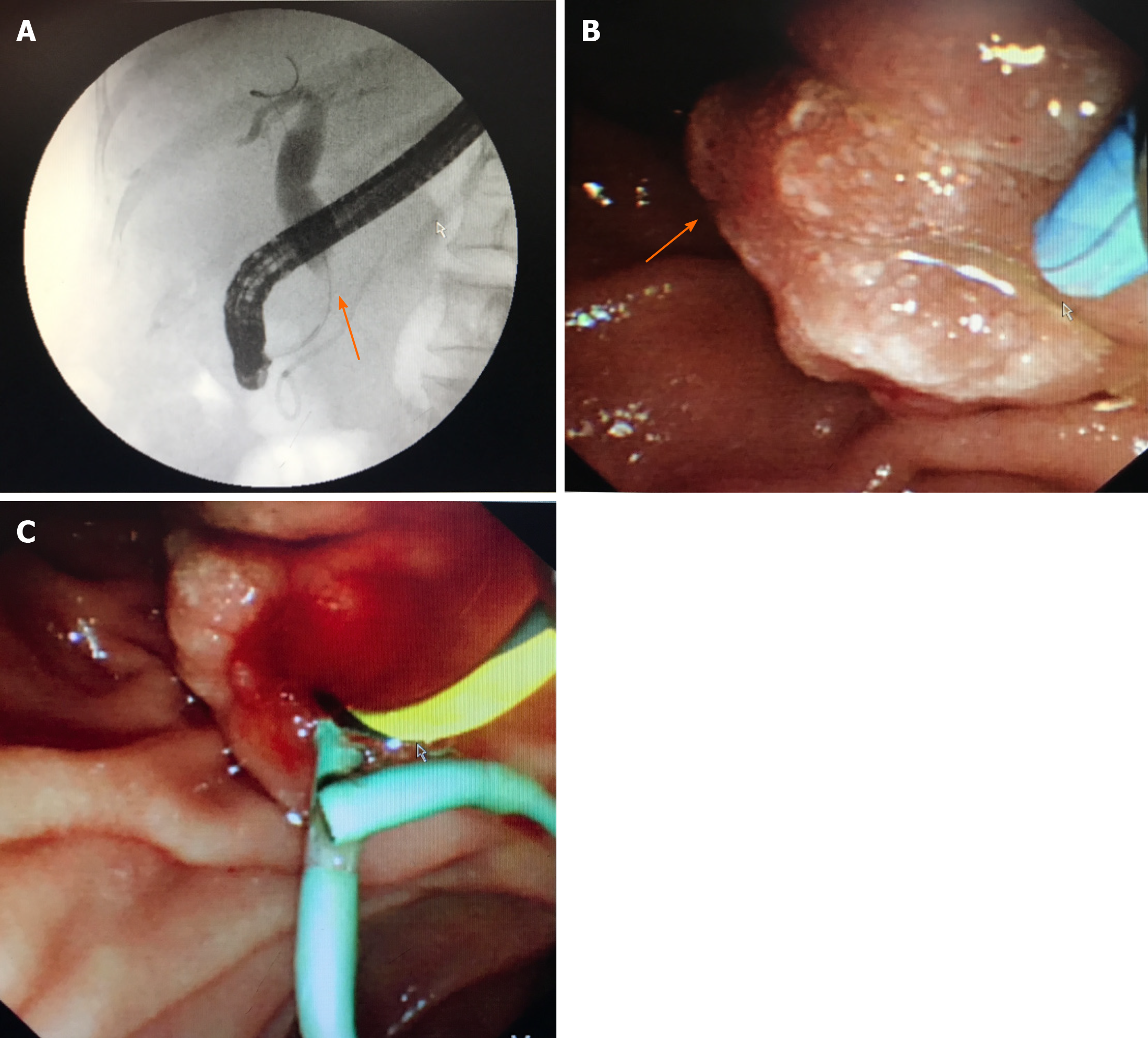
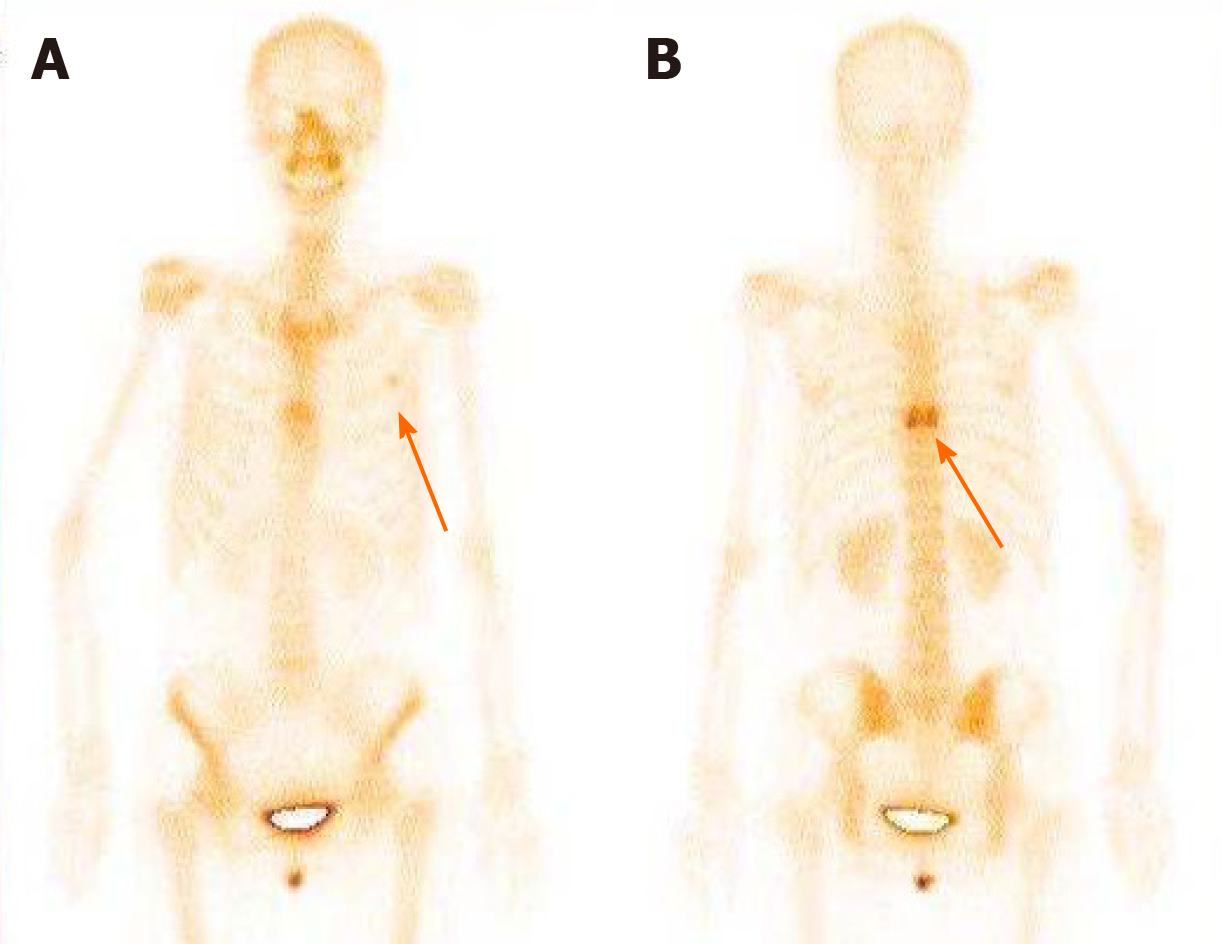
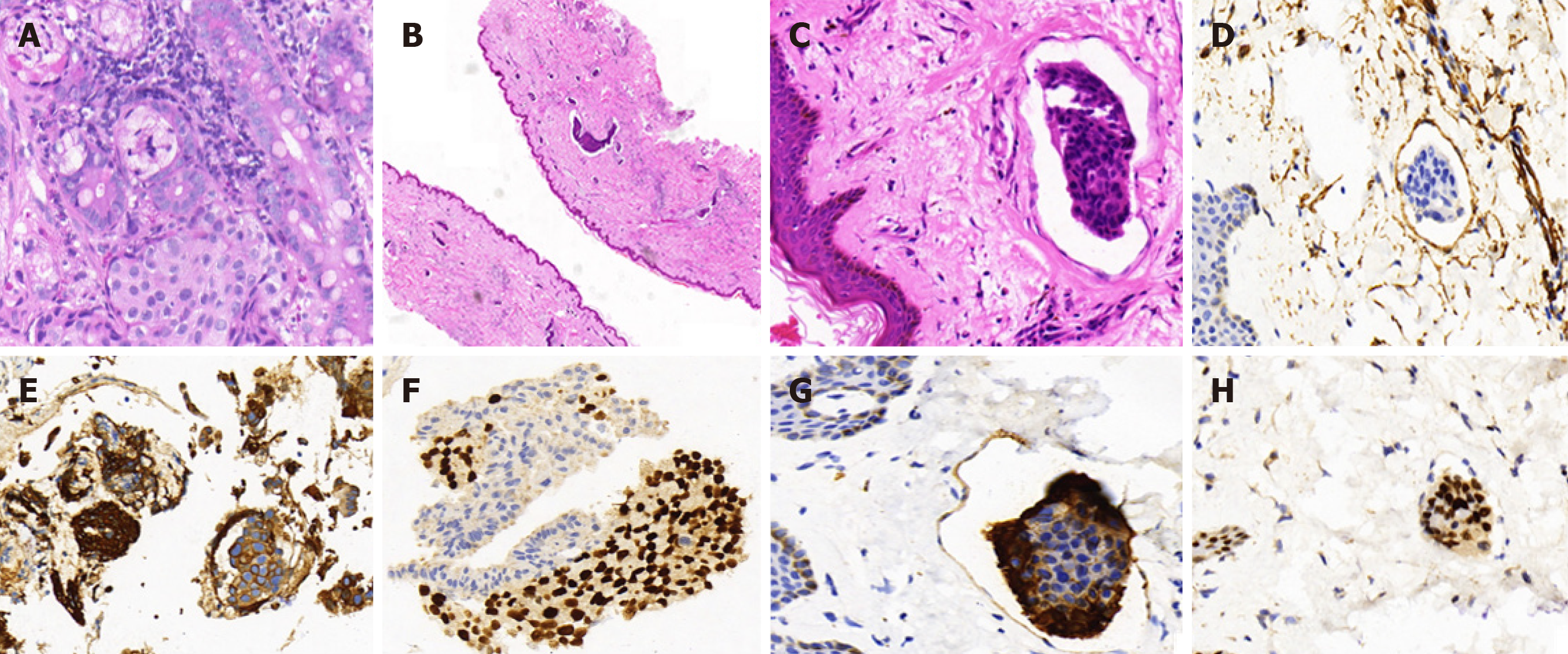
Abdominal enhanced magnetic resonance imaging showed that the wall of the CBD was thickened and became obviously enhanced with contrast agent. The coronal view showed dilation of the upper segment of the CBD, with rough tissue wall and narrowing of the lower segment. Nodular thickening was also seen (localized) in the lower segment of the CBD (Figure 3). Endoscopic retrograde cholangiopancre-atography (ERCP) showed an obstruction at the end of the CBD. Biopsies were taken from the affected tissues (CBD end and symptomatic skin lesions; Figure 4). Bone scan showed metastases in the left third rib and the eighth vertebral body (Figure 5).
Histopathology results for the biopsied CBD and skin of left neck and chest were: Cytokeratin 7 (+); trans-acting T-cell-specific transcription factor (+); estrogen receptor (ER) (-); progesterone receptor (PR) (-); and human epidermal growth factor receptor-2 (HER2) (-). Morphological characteristics were assessed, and a cancer embolus was found in the skin vasculature (Figure 6).
Metastases of breast cancer to the skin and biliary ducts.
After undergoing ERCP placement of a plastic stent for systemic chemotherapy, the patient was referred to an oncologist who ordered a treatment course of gemcitabine (1000 mg, administered on day 1 and day 8) and cisplatin (30 mg, administered on day 1 to day 3).
The patient eventually died of multiple organ failure caused by severe infection after systemic chemotherapy.
Invasive breast carcinoma of no special type is the most common histological type of breast cancer, accounting for 75%-80% of all invasive breast cancer cases. Gastrointestinal tract involvement is rare, accounting for only about 10% of all cases, but usually occurs in invasive lobular carcinoma[11]. Through literature review, we found reports of 30 cases of metastatic breast cancer involving the bile ducts, including 7 cases involving CBD[11-16], 20 cases involving extrahepatic duct[9,17-21] or lymph nodes extending directly into bile ducts[12,22] and ampulla of Vater, and 3 cases classified as uncertain[23] (Table 1). In these cases, the time interval between the diagnosis of breast cancer and the secondary tumor was as long as 21 years and as short as 2 years. Preoperative diagnosis was achieved in only 4 cases, and the others were treated surgically. Rego et al[15] reported on 2 patients with abnormal ampulla and distal CBD stenosis detected by ERCP, with a diagnosis of breast metastasis confirmed by immunohistochemistry before surgical treatment. Budimir et al[11]reported on another case in which ERCP detected CBD stenosis, after which brush aspiration was performed for subsequent cytological analysis. Finally, Cochrane et al[16] reported on a case in which endoscopic ultrasound revealed a mass in the mid-portion of the CBD, with fine-needle aspiration biopsy demonstrating metastatic breast cancer.
| Ref. | Cases, n | Location of secondary tumor | Therapy | Time interval between diagnosis of primary tumor and metastasis |
| Popp et al[12], 1979 | 1 | CBD | Surgical bypass plus chemotherapy | Average 40 mo |
| Titus et al[13], 1997 | 1 | Distal bile duct | Pancreaticoduodenectomy | Undetermined |
| Stoeckler et al[14], 2007 | 1 | Distal bile duct | Pancreaticoduodenectomy | Undetermined |
| Rego et al[15], 2009 | 2 | Ampulla of Vater and CBD | Surgery and palliative chemotherapy | 2 yr |
| Cochrane et al[16], 2015 | 1 | CBD | Aplastic stent placed, chemotherapy and endocrine therapy | 21 yr |
| Budimir et al[11], 2015 | 1 | CBD | Metal stent implantation and aromatase inhibitors | 6 yr |
| Popp et al[12], 1979 | 6 | Extrahepatic lymph nodes | Radical and palliative surgery, transhepatic drainage, radiation, chemotherapy | Average 40 mo |
| Kopelson et al[17], 1980 | 6 | Extrahepatic duct | Radical and palliative surgery, radiation, chemotherapy | Undetermined |
| Engel et al[22], 1980 | 2 | Extrahepatic duct, lymph nodes | Biliary tract resection and choledochojejunostomy | Undetermined |
| Franco et al[9], 1987 | 2 | Proximal bile duct, bifurcation | Bile duct resection, double choledochojejunostomy | 6 and 8 yr respectively |
| Pappo et al[18], 1991 | 1 | Extrahepatic, intra and extraluminal | Bile duct resection, choledochojejunostomy, cholecystectomy | 2 yr |
| Feliu Villaró et al[19], 1995 | 1 | Extrahepatic intraluminal | Undetermined | Undetermined |
| Papo et al[20], 1996 | 1 | Extrahepatic intraluminal | Biliary tract resection and choledochojejunostomy | Undetermined |
| Coletta et al[21], 2014 | 1 | Extrahepatic bile ducts | Cholecystectomy, extrahepatic biliary resection, and double hepaticojejunostomy | 13 yr |
| Rabin et al[23], 1979 | 3 | Undetermined | Undetermined | Undetermined |
Patients with metastatic breast cancer involving the bile ducts may experience abdominal discomfort, upper gastrointestinal bleeding, jaundice and related symptoms, such as pruritus or alterations in stool and urine[24]; these nonspecific clinical symptoms, being similar to the presentation of primary tumors, complicate the diagnostic process. Moreover, in most cases, there is a long interval between the diagnosis of the primary tumor and the development of metastatic tumors affecting biliary tract function, which poses a further challenge to suspicion of a relationship between the historical breast cancer event and the presenting biliary disease[13,15,25,26]. Endoscopic diagnosis may be difficult, due to implantation of the metastatic cells in the submucosa. Coletta et al[21] reported that breast cancer metastasis could target the extrahepatic bile duct without involvement of the lumen or duct mucosa. ERCP sensitivity ranges from 25% to 50% for masses near the hepatic hilum, and negative finding for biopsied tissues is estimated to occur in > 30% of cases[24]. Thus, the combination of ultrasound-guided biopsy and immunohistochemical analysis plays a key role in the diagnosis of this rare type of secondary tumor.
Early identification and surgical resection of cholangiocarcinoma have been shown to improve the 5-year overall survival[2]. In the case of primary malignant bile duct tumors, clinicians are likely to recommend surgery, but for patients with metastatic breast cancer, surgery is generally not considered clinically beneficial[27]. Treatments for metastatic breast cancer are guided by multiple factors, most importantly the expression status of ER, PR and HER2, treatment history, and prognostic indicators (i.e. short disease-free interval, presence of visceral metastases, performance status, and degree of symptoms)[28]. For example, patients with hormone receptor-positive status are expected to benefit from hormone therapy, whereas those with HER2-positive disease are treated with targeted agents (trastuzumab and lapatinib) in conjunction with chemotherapy. In contrast, treatments of patients with triple-negative breast cancer only depend on cytotoxic chemotherapy[28].
Discordant ER, PR, or HER2 expression status between primary tumors and metastases has been reported[29]. In addition, the conversion to negative receptor status is, on average, higher than that of positive conversion (24% vs 14% for ER; 46% vs 15% for PR; 13% vs 5% for HER2)[30]. Two explanations have been put forth for this observation. The first involves technical issues that result in poor reproducibility of immunohistochemistry techniques. The second involves tumor heterogeneity (i.e. subpopulations of cells within the primary tumor and its metastases, having distinct genotypes and phenotypes and possibly different biological behaviors)[31]. Receptor status of breast cancer may indicate both the prognosis and risk of recurrence; for example, ER-positive tumors display protracted metastasis latency periods and frequently metastasize to the bone[32-35]. In our case, CBD metastasis of breast cancer occurred 3 years after surgical treatment, with multiple bone metastases in addition. Expression of the nuclear protein Ki-67 has been shown to correlate with the proliferative rate of tumor cells and this biomarker is used clinically as an independent prognostic biomarker in primary breast cancer, especially among patients with ER (+) tumors[36,37]. Therefore, a biopsy of suspected breast cancer metastases would be necessary to determine the appropriate management of the disease.
In general, patients with pancreaticobiliary malignancies, metastatic disease and external biliary compression by lymph nodes should be implanted with plastic or self-expanding metal stents to both relieve related jaundice and abdominal pain symptoms and to improve the overall prognosis[11]. Surgical bypass or biliary stenting is reported to extend survival to over 1 year, in comparison to un-intervened cases of liver metastases, for whom mean survival is only about 1 mo[11].Although many previous reports have indicated the superiority of a metal stent for malignant distal biliary obstruction[38], we generally prefer to use plastic stents to relieve symptoms before pathological diagnosis is obtained. Thus, only after our patient underwent ERCP placement of a plastic stent was she referred to the oncology department for systemic chemotherapy.
In conclusion, we report a special breast cancer case of direct metastasis to the CBD. Although this situation is rare, the possibility of biliary metastasis should be considered in the differential diagnosis of the bile duct stenosis for patients with a history of breast cancer, to avoid unnecessary hepatectomy. The differential diagnosis of cholangiocarcinoma is very important, with advanced endoscopic techniques and pathological diagnosis playing an important role in such.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kimura K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015;72:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 2. | Wong K, Henderson IC. Management of metastatic breast cancer. World J Surg. 1994;18:98-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol. 2015;35:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N, Pagani O, Senkus E, Thomssen C, Aapro M, Bergh J, Di Leo A, El Saghir N, Ganz PA, Gelmon K, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Leadbeater M, Mayer M, Rodger A, Rugo H, Sacchini V, Sledge G, van't Veer L, Viale G, Krop I, Winer E. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2932] [Cited by in RCA: 3209] [Article Influence: 168.9] [Reference Citation Analysis (0)] |
| 6. | Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1537] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 7. | Giestas S, Lopes S, Souto P, Agostinho C, Camacho E, Cipriano M, Sofia C. Ampullary Metastasis From Breast Cancer: A Rare Cause of Obstructive Jaundice. GE Port J Gastroenterol. 2016;23:300-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Stellato TA, Zollinger RM Jr, Shuck JM. Metastatic malignant biliary obstruction. Am Surg. 1987;53:385-388. [PubMed] |
| 9. | Franco D, Martin B, Smadja C, Szekely AM, Rougier P. Biliary metastases of breast carcinoma. The case for resection. Cancer. 1987;60:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest Endosc. 1992;38:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Budimir I, Sabol Pusic M, Nikolic M, Dorosulic Z, Ljubicic N, Stajduhar E, Mise I, Vazdar L, Sarcevic B. Obstructive Jaundice as an Uncommon Manifestation of Metastatic Breast Cancer. World J Oncol. 2015;6:297-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Popp JW Jr, Schapiro RH, Warshaw AL. Extrahepatic biliary obstruction caused by metastatic breast carcinoma. Ann Intern Med. 1979;91:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Titus AS, Baron TH, Listinsky CM, Vickers SM. Solitary breast metastasis to the ampulla and distal common bile duct. Am Surg. 1997;63:512-515. [PubMed] |
| 14. | Stoeckler F, Hagmüller E, Rumpelt HJ, Weickert U. A rare cause of distal bile duct stenosis. J Gastrointest Cancer. 2007;38:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Rego RF, Atiq M, Velchala N, Nevin D, McElreath DP, McKnight WD, Aduli F. Ampullary metastasis from breast cancer: an unusual finding. Endoscopy. 2009;41 Suppl 2:E278-E279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Cochrane J, Schlepp G. Metastatic Breast Cancer to the Common Bile Duct Presenting as Obstructive Jaundice. Case Rep Gastroenterol. 2015;9:278-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 17. | Kopelson G, Chu AM, Doucette JA, Gunderson LL. Extra-hepatic biliary tract metastases from breast cancer. Int J Radiat Oncol Biol Phys. 1980;6:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Pappo I, Feigin E, Uziely B, Amir G. Biliary and pancreatic metastases of breast carcinoma: is surgical palliation indicated? J Surg Oncol. 1991;46:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Feliu Villaró F, Luengo Rodríguez de Ledesma L, Gómez Sugrañés JR, Castellote Caixal M, Ros López S, García Rubio B. [Obstructive jaundice due to the intraluminal biliary metastasis of a breast carcinoma]. Rev Esp Enferm Dig. 1995;87:482-483. [PubMed] |
| 20. | Papo M, Fernandez J, Quer JC, Sirvent JJ, Richart C. Metastatic breast carcinoma presenting as obstructive jaundice. Am J Gastroenterol. 1996;91:2240-2241. [PubMed] |
| 21. | Coletta M, Montalti R, Pistelli M, Vincenzi P, Mocchegiani F, Vivarelli M. Metastatic breast cancer mimicking a hilar cholangiocarcinoma: case report and review of the literature. World J Surg Oncol. 2014;12:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Engel JJ, Trujillo Y, Spellberg M. Metastatic carcinoma of the breast: a cause of obstructive jaundice. Gastroenterology. 1980;78:132-135. [PubMed] |
| 23. | Rabin MS, Richter IA. Metastatic breast carcinoma presenting as obstructive jaundice. A report of 3 cases. S Afr Med J. 1979;55:388-390. [PubMed] |
| 24. | Sarocchi F, Gilg MM, Schreiber F, Langner C. Secondary tumours of the ampulla of Vater: Case report and review of the literature. Mol Clin Oncol. 2018;8:274-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Ferrari AB, Pulcini G, Gheza F, Vinco A, Manenti S, Cervi E, Villanacci V, Cervi G. Duodenal metastasis from male breast cancer: a case report and review of the literature. J Med Case Rep. 2009;3:8331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Bastos T, Souza TF, Otoch JP, Grecco E, Àvila F, Artifon EL. Metastasis of breast cancer to major duodenal papilla. Rev Gastroenterol Peru. 2014;34:149-150. [PubMed] |
| 27. | Abid A, Moffa C, Monga DK. Breast cancer metastasis to the GI tract may mimic primary gastric cancer. J Clin Oncol. 2013;31:e106-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Smith NZ. Treating metastatic breast cancer with systemic chemotherapies: current trends and future perspectives. Clin J Oncol Nurs. 2012;16:E33-E43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Vignot S, Besse B, André F, Spano JP, Soria JC. Discrepancies between primary tumor and metastasis: a literature review on clinically established biomarkers. Crit Rev Oncol Hematol. 2012;84:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Aurilio G, Monfardini L, Rizzo S, Sciandivasci A, Preda L, Bagnardi V, Disalvatore D, Pruneri G, Munzone E, Della Vigna P, Renne G, Bellomi M, Curigliano G, Goldhirsch A, Nolè F. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013;52:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 682] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 32. | Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 608] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 33. | Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1582] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 36. | Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF; International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1326] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 37. | Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1415] [Cited by in RCA: 1574] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 38. | Fernandez Y Viesca M, Arvanitakis M. Early Diagnosis And Management Of Malignant Distal Biliary Obstruction: A Review On Current Recommendations And Guidelines. Clin Exp Gastroenterol. 2019;12:415-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (1)] |