Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1453
Peer-review started: March 6, 2021
First decision: June 4, 2021
Revised: July 9, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: October 15, 2021
Processing time: 221 Days and 2.1 Hours
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is a rare disease which is often associated with Helicobacter pylori (H. pylori) infection. First-line treatment of stage IE and IIE localized gastric MALT lymphoma is based on the eradication of H. pylori. The presence of H. pylori resistance factors such as translocation t (11;18), peri-gastric lymph node involvement and the degree of tumor infiltration of the gastric wall; or lack of response to antibiotic therapy are two main indications to treat with definitive radiotherapy (RT). RT is an effective treatment in localized gastric MALT lymphoma. A moderate dose of 30 Gy allows a high cure rate while being well tolerated. After treatment, regular gastric endoscopic follow-up is necessary to detect a potential occurrence of gastric adenocarcinoma.
Core Tip: In this review, after Helicobacter pylori eradication failure, radiotherapy is an effective and well tolerated treatment in localized gastric mucosa-associated lymphoid tissue lymphoma. A moderate dose of 30 Gy allows a high cure rate while being well tolerated. Long-term gastric endoscopy follow-up is necessary to detect a possible occurrence of a stomach carcinoma.
- Citation: Quéro L, Labidi M, Bollet M, Bommier C, Guillerm S, Hennequin C, Thieblemont C. Radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma. World J Gastrointest Oncol 2021; 13(10): 1453-1465
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1453.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1453
Extranodal marginal zone lymphoma (EMZL) is an indolent non-Hodgkin's B-cell lymphoma. It belongs to the heterogeneous group of MZLs, beside splenic MZL and nodal MZL. It has an extra-nodal origin. EMZL can develop from mucosal, called also mucosa-associated lymphoid tissue (MALT) lymphoma, and non-mucosal sites, in various organs such as the stomach, small intestine, bronchi, thyroid gland, liver, kidney, breast, salivary glands, orbit/orbital adnexa, or skin. It can be associated with autoimmune diseases such as Hashimoto's thyroiditis or Sjögren's syndrome. The most common sites of EMZL are the stomach, the orbit/orbital adnexa and the skin, and gastric MALT-lymphoma accounts for 30%–50% of all MALT-lymphoma locations[1,2].
