Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1616
Peer-review started: February 23, 2021
First decision: April 19, 2021
Revised: April 25, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: November 15, 2021
Processing time: 261 Days and 14.5 Hours
Liver cancer is a leading cause of death worldwide, and hepatocellular carcinoma (HCC) is the most frequent primary liver tumour, followed by cholangiocarcinoma. Notably, secondary tumours represent up to 90% of liver tumours. Chronic liver disease is a recognised risk factor for liver cancer development. Up to 90% of the patients with HCC and about 20% of those with cholangiocarcinoma have an underlying liver alteration. The gut microbiota-liver axis represents the bidirectional relationship between gut microbiota, its metabolites and the liver through the portal flow. The interplay between the immune system and gut microbiota is also well-known. Although primarily resulting from experiments in animal models and on HCC, growing evidence suggests a causal role for the gut microbiota in the development and progression of chronic liver pathologies and liver tumours. Despite the curative intent of “traditional” treatments, tumour recurrence remains high. Therefore, microbiota modulation is an appealing therapeutic target for liver cancer prevention and treatment. Furthermore, microbiota could represent a non-invasive biomarker for early liver cancer diagnosis. This review summarises the potential role of the microbiota and immune system in primary and secondary liver cancer development, focusing on the potential therapeutic implications.
Core Tip: Liver cancer is a worldwide leading cause of death. Growing evidence suggested a pathogenetic role of the gut microbiota and immune system in liver cancer development. Although there have been rapid developments in metagenomic science, definitive and complete knowledge of these processes is still far from being acquired. However, targeting both microbiota and the immune system could represent an appealing therapeutic option alone or as a boost of conventional treatments. Finally, the microbiota signature evaluation could represent a potential novel, non-invasive biomarker for early diagnosis.
- Citation: Bartolini I, Risaliti M, Tucci R, Muiesan P, Ringressi MN, Taddei A, Amedei A. Gut microbiota and immune system in liver cancer: Promising therapeutic implication from development to treatment. World J Gastrointest Oncol 2021; 13(11): 1616-1631
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1616.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1616
Primary liver cancer is a leading cause of death worldwide. Hepatocellular carcinoma (HCC) is the most common primary liver tumour, accounting for about 80% of the cases. Cholangiocarcinoma (CCA) is the second primary liver tumour, representing approximately 15% of the malignancies[1]. Finally, the secondary tumours represent up to 90% of liver tumours, and the liver is the most frequent metastatic site[2].
Chronic liver disease is a recognised risk factor for HCC. Up to 90% of the patients with HCC have an underlying liver alteration, and about 30% of the patients with cirrhosis will suffer from HCC[3]. Several pathologies may cause liver cirrhosis, including viral hepatitis, alcohol abuse, diabetes and non-alcoholic fatty liver disease (NAFLD)[4]. Although most CCAs occur without any specific predisposing factors, about 20% of the patients harbour some of the same causal pathologies as HCC[5].
The gut microbiota consists of the biological community of bacteria, Archaea, fungi and viruses harboured within a host showing a commensal, symbiotic or pathogenetic attitude[6,7]. The liver is exposed to both microbiota and microbial metabolites through the portal flow. There is a bidirectional relationship between gut microbiota and liver through signalling-sensing pathways, known as the “gut microbiota-liver axis”[3,7,8].
The intestinal barrier has a crucial role in preserving the host from the environment. Intestinal barrier and gut microbiota can influence each other in a dynamic process, and alterations in each of them may impair this balance[9].
The interplay between the immune system and gut microbiota is also well-known. The liver immune system is a dynamic microenvironment subjected to changes related to the received stimuli[10]. In the liver, there are cells of the innate immune system, including Kupffer cells, natural killer cells, natural killer T (NKT) cells and cells belonging to the adaptive immune system, including T lymphocytes[11]. The γδT cells are unconventional T lymphocytes and act as a bridge between innate and adaptive immunity. γδT cells are getting attention due to their pleiotropy and their potential causal or beneficial role in the different aspects of tumour progression[12,13].
Metagenomic analysis, polymerase chain reaction and 16S ribosomal RNA seque
Several guidelines propose the best treatment strategies for liver cancer. Traditional treatments include surgery, transplantation, locoregional therapies, chemotherapy or chemoradiotherapy[15]. Despite curative treatments, tumour recurrence remains high. Therefore, gut microbiota modulation may represent a promising therapeutic target for liver cancer prevention and therapy[7]. Since complete prevention is not achievable, as with any other cancer, an early diagnosis may allow better patient outcomes, and the gut microbiota may represent a novel, non-invasive biomarker[16-18].
This review aims to provide the actual state of the art of the potential role of microbiota-immunity in every step of both primary and secondary liver cancer, focusing on the potential therapeutic implications.
Several studies reported the progressive increase in the number of pathogenic bacteria with the decrease of those showing a healthy behaviour and the different stages of chronic liver disease and HCC development[7,19]. Furthermore, faecal biodiversity seems to decrease along with the cirrhosis progression, but it seems to increase again along with early HCC progression[20]. Tables 1 and 2 summarise the most important changes in chronic liver disease and HCC, respectively.
| Chronic hepatitis B[21] | Chronic hepatitis C[22] | NAFLD[3,24] | Cirrhosis[25,26] |
| ↓: Alistipes, Bacteroides, Asaccharobacter, Butyricimonas, Ruminococcus, Clostridium cluster IV, Parabacteroides, Escherichia/Shigella | ↓: Clostridiales Bifidobacterium | ↓: Prevotella | ↓: Lachnospiraceae |
| ↑: Megamonas, Lachnospiraceae, Clostridium sensustricto, Actinomyces | ↑: Bacteroidetes, Streptococcus, Lactobacillus | ↑: Proteobacteria, Fusobacteria, Erysipelotrichaceae,Enterobacteriaceae, Lachnospiraceae, Streptococcaceae, Escherichia, Shigella | ↑:Enterobacteriaceae, Veillonellaceae, Streptococcaceae |
| Hepatitis B-related HCC[27] | NASH-related HCC[29] | Cirrhosis-related HCC[17] |
| ↓: Faecalibacterium, Ruminococcus, Ruminoclostridium | ||
| ↑: Escherichia, Shigella, Enterococcus | ↑: Clostridium, Corynebacterium Bacillus, Desulfovibrio, Rhodococcus | ↑: Epsilonproteobacteria, Actinobacteria, Clostridia, Fusobacterium, Oribacterium |
Studies in humans reported a significant difference between the gut microbiota of patients with chronic viral hepatitis and healthy volunteers. In particular, a significant decrease in the number of Alistipes, Bacteroides, Asaccharobacter, Butyricimonas, Ruminococcus, Clostridium cluster IV, Parabacteroides and Escherichia/Shigella was found together with a significant increase in the number of Megamonas, unclassified Lachnospiraceae, Clostridium sensu stricto and Actinomyces in patients with chronic hepatitis B[21].
Patients with chronic hepatitis C presented in their stool samples a lower bacterial diversity, a higher presence of Streptococcus, Lactobacillus and Bacteroidetes, and a lower presence of Clostridiales and Bifidobacterium[22].
Alcoholic patients showed an increased number of gram-negative bacteria[6], but also the contributing role of the Enterococcus faecalis in alcoholic hepatitis has been described[23].
Dysbiosis has also been found in patients with NAFLD, though different studies reported a different relative abundance of bacteria in the gut of this subgroup of patients[3]. For example, compared with healthy controls, NAFLD patients’ microbiota presented enriched in Proteobacteria and Fusobacteria with higher representations of the bacteria belonging to the family Erysipelotrichaceae, Enterobacteriaceae, Lachnospiraceae and Streptococcaceae. An increased number of Escherichia and Shigella were also found together with a reduced number of Prevotella[24].
Independently from the aetiology, patients with cirrhosis presented with a progr
Patients with hepatitis B-related HCC resulted in having a higher level of pro-inflammatory bacteria, including Escherichia, Shigella (Enterobacteriaceae) and Enterococcus, with a reduced amount of Faecalibacterium, Ruminococcus and Ruminoclostridium compared with healthy subjects[27].
The presence of Veillonella parvula and Bacteroides caecimuris seems to allow the differentiation of patients with NAFLD-related HCC from those with NAFLD-related cirrhosis only[28].
In a murine model of non-alcoholic steatohepatitis-induced HCC, Clostridium, Corynebacterium, Bacillus, Desulfovibrio, and Rhodococcus were highly represented in male mice and were associated with a higher risk of HCC development[29].
A particular abundance of bacteria, including Clostridium and CF231, were uniquely observed in HCC patients, independently from the cirrhosis stage or other environmental factors[30].
Interestingly, Western and Eastern people showed different gut microbiota, but they shared similar pathogenic microbial signatures[31]. Lu et al[17] analyzed the tongue coating microbiota in cirrhosis-related HCC patients. They found significantly higher biodiversity and dysbiosis in patients compared with healthy controls. Epsilonproteobacteria, Actinobacteria, Clostridia and Fusobacteria were increased in the patients, while there was a higher presence of Gammaproteobacteria and Bacteroidetes in the volunteers. In particular, the number of Fusobacterium and Oribacterium seems to differentiate HCC patients from healthy people[17].
Data about CCAs have been rarely reported. Lactobacillus, Actinomyces, Alloscardovia and Peptostreptococcaceae increased in stool samples from patients with intrahepatic CCA compared to those with HCC or healthy people. Furthermore, the overgrowth of bacteria belonging to the family of the Ruminococcaceae, together with higher levels of interleukin-4 (IL-4) and lower levels of IL-6, correlated with vascular invasion and, thus, with patients’ prognosis. Furthermore, Lactobacillus and Alloscardovia are directly related to the tauroursodeoxycholic acid levels, and tauroursodeoxycholic acid levels showed a negative association with survival[1].
In a small study on bile samples taken during endoscopic retrograde cholangiopancreatography of patients with extrahepatic CCA, first episodes of bile duct stones and recurrent bile duct stones, Chen et al[32] showed a significant increase in the presence of Gemmatimonadetes, Latescibacteria, Planctomycetes and Nitrospirae in the patients with extrahepatic CCA. At the same time, they were absent in patients with the first episode of bile duct stones.
Similarly, in another different study on bile samples of patients with gallbladder cancer and gallbladder lithiasis, Tsuchiya et al[33] found the predominance of Fusobacterium nucleatum, Escherichia coli and Enterobacter species in cancer patients and a predominance of Escherichia coli, Salmonella species and Enterococcus gallinarum in the patients with gallbladder lithiasis.
Despite these data, it remains challenging to assess whether these modifications in microbiota composition are related to liver disease rather than the medications used in these patients[3]. Furthermore, some results may appear conflicting. Many reasons could explain these differences, including: (1) The different study models (in vitro, animal, human); (2) The influence of the environment, diet, lifestyle, and, eventually, other comorbidities; (3) The different methods to take and manage the samples; (4) The potential confounding effects of known or unknown factors; and (5) The relationship between testable and untestable microbiota.
Further large-scale human studies are needed. Complete knowledge of the relati
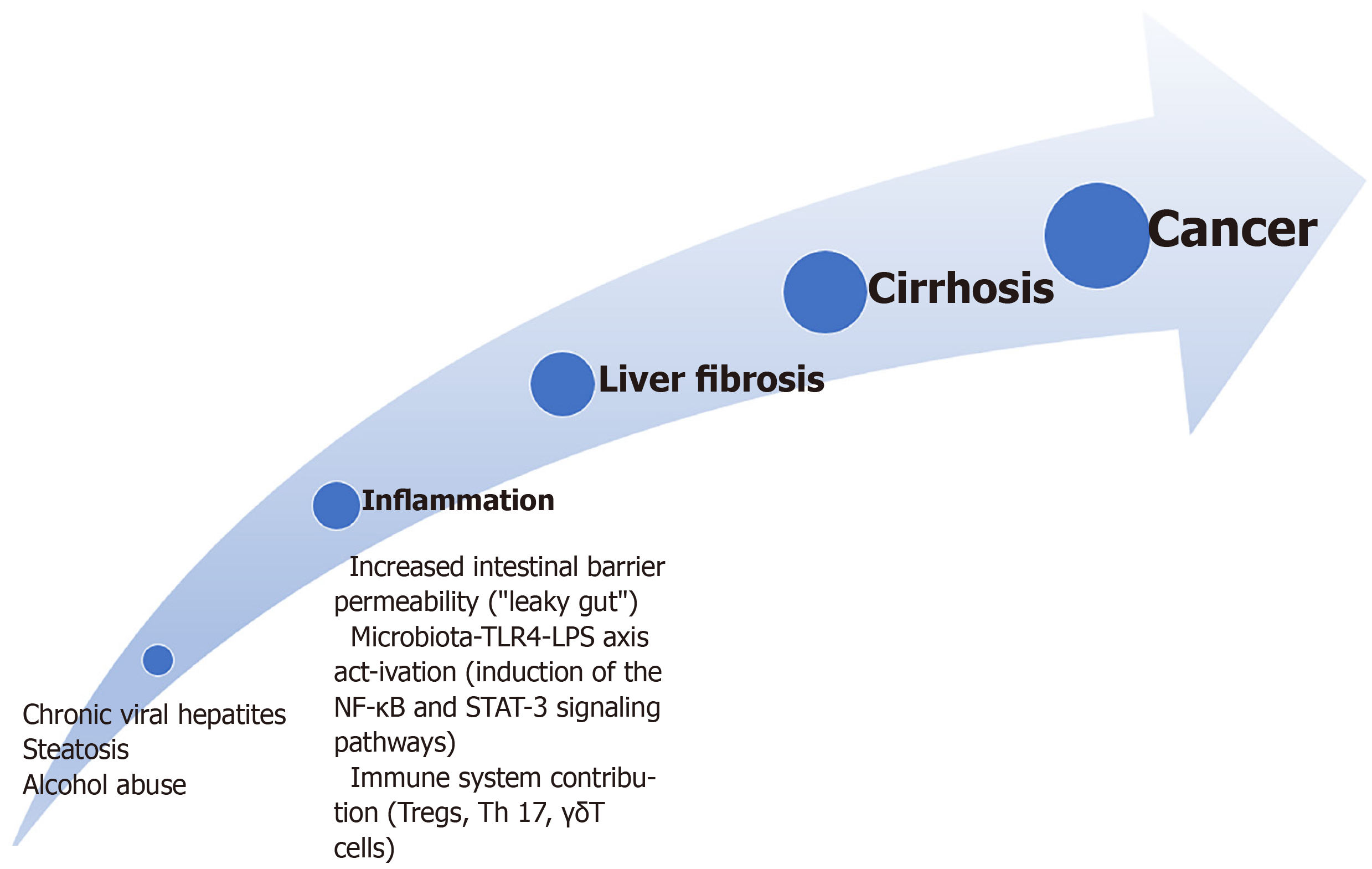
Inflammation-liver fibrosis-cirrhosis-cancer: The pathway comprehending inflammation-liver fibrosis-cirrhosis-cancer is one of the most commonly recognised for HCC development[7]. Figure 1 summarises this pathway. On the contrary, most studies reported these alterations as a protective factor for liver metastasis development. However, some papers showed similar pathogenesis for both primary and secondary liver cancers[34].
In addition, bacterial dysbiosis causes a higher release of inflammatory cytokines and an increased intestinal barrier permeability[35].
Finally, there are growing data about the role of the microbiota toll-like receptor (TLR) 4 axis and of the lipopolysaccharide (LPS)-TRL4 axis in the development of inflammation and liver fibrosis from experimental and in clinical settings[36-38].
The TLR4 is expressed in the Kupffer, hepatic stellate, endothelial cells and hepatocytes[3]. TLR4 activation causes the upregulation of the epidermal growth factor epiregulin that shows a mitogenic effect on hepatocytes causing HCC promotion[39].
LPS, a component of the gram-negative bacteria wall, is a well-recognised inflammation inducer. It binds to the transmembrane TLR4 causing the expression of the Hepcidin (an inflammatory molecule), showing an anti-apoptotic effect on the hepatocytes via the activation of the nuclear factor-κB and signal transducer and activator of transcription 3 signalling and the production of IL-17, IL-6, IL-1β, and tumour necrosis factor (TNF)-α[3,40]. Furthermore, the binding between LPS and TLR4 in the Kupffer cell causes hepatocyte proliferation due to reducing TNF and IL-6 release[41].
Higher levels of LPS, together with a higher presence of bacterial unmethylated CpG DNA that binds to the TLR9, have been found in peripheral blood of patients with chronic liver disease and liver metastasis[42,43]. While there is little specific data about modification of the microbiota in the subgroup of viral hepatitis-related cirrhosis[44], a synergic action between TLR4 signalling pathway and hepatitis C infection in promoting HCC has been reported[45].
Alcohol intake may also cause increased blood LPS levels by increasing gram-negative bacteria numbers[6]. Furthermore, alcohol abuse may interfere with the tight junctions enabling intestinal translocation[46]. Similarly, a high-fat diet can increase LPS levels up to three-fold and increase intestinal barrier permeability[47].
On the other hand, mouse models of HCC demonstrated that the overexpression of the granulocyte-macrophage colony-stimulating factor (GM-CSF) promoted by the microbiota might help reduce the inflammatory status through the modulation of the immune system. In particular, GM-CSF downregulated the pro-inflammatory cytokines IL-1β and IL-2 and TLR4 expression while increasing levels of the anti-inflammatory cytokines IL-4 and IL-10. Furthermore, mice with HCC and the overexpression of GM-CSF showed a different microbiota composition, with an increased anti-inflammatory genera Roseburia, Blautia and Butyricimonass and a significantly reduced presence of Prevotella, Parabacteroides, Anaerotruncus, Streptococcus, Clostridium and Mucispirillum, together with modification in microbial metabolites. In particular, mice with HCC and GM-CSF overexpression showed higher biotin levels, reduced level of IL-2, and a low level of succinic acid levels together with an increased level of IL-4 and IL-10, thus showing a decreased intestinal barrier function and dysbiosis[48].
Alteration of the intestinal barrier has a role in the inflammation-cirrhosis-cancer pathway. The intestinal barrier is composed of a high turnover epithelium; a double layer mucus covers the epithelium and allows the microbes not to be carried away by the peristaltic movements; immunoglobulin A and defensins are secreted within the mucus layer; Paneth cells can produce antibacterial peptides; lastly, there is mucosa-associated lymphoid tissue. At the apical side of the cells, there are tight junctions that harbour signalling molecules[6,9,49].
The status of increased intestinal barrier permeability is known as “leaky gut”[8]. With increased gut permeability, microbiota and toxins, including endotoxins or flagellin, may reach the liver through the portal vein stimulating an inflammatory reaction[50]. Although the exact pathogenetic mechanism under this alteration is not yet wholly explained, both acute and chronic liver pathologies may impair the intestinal barrier function[3]. For example, excessive alcohol intake and its metabolism derive high toxic acetaldehyde levels that increase gut permeability, other than hepatocyte impairment[6]. Furthermore, mucus represents a nutrient for some bacteria, including Akkermansia municiphila, and, in the presence of a low-fibre diet, these species may overgrow, reducing the mucus thickness[51].
The immune system has a crucial role in cancer development, and the interplay with the gut microbiota is well-known. Regulatory T cells (Tregs) can suppress the host antitumour immunity and cause tumour progression, worsening CD8+ T cells function. High levels of Tregs have been found in the HCC patients’ peripheral blood[52].
In vitro studies demonstrated that the microbiota of patients with NAFLD-related HCC, and not that of patients with NAFLD-related cirrhosis, stimulated a T cell immunosuppressive environment to reduce CD8+ T cells and an increased number of IL-10+ Tregs[53]. T helper (Th) 17 cells showed pro-inflammatory effects through the secretion of IL-17A and IL-22. Increased blood and tumour levels of Th17 have been found in HCC patients, and these levels were directly related to poor survival[54].
The γδT cells are getting attention because of their pleiotropy, with both Th1 and Th2 phenotypes, different behaviour in distinct liver pathologies and interplay with the microbiota[12,13,55]. γδT cells are scarcely represented in the peripheral blood but are highly expressed in the liver[12,56].
γδT cells are pathogenic in patients affected by hepatitis C infection and worsen the steatohepatitis in NAFLD patients[12,57]. In early-stage cirrhotic patients, γδT cells produce IL-17 causing fibrosis by stimulating the stellate and Kupffer cells[58]. On the contrary, in the late stages, γδT cells limit fibrosis and induce stellate cell apoptosis[12,59]. In vitro studies reported the cytotoxic activity of the γδT cells through the secretion of IFN-γ, TNF-α, perforin, and granzymes in the presence of HCC[12,60]. The ratio of peritumoural HSC to γδT cells resulted in a prognostic factor for resected HCC[61]. Enhancing this immunity could represent a potential therapeutic target[12].
γδT cells also play a role in intestinal barrier homeostasis and interplay with the microbiota[12,55]. Tumour-associated antigens elicit antitumour T lymphocyte response. There are many tumour-infiltrating lymphocytes in the interface between HCC and liver (CD4+ T cells) or within the tumour (CD8+ T cells), but tumour cells may induce Tregs, causing immunosuppression[62]. Interestingly, no differences in the tumour-infiltrating pattern have been found between HCC and CCA[63].
Neutrophils can induce cancer cell proliferation and remodel the extracellular matrix. High levels of neutrophils have been found in metastatic sites, including the liver[64].
The pathways involving the peroxisome proliferator-activated receptors (PPARs) could have a role in the HCC development. Published data reported their protective role in chronic liver disease development through an interplay with the microbiome and their ability to reverse leaky gut conditions and dysbiosis[65].
Li et al[66] reported that the tumour-released secretory protein cathepsin K (CTSK) represented a link between altered gut microbiota and metastatic behaviour of colorectal cancer. In particular, experiments in vitro and on mice models showed a direct correlation between Escherichia coli, high LPS levels, CTSK overexpression (stimulated by the LPS) and liver metastasis compared to the control group. Furthermore, the CTSK could activate an m-TOR-dependent pathway by binding to TLR4 and inducing macrophages’ M2 polarisation. These macrophages could promote cancer metastasisation through the secretion of IL-10 and IL-17 and the activation of the nuclear factor-κB pathway. The CTSK silencing or the administration of the CTSK inhibitor Odanacatib abolished colorectal cancer cell migration[66]. Consequently, CTSK could represent a therapeutic target and a biomarker for the diagnosis and prognosis of metastasis from colorectal cancer. Similarly, an engineered LPS trap protein showed the ability to reduce the chance of colorectal cancer liver metastasis development[67].
More generally, enhancing immune activity may represent a potential therapeutic target.
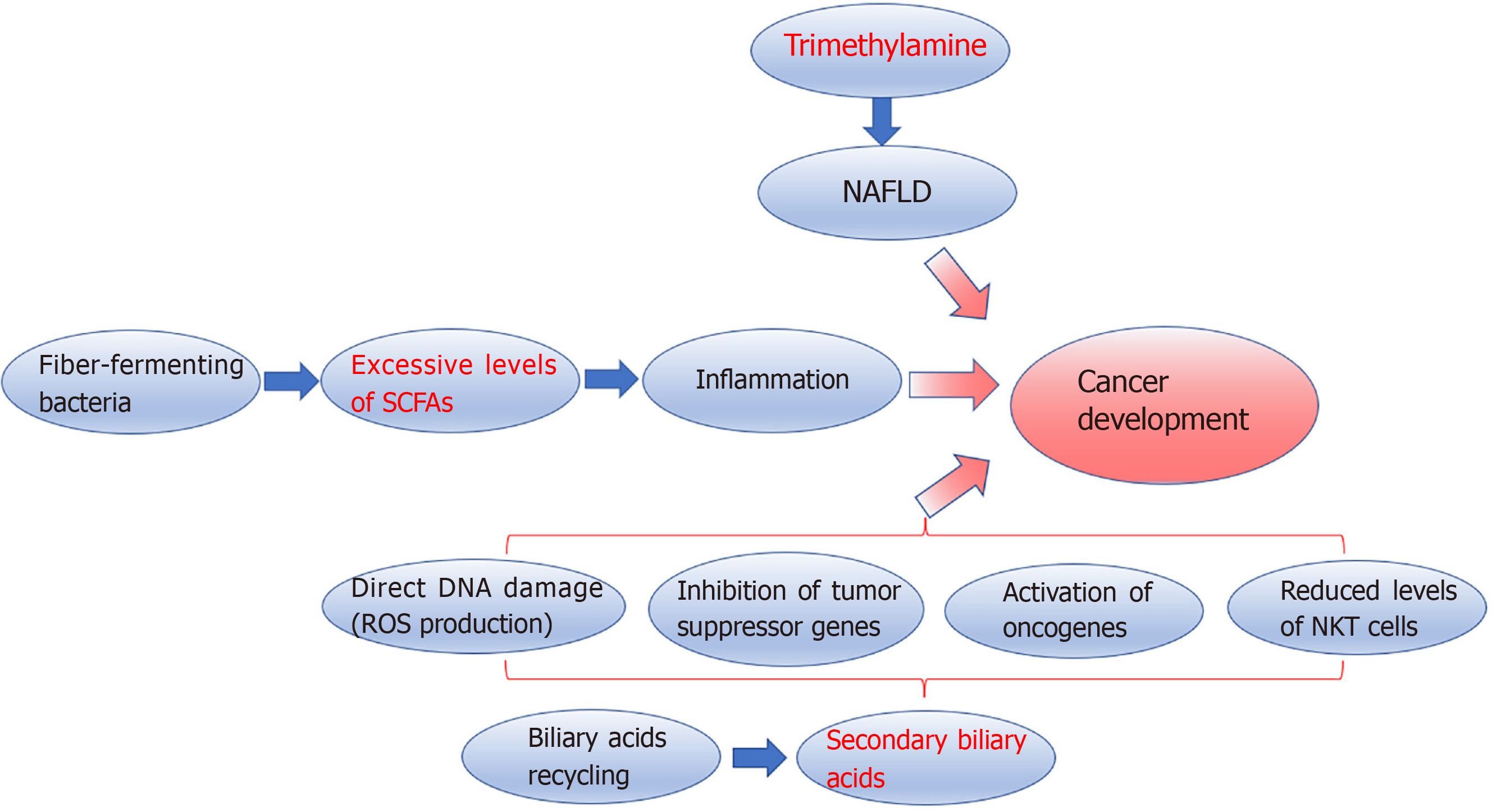
Microbial metabolites: Microbiota metabolites may also have a causal role in liver cancer development (Figure 2). Trimethylamine (TMA) is an example of microbial metabolites involved in the pathogenesis of NAFLD[8]. Experimental studies on mice showed that excessive intake of soluble dietary fibre is associated with excessive proliferation of fibre-fermenting bacteria, including Clostridium that produces short-chain fatty acids (SCFAs), which showed immunomodulatory functions[68,69]. Excessive levels of SCFAs, particularly butyrate, promote inflammation having a causal role in cholestasis, NAFLD, and HCC development, as reported in metabolomic studies[8,53,70]. Faeces and serum levels of butyrate resulted higher in patients with NAFLD-related HCC than those with NAFLD-related cirrhosis. Furthermore, butyrate can impair cytotoxic CD8+ T cell activity[53]. Conversely, propionate seems able to inhibit cancer progression[71]. Gut microbiota metabolises choline into several metabolites, including TMA, that the liver metabolises into TMA oxide, and TMA oxide is related to liver inflammation[72].
The intestinal microbiota also has a fundamental role in bile acids (BA) production and recycling. BAs are synthesised by the liver and metabolised by gut bacteria into secondary BAs, which are sensed by the epithelial cells’ farnesoid X-activated receptor (FXR). FXR provides feedback to the liver[73]. BA excess is another recognised pathogenetic factor in carcinogenesis. Secondary BAs can cause direct DNA damage by producing reactive oxygen species, inhibiting tumour suppressor genes, and activating oncogenes[74]. Furthermore, the deoxycholic acid, a secondary BA, binding to the TLR2 in hepatic stellate cells, can induce cyclooxygenase-2 expression, enhancing the inhibition of the antitumour activity prostaglandin E2-mediated[75]. Obesity can increase BA conversions[30]. On the contrary, the inhibition of 7α-dehydroxylation responsible for secondary BA metabolization is associated with a lower incidence of HCC in mice[76]. In both animal models and humans, conversion of primary BA to secondary BA is also negatively related to NKT cell infiltration. NKT cells can control both primary and secondary cancer development[77,78].
Complete knowledge of these pathways may allow the design of further studies on several appealing preventive options, including agents able to reestablish a correct balance between the different microbial species, selective agents against pathogenic bacteria, inhibitors of bacterial pathogenic metabolites production and gut barrier improvement[3].
Specific pathways in CCA: Infection of Opisthorchis viverrini and Clonorchis sinensis is a well-known risk factor for CCA development. Besides direct mechanical damage on the biliary tract epithelium and sustained inflammation, dysbiosis in local microbiota with bacterial translocation from the duodenum may contribute to CCA development[79].
Surgical resection is the elective treatment for HCC, CCA or liver metastasis from several primary cancers, mostly colorectal adenocarcinoma, whenever possible. In the setting of advanced disease, chemotherapy and novel pharmacologic treatments, including immunotherapy and targeted therapies, should be preferred.
Immune checkpoint inhibitors, including the tremelimumab, a monoclonal antibody against cytotoxic T-lymphocyte-associated antigen 4, and nivolumab or pembrolizumab that are monoclonal antibodies against programmed cell death ligand 1[7], show a response rate in HCC patients that is reported to be up to 20%[80]. Immunotherapy may also be combined with locoregional therapies, showing a synergistic effect[81]. Tremelimumab showed greater efficacy in hepatitis C-related HCC since it can enhance CD8+ T cell infiltration and, consequently, lower the viral load[81].
Since gut microbiota seems to impact these systemic treatments' efficacy, the microbiota’s modulation to enhance treatments’ response appears as a promising therapeutic target[7]. It has been reported that while antibiotics may reduce the efficacy of the checkpoint inhibitors lowering the gut microbiota biodiversity, there are specific overrepresented taxa associated with more significant responses[82].
Zheng et al[83] showed higher levels of Akkermansia muciniphila and bacteria from the family of the Ruminococcaceae in faecal samples of anti-programmed cell death ligand 1 immunotherapy responders. Conversely, in non-responders patients, higher Proteobacteria levels were found from week 3 of therapy, and a predominance of Proteobacteria was found at week 12[83].
The use of epigenetic drugs, including DNA methyltransferase enzymes-mediated hypermethylation and histone deacetylases-mediated histone modification, is under evaluation showing promising results in combination with conventional immunotherapy in murine models[84].
The microbiota evaluation may help in better selecting the candidate for a specific treatment hypothesising the response rate. Furthermore, the possibility to target both innate and adaptive immune systems could represent an appealing therapeutic option. In particular, actions on the innate arms may allow improvements in cytotoxic effect, stimulate the adaptive immune system and reduce the tumour-promoting effect[10].
Antimicrobial peptides (AMPs) are constitutively or inducibly expressed in the tissues, which may be in contact with pathogens[85]. AMPs are present in the great majority of vertebrates, invertebrates and vegetables. The antimicrobial effect of the AMPs can be exerted through cellular membrane damages, inhibition of cellular replication and through their immunomodulatory abilities[85-87].
Some AMPs showed anticancer properties, also causing cancer cell apoptosis. Furthermore, the healthy or cancer cell membrane composition differs, and tumour cells are more easily damaged by the anticancer peptides (ACPs). ACPs can interact with LPS or other bacterial products resulting in an anti-inflammatory effect[50]. The use of ACPs, including TLR agonist and tumour-associated antigens-derived peptides, may represent a promising therapeutic option in HCC treatment[50,63,88]. To be effective, some ACPs would have to be delivered. Delivery systems may include peptide-derived vaccines, nanoparticles and liposomes, each related to advantages and limitations[50].
More specific details about the design and the delivery of these molecules are reviewed elsewhere[50,89].
Sorafenib is a tyrosine kinase inhibitor worldwide used in advanced HCC that can suppress abnormal cell proliferation and angiogenesis. Microbiota can influence sorafenib’s blood levels, affecting enterohepatic recirculation. Drug blood levels are related to the chance of suffering from the side effects[90]. Two common side effects include diarrhoea and hand-foot syndrome and require reducing the administered drug[91]. Butyric acid showed a protective action toward the inflamed intestinal mucosa by stimulating the Tregs and IL-10 secretion. Increased Butyricimonas, a butyric acid producer, have been found in patients not experiencing diarrhoea[91,92]. Dysbiosis and increased levels in the gut of bacteria typically found in the mouth (Veillonella, Bacillus, Enterobacter) have been found in patients not experiencing the hand-foot syndrome[91].
On the contrary, reduced Treg levels allow the achievement of better outcomes through the enhancement of the CD8+ T cell antitumour activity. Furthermore, the baseline CD4+ T effector/Tregs ratio has a prognostic value[93].
Complete knowledge of the interaction between gut microbiota and liver cancer steps may help design new and tailored therapeutic options[3].
The only actual method to prevent primary liver cancer development is to prevent and cure the underlying chronic liver disease whenever present. Although the gut microbiota role in these pathologies is still not wholly understood, microbiota modulation may be a promising target to reduce cancer. There are conditions in which microbiota modulation would have a marginal role, including perinatal viral hepatitis infections or cancers occurring on “healthy” livers[3].
The environment, diet, lifestyle, the use of antibiotics or pre/probiotics and several diseases may change the gut microbiota composition. It has been reported that a vegetable-enriched diet may lower the incidence of primary liver cancers, mainly in the male population. Conversely, a high-fat diet favours gram-negative bacteria overgrowth with increased LPS levels, and a high-fructose diet reduces the population of Bifidobacterium and Lactobacillus[94,95].
On a theoretical basis, using non-selective antibiotics may lower the entire gut microbiota population reducing the chance of bacterial translocation and the induction of a pro-inflammatory status. Treatments with selective antibiotics, if available, could reduce only those species producing cancer-promoting metabolites[3,76].
Experiments on rats demonstrated that non-absorbable antibiotic administration could positively affect steatosis and the inflammatory status. Studies on murine models showed that metronidazole administration might decrease the risk of cholestasis and HCC development by reducing the population of bacteria production of butyrate, which shows a health-promoting effect in other circumstances[70]. Similarly, vancomycin may reduce gram-positive bacteria producing secondary BAs[96]. The administration of the combination of ampicillin, neomycin, metronidazole and vancomycin showed a more powerful effect against late stages of HCC carcinogenesis compared to earlier stages[39]. On the contrary, penicillin intake was reported to be related to a higher risk of HCC development in rats[97].
The non-absorbable oral norfloxacin and rifaximin showed a good safety profile and microbiota-related positive effects in cirrhotic patients and mice with HCC[3]. An experimental study on the subcutaneous implantation model of thymoma on mice showed that an antibiotic combination of vancomycin, neomycin and primaxin reduced the chance of developing liver metastasis, though without affecting the primary tumour[77].
Despite the lack of much data on humans, it is reasonable that a long-term antibiotic assumption may be burdened with several side effects, including depletion of beneficial bacteria, kidney damages or antibiotic resistance[3]. Consequently, further studies are needed.
The use of probiotics may help resolve dysbiosis, increase the number of bacteria with favourable properties, improve the intestinal barrier functions, absorb carcinogens and interact with the immune system, causing a reduction of Th17[98] cells. While ongoing human trials evaluate the effects of probiotic administrations in patients suffering from chronic liver diseases, evidence-based data about HCC comes only from murine models[3].
The assumption of the so-called VSL#3, a mixture of Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subspecies and Bulgaricus seemed to have positive effects on the pathway inflammation-fibrosis-HCC development being associated with an enriched population of Prevotella and Oscillibacter and with Th12 cell differentiation[97,99].
Similarly, prebiotics are substances able to stimulate the overgrowth of beneficial bacteria. Some examples include prebiotics of fructooligosaccharides reported to re-establish eubiosis, improve intestinal barrier function and reduce inflammation. Lactulose is related to an overgrowth of Bifidobacterium that shows a healthy behaviour by reducing LPS serum levels. Therapies with synbiotics are based on the combined use of probiotics and prebiotics[100].
Finally, faecal microbiota transplantation (FMT) is another treatment option that can reduce the risk of HCC development. It has been reported that FMT may reduce steatohepatitis in mice[57]. However, several concerns have been raised, including the possibility of a long-lasting efficacy and the risk of infection transmission. The opportunity to transplant only beneficial bacteria could represent an appealing option[3].
The LPS-TLR4 axis has a crucial role in the inflammation-fibrosis-cirrhosis-cancer pathway. Consequently, several antagonists of the TLR4 have been proposed. Some examples include polymyxin B, able to bind and sequestrate LPS; E5531 or eritoran, molecules interacting with other steps of this signalling pathway; resatorvid, able to target the TLR4; thalidomide, a TLR inhibitor[3]. Further details are not the object of this review and can be found elsewhere[3,88].
However, the primary concern is the consequent status of immunosuppression that could be detrimental in patients with chronic liver disease or HCC[3]. Furthermore, the results of published studies are sometimes controversial due to the complexity of the known and unknown interactions[88]. Consequently, further long-term studies are needed.
Several studies reported the beneficial effects of both natural and synthetic PPARs agonists in chronic liver disease development through microbiota modulation. Although specific studies on cancer progression are lacking, targeting the PPARs could represent, at least, a cancer prevention strategy. Further details can be found elsewhere[65].
The integrity of the gut barrier is vital for healthy individuals. A high caloric diet seems to impair the intestinal barrier[101]. Conversely, physical exercise improves short-term and long-term gut permeability through effects on the immune system and the microbiota, increasing the Bacteroidetes/Firmicutes ratio[6,102].
Cisapride is a prokinetic medication that resulted in reducing both bacterial overgrowth and translocation, fastening the intestinal transit time[103]. Some nonselective β-adrenergic blockers showed similar properties.
BA influence the function of the gut barrier, and the FXRs are crucial in BA synthesis, other than in the regeneration of the liver and tumour growth suppression. The obeticholic acid is an FXR agonist and showed beneficial effects on damaged mucosa and reduced the gut barrier permeability, the inflammatory status, bacterial overgrowth and preventing the progression from non-alcoholic steatohepatitis to other complications, thus becoming an attractive potential treatment option[104].
Excessive TNF production is associated with increased gut barrier permeability reducing the tight junction proteins[105]. Consequently, anti-TNF-based therapies could represent potential therapeutic options, but as previously stated, the related immunosuppression may be detrimental[3]. However, n-3 polyunsaturated fatty acids (PUFA) showed anti-inflammatory properties in experimental models reducing the level of TNF and IL-1, thus resulting in an appealing option[106,107]. Furthermore, in vitro experiments demonstrated the ability of the n-3 PUFA to block β-catenin and cyclooxygenase-2[108]. On the contrary, n-6 PUFA seems related to a pro-inflammatory status[109].
There is a continuous search for new, non-invasive biomarkers for diagnosis, and microbiota seems promising even in this field. Since there are different microbial signatures along with disease progression, microbial samples could represent appealing non-invasive biomarkers for an early diagnosis[16].
Furthermore, Ponziani et al[110] demonstrated an inverse relation between Akkermansia and Bifidobacterium and the well-known inflammatory marker calprotectin. Analysis on faecal samples of patients with primary liver cancers showed a significant link between Veillonella and alpha-fetoprotein levels together with a negative connection between Subdoligranulum and alpha-fetoprotein levels[50].
Along with faecal samples, analysis of the tongue microbiota could represent another non-invasive biomarker. In particular, Oribacterium and Fusobacterium presence could differentiate HCC patients from healthy subjects[17].
Jia et al[1] reported that the plasma-stool ratio of two BAs, tauroursodeoxycholic and glycoursodeoxycholic acids, demonstrated the ability to identify patients with intrahepatic CCA from those with HCC or healthy people with an area under the curve of 0.801 and 0.906, respectively[1]. Although some methodological and cause-effects concerns have been raised[111], this potential biomarker is appealing. Again, further studies are needed to obtain new markers that could be used independently or within algorithms[18,111].
In conclusion, a growing body of literature demonstrates a pathogenetic role of the gut microbiota-immunity axis in liver cancer development. Although there is an ongoing rapid development of metagenomic science, definitive and complete knowledge of this process is still far from being wholly acquired. However, targeting microbiota and the immune system may represent appealing therapeutic options alone or boost conventional treatments. Finally, the gut microbiota signature evaluation could represent a potential novel, non-invasive biomarker for early diagnosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du Z S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yu HG
| 1. | Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, Zhu Z, Hong Z, Zhang T, Du G, Xiang J, Wu D, Bai W, Yang B, Li Y, Huang J, Li H, Safadi R, Lu Y. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology. 2020;71:893-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 4. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 839] [Article Influence: 104.9] [Reference Citation Analysis (2)] |
| 5. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 969] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 6. | Plaza-Díaz J, Solís-Urra P, Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadía-Molina F, Álvarez-Mercado AI. The Gut Barrier, Intestinal Microbiota, and Liver Disease: Molecular Mechanisms and Strategies to Manage. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Yang M, Ericsson AC. The Potential Gut Microbiota-Mediated Treatment Options for Liver Cancer. Front Oncol. 2020;10:524205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1259] [Article Influence: 251.8] [Reference Citation Analysis (1)] |
| 9. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2137] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 10. | Ruf B, Heinrich B, Greten TF. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol Immunol. 2021;18:112-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 11. | Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 599] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 12. | Zhou QH, Wu FT, Pang LT, Zhang TB, Chen Z. Role of γδT cells in liver diseases and its relationship with intestinal microbiota. World J Gastroenterol. 2020;26:2559-2569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 967] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 14. | Arteta AA, Milanes-Yearsley M, Cardona-Castro N. Cholangiocyte derived carcinomas and local microbiota. J Hepatobiliary Pancreat Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2874] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 16. | Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919848184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Lu H, Ren Z, Li A, Zhang H, Jiang J, Xu S, Luo Q, Zhou K, Sun X, Zheng S, Li L. Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. 2016;6:33142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Rao BC, Lou JM, Wang WJ, Li A, Cui GY, Yu ZJ, Ren ZG. Human microbiome is a diagnostic biomarker in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2020;19:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wei X, Jiang S, Chen Y, Zhao X, Li H, Lin W, Li B, Wang X, Yuan J, Sun Y. Cirrhosis related functionality characteristic of the fecal microbiota as revealed by a metaproteomic approach. BMC Gastroenterol. 2016;16:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 508] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 21. | Wang J, Wang Y, Zhang X, Liu J, Zhang Q, Zhao Y, Peng J, Feng Q, Dai J, Sun S, Zhao L, Zhang Y, Hu Y, Zhang M. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front Microbiol. 2017;8:2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 23. | Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, Shao Y, Liu J, Hernandez-Morales A, Lessor L, Rahman IR, Miyamoto Y, Ly M, Gao B, Sun W, Kiesel R, Hutmacher F, Lee S, Ventura-Cots M, Bosques-Padilla F, Verna EC, Abraldes JG, Brown RS Jr, Vargas V, Altamirano J, Caballería J, Shawcross DL, Ho SB, Louvet A, Lucey MR, Mathurin P, Garcia-Tsao G, Bataller R, Tu XM, Eckmann L, van der Donk WA, Young R, Lawley TD, Stärkel P, Pride D, Fouts DE, Schnabl B. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 659] [Cited by in RCA: 586] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 24. | Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 411] [Article Influence: 51.4] [Reference Citation Analysis (1)] |
| 25. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1536] [Article Influence: 139.6] [Reference Citation Analysis (40)] |
| 26. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 27. | Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, Singh S, Madamba EV, Bettencourt R, Richards L, Yu RT, Atkins AR, Huan T, Brenner DA, Sirlin CB, Downes M, Evans RM, Loomba R. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020;32:901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Xie G, Wang X, Zhao A, Yan J, Chen W, Jiang R, Ji J, Huang F, Zhang Y, Lei S, Ge K, Zheng X, Rajani C, Alegado RA, Liu J, Liu P, Nicholson J, Jia W. Sex-dependent effects on gut microbiota regulate hepatic carcinogenic outcomes. Sci Rep. 2017;7:45232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Lapidot Y, Amir A, Nosenko R, Uzan-Yulzari A, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, Koren O, Safran M, Ben-Ari Z. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Fukui H. Gut Microbiome-based Therapeutics in Liver Cirrhosis: Basic Consideration for the Next Step. J Clin Transl Hepatol. 2017;5:249-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Chen B, Fu SW, Lu L, Zhao H. A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma. Biomed Res Int. 2019;2019:1092563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Tsuchiya Y, Loza E, Villa-Gomez G, Trujillo CC, Baez S, Asai T, Ikoma T, Endoh K, Nakamura K. Metagenomics of Microbial Communities in Gallbladder Bile from Patients with Gallbladder Cancer or Cholelithiasis. Asian Pac J Cancer Prev. 2018;19:961-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 34. | Rossetto A, De Re V, Steffan A, Ravaioli M, Miolo G, Leone P, Racanelli V, Uzzau A, Baccarani U, Cescon M. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 36. | Song IJ, Yang YM, Inokuchi-Shimizu S, Roh YS, Yang L, Seki E. The contribution of toll-like receptor signaling to the development of liver fibrosis and cancer in hepatocyte-specific TAK1-deleted mice. Int J Cancer. 2018;142:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S. Toll like receptor 4 and hepatocellular carcinoma; A systematic review. Life Sci. 2017;179:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1556] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 39. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1028] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 40. | Lee YS, Kim YH, Jung YS, Kim KS, Kim DK, Na SY, Lee JM, Lee CH, Choi HS. Hepatocyte toll-like receptor 4 mediates lipopolysaccharide-induced hepcidin expression. Exp Mol Med. 2017;49:e408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Gao C, Qiao T, Zhang B, Yuan S, Zhuang X, Luo Y. TLR9 signaling activation at different stages in colorectal cancer and NF-kappaB expression. Onco Targets Ther. 2018;11:5963-5971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Bajaj JS, Sterling RK, Betrapally NS, Nixon DE, Fuchs M, Daita K, Heuman DM, Sikaroodi M, Hylemon PB, White MB, Ganapathy D, Gillevet PM. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment Pharmacol Ther. 2016;44:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Semin Liver Dis. 2009;29:188-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 48. | Wu YN, Zhang L, Chen T, Li X, He LH, Liu GX. Granulocyte-macrophage colony-stimulating factor protects mice against hepatocellular carcinoma by ameliorating intestinal dysbiosis and attenuating inflammation. World J Gastroenterol. 2020;26:5420-5436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Johansson ME. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS One. 2012;7:e41009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Zhang L, Wu YN, Chen T, Ren CH, Li X, Liu GX. Relationship between intestinal microbial dysbiosis and primary liver cancer. Hepatobiliary Pancreat Dis Int. 2019;18:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167:1339-1353.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1909] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 52. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 692] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 53. | Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 54. | Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Li M, Gao J, Tang Y, Liu M, Wu S, Qu K, Long X, Li H, Liu Y, Yuan J, Mao L, Zheng X, Wang E, Wang J, Yang Y. Traditional Herbal Medicine-Derived Sulforaphene LFS-01 Reverses Colitis in Mice by Selectively Altering the Gut Microbiota and Promoting Intestinal Gamma-Delta T Cells. Front Pharmacol. 2017;8:959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 57. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 58. | Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 59. | Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. γδT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front Immunol. 2019;10:477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Lança T, Costa MF, Gonçalves-Sousa N, Rei M, Grosso AR, Penido C, Silva-Santos B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J Immunol. 2013;190:6673-6680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 61. | Zhou BY, Gong JH, Cai XY, Wang JX, Luo F, Jiang N, Gong JP, Du CY, Liao R. An imbalance between stellate cells and γδT cells contributes to hepatocellular carcinoma aggressiveness and recurrence. Hepatol Int. 2019;13:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Nishida N, Kudo M. Immunological Microenvironment of Hepatocellular Carcinoma and Its Clinical Implication. Oncology. 2017;92 Suppl 1:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. 2009;15:5053-5057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1023] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 65. | Yu Q, Wu L, Ji J, Feng J, Dai W, Li J, Wu J, Guo C. Gut Microbiota, Peroxisome Proliferator-Activated Receptors, and Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2020;7:271-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Li R, Zhou R, Wang H, Li W, Pan M, Yao X, Zhan W, Yang S, Xu L, Ding Y, Zhao L. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26:2447-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 67. | Song W, Tiruthani K, Wang Y, Shen L, Hu M, Dorosheva O, Qiu K, Kinghorn KA, Liu R, Huang L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv Mater. 2018;30:e1805007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 68. | Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, Cong Y. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 69. | Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gómez L, Verginadis I, Bittinger K, Pustylnikov S, Pierini S, Perales-Linares R, Blair IA, Mesaros CA, Snyder NW, Bushman F, Koumenis C, Facciabene A. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest. 2020;130:466-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 70. | Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD Jr, Zhang L, Wang WB, Hao S, Flythe MD, Gonzalez DJ, Cani PD, Conejo-Garcia JR, Xiong N, Kennett MJ, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell. 2018;175:679-694.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 71. | Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer. 2012;107:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 72. | Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 73. | Dossa AY, Escobar O, Golden J, Frey MR, Ford HR, Gayer CP. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol. 2016;310:G81-G92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Wang X, Fu X, Van Ness C, Meng Z, Ma X, Huang W. Bile Acid Receptors and Liver Cancer. Curr Pathobiol Rep. 2013;1:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 76. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1649] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 77. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1037] [Article Influence: 148.1] [Reference Citation Analysis (0)] |
| 78. | Jia B. Commentary: Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Front Immunol. 2019;10:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Saltykova IV, Petrov VA, Brindley PJ. Opisthorchiasis and the Microbiome. Adv Parasitol. 2018;102:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (1)] |
| 81. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 639] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 82. | Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. 2018;359:1366-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 83. | Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, Li P, Qin N, Fang W. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 370] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 84. | Vaziri F, Colquhoun S, Wan YY. Hepatocellular carcinoma immunotherapy: The impact of epigenetic drugs and the gut microbiome. Liver Res. 2020;4:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Yang M, Zhang C, Zhang X, Zhang MZ, Rottinghaus GE, Zhang S. Structure-function analysis of Avian β-defensin-6 and β-defensin-12: role of charge and disulfide bridges. BMC Microbiol. 2016;16:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Yang M, Zhang C, Zhang MZ, Zhang S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol. 2018;18:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel). 2013;6:1543-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 759] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 88. | Zou H, Wang WK, Liu YL, Braddock M, Zheng MH, Huang DS. Toll-like receptors in hepatocellular carcinoma: potential novel targets for pharmacological intervention. Expert Opin Ther Targets. 2016;20:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Felício MR, Silva ON, Gonçalves S, Santos NC, Franco OL. Peptides with Dual Antimicrobial and Anticancer Activities. Front Chem. 2017;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 90. | Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570:462-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 747] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 91. | Yamamoto K, Kuzuya T, Honda T, Ito T, Ishizu Y, Nakamura M, Miyahara R, Kawashima H, Ishigami M, Fujishiro M. Relationship Between Adverse Events and Microbiomes in Advanced Hepatocellular Carcinoma Patients Treated With Sorafenib. Anticancer Res. 2020;40:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Yu W, Su X, Chen W, Tian X, Zhang K, Guo G, Zhou L, Zeng T, Han B. Three types of gut bacteria collaborating to improve Kui Jie'an enema treat DSS-induced colitis in mice. Biomed Pharmacother. 2019;113:108751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 93. | Kalathil SG, Hutson A, Barbi J, Iyer R, Thanavala Y. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 94. | Safari Z, Monnoye M, Abuja PM, Mariadassou M, Kashofer K, Gérard P, Zatloukal K. Steatosis and gut microbiota dysbiosis induced by high-fat diet are reversed by 1-week chow diet administration. Nutr Res. 2019;71:72-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Jegatheesan P, Beutheu S, Ventura G, Sarfati G, Nubret E, Kapel N, Waligora-Dupriet AJ, Bergheim I, Cynober L, De-Bandt JP. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin Nutr. 2016;35:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 96. | Singh V, Yeoh BS, Abokor AA, Golonka RM, Tian Y, Patterson AD, Joe B, Heikenwalder M, Vijay-Kumar M. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes. 2020;11:1077-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, Chen HY, Dai RY, Qiu BJ, He YQ, Wang C, Zheng LY, Li YQ, Wu FQ, Li Z, Yan HX, Wang HY. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 98. | Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 99. | Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 100. | Wang L, Wan YY. The role of gut microbiota in liver disease development and treatment. Liver Res. 2019;3:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | Álvarez-Mercado AI, Navarro-Oliveros M, Robles-Sánchez C, Plaza-Díaz J, Sáez-Lara MJ, Muñoz-Quezada S, Fontana L, Abadía-Molina F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 102. | Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc Sport Sci Rev. 2019;47:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 300] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 103. | Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 518] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 105. | Muñoz L, Albillos A, Nieto M, Reyes E, Lledó L, Monserrat J, Sanz E, de la Hera A, Alvarez-Mon M. Mesenteric Th1 polarization and monocyte TNF-alpha production: first steps to systemic inflammation in rats with cirrhosis. Hepatology. 2005;42:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S-348S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 655] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 107. | Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 108. | Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 109. | Monteiro J, Leslie M, Moghadasian MH, Arendt BM, Allard JP, Ma DW. The role of n - 6 and n - 3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014;5:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 473] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 111. | Herraez E, Romero MR, Macias RIR, Monte MJ, Marin JJG. Clinical relevance of the relationship between changes in gut microbiota and bile acid metabolism in patients with intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2020;9:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |