Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.782
Peer-review started: March 4, 2020
First decision: March 28, 2020
Revised: April 21, 2020
Accepted: May 26, 2020
Article in press: May 26, 2020
Published online: July 15, 2020
Processing time: 133 Days and 0.7 Hours
Gastric cancer is the second most common malignant tumor in China, ranking third among all malignant tumor mortality rates. Hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to increase significantly the effectiveness of intraperitoneal chemotherapeutic drugs, prolong the action time of these drugs on intraperitoneal tumor cells, and enhance their diffusion in tumor tissues. HIPEC may be one of the best choices for the eradication of residual cancer cells in the abdominal cavity.
The aim of this study was to study the role of preventive HIPEC after radical gastrectomy.
A prospective analysis was performed with patients with cT4N0-3M0 gastric cancer to compare the effects of postoperative prophylactic HIPEC plus intravenous chemotherapy with those of routine adjuvant chemotherapy. Patients’ medical records were analyzed, and differences in the peritoneal recurrence rate, disease-free survival time, and total survival time between groups were examined.
The first site of tumor recurrence was the peritoneum in 11 cases in the conventional adjuvant chemotherapy group and in 2 cases in the HIPEC group (P = 0.020). The 1-year and 3-year disease-free survival rates were 91.9% and 60.4%, respectively, in the conventional adjuvant chemotherapy group and 92.1% and 63.0%, respectively, in the HIPEC group. The 1-year and 3-year overall survival rates were 95.2% and 66.3%, respectively, in the conventional adjuvant chemotherapy group and 96.1% and 68.6%, respectively, in the HIPEC group. No significant difference in postoperative or chemotherapy complications was observed between groups.
In patients with cT4N0-3M0 gastric cancer, prophylactic HIPEC after radical tumor surgery is beneficial to reduce peritoneal tumor recurrence and prolong survival.
Core tip: This was a prospective analysis performed with patients with cT4N0-3M0 gastric cancer to compare the effects of postoperative prophylactic hyperthermic intraperitoneal chemotherapy plus intravenous chemotherapy with those of routine adjuvant chemotherapy. Prophylactic hyperthermic intraperitoneal chemotherapy after radical tumor surgery is beneficial to reduce peritoneal tumor recurrence and prolong survival.
- Citation: Xie TY, Wu D, Li S, Qiu ZY, Song QY, Guan D, Wang LP, Li XG, Duan F, Wang XX. Role of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer. World J Gastrointest Oncol 2020; 12(7): 782-790
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/782.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.782
China is the country with the largest number of new cases of gastric cancer in the world. Nearly 500000 new cases are diagnosed each year, accounting for 47% of cases worldwide[1]. Gastric cancer is the second most common malignant tumor in China, with a mortality rate of 22.04/100000, ranking third among all malignant tumor mortality rates[2]. The diagnostic efficacy for early gastric cancer is lesser in China than in Japan, Europe, and the United States[3], and most patients are diagnosed at an advanced stage[4]. Therefore, the 5-year survival rate for patients with gastric cancer in China is relatively low.
Local tumor recurrence significantly affects survival time, and the peritoneum is one of the most common sites of gastric cancer recurrence[5,6]. Peritoneal recurrence after radical surgery occurs in approximately 10%–54% of patients with gastric cancer[7,8]. Thus, improved removal of residual cancer cells in the peritoneal cavity represents a breakthrough for prolongation of the survival of patients with gastric cancer.
Hyperthermic intraperitoneal chemotherapy (HIPEC) involves the continuous pumping of low-tension liquid containing chemotherapeutic drugs heated to 42 °C-43 °C into the abdomen as a peritoneal lavage[9]. This treatment method has been shown to increase significantly the effectiveness of intraperitoneal chemotherapeutic drugs, prolong the action time of these drugs on intraperitoneal tumor cells, and enhance their diffusion in tumor tissues[10]. In addition, the toxicity of chemotherapeutic drugs towards tumor cells can be enhanced under heated conditions. For patients with residual peritoneal cancer after radical gastrectomy, systemic intravenous administration is not an efficacious means of delivering chemotherapy to tumor cells because residual cancer cells have not yet established a complete nourishing blood vessel[11]. At this time, HIPEC may be one of the best choices for the eradication of residual cancer cells in the abdominal cavity.
To determine whether HIPEC can reduce peritoneal recurrence in patients with gastric cancer after radical resection, we prospectively analyzed the medical records of patients with cT4N0-3M0 gastric cancer who underwent prophylactic HIPEC plus intravenous adjuvant chemotherapy and those who underwent postoperative routine adjuvant chemotherapy. Differences in the peritoneal recurrence rate, disease-free survival time, and total survival time were analyzed.
To study the role of preventive HIPEC after radical gastrectomy, a randomized, parallel prospective registry trial was conducted at the Chinese PLA General Hospital. Patients from the hospital’s Department of General Surgery were enrolled and allocated to the HIPEC and conventional adjuvant chemotherapy groups by envelope selection. Patients received postoperative HIPEC and intravenous (with or without oral) adjuvant chemotherapy or conventional adjuvant chemotherapy in addition to the base treatment. They were followed for 36 mo.
Patients with gastric adenocarcinoma were invited to participate in the present study, and the eligibility of willing patients was evaluated. Detailed information about the trial was provided to eligible patients, and written informed consent was obtained from all patients prior to enrollment. The stage of gastric cancer was confirmed using medical records, including data from endoscopic ultrasound (EUS), enhanced computed tomography (CT), magnetic resonance imaging (MRI), and diagnostic laparoscopic exploration. The 8th edition of the American Joint Committee on Cancer staging system guidelines were used for clinical stage classification. Patients with cancer stages cT4N0-3M0 were enrolled and allocated to the study groups.
The inclusion criteria were non-bedridden status; age 18–80 years; Eastern Cooperative Oncology Group physical condition score 0–1; preoperative histopathological confirmation of gastric adenocarcinoma; preoperative completion of enhanced CT/MRI, EUS, and/or diagnostic laparoscopic exploration showing cT4N0-3M0 clinical stage per the 8th edition of the American Joint Committee on Cancer staging guidelines; lack of severe underlying disease with expected survival < 3 years; and provision of written informed consent.
Exclusion criteria were: Pregnancy or lactation (pregnancy tests were administered to women of childbearing age); contraceptive use during the study period; receipt of chemotherapy, radiotherapy, or immunotherapy prior to study participation; history of other malignancies in the past 5 years; history of uncontrolled central nervous system disease, epilepsy, or mental disorder; refusal of treatment continuation; presence of adverse symptoms such as toxicity after treatment; risk of anastomotic leakage, anastomotic bleeding, or major abdominal bleeding; previous history of hematological disease; poor general condition; intolerance of HIPEC; severe neutropenia or myelosuppression; and intraoperative detection of multiple metastases or other causes of radical resection failure.
Patients were free to withdraw from the study at any time without having to provide a reason but with allowance of continued data collection.
In total, 137 patients were enrolled in this study. Seven patients were excluded due to previous histories of tumor outbreaks, and 17 patients were excluded due to intolerance of chemotherapy excretion studies. Thus, 113 patients were ultimately included; 51 patients were assigned to the postoperative HIPEC and intravenous (or oral and intravenous) adjuvant chemotherapy group and 62 patients were assigned to the conventional adjuvant chemotherapy group. Age, sex, and depth of tumor invasion did not differ significantly between groups (P > 0.05; Table 1). The study began in 2014. Patients with gastric cancer first underwent EUS, enhanced CT, MRI, and/or diagnostic laparoscopic exploration. Patients provided written consent to HIPEC after being informed about the need for the procedure and related risks.
| Characteristic | Conventional chemotherapy group, n = 62 | HIPEC group, n = 51 | P value |
| Sex | 0.887 | ||
| Male | 43 | 36 | |
| Female | 19 | 15 | |
| Age in yrmean ± SD | 61.5 ± 8.6 | 60.9 ± 7.1 | 0.682 |
| Operation method | 0.767 | ||
| Proximal gastrectomy | 4 (6.45) | 2 (3.92) | |
| Distal gastrectomy | 34 (54.84) | 24 (47.06) | |
| Total gastrectomy | 24 (38.71) | 25 (49.02) | |
| Pathological T staging | 0.502 | ||
| T3 | 6 (9.68) | 7 (13.72) | |
| T4 | 56 (90.32) | 44 (86.27) | |
| Pathological N staging | 0.656 | ||
| N0 | 8 (12.90) | 6 (11.76) | |
| N1 | 21 (33.87) | 17 (33.33) | |
| N2 | 26 (41.94) | 20 (39.22) | |
| N3 | 7 (11.29) | 8 (15.69) | |
| Tumor perforation | 0.276 | ||
| Yes | 23 (3.22) | 1 (1.96) | |
| No | 57 (91.94) | 43 (84.31) | |
| Chemotherapy | 0.458 | ||
| XELOX | 6 (9.68) | 3 (5.89) | |
| SOX | 56 (90.32) | 48 (94.12) | |
| Hospital stay, mean ± SD | 11.8 ± 2.8 | 13.4 ± 3.5 | 0.008 |
Patients in the two groups underwent laparoscopic-assisted radical gastrectomy prior to treatment. In the HIPEC group, four special drainage tubes were placed for peritoneal hyperthermic perfusion during surgery: One tube was placed in the liver and kidney crypt, one was placed in the splenic fossa, and two tubes were placed in the pelvic cavity. For patients in the HIPEC group with stable postoperative vital signs, HIPEC was started on the 1st or 2nd d after surgery.
A panel of trained and experienced surgeons conducted intraoperative HIPEC using the RanD Performer® HT perfusion device (RanD Co. Ltd., Florence, Italy). The open coliseum technique was adopted for optimal thermal homogeneity and spatial diffusion, with 50 mg cisplatin per liter of saline perfusate. The perfusion solution containing the chemotherapeutic drugs was heated to 42 °C–43 °C using an abdominal perfusion instrument. After the drainage tubes had been checked for peritoneal heat perfusion, the perfusate was injected into the abdominal cavity using the liver and kidney crypt or spleen drainage tube. At the same time, the perfusate was aspirated through the pelvic drainage tubes into the perfusion apparatus to be heated and subsequently pumped back into the abdominal cavity, creating a closed circuit. The entire lavage process lasted approximately 60 min. At the end of perfusion, the patency of the perfusion drainage tubes was checked, and the perfusate remaining in the abdominal cavity was aspirated through a pelvic drainage tube. Electrocardiography was performed, and oxygen saturation was monitored during and after perfusion. Blood gas analysis was performed, and patients’ coagulation profiles, electrolyte levels, and liver and kidney functions were assessed on the second day following abdominal perfusion.
Patients in the HIPEC group started oral and intravenous chemotherapy (capecitabine and oxaliplatin, XELOX) or tegafur gimeracil and oxaliplatin (SOX) combined oral–intravenous chemotherapy 6–8 wk after surgery. Patients in the conventional adjuvant chemotherapy group started XELOX or SOX chemotherapy at 4–6 wk after surgery and received a total of 6–8 cycles every 3 wk (Regimen: Oxaliplatin 130 mg/m2 ivgtt d1 + xeloda 1500 mg/m2 BID PO d1–15).
The following indicators were assessed: (1) Probability of peritoneal recurrence; (2) Disease-free survival time; (3) Total survival time; and (4) Complications. Recent complications were assessed during patients’ initial hospital stays, and long-term complications were assessed during the follow-up period. The other indicators were evaluated during outpatient and telephone follow-up consultations.
Whole-abdominal enhanced CT was performed every 3–6 mo during the first 2 years after surgery, every 6–9 mo at 2–3 years after surgery, and every year at 3–5 years after surgery. Endoscopy was performed once per year after surgery. Telephone follow-up consultation was performed once per month after surgery.
All data analyses were performed using the SPSS 22.0 software (Armonk, NY, United States). Continuous data are expressed as means and standard deviations and compared between groups using the t test. Categorical data are expressed as ratios or composition ratios and compared between groups using the χ2 test or Fisher’s exact test. Survival curves were generated and analyzed using the Kaplan–Meier and log rank tests. P < 0.05 was considered to indicate statistical significance.
The mean follow-up periods in the conventional adjuvant chemotherapy and HIPEC groups were 25.5 ± 11.4 and 27.3 ± 10.5 mo, respectively. In the conventional adjuvant chemotherapy group, 29 cases of tumor recurrence (11 in the peritoneum) occurred; in the HIPEC group, 11 cases of recurrence (2 in the peritoneum) occurred. The probability of peritoneal recurrence was significantly lesser in the HIPEC group than in the conventional adjuvant chemotherapy group (P = 0.020).
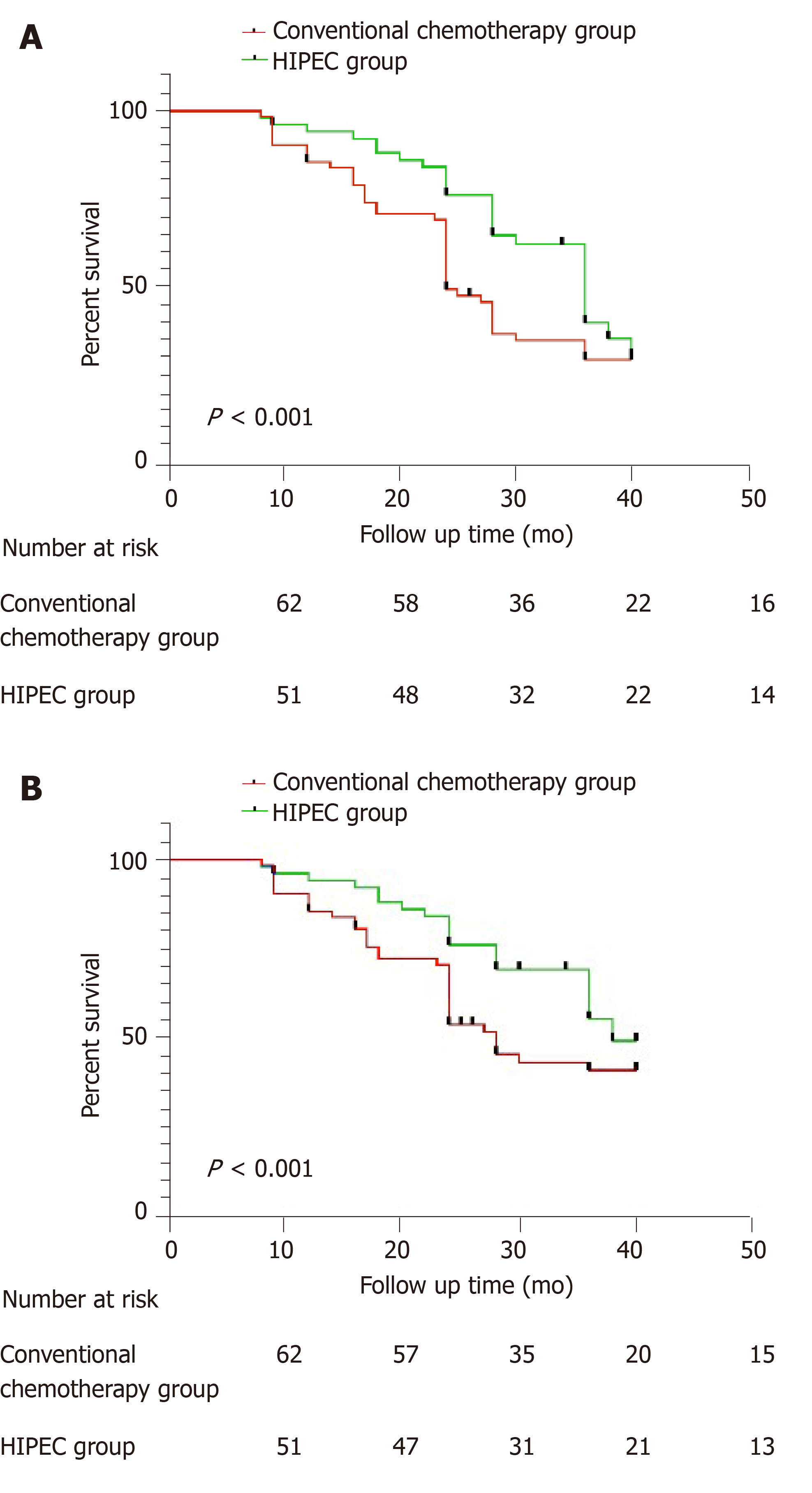
The 1-year and 3-year disease-free survival rates in the conventional adjuvant chemotherapy group were 91.9% and 60.4%, respectively, and those in the HIPEC group were 92.1% and 63.0%, respectively. The disease-free survival rate was significantly higher in the HIPEC group (P = 0.037; Figure 1A).
During the follow-up period, 24 patients in the conventional adjuvant chemotherapy group and 9 in the HIPEC group died. All deaths were due to tumor recurrence and multiple organ failure. The 1-year and 3-year overall survival rates in the conventional adjuvant chemotherapy group were 95.2% and 66.3%, respectively, and those in the HIPEC group were 96.1% and 68.6%, respectively. The overall survival rate was significantly higher in the HIPEC group (P = 0.044; Figure 1B).
No anastomotic leakage occurred in either group. Three cases of anastomotic hemorrhage occurred in the conventional adjuvant chemotherapy group, and two cases occurred in the HIPEC group (P > 0.05). Two cases of hemorrhage in the surgical field occurred in the conventional adjuvant chemotherapy group, and one case occurred in the HIPEC group (P > 0.05). Two cases of abdominal abscess were reported in the conventional adjuvant chemotherapy group, and one case was reported in the HIPEC group (P > 0.05). All patients with severe postoperative complications recovered successfully after conservative treatment, and none died. All complications occurred within 30 d of treatment. The incidences of other surgical and chemotherapy-related complications did not differ between groups (P > 0.05; Table 2).
| Complication | Conventional chemotherapy group | HIPEC group | P value |
| Anastomotic fistula | 0 (0.00) | 0 (0.00) | - |
| Anastomotic bleeding | 1 (1.61) | 1 (1.96) | > 0.05 |
| Abdominal hemorrhage | 1 (1.61) | 1 (1.96) | > 0.05 |
| Abdominal abscess | 2 (3.23) | 1 (1.96) | > 0.05 |
| Intestinal obstruction | 3 (4.84) | 4 (7.84) | > 0.05 |
| Diarrhea | 5 (8.06) | 6 (11.76) | > 0.05 |
| Cardiovascular abnormalities | 5 (8.06) | 7 (13.73) | > 0.05 |
| Pulmonary infection | 1 (1.61) | 2 (3.92) | > 0.05 |
| Urinary tract infection | 2 (3.23) | 3 (5.88) | > 0.05 |
| Electrolyte disturbance | 6 (9.68) | 8 (15.69) | > 0.05 |
| Myelosuppression | 7 (11.29) | 7 (13.73) | > 0.05 |
| Clavien-Dindo classification | |||
| I | 0 | 0 | - |
| II | 29 (46.8) | 37 (72.5) | > 0.05 |
| IIIa | 3 (4.84) | 2 (3.92) | > 0.05 |
| IIIb | 1 (1.61) | 1 (1.96) | > 0.05 |
| IVa | 0 | 0 | - |
| IVb | 0 | 0 | - |
| V | 0 | 0 | - |
HIPEC was first described in 1980 for the treatment of peritoneal tumors[11]. Compared with intravenous chemotherapy, its main advantages are that it increases the levels, duration, and infiltration of intraperitoneal chemotherapeutic drugs under heated conditions[12]. DNA denaturation and apoptosis of tumor cells are achieved without significant damage to normal cells[13]. Several randomized controlled trials have demonstrated that tumor depletion in conjunction with HIPEC for advanced gastric cancer with peritoneal metastasis can prolong survival. Moreover, the combination of cytoreductive surgery and HIPEC has been added to treatment guidelines for gastric cancer in recent years[14].
Although the therapeutic effect of HIPEC on advanced peritoneal tumors has been recognized, whether prophylactic HIPEC can reduce the probability of peritoneal recurrence in patients with gastric cancer and possible peritoneal metastasis has not been studied[15]. In the current study, a prospective trial design was used to determine the effects of HIPEC in patients at high risk of peritoneal metastases. In this population, HIPEC reduced peritoneal recurrence rate compared with conventional adjuvant chemotherapy alone. Moreover, the disease-free and total survival times were longer in the HIPEC group. These results suggest that prophylactic HIPEC is beneficial for patients with gastric cancer who are at risk of peritoneal metastasis.
Previous findings suggest that the use of cytoreductive surgery plus HIPEC increases the risks of postoperative anastomotic leakage, intestinal fistula development, and abdominal bleeding[16]. However, in this study, the incidence of complications did not differ significantly between groups. The underlying reason for this effect may be related to the general physical conditions of the included patients. Our patients experienced no significant systemic complication, and their tumors were deemed curable. The GASTRICHIP trial, which included 249 patients, yielded similar results. In the gastrectomy and HIPEC group, two patients died within 60 d, and the incidence of adverse events was 28.4%. In the radical gastrectomy group, three patients died within 60 d, and the incidence of adverse events was 26.2%. No significant difference was observed between groups, suggesting that HIPEC is safe and did not increase the incidence of perioperative mortality or adverse events. In contrast, previous studies have included patients with no chance of cure and poor overall general conditions. The findings of the present study suggest that HIPEC is safe and effective after radical surgery in strictly screened cases.
The main limitations of this study are as follows. First, the non-randomized controlled design may have led to patient selection bias. Moreover, the sample was relatively small. A prospective randomized controlled trial with a large sample is warranted and will be conducted to verify the conclusions of this study. We hope that this study will stimulate peers to design scientific experiments to study the therapeutic effects of preventive HIPEC.
In summary, for patients with cT4N0-3M0 gastric cancer, prophylactic HIPEC after radical surgery can reduce the probability of peritoneal recurrence and prolong disease-free and overall survival.
Gastric cancer is the second most common malignant tumor in China, with a mortality rate of 22.04/100000, ranking third in all malignant tumor mortality rates. Local recurrence of tumors seriously affects the survival time of patients, and the peritoneum is one of the most common sites of gastric cancer recurrence. Hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to increase significantly the effectiveness of intraperitoneal chemotherapeutic drugs, prolong the action time of these drugs on intraperitoneal tumor cells, and enhance their diffusion in tumor tissues. At this time, HIPEC may be one of the best choices for the eradication of residual cancer cells in the abdominal cavity.
To determine whether HIPEC can reduce peritoneal recurrence in patients with gastric cancer after radical resection, more multicenter prospective clinical trials should be completed to verify the role of HIPEC.
The aim of this study was to study the role of preventive HIPEC after radical gastrectomy.
The effects of postoperative prophylactic HIPEC plus intravenous chemotherapy and routine adjuvant chemotherapy for patients with cT4N0-3M0 gastric cancer were compared. Patients’ medical records were analyzed and differences in the peritoneal recurrence rate, disease-free survival time, and total survival time between groups were examined.
The first site of tumor recurrence was the peritoneum in 11 cases in the conventional adjuvant chemotherapy group and in 2 cases in the HIPEC group. In the conventional adjuvant chemotherapy group, the 1-year and 3-year disease-free survival rates were 91.9% and 60.4%, respectively, and they were 92.1% and 63.0% in the HIPEC group. In the conventional adjuvant chemotherapy group, the 1-year and 3-year overall survival rates were 95.2% and 66.3%, respectively, and they were 96.1% and 68.6% in the HIPEC group. No significant difference in postoperative or chemotherapy complications was observed between groups.
Prophylactic HIPEC after radical tumor surgery is beneficial to reduce peritoneal tumor recurrence and prolong survival for patients with cT4N0-3M0 gastric cancer.
We hope that this study will stimulate peers to design scientific experiments to study the therapeutic effects of preventive HIPEC. Considering the limitations of this study, more prospective randomized controlled trials with large sample sizes is warranted and will be conducted to verify the conclusions of this study.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Georgescu EF, Grotz TE, Rukavina M S-Editor: Wang JL L-Editor: Filipodia E-Editor: Liu MY
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12187] [Article Influence: 1523.4] [Reference Citation Analysis (3)] |
| 2. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 4. | Horiuchi Y, Fujisaki J, Yamamoto N, Ida S, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Yamamoto Y, Nagahama M, Takahashi H, Tsuchida T. Pretreatment diagnosis factors associated with overtreatment with surgery in patients with differentiated-type early gastric cancer. Sci Rep. 2019;9:15356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kashihara H, Shimada M, Yoshikawa K, Higashijima J, Tokunaga T, Nishi M, Takasu C. Risk factors for recurrence of gastric cancer after curative laparoscopic gastrectomy. J Med Invest. 2017;64:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, Weitz J, Rahbari NN. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6:35564-35578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wang H, Hu L, Zang M, Zhang B, Duan Y, Fan Z, Li J, Su L, Yan M, Zhu Z, Liu B, Yang Q. REG4 promotes peritoneal metastasis of gastric cancer through GPR37. Oncotarget. 2016;7:27874-27888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol. 2016;22:1114-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, Parisi A, Woo Y. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 11. | Ji ZH, Peng KW, Yu Y, Li XB, Yonemura Y, Liu Y, Sugarbaker PH, Li Y. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int J Hyperthermia. 2017;33:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Mielko J, Rawicz-Pruszyński K, Skórzewska M, Ciseł B, Pikuła A, Kwietniewska M, Gęca K, Sędłak K, Kurylcio A, Polkowski WP. Conversion Surgery with HIPEC for Peritoneal Oligometastatic Gastric Cancer. Cancers (Basel). 2019;11:1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Somashekhar SP, Yethadka R, Kumar C R, Ashwin KR, Zaveri S, Rauthan A. Toxicity profile of chemotherapy agents used in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies. Eur J Surg Oncol. 2020;46:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Brenkman HJF, Päeva M, van Hillegersberg R, Ruurda JP, Haj Mohammad N. Prophylactic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer-A Systematic Review. J Clin Med. 2019;8:1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Macrì A, Morabito F. The use of intraperitoneal chemotherapy for gastric malignancies. Expert Rev Anticancer Ther. 2019;19:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Beeharry MK, Zhu ZL, Liu WT, Yao XX, Yan M, Zhu ZG. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. 2019;19:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (2)] |