The incidence of non-Hodgkin lymphoma (NHL) has increased by 70% between 1975 and 2017[3]. MALT gastric lymphoma is rare: It represents 4% of all NHL and approximately 5% of primary gastric neoplasms[4]. According of the SEER United States database, new cases of NHL and gastric cancer were respectively 77240 and 27600 in 2020 in the United Stade of America[3], which corresponds to between 1380 and 3090 new cases of gastric MALT lymphoma.
The MALT-IPI index was developed to identify patients with a poor prognosis and thus to allow for appropriate treatment of patients with EMZL. This index classified patients into three prognostic groups (low, intermediate, and high) that are predictive for event-free, progression-free and overall survival (OS), whatever the extranodal site of the lymphoma, gastric or non-gastric. The 5-year event-free survival (EFS) rates in the low-, intermediate-, and high-risk groups were 70%, 56%, and 29%, respectively. This index was based on: Age ≥ 70 years, Ann Arbor stage III or IV and elevated lactate dehydrogenase (LDH). The MALT-IPI has been developed from 401 patients treated with chlorambucil, rituximab, or both in the international randomized trial IELSG-19. The index was subsequently validated by merging three independent cohorts of MALT lymphoma patients (n = 633 patients)[5].
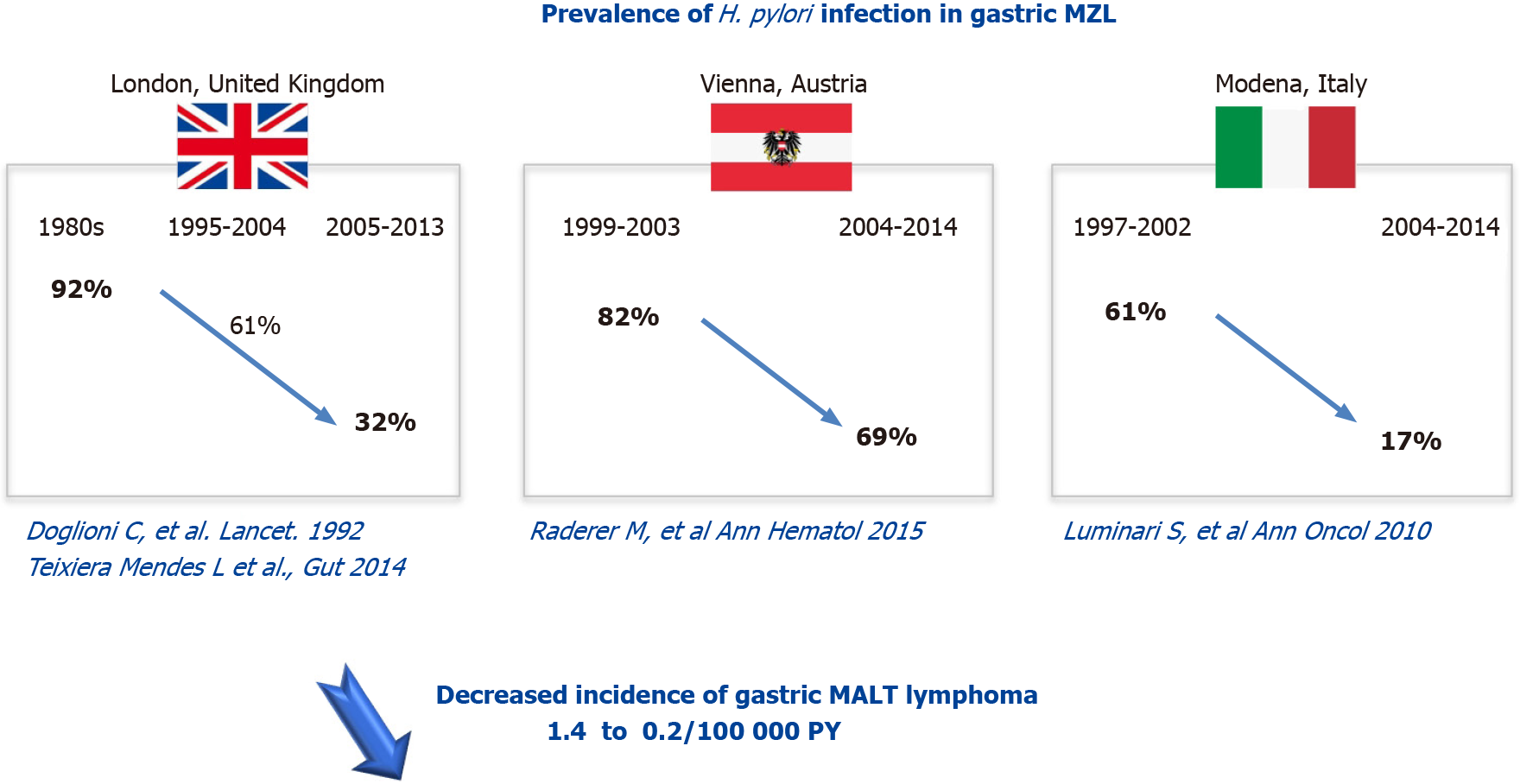
In 90% of cases, gastric MALT lymphoma is associated with a Helicobacter pylori (H. pylori) infection. H. pylori prevalence in developed countries decreased after 2000: In Europe from 48.8% to 39.8%, in Northern America from 42.7% to 26.6%, and in Oceania from 26.6% to 18.7% whereas it remained stable elsewhere: In Asia (53.6% before 2000 vs 54.3% after 2000) and South America and the Caribbean (62.8% before 2000 vs 60.2% after 2000)[6]. In Europe, the prevalence of H. pylori infection decreased from 92% in the 80’s to 61% between 1995-2004 and then to 32% between 2005 -2013 for the United Kingdom, from 82% between 1999-2003 to 69% between 2004-2014 in Austria and from 61% between 1997-2002 to 17% between 2004-2014 for Italy[7-10] (Figure 1).
First-line treatment of stage IE and IIE localized gastric MALT lymphoma is based on the eradication of H. pylori. Recommended anti-H. pylori therapy includes a proton pump inhibitor (or ranitidine bismuth citrate), clarithromycin and amoxicillin (or metronidazole).
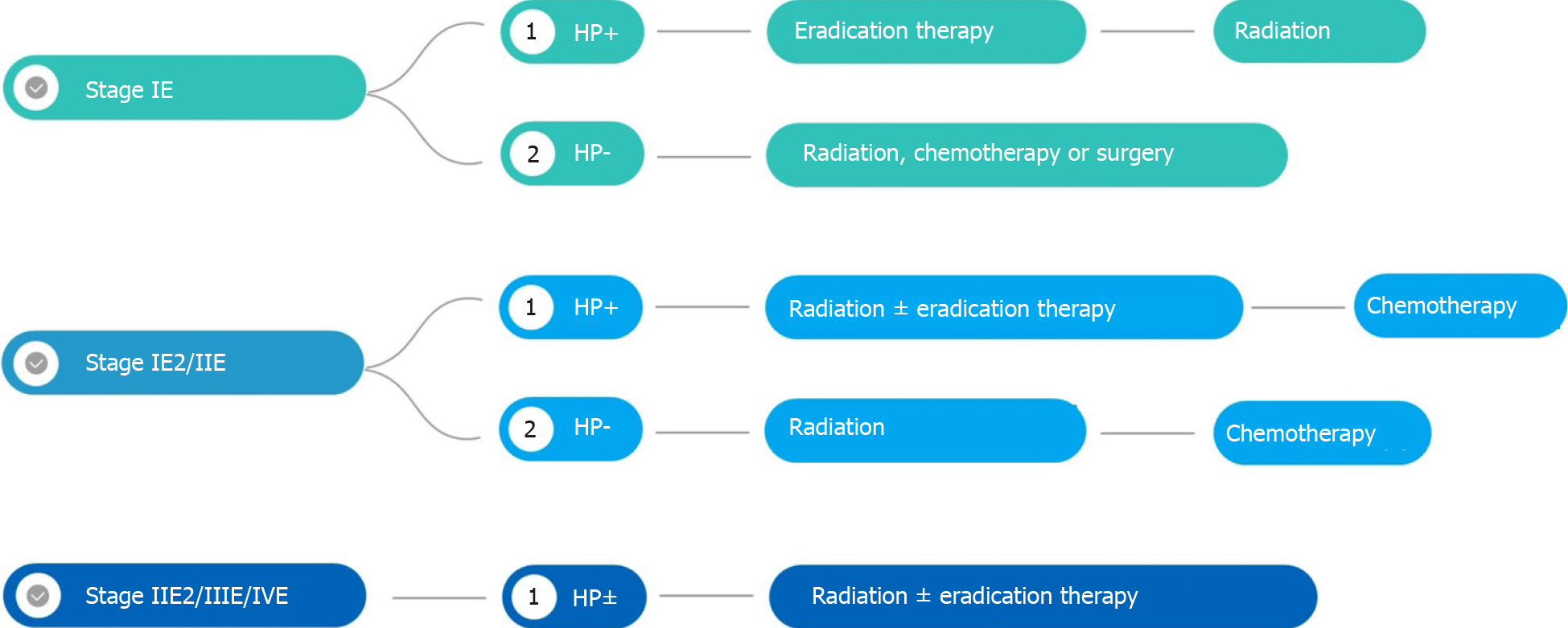
Because MALT lymphoma is rare, treatment recommendations are based on studies with low levels of evidence (Figure 2). The aim of this review is to assess the effectiveness and tolerance of radiotherapy (RT) and the alternative treatments.
Previously, staging of MALT gastric lymphoma was based on Lugano staging system[11] which is a modification of the Ann Arbor staging system. More recently, Paris staging system[12] which corresponds to the tumor, node, metastasis system for gastric cancer, has been proposed. The Paris system has the advantage, over the Lugano staging system, of better describing the depth of invasion into the gastric wall (Tables 1 and 2).
| Tumour extension | |
| T1m N0 M0 | Mucosa |
| T1sm N0 M0 | Submucosa |
| T2 N0 M0 | Muscularis propria |
| T3 N0 M0 | Serosa |
| T1–3 N1 M0 | Perigastric lymph nodes |
| T1–3 N2 M0 | More distant regional nodes |
| T4 N0–2 M0 | Invasion of adjacent structures with or without abdominal lymph nodes |
| T1–4 N3 M0 | Extra-abdominal lymph nodes |
| T1–4 N0–3 M1 | Distant (non-contiguous) GI sites involvement |
| T1–4 N0–3 M2 | Non-GI sites involvement |
| Stage | |
| Stage I | Confined to the GI tract (single primary or multiple, non-contiguous) |
| Stage II | Extension into abdomen |
| II1 | Local nodal involvement |
| II2 | Distant nodal involvement |
| Stage IIE | Penetration of serosa to involve adjacent organs or tissues |
| Stage IV | Disseminated extranodal involvement or concomitant supra-diaphragmatic nodal involvement |
According to ESMO guidelines, initial staging should include history and physical examination, full blood and differential counts, biochemistry including renal and liver function tests, protein electrophoresis, LDH and beta2-microglobulin, serum and urine immunofixation, serology for hepatitis B virus, hepatitis C virus (HCV) and human immunodeficiency virus and cryoglobulins and cryocrit if HCV-positive.
Gastroduodenal endoscopy of any abnormal lesion associated with systematic multiple biopsies. The following tumor analyses from biopsies, performed by an expert pathologist in hematology, are mandatory: (1) Immunohistochemistry panel analysis: CD20, CD3, CD5, CD10, BCL2, kappa/Lambda, CD21 or CD23, BCL6, cyclin D1 and immunoglobulin D; and (2) H. pylori testing: The detection of the t (11;18) (q21;q21) translocation by Fluorescence in situ hybridization is only recommended in case of failure of anti-H. pylori antibiotic treatment.
Endoscopic ultrasound is recommended to evaluate gastric wall infiltration and perigastric lymph node involvement.
Imaging includes chest and abdominal computed tomography (CT): Positron emission tomography (PET)/CT is not recommended before the treatment.
In a study of 103 patients with stage IE and IIE gastric MALT lymphoma, Moleiro et al[13] reported 83% complete response (CR) (78/103) with a mean follow-up of 7 mo (2–63 mo) after H. pylori eradication treatment and 86% (67/103) with a mean follow-up time of 105 mo. H. pylori infection (P = 0.004) and lymphoma location to the antrum/body (P = 0.016) were significantly associated with higher and lower CR rates, respectively. Relapse occurred in 11/78 (14%) patients after a mean time-lapse of 21 mo. The absence of H. pylori re-infection (P = 0.038), the need for only one eradication regimen (P = 0.009) and antrum lymphomas (P = 0.031) correlated with lower relapse rates. At stage IIE, H. pylori eradication was performed in 17/24 patients but only five experienced CR (30%)[13].
A systematic review of 32 publications revealed that CR was achieved in 78% of 1408 patients with low-grade stage I/II1 gastric MALT lymphoma in treated with H. pylori eradication[14].
In a Japanese multicenter cohort study, 420 patients with gastric MALT had undergone successful H. pylori eradication. Seventy-seven percent (323 patients) responded to H. pylori eradication. Absence of H. pylori, submucosal invasion and t (11;18)/API2-MALT1 were independent predictors of resistance to H. pylori eradication. Among responders, MALT relapsed in 3% of patients while progressive disease occurred in 27% of non-responders (27/97). After a median follow-up of 6 years, freedom from treatment failure (FFTF), OS and EFS at 10 years were 90%, 95% and 86%, respectively. Peri-gastric lymph node involvement and the depth of tumor infiltration in the gastric wall are factors also associated with a poor tumor response after H. pylori eradication[15].
H. pylori disappears usually 6 wk after the start of H. pylori eradication, soon after the completion of drug therapy, whereas MALT lymphoma usually takes several months to disappear (median 6 mo; extremes 3-24 mo).
However, if there is no H. pylori infection, the remission rate after antibiotic therapy is low (between 15% and 30%).
The translocation t (11;18) (q21;q21)/(API2-MALT1) is observed in 20 to 30% of gastric MALT lymphomas. It is associated with a higher rate of recurrence after eradication. This translocation is also associated with a lower response rate after alkylating-agent-based chemotherapy (90% vs 40% approximately), but not with rituximab.
According to ESMO clinical practice guidelines, the presence of H. pylori resistance factors, or lack of response to antibiotic therapy are the two main indications to treat with definitive RT. Given the possibility of a delayed response, RT should not be recommended for asymptomatic patients with non-progressive residual disease within the 9–12 mo following successful H. pylori eradication therapy. Patients with asymptomatic minimal residual disease may have an indolent course and may not require further treatment.
Some expert groups recommend RT in the case of localized disease and chemotherapy with or without immunotherapy in the case of advanced disease.
Indolent NHLs are sensitive to moderate doses of RT. In this literature review, the median delivered dose was 30 Gy (Table 3). For localized gastric lymphomas, exclusive RT in the case of failure of H. pylori eradication gives very good results with a complete remission rate of 96% to 100% for a median follow-up of between 1.3 and 4.1 years without long-term side effects. The local tumor control is excellent after exclusive RT with fewer than 5% of patients experiencing local relapse after treatment. Gastric RT is well tolerated with hardly any severe (G3+) acute or late toxicities. Some studies have reported only mild (G1-2) and transient acute gastro-intestinal toxicities.
| Ref. | Country | Patients, n (%) | Stage | Failure of H. pylori eradication or H. pylori | H. pylori | Median [Total RT dose] | Median F/U (mo) | Survival | Relapse (Local/distant) | Toxicity G3+ |
| Schechter et al[31], 1998 | NY, United States | 17 | IE = 71%; II1 = 24%, II2 = 5% | 17/17 | 12/17 | 30 Gy [28.5–43.5] | 27 [11–68] | NR | 0/0 | 0 |
| Park et al[32], 2002 | Korea | 6 | IE = 100% | 4/6 | 1/6 | 30.6 Gy [30–39] | 12 | 100% | 0/0 | 0 |
| Tsang et al[33], 2003 | Canada | 13/93 (gastric and RT subgroup) | IA = 88%; IIA = 10%, IIB = 2% | 13/13 | 7/15 | 26.2 [20–35] | 61 | 98% at 5 yr for the entire cohort | 0/0 | 0 |
| Koch et al[24], 2005 | Germany | 144/332 (indolent subgroup with conservative management) | I = 66%; II1 = 17%; II2 = 17% | NR | NR | 40 Gy | 42 [0.07–97.4] | 93% at 3.5 yr | 6 | 9 |
| Avilés et al[34], 2005 | Mexcio | 78/241 (RT group) | IE = 73%; IIE = 27% | NR | NR | 40 Gy | 90 [58–139] | 75% at 10 yr | 0/30 | 0 |
| Sugimoto et al[35], 2006 | Japan | 3 | I = 3 | 3/3 | 0 | 39 Gy [36–40] | 42 [24–72] | 100% | 0/0 | 0 |
| Tsai et al[36], 2007 | MA, United Sttaes | 18/77 gastric and RT subgroup | IE = 100% | 6/18 | NR | 30 Gy [18–40] | 61 [2–177] | 91% at 5 yr for the entire cohort | 0 over 11 patients treated by RT | NR |
| Yamashita et al[37], 2008 | Japan | 11/41 gastric subgroup | IE = 90%; IIE = 10% (whole cohort) | 6/11 | 5/11 | 30 Gy | 32 [2–162] | 100% | 0/0 | 0 |
| Vrieling et al[38], 2008 | The Netherlands | 68/115 (RT subgroup) | I = 79%; II1 = 12%, II2 = 9% | 85/115 | 13/115 | 40 Gy [31–44] | 150 [7–301] (radiotherapy only cohort) | 78% at 5 yr (entire cohort) | 3/4 | NR |
| Gobbi et al[39], 2009 | Italia | 5 | IE = 4; IIE = 1 | 5/5 | 0/5 | 30 Gy | 48 [21–70] | 80% | 0 | 0 |
| Tomita et al[40], 2009 | Japan | 20/50 (gastric subgroup) | IE = 96%; IIE = 4% (entire cohort) | 20/20 | 7/20 | 32 Gy [25.6–50] | 92 | 97% at 5 yr (entire cohort) | 0 | 2% for all cohort |
| Goda et al[41], 2010 | Canada | 25/167 (gastric subgroup) | IA = 90%; IIA = 9%; IIB = 1% | 19/19 | 5/19 | 30 Gy [20-35] | 89 [8–194] | 95% at 5-yr (entire cohort) | 0 | NR |
| Okada et al[42], 2012 | Japan | 22 | IE = 19; IIE = 3 | 22/22 | 14/22 | 30 Gy | 74 [27–159] | 91% at 5-yr | 0/3 | 0 |
| Kim et al[43]; 2013 | Korea | 64 | IE = 53 (83%); IIE = 11 (17%) | 56/60 | 33/64 | 36 Gy | 39 [9–131] | 94% at 5-yr | 5 | 0 |
| Wirth et al[16], 2013 | International (IELSG) | 102 | IE = 84%; IIE = 16% | 80/102 | 45/90 | 40 Gy [26–46] | 95 [4–528] | 70% at 10 yr | 7/5 | NR |
| Nam et al[44], 2014 | Korea | 41 (MALT subgroup) | IE = 77%; IIE = 23% | 29/41 | 29.6%(whole cohort) | 30.6 Gy [30.6-43.2] | 48 [6– 158] | 90% at 5-yr (entire cohort) | 1/0 | 0 |
| Abe et al[45], 2013 | Japan | 34 | IE = 34 (100%) | 34/34 | 17/34 | 30 Gy | 90 (14–156) | 100% at 5-yr | 1 (emergency gastrectomy during RT at 15 Gy for acute bleeding)/0 | 1 |
| Ruskoné-Fourmestraux et al[18], 2015 | France | 53 | IE = 45 (85%); IIE = 8 (15%) | 53/53 | 15/53 | 30 Gy | 59 (4–199) | 94% at 5-yr | 0/0 | 0 |
| Lim et al[46], 2016 | Korea | 33 | IE = 30 (91%); IIE = 3 (9%) | 33/33 | 17/33 | 30.6 Gy | 50 (12–145) | 97% at 5-yr | 0 | 0 |
| Ohkubo et al[47], 2017 | Japan | 27 | IE = 27 (100%) | 24/27 | 15/27 | 30 Gy [30–39.5] | 121 (8–176) | 92% at 5-yr; 87% at 10-yr | 0/2 | 2/27 (White blood cell count decreases) |
| Teckie et al[48], 2017 | NY, United States | 122/225 (gastric and RT subgroup) | IE = 225 (92%); IIE = 19 (8%) | 115/122 | 78/122 | 30 Gy | 62 [2–256] | 92% at 5-yr; 79% at 10-yr for all the subgroups | 7 (gastric subgroup) | NR |
| Pinnix et al[17], 2019 | TX, United States | 32 | IE = 25(78%); IIE = 5 (16%); IV = 2 (6%) | 32/32 | 25/32 | 36 Gy (2; 6%); 30 Gy (n = 19; 59%); 24 Gy (n = 11; 34%) | 55 [32–78] | 97% at 2-yr | 1/1 | NR |
| Schmelz et al[49], 2019 | Germany | 22 | IE = 20; IIE = 2 | 22/22 | 0/22 | 25.2 Gy/36 Gy | 79 [36.4 –143.8] | 100% at 56.6 yr | 0 | NR |
| Watanabe et al[50], 2020 | Japan | 30/34 (gastric subgroup) | IE = 100% | 26/30 | 16/30 | 30 Gy [30–40] | 61 [9–136] | 94.7% at 5 yr | 0/2 | 0 |
In the IELSG retrospective multicentric international study, Wirth et al[16] reported in 102 patients treated by extended field or whole abdomen RT for gastric MZL and persistent H. pylori infection after eradication or H. pylori, 88% of FFTF at 10-years. RT median dose delivered to the stomach was 40 Gy (26–46). In this study, RT field size, RT dose, and failure of prior therapy (H. pylori eradication, chemotherapy or surgery) were not associated with inferior RT efficacy[16]. Second malignancies were reported in 5 of 41 patients who had gastric/nodal irradiation only, of which two were likely in-field (2 colon), one was likely out of field (bladder) and two had uncertain relationship to field (1 breast, 1 lung).
In a retrospective monocentric study that evaluated the outcome of 32 patients treated for gastric MALT lymphoma with involved site radiation therapy using intensity modulated radiation therapy (IMRT), Pinnix et al[17] have reported excellent outcome with 2-year Freedom from local treatment failure, FFTF, and OS of 100%, 100% and 97%, respectively. Moreover, they did not observe any association between the lower 24 Gy dose with FFLTF (P = 0.819), FFTF (P = 0.819) or OS (P = 0.469)[17].
The excellent results and safety of moderate-dose RT, 30 Gy, have been confirmed by the GELD/FFCD prospective study that included 53 patients, all well selected (H. pylori eradication failure, H. pylori negative). The response rate was very high (98%). After 4.9-years median follow-up, the overall lymphoma survival was 94%[18]. Neither local or distant relapses nor severe acute or late G3+ toxicities were observed.
In a large retrospective monocentric study from the Memorial Sloan Kettering Hospital New York, Teckie et al[19] reported very favorable outcome in patients treated for gastric MALT lymphoma by moderate dose of RT (30 Gy). Indeed, gastric location was associated with the best relapse free-survival among patients treated by RT for MZL. After a long follow-up [62 mo (2–256)], local failures occurred in only 7 out of 102 treated patients[19].
The main weaknesses of most of the studies reported in this review are, their retrospective nature, often monocentric, their small sample size, their short follow-up and that some studies have included patients who had not failed to H. pylori eradication.
The median delivered dose in this literature review is in accordance with the ILROG guidelines: 30-30.6 Gy in conventional fractionation (1.8 to 2 Gy/d and 5 d per week) on gastric and peri gastric lymph node volumes (involved site RT)[20]. During RT, proton pump inhibitor and 5-HT3 serotonin receptor antagonist are recommended to prevent acute side effects.
CT Simulation: Patients must be simulated and treated with an empty stomach after a fast of at least 4 h or overnight.
Patients should be simulated supine with arms up using customized immobilization device.
A small volume (50 cc) of oral contrast medium should be used.
CT images should be acquired before and after oral contrast medium.
Intravenous contrast medium injection is recommended in case of lymph nodes involvement.
In order to reduce margins, respiratory motion should be assessed with 4D-CT.
The gross tumor volume corresponds to the gastric tumor and the macroscopically invaded lymph nodes determined on CT or PET-CT.
Because gastric MALT lymphoma is often associated with multifocal involvement of the stomach and may spread to regional lymph nodes, radiation volumes should include the entire stomach as well as the perigastric lymph nodes of the lesser and greater curvatures.
The clinical target volume (CTV) corresponds to the entire stomach from the gastroesophageal junction to the duodenal bulb; the whole gastric wall and the perigastric lymph nodes must be included.
The internal target volume needs to be determined by 4D-CT in order to take into account stomach mobility during breathing.
In the absence of 4D-CT, 2 cm margins should be added to the CTV to take into account the respiratory movement of the stomach.
For the planning target volume (PTV), an additional margin of 0.5 to 1 cm should be added, to take into account setup variation.
In order to reduce the margins due to respiratory movements, modern irradiation techniques such as deep-inspiratory breath-hold (DIBH) and 4D-Cone Beam Computed Tomography (CBCT)-Image Guided Radiation Therapy, should be used. In a retrospective study of 10 patients treated for gastric NHL including 5 MALT and 5 DLBCL, Christopherson et al[21] have reported that DIBH led to reduced dose to the heart and the kidneys, through improved spatial separation between the heart and the stomach, while simultaneously allowing for reduced target volumes, without compromising target coverage or increasing dose to other organs at risk (OAR)[21].
Shimohigashi et al[22] reported that the use of 4D-CBCT reduced the PTV when applying 4D soft-tissue matching, compared to skin and bone matchings. Based on these results, authors recommended daily 4D-CBCT during RT treatment[22].
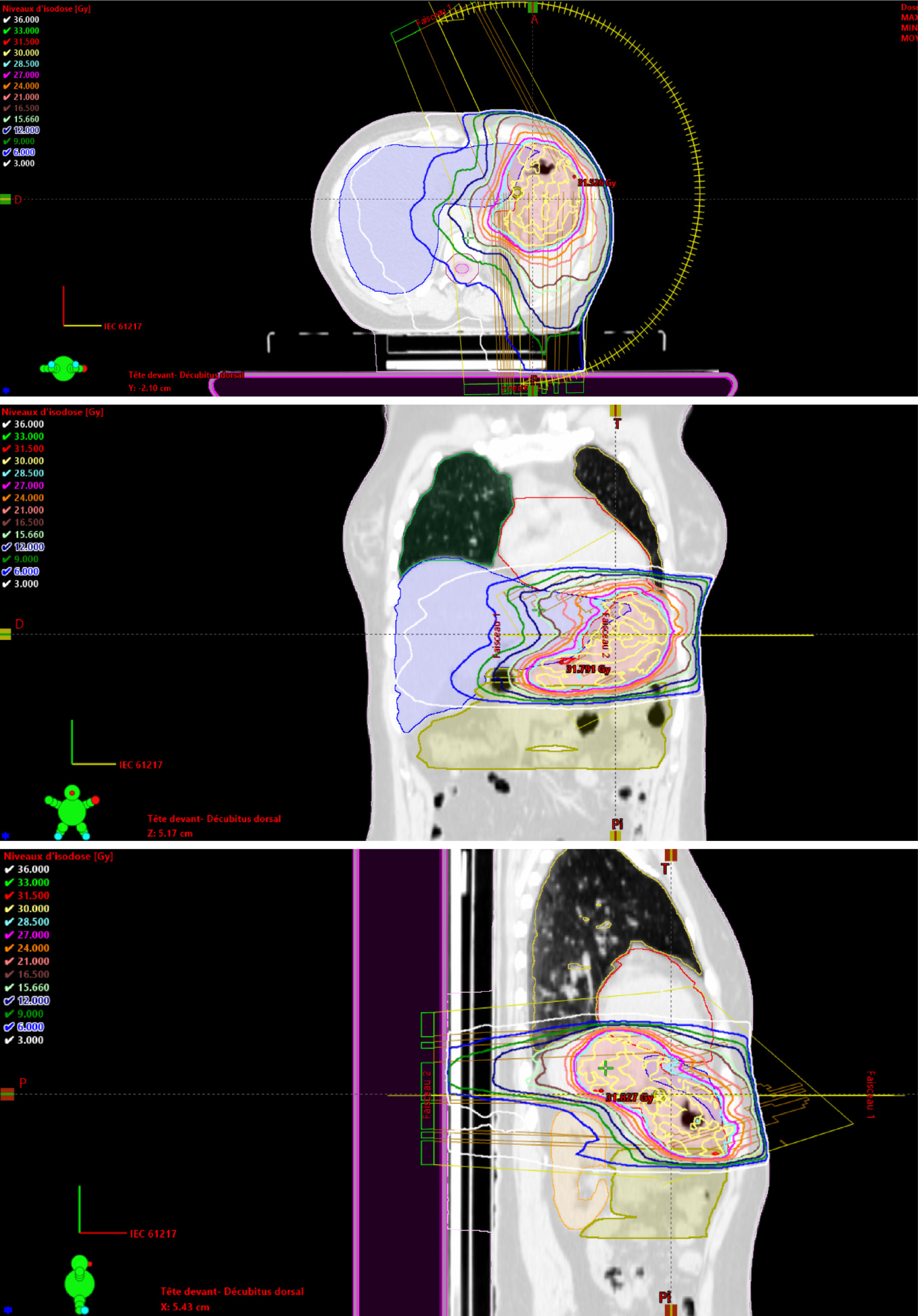
The following OAR should be delineated: Liver, kidneys, bowels, heart, lungs and spinal cord. 3D conformal RT, IMRT or VMAT (Figure 3), are recommended to reduce the dose delivered to the kidneys and liver.
The median delivered dose in this literature review is in agreement with the ILROG guidelines: 30-30.6 Gy in conventional fractionation (1.8 to 2 Gy/d and 5 d per week) on gastric and perigastric lymph node volumes (involved site RT)[23].
During RT, proton pump inhibitor and 5-HT3 serotonin receptor antagonist are recommended to prevent acute side effects.
In the GIT NHL 02/96 multicentric non-randomized German study, Koch et al[24] have reported no survival advantage at 42 mo of surgery ± adjuvant RT in case of R1/R2 resection grade or stage II disease in comparison with exclusive RT without surgery in 144 patients treated for localized primary gastric indolent lymphoma (86% vs 91%)[24].
In clinical practice, surgical treatment is rarely indicated. The only current indications are emergency situations such as perforation or uncontrolled bleeding in endoscopy.
In the IELSG-19 randomized trial that included 401 patients with localized or disseminated gastric and non-gastric MALT lymphoma, the combination of rituximab and chlorambucil was significantly superior to chlorambucil monotherapy and rituximab monotherapy in terms of response rate [79% vs 63% vs 56%, respectively (P < 0.001)], EFS (68% vs 51% vs 51%, P = 0.0009) and progression-free survival at 5 years (72% vs 59% vs 57%, P = 0.0119). Five-year OS was approximately 90% in each arm. All treatments were well tolerated. No unexpected toxicities were reported[23].
In the MALT2008-01 phase II Spanish multicentric study that included 57 patients with localized or disseminated gastric and non-gastric MALT lymphoma, the combination of bendamustine and rituximab for first-line systemic treatment resulted in a complete or unconfirmed CR rates of 98% and an EFS at 4 years of 88%. Progression-free survival at 4 years was 93% with no differences between patients with gastric and non-gastric sites[25].
Long-term complications after treatment of indolent lymphomas have been reported, including a significant increase in secondary cancers. The risk of recurrence in patients with minimal residual gastric MALT lymphoma after successful eradication of H. pylori without oncological treatment, is low. Watchful waiting with regular endoscopies and biopsies could therefore be considered, particularly in elderly patients or in patients with comorbidity[26]. This decision to treat or not, should be discussed in the setting of a multidisciplinary meeting.
Follow-up after treatment of localized gastric-MALT is based on upper gastrointestinal endoscopy. The follow-up differs slightly between expert groups.
Endoscopic control 3 mo after RT: In case of CR: Clinical follow-up every 3–6 mo for 5 years and then yearly or as clinically indicated.
The timing of endoscopy and imaging is not determined: They should be decided on according to clinical symptoms.
Following RT treatment, endoscopic controls with gastric biopsies and systemic follow-up (clinical examination, blood counts) every 12-18 mo are recommended.
Following the documentation of H. pylori eradication, strict endoscopic follow-up is recommended, with multiple biopsies taken 2-3 mo after treatment to rule out tumor progression, and subsequently every 6 mo for 2 years to monitor the histological regression of the gastric lymphoma.
Because of its reliability, it is recommended to use the GELA histological scoring system for post-therapy biopsies evaluation[27]. Comparison with previous biopsies is helpful to assess response.
In spite of the excellent cure rate, long-term follow-up by endoscopy is required as cases of gastric adenocarcinomas have been reported during follow-up of cured lymphomas[26,28,29]. Moreover, the Dutch epidemiological study by Capelle et al[30] found a 6-fold increase in the risk of gastric adenocarcinoma compared to the general population in patients with a history of gastric lymphoma regardless of treatment. This risk was 16.6 times higher in patients with gastric MALT lymphoma aged between 45 and 59 years than in the Dutch population (P < 0.001)[30].
RT is a highly effective treatment in the management of localized gastric MALT lymphomas resistant to H. pylori eradication with a cure rate close to 100% and no major toxicity. Long follow-up with gastric endoscopy monitoring is necessary to diagnose at an early stage the potential occurrence of gastric adenocarcinoma.
The authors would like to thank Mr. Abdellah El Mouqaddem, a physicist from our department, for the dosimetry using VMAT technique presented in this article.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Nath J, Pruthi DS, Sun Y, Wang SY S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127:2082-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Khalili M, Sakhaee E, Aflatoonian MR, Abdollahpour G, Tabrizi SS, Damaneh EM, Hossini-Nasab S. Seroprevalence of bovine leptospiral antibodies by microscopic agglutination test in Southeast of Iran. Asian Pac J Trop Biomed. 2014;4:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | NIH. SEER Preliminary Cancer Incidence Rate Estimates for 2017, and diagnosis years 2000 to 2017: Cancer Stat Facts: Non-Hodgkin Lymphoma. Surveillance Epidemiology and End Results Program: National Cancer Institute Bethesda MD, 2020. [cited 10 February 2021]. Available from: https://seer.cancer.gov/statistics/preliminary-estimates/. |
| 4. | Müller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, Laszlo D, Coiffier B, Lopez Guillermo A, Torri V, Cavalli F, Johnson PW, Zucca E. A MALT lymphoma prognostic index. Blood. 2017;130:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2049] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 7. | Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet. 1992;339:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 167] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Luminari S, Cesaretti M, Marcheselli L, Rashid I, Madrigali S, Maiorana A, Federico M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2010;21:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Raderer M, Wöhrer S, Kiesewetter B, Dolak W, Lagler H, Wotherspoon A, Muellauer L, Chott A. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol. 2015;94:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sena Teixeira Mendes L, D Attygalle A, C Wotherspoon A. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut. 2014;63:1526-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 13. | Moleiro J, Ferreira S, Lage P, Dias Pereira A. Gastric malt lymphoma: Analysis of a series of consecutive patients over 20 years. United European Gastroenterol J. 2016;4:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 16. | Wirth A, Gospodarowicz M, Aleman BM, Bressel M, Ng A, Chao M, Hoppe RT, Thieblemont C, Tsang R, Moser L, Specht L, Szpytma T, Lennard A, Seymour JF, Zucca E. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol. 2013;24:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Pinnix CC, Gunther JR, Milgrom SA, Cruz Chamorro RJ, Medeiros LJ, Khoury JD, Amini B, Neelapu S, Lee HJ, Westin J, Fowler N, Nastoupil L, Dabaja B. Outcomes After Reduced-Dose Intensity Modulated Radiation Therapy for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Int J Radiat Oncol Biol Phys. 2019;104:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Ruskoné-Fourmestraux A, Matysiak-Budnik T, Fabiani B, Cervera P, Brixi H, Le Malicot K, Nion-Larmurier I, Fléjou JF, Hennequin C, Quéro L; Groupe d’Étude des Lymphomes Digestifs (GELD) & Fédération Francophone de Cancérologie Digestive. Exclusive moderate-dose radiotherapy in gastric marginal zone B-cell MALT lymphoma: Results of a prospective study with a long term follow-up. Radiother Oncol. 2015;117:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Teckie S, Qi S, Lovie S, Navarrett S, Hsu M, Noy A, Portlock C, Yahalom J. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys. 2015;92:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Wirth A, Mikhaeel NG, Aleman BMP, Pinnix CC, Constine LS, Ricardi U, Illidge TM, Eich HT, Hoppe BS, Dabaja B, Ng AK, Kirova Y, Berthelsen AK, Dieckmann K, Yahalom J, Specht L. Involved Site Radiation Therapy in Adult Lymphomas: An Overview of International Lymphoma Radiation Oncology Group Guidelines. Int J Radiat Oncol Biol Phys. 2020;107:909-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Christopherson KM, Gunther JR, Fang P, Peterson SL, Roach KE, Wong PF, Mirkovic D, Lim TY, Wang H, Wang XA, Wang C, Garcia J, Dabaja BS, Pinnix CC. Decreased heart dose with deep inspiration breath hold for the treatment of gastric lymphoma with IMRT. Clin Transl Radiat Oncol. 2020;24:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Shimohigashi Y, Toya R, Saito T, Kono Y, Doi Y, Fukugawa Y, Watakabe T, Matsumoto T, Kai Y, Maruyama M, Oya N. Impact of four-dimensional cone-beam computed tomography on target localization for gastric mucosa-associated lymphoid tissue lymphoma radiotherapy: reducing planning target volume. Radiat Oncol. 2021;16:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Zucca E, Conconi A, Martinelli G, Bouabdallah R, Tucci A, Vitolo U, Martelli M, Pettengell R, Salles G, Sebban C, Guillermo AL, Pinotti G, Devizzi L, Morschhauser F, Tilly H, Torri V, Hohaus S, Ferreri AJM, Zachée P, Bosly A, Haioun C, Stelitano C, Bellei M, Ponzoni M, Moreau A, Jack A, Campo E, Mazzucchelli L, Cavalli F, Johnson P, Thieblemont C. Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival With Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J Clin Oncol. 2017;35:1905-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Koch P, Probst A, Berdel WE, Willich NA, Reinartz G, Brockmann J, Liersch R, del Valle F, Clasen H, Hirt C, Breitsprecher R, Schmits R, Freund M, Fietkau R, Ketterer P, Freitag EM, Hinkelbein M, Heinecke A, Parwaresch R, Tiemann M. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol. 2005;23:7050-7059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Salar A, Domingo-Domenech E, Panizo C, Nicolás C, Bargay J, Muntañola A, Canales M, Bello JL, Sancho JM, Tomás JF, Rodríguez MJ, Peñalver FJ, Grande C, Sánchez-Blanco JJ, Palomera L, Arranz R, Conde E, García M, García JF, Caballero D, Montalbán C; Grupo Español de Linfomas/Trasplante de Médula Ósea (GELTAMO). First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2014;1:e104-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, Raderer M, Savio A; EGILS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Copie-Bergman C, Wotherspoon AC, Capella C, Motta T, Pedrinis E, Pileri SA, Bertoni F, Conconi A, Zucca E, Ponzoni M, Ferreri AJ. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br J Haematol. 2013;160:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Copie-Bergman C, Locher C, Levy M, Chaumette MT, Haioun C, Delfau-Larue MH, Leroy K, Gaulard P, Delchier JC. Metachronous gastric MALT lymphoma and early gastric cancer: is residual lymphoma a risk factor for the development of gastric carcinoma? Ann Oncol. 2005;16:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Zullo A, Hassan C, Andriani A, Cristofari F, Cardinale V, Spinelli GP, Tomao S, Morini S. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Capelle LG, de Vries AC, Looman CW, Casparie MK, Boot H, Meijer GA, Kuipers EJ. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Park HC, Park W, Hahn JS, Kim CB, Lee YC, Noh JK, Suh CO. Low grade MALT lymphoma of the stomach: treatment outcome with radiotherapy alone. Yonsei Med J. 2002;43:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Patterson BJ. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Avilés A, Nambo MJ, Neri N, Talavera A, Cleto S. Mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach: results of a controlled clinical trial. Med Oncol. 2005;22:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Sugimoto M, Kajimura M, Shirai N, Furuta T, Kanaoka S, Ikuma M, Sato Y, Hishida A. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. 2006;45:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Tsai HK, Li S, Ng AK, Silver B, Stevenson MA, Mauch PM. Role of radiation therapy in the treatment of stage I/II mucosa-associated lymphoid tissue lymphoma. Ann Oncol. 2007;18:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Yamashita H, Nakagawa K, Asari T, Murakami N, Igaki H, Ohtomo K. Radiotherapy for 41 patients with stages I and II MALT lymphoma: a retrospective study. Radiother Oncol. 2008;87:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Vrieling C, de Jong D, Boot H, de Boer JP, Wegman F, Aleman BM. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiother Oncol. 2008;87:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Gobbi PG, Corbella F, Valentino F, Bergonzi M, Sangalli C, Perfetti V, Corazza GR. Complete long-term response to radiotherapy of gastric early-stage marginal zone lymphoma resistant to both anti-Helicobacter pylori antibiotics and chemotherapy. Ann Oncol. 2009;20:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Tomita N, Kodaira T, Tachibana H, Nakamura T, Mizoguchi N, Takada A. Favorable outcomes of radiotherapy for early-stage mucosa-associated lymphoid tissue lymphoma. Radiother Oncol. 2009;90:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Goda JS, Gospodarowicz M, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Tsang RW. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010;116:3815-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Okada H, Takemoto M, Kawahara Y, Nasu J, Takenaka R, Kawano S, Inoue M, Ichimura K, Tanaka T, Shinagawa K, Yoshino T, Yamamoto K. A prospective analysis of efficacy and long-term outcome of radiation therapy for gastric mucosa-associated lymphoid tissue lymphoma. Digestion. 2012;86:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Kim SW, Lim DH, Ahn YC, Kim WS, Kim SJ, Ko YH, Kim KM. Clinical outcomes of radiation therapy for early-stage gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol. 2013;19:6062-6068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Nam TK, Ahn JS, Choi YD, Jeong JU, Kim YH, Yoon MS, Song JY, Ahn SJ, Chung WK. The role of radiotherapy in the treatment of gastric mucosa-associated lymphoid tissue lymphoma. Cancer Res Treat. 2014;46:33-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Abe S, Oda I, Inaba K, Suzuki H, Yoshinaga S, Nonaka S, Morota M, Murakami N, Itami J, Kobayashi Y, Maeshima AM, Saito Y. A retrospective study of 5-year outcomes of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Jpn J Clin Oncol. 2013;43:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Lim HW, Kim TH, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom HS, Moon SH, Kim DY. Radiation therapy for gastric mucosa-associated lymphoid tissue lymphoma: dose-volumetric analysis and its clinical implications. Radiat Oncol J. 2016;34:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Ohkubo Y, Saito Y, Ushijima H, Onishi M, Kazumoto T, Saitoh JI, Kubota N, Kobayashi H, Maseki N, Nishimura Y, Kurosumi M. Radiotherapy for localized gastric mucosa-associated lymphoid tissue lymphoma: long-term outcomes over 10 years. J Radiat Res. 2017;58:537-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Teckie S, Qi S, Chelius M, Lovie S, Hsu M, Noy A, Portlock C, Yahalom J. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol. 2017;28:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Schmelz R, Miehlke S, Thiede C, Brueckner S, Dawel M, Kuhn M, Ruskoné-Formestraux A, Stolte M, Jentsch C, Hampe J, Morgner A. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages IE & II1E: a prospective randomized trial. J Gastroenterol. 2019;54:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Watanabe S, Ogino I, Hata M. Radiotherapy for non-gastric intestinal vs gastric MALT lymphoma: a comparison of treatment outcomes. Blood Res. 2020;55:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |