Published online Jun 15, 2020. doi: 10.4251/wjgo.v12.i6.604
Peer-review started: February 24, 2020
First decision: April 9, 2020
Revised: May 8, 2020
Accepted: May 29, 2020
Article in press: May 29, 2020
Published online: June 15, 2020
Processing time: 111 Days and 15.4 Hours
Colon cancer represents one of the most common cancers diagnosed in older adults worldwide. The standard of care in resected stage II and stage III colon cancer continues to evolve. While there is unequivocal evidence to suggest both disease free and overall survival benefits with the use of combination chemotherapy in patients with stage III colon cancer, data regarding its use in patients with stage II colon cancer are less clear. Further, although colon cancer is a disease that affects older adults, there is considerable debate on the value of adjuvant chemotherapy in the aging population. In particular, many older patients are undertreated when compared to their younger counterparts. In this review, we will describe the clinical trials that contributed to the current adjuvant chemotherapy approach in colon cancer, discuss representation of older adults in trials and the specific challenges associated with the management of this sub-population, and highlight the role of comprehensive geriatric assessments. We will also review how real-world evidence complements the data gaps from clinical trials of early stage colon cancer.
Core tip: Use of adjuvant chemotherapy consisting of fluoropyrimidines with/without oxaliplatin has improved survival outcomes for patients with resected colon cancer, especially in those with stage III disease. However, older adults are often not given the opportunity to benefit from chemotherapy after surgery because undertreatment may occur due to various patient and physician related factors. The issue is further compounded by the limited representation of older adults in clinical trials, resulting in underpowered subgroup analyses that do not provide conclusive answers regarding the utility of adjuvant chemotherapy in the advanced age group. We herein review the role of adjuvant chemotherapy in early stage colon cancer, specifically in the context of the older subpopulation, by focusing on both data from clinical trials and data from real-world evidence sources.
- Citation: Batra A, Rigo R, Sheka D, Cheung WY. Real-world evidence on adjuvant chemotherapy in older adults with stage II/III colon cancer. World J Gastrointest Oncol 2020; 12(6): 604-618
- URL: https://www.wjgnet.com/1948-5204/full/v12/i6/604.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i6.604
Colon cancer is the fourth most frequently diagnosed cancer in the world, with approximately 1.1 million new cases diagnosed annually[1]. The median age at diagnosis is 67 years, making it the third most common cancer diagnosis in older patients[1,2].
The definitive treatment for localized colon cancer is surgery, whereby the risk of recurrence increases as the local extent of disease increases[3]. Adjuvant chemotherapy is targeted at occult metastasis and it is recommended in stage II disease with high-risk features, and stage III colon cancer[4-6]. Available treatment options include single agent fluoropyrimidine (5-FU or capecitabine) and combination chemotherapy consisting of a fluoropyrimidine with oxaliplatin (FOLFOX or CAPOX) for 3 to 6 months. These guidelines are based on data from phase III randomized controlled trials[7-11]. However, most of these clinical trials were limited by stringent inclusion and exclusion criteria, which precluded the broad participation of older adults[12].
Apart from age related decline in organ functions, older adults are more likely to have comorbid conditions, which can potentially complicate the administration of adjuvant chemotherapy[13,14]. However, age is not a robust surrogate marker for functional status and should not be used as a sole criterion to exclude patients from receiving treatment. A comprehensive geriatric assessment (CGA) can provide a better overall assessment of the functional status of older adults to guide the use of chemotherapy[15].
Meanwhile, real-world evidence refers to the analysis of data collected from routine clinical practice, outside the context of trials[16]. To date, real-world studies have also identified that older adults are less likely to receive adjuvant chemotherapy for colon cancer even though they stand to derive some benefit from treatment[17-20].
In this review, we will discuss the standard of care for stage II and III colon cancer, participation of older adults in clinical trials, specific challenges in the management of this population, and summarize data from clinical trials and data from real-world evidence on the use of adjuvant chemotherapy in older adults.
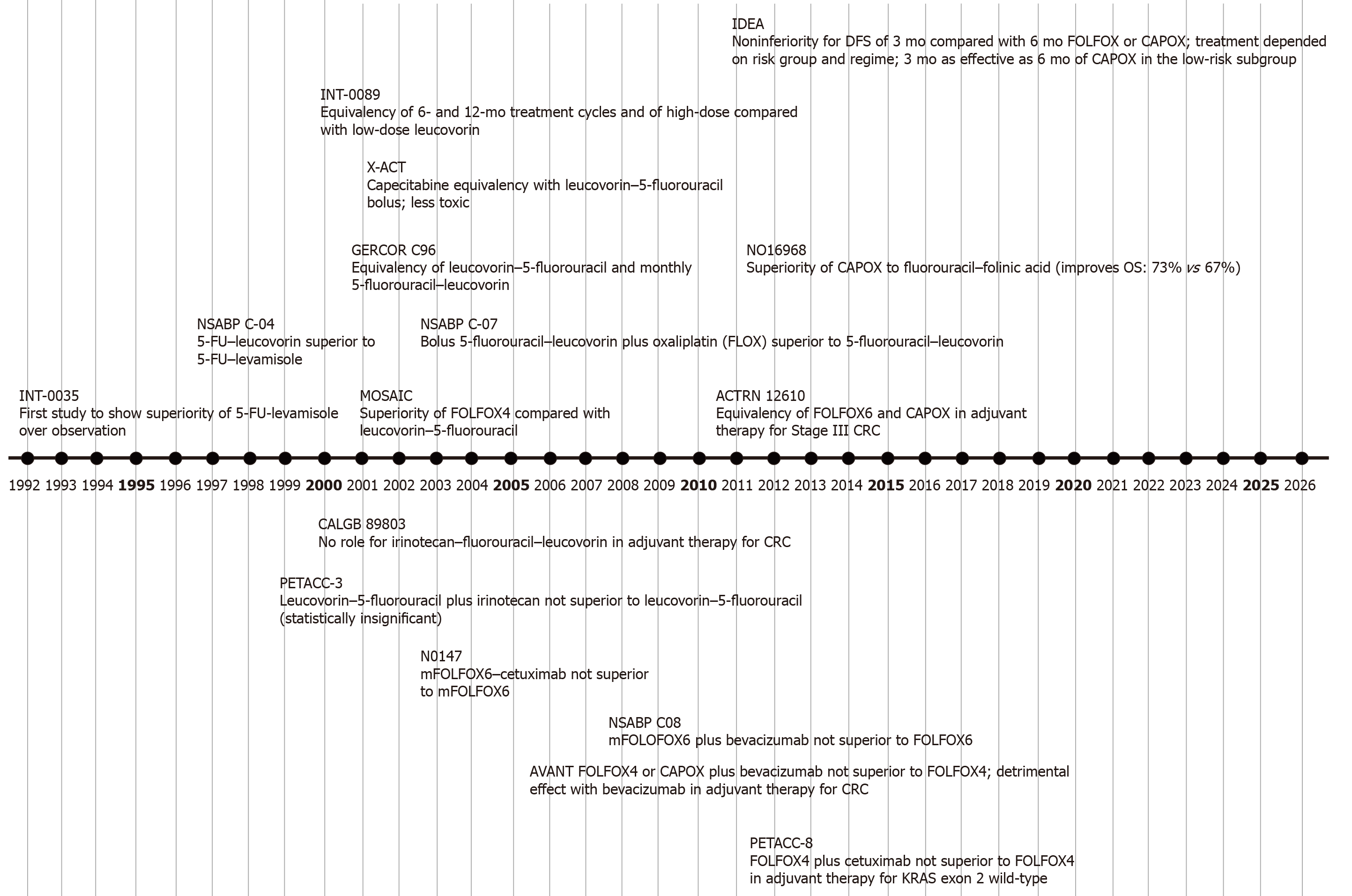
The benefit of adjuvant chemotherapy with 5-FU and levamisole was first apparent in a small randomized trial from the late 1980s[21,22]. The National Surgical Adjuvant Breast and Colon Project (NSABP) C-01 study and a subsequent meta-analysis further confirmed this disease free survival (DFS) benefit[23,24]. This was followed by another study that observed a 40% reduction in recurrence and a 33% improvement in overall survival (OS) among patients with stage III colon cancer who were treated with 12 months of 5-FU and levamisole[25], resulting in this being established as the standard adjuvant chemotherapy for colon cancer (Figure 1).
The next decade of treatment was marked by the eventual omission of levamisole due to its inefficacy, and the modulation of 5-FU and leucovorin into different regimens, including the “Mayo” (bolus low-dose LV and 5-FU daily × 5), “Roswell Park” (weekly high-dose LV and bolus 5-FU), and “de Gramont’s LV5FU2” (LV and bolus 5-FU plus infusion)[26-29]. Although none of the three regimens proved to be superior, LV5FU2 became the most preferred regimen because it was significantly better tolerated than the bolus regimens[28,29].
The standard duration of adjuvant chemotherapy was subsequently reduced from 12 to six months after the Intergroup-0089 and the GERCOR (French Oncology Research Group) C97-2 trials established non-inferiority of 6 months bolus 5FU regimens or LV5FU2, when compared to 12 months of 5FU/levamisole and 9 months of LV5FU2, respectively.
The X-ACT (Xeloda in Adjuvant Colon Cancer Therapy) and NSABP C-06 studies evaluated the efficacy of oral fluoropyrimidines as compared to bolus 5-FU regimens. Both capecitabine and tegafur had similar DFS and OS, and they were also better tolerated than bolus 5-FU[9,30].
Three landmark trials established the role of combination chemotherapy with oxaliplatin and a fluoropyrimidine (Table 1)[8,10,31]. The MOSAIC (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin) study compared six months of adjuvant FOLFOX4 with LV5FU2 in patients with stage II and III colon cancer, and showed a 7.5% improvement in five-year DFS [hazard ratio (HR) = 0.78; P = 0.005], and 4.2% improvement in six-year OS (HR = 0.80; P = 0.023) with the addition of oxaliplatin in those with stage III disease[32]. Although there was a modest benefit in DFS, there was no OS benefit in stage II colon cancer at six and ten years, respectively[7,32]. Based on this trial, six months of adjuvant chemotherapy with FOLFOX became the new standard of care for patients with stage III colon cancer.
| Clinical Trial | Regimen | Patients (n/age) | Stage | DFS | OS |
| 5-yr | 6-yr | ||||
| MOSAIC, 2004 | FOLFOX4 vs LV5FU2 | 2246 | II/III | 73.3% vs 67.4% | 72.9% vs 68.7% |
| Age 18-75 | 40% Stage II | For stage III | |||
| 778, > 65 yr (34.6%) | No benefit for stage II at 6-yr and 10-yr | ||||
| 4-yr | 5-yr | ||||
| NSABP-C-07, 2007 | Weekly bolus 5-FU/LV ± Oxaliplatin (FLOX) | 2407 | II/III | 73.2% vs 67.0% | N/A |
| 396, > 70 yr (16.4%) | 695 Stage II (28.8%) | Unplanned subset analyses suggested no benefit for patients > 70 yr (71.6% vs 76.3%) | |||
| 7-yr | 7-yr | ||||
| NO16968, 2015 | CAPOX (XELOX) vs 5-FU/Leucovorin (C. Mayo/R. Park) | 1886 | III | 63% vs 56% | 73% vs 67% |
| 409, > 70 yr (21.6%) | HR = 0.86 (95%CI: 0.64-1.16) | HR = 0.91 (95%CI: 0.66 TO 1.26) | |||
The NSABP C-07 and NO16968 trials demonstrated similar DFS and OS benefits with the addition of oxaliplatin to bolus 5-FU/leuvovorin or capecitabine (CAPOX), when compared to bolus 5-FU/leucovorin[8,10]. While the C-07 study included patients with stage II and III colon cancer, the latter study exclusively focused on those with stage III disease.
Although irinotecan is active in advanced settings, its addition to 5-FU/leucovorin as adjuvant treatment in stage II and III colon cancer did not show any incremental benefit across three different randomized trials[33-36]. Furthermore, the addition of bevacizumab or cetuximab to FOLFOX was also not associated with any improvements in DFS or OS[37-40].
The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration performed a pooled analysis of 12834 patients enrolled across six randomized trials in order to evaluate the non-inferiority of three months of adjuvant CAPOX or FOLFOX therapy as compared to six months of treatment in stage III colon cancer[11]. Although non-inferiority could not be confirmed in the overall population, three months of therapy was non-inferior to six months in lower risk patients (T1-3 and N1), particularly with CAPOX. Notably, in patients with T4 and/or N2 disease, six months of chemotherapy was superior to three months. Since these results were preliminary and OS data are not yet mature, both three and six months of adjuvant chemotherapy in low risk stage III colon cancer remain reasonable options. However, six months continues to be the standard duration when using single agent fluoropyrimidines.
Although the benefit of adjuvant chemotherapy has been proven in clinical trials of patients with stage III colon cancer, the more modest DFS and OS benefits seen in stage II disease has prompted the need to categorize patients into low and high risk groups based on clinico-pathological factors[23,41]. The four randomized clinical trials that included predominantly stage II colon cancer patients failed to show consistent benefits of adjuvant chemotherapy[42-45]. A detailed description of the role of adjuvant chemotherapy for stage II colon cancer is beyond the scope of this review. However, there is general agreement that adjuvant treatment with a fluoropyrimidine may benefit some patients with resected stage II colon cancer when specific high features are present. These high-risk features typically include, but are not limited to, colonic obstruction or localized perforation at presentation, poorly differentiated histology, T4 lesion, fewer than 12 nodes sampled, perineural, vascular or lymphatic invasion, and close/indeterminate or positive margins[4-6]. The role of microsatellite instability (MSI) in decision-making for adjuvant chemotherapy is still emerging; however, data suggest a high risk of treatment resistance to single agent fluoropyrimidines in MSI-high tumors[46-48]. While additional benefit of oxaliplatin has not been proven in patients with stage II colon cancer, this is likely to overcome fluoropyrimidine resistance in those with MSI-high status, based on a small exploratory analysis from the MOSAIC trial[7].
Despite cancer being a disease of advanced age, older patients are under-represented in the majority of clinical trials[49-52]. A paradox exists whereby patients aged greater than 65 years constitute approximately 70% of the overall population with colorectal cancer, but this age group represents only 30% of clinical trial participants[52]. The main reasons that many older patients with colorectal cancer are excluded from studies include: previous or concomitant cancer treatments, comorbid conditions or medications, and poor performance status[53]. Interestingly, one-third of “eligible” older patients are still not invited to participate in trials, driven by the observation that physicians are less likely to discuss and/or offer clinical trials to older patients with cancer[53,54].
There has been little change in this regard over last two decades, even though representation of other marginalized sub-groups such as women and ethnic minorities has improved[55-58]. An upper numerical age limit continues to be a common exclusion factor across contemporary clinical trials, despite recommendations by many major international societies that encourage the recruitment of older patients and the adoption of criteria based on physiological age rather than chronological age[59,60].
There are well described age-related deteriorations in organ function including in the hepatic, renal, cardiovascular, central nervous, and hematopoietic systems[61,62]. This leads to loss of physiologic reserve, which decreases the threshold for decompen-sation when faced with stressors, such as chemotherapy. Declining hepatic and renal function can potentially expose older patients to oxaliplatin and capecitabine at higher peak concentrations for a longer duration, respectively[62]. Moreover, due to potential muscle loss associated with aging, serum creatinine is a less reliable marker to assess renal function[63,64]. Similar deteriorations in bone marrow reserve that come with aging can potentiate chemotherapy dose reductions and delays, and also exacerbate the risk of febrile neutropenia in older adults[61].
Patients older than 75 years have a median of five comorbid medical conditions. These may include anemia, cardiovascular disease, chronic obstructive airway disease, diabetes, previous cancer, and renal disease[13,14]. Charlson’s comorbidity index is one of the available tools to quantify these comorbidities, and a score of more than 2 has been independently associated with an increase in mortality among patients with colorectal cancer[65,66]. The interaction of comorbid conditions with the administration of adjuvant chemotherapy is rather complex. While the presence of severe comorbid conditions may outweigh the risk associated with adjuvant chemotherapy, patients with multiple well-control medical conditions are still likely to benefit from cancer treatment[67]. The presence of comorbid conditions can also increase the toxicity due to chemotherapy. Capecitabine, for example, can cause severe side effects in the presence of renal dysfunction, as it is excreted by the kidneys[68]. Moreover, capecitabine and 5-FU can lead to life-threatening coronary vasospasms that can result in further deterioration of cardiac function in those with pre-existing cardiovascular disease[69,70].
There are significant age-based alterations in pharmacokinetics of chemotherapeutic drugs. For example, the absorption of capecitabine may be affected due to worsening splanchnic blood flow, secretion of digestive enzymes, and gut motility among older patients[71]. The distribution of drugs is also affected by an increase in fat content and a decrease in intracellular water with advanced age, leading to a reduction in the peak concentration and contributing to longer half-lives of drugs[72]. Further, declining hepatic and renal function can affect the metabolism and excretion of chemotherapeutic drugs[61,62].
Cancer in older individuals develops from senescent cells, which are unable to undergo apoptosis, and hence, these cells are more likely to be resistant to chemotherapeutic agents that act by apoptosis[73]. Decreased angiogenesis in tumors of older adults may also lead to decreased delivery of drugs to the neoplastic cells[74]. Moreover, reduced levels of dehydropyrimidine dehydrogenase in older patients may cause delayed clearance of fluoropyrimidines, subsequently leading to increased adverse events with the use of capecitabine or 5-FU[75].
While 90% of the older population uses at least one medication, the average number of drugs is four per patient[76]. This predisposes older patients to potentially harmful drug interactions, which can negatively impact adherence to additional chemotherapy pills (capecitabine), as well as oral supportive medications that combat chemotherapy related adverse effects[77,78].
Quality of life should always be considered in treatment decision making, but it is particularly prioritized in older patients since aggressive use of chemotherapy may compromise their daily activities and overall wellbeing. There are limited data that accurately describe the effect of chemotherapy on the quality of life of older patients with colorectal cancer[79]. However, there is recognition that older adults are less willing to endure the side-effects of chemotherapy, as compared to younger patients[80]. This should not be presumed for all older patients with cancer and individual preferences must be considered when discussing the benefits and risks of chemotherapy. Further, increased anxiety and depression have been reported in older individuals at diagnosis of cancer[81]. However, older adults who know about the diagnosis and prognosis of cancer are able to better cope with the anxiety compared with those who do not know the details[82] Therefore, a well-informed decision-making process with older adults is likely to improve the psychosocial aspects and better maintain quality of life.
Chronological age is a poor surrogate of physiologic and functional status of older patients[83]. Performance status (PS) as assessed by physicians is commonly employed in oncology to ascertain the functional status of patients; the most validated scales include the Eastern Cooperative Oncology Group PS (ECOG PS), and Karnofsky PS (KPS)[84,85]. In general, patients with ECOG PS > 2 and KPS < 60 are considered to be poor candidates for chemotherapy. However, data suggest that clinician assessments of functional status tend to underestimate the true functional status of older patients[86].
A CGA involves a multidimensional evaluation of older patients, including their functional status, comorbid medical conditions, cognition, nutritional status, psychological state, socio-economic condition, and a record of the patient's medications[87]. The purpose of a CGA is to identify frail older patients who may be able to tolerate chemotherapy, as well as fit older patients who may have a higher likelihood of developing chemotherapy related side effects. Several studies have demonstrated how CGA can add information to the conventional ECOG PS in older patients[15,88,89], help in cancer treatment decision making[15,90], predict complications and adverse events from chemotherapy[91-94], and improve pain control, mental health, and well-being[95]. Although there is no uniform CGA tool, various models have been implemented in different settings that encompass the core domains of the assessment[96]. A combination of a self-administered questionnaire and a healthcare provider assessment is most often utilized. There is considerable debate as to which older patients actually benefit from the resource intensive CGA. In response to this, pre-CGA screening tools have also been developed[97-99]. However, a systematic review concluded that there is limited sensitivity and specificity of these tools[100]. The consensus guidelines by the American Society of Clinical Oncology (ASCO) and the International Society of Geriatric Oncology (SIOG) recommend the routine use of CGA in patients aged more than 65 years with cancer, but to date there has been variable adoption of CGA in busy routine clinical practices[101,102].
Due to poor representation in clinical trials, evidence for the efficacy of fluoropyrimidines as adjuvant chemotherapy in stage III colon cancer among older adults is derived mainly from pooled analysis of individual patient-level data from randomized trials, and data from population-based real-world studies. In particular, there was a large pooled analysis that included information from 3351 patients with stage II or III colon cancer from seven randomized phase III clinical trials evaluating the role of 5-FU (five with leucovorin, and two with levamisole), which categorized patients into four groups according to age (< or = 50, 51 to 60, 61 to 70, and > 70 years)[103]. Adjuvant treatment was associated with improved OS (HR = 0.76; 95%CI: 0.68-0.85, P < 0.001), superior time to recurrence (HR = 0.68; 95%CI: 0.60-0.76; P < 0.001), and better 5-year OS (64% vs 71%). There were 506 patients aged more than 70 years, and no significant interaction was observed between age and treatment efficacy. Moreover, the incidence of adverse events was similar in the four age groups, except there was a higher incidence of leukopenia in patients older than 70 years. Although this analysis suggests similar benefits of chemotherapy in older patients as compared to their younger counterparts, findings were limited by the representation of only fit older patients who met the stringent inclusion criteria of clinical trials[22,25,41,104,105].
Population-based studies complement clinical trials by providing information regarding the efficacy of adjuvant chemotherapy with 5FU in unselected patients in the real-world setting (Table 2). One such study analyzed data of patients aged more than 65 years diagnosed with stage III colon cancer from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and Medicare[17]. Of the 4738 patients included in that analysis, 52% received adjuvant chemotherapy, and there was a 34% (HR = 0.66; 95%CI: 0.60-0.73) relative reduction in mortality with 5-FU based treatment. In a subsequent published study that used the same database, 51% (3672 out of 7182) patients aged more than 65 years received 5FU based adjuvant chemotherapy within 6 months of surgery[106]. In that study, patients were categorized into five age groups (66-69, 70-74, 75-79, 80-84, and > 85 years) to analyze the effect of increasing age. While patients in all age groups derived benefit from adjuvant chemotherapy, the magnitude was not uniform across all ages whereby patients aged 70-74 years experienced 14% greater survival when compared to 8% in patients aged 80 to 84 years.
| Ref. | Study design | n/stage | Age selection | Treatment arms/parameters | Conclusion |
| Sargent et al[103], 2001 | Pooled analysis 7 trials | 3351, stage II/III | ≤ 50, 51- 60, 61-70, and > 70 yr | Fluorouracil plus leucovorin or levamisole vs surgery alone | Same benefit from fluorouracil-based adjuvant therapy as their younger counterparts |
| Sundararajan et al[17], 2002 | Retrospective cohort | 4768, node-positive | 65 years of age or older | Association of 5-FU adjuvant therapy with survival | 5-FU therapy is significantly associated with reduced mortality in older patients |
| Zuckerman et al[106], 2009 | Observational, retrospective cohort | 3016, stage III | aged 66 and older | 5-FU or leucovorin within 6 mo after surgery | Elderly patients had a significant survival benefit associated with adjuvant chemotherapy |
| Jessup et al[107], 2005 | Prospective data | 85934, stage III | < 60, 60-69. 70-79, > 80 yr | Adjuvant chemotherapy usage and 5-yr survival | Elderly patients have the same benefit as younger patients but are less frequently treated |
| Neugut et al[108], 2006 | Retrospective database | 1722, stage III | ≥ 65 years of age | Early discontinuation of FU-based chemotherapy | High percentage of early treatment discontinuation in the elderly population |
| Dobie et al[109], 2006 | Retrospective SEER analysis | 3193, stage III | 65 years and older | Adjuvant chemotherapy completion and its relation to 3-year cancer mortality | Incomplete adjuvant chemotherapy associated to physical frailty and treatment complications |
| McCleary et al[12], 2013 | Pooled analysis, ACCENT group | 14528, stage II/III | age < 70 and age ≥ 70 years | Impact of age on colon cancer recurrence and mortality after adjuvant therapy | Reduced benefit from adding oxaliplatin to fluoropyrimidines in the adjuvant setting for patients > 70 years |
| Meyers et al[114], 2017 | Systematic review | Stage II and III | age < 70 and age ≥ 70 years | Benefit from adjuvant chemotherapy (5-fluorouracil /leucovorin/ oxaliplatin or capecitabine/ oxaliplatin) | Patients with high-risk stage II disease may benefit from adjuvant chemotherapy |
| Green et al[117], 2019 | Retrospective SEER-Medicare database analysis | 31990, stage II/III | Aged 66-69, 70-74, 75-79, 80-84, 85-89 and 90+ | Use and outcomes of adjuvant chemotherapy | Administration of adjuvant chemotherapy for colon cancer decreases with advancing age, but improved outcomes are seen in stage III patients under 90 years of age |
| Sanoff et al[121], 2012 | Retrospective data source analysis | 4060, stage III | < 50, 50-64, 65-69, 70-74 yr | Oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy | The addition of oxaliplatin to 5-FU appears to be associated with better survival among patients receiving adjuvant colon cancer treatment in the community |
Another study that analyzed the use and efficacy of adjuvant chemotherapy in 85,934 patients across all ages with stage III colon cancer residing in community settings concluded that the benefit of adjuvant chemotherapy was similar in patients aged more than 80 years[107].
Data from similar studies that examined the proportion of patients who completed the planned duration of chemotherapy suggest that one-third of older patients discontinued adjuvant treatment prematurely[108,109]. Apart from advanced age, other factors associated with early termination of chemotherapy include physical frailty, treatment complications, and lack of social and psychological support.
In the previously described X-ACT trial that randomized stage III colon cancer patients to either capecitabine or 5-FU/leucovorin, 396 of 1987 enrolled patients were older than 70 years. Although older patients required more frequent dose modifications of capecitabine when compared to younger patients (65% vs 55%), capecitabine was non-inferior to 5-FU/leucovorin in the subgroup analysis involving patients aged more than 70 years [5-year OS, 68.8% and 65.0% (HR = 0.91, 95%CI: 0.65-1.26)][9]. However, capecitabine should still be used with caution in very old patients because of the increased toxicity associated with declining renal function[110]. The efficacy and safety data for UFT in older adults are very limited. A small trial of 63 patients aged greater than 70 years suggests a favorable toxicity profile[111].
Unlike fluoropyrimidines, the benefit of adding oxaliplatin in the adjuvant treatment of older patients with resected colon cancer is more controversial. The main driver for this is the under-representation of the older population in the landmark MOSAIC and NSABP C-07 trials[112,113]. The MOSAIC trial excluded those older than 75 years, and only 14% (315 of 2246) of patients were aged 70 to 75 years so the subgroup analysis was underpowered to show any benefit of FOLFOX as compared to 5FU/leucovorin. The HRs for DFS and OS were 0.93 (95%CI: 0.64 to 1.35) and 1.10 (95%CI: 0.73 to 1.65), respectively[112]. While the NSABP C-07 study did not apply an upper age limit to enrollment, only 14% of the participants were aged more than 70 years. Similar to MOSAIC’s subgroup analysis, there was no observed DFS and OS advantage with the addition of oxaliplatin among patients older than 70 years[113]. Moreover, the Adjuvant Colon Cancer End Points (ACCENT) group analyzed the pooled data of 14528 patients from seven randomized trials. Of these, 2575 patients were older than 70 years. Although there was a 3-year DFS benefit with combination chemotherapy, no incremental OS benefit was seen in older patients[12]. Likewise, a systematic review published by Cancer Care Ontario concluded little additional benefit of oxaliplatin in patients older than 70 years of age[114].
In contrast, the NO16968 trial randomized 1886 patients with stage III colon cancer to CAPOX or 5-FU/leucovorin. There was a preplanned subgroup analysis which demonstrated a similar magnitude of benefit in younger and older patients [10]. Furthermore, a pooled analysis of four clinical trials evaluated the role of oxaliplatin in patients aged more than 70 years[115]. This particular analysis excluded the MOSAIC study because of inaccessibility to patient level data and the NSABP C-07 study due to its use of an outdated regimen. A total of 4819 patients from four randomized trials were examined of whom 904 patients were more than 70 years old[9,10,37,38]. It concluded that the addition of oxaliplatin improved DFS and OS in both younger and older patients, although there was a modest attenuation in the survival benefit (DFS, < 70 years: HR = 0.68; 95%CI: 0.61-0.76; P < 0.0001; > 70 years: HR = 0.77; 95%CI: 0.62-0.95; P = 0.014).
With the increasing number of patients that are considered “older”, the significance of real-world evidence and its capacity to complement randomized trials are increasingly being recognized[116]. However, similar to the case with clinical trial data, results from real-world evidence studies that analyzed the outcomes of oxaliplatin in older patients are conflicting. The largest contemporary real world study of patients older than 65 years included 31990 patients with stage II/III colon cancer from the SEER/Medicare database and grouped patients by age at five year intervals[117]. Overall, there was a gradual decline in the use of adjuvant chemotherapy, ranging from 57% in 66-69 years old to 1% in those aged more than 90 years. While the benefit of adjuvant chemotherapy was seen in all patients less than 90 years, those with stage II disease had increased mortality with adjuvant chemotherapy. Of note, the SEER/Medicare does not include data on high risk features of stage II colon cancer, so this finding should be interpreted with caution. Likewise, the authors did not perform an analysis based on type of chemotherapy because there was poor sensitivity in identifying specific chemotherapeutic agents[118]. In other studies that linked with SEER/Medicare database, oxaliplatin in stage III colon cancer demonstrated OS benefit in the age group of 70-74 years old (HR = 0.66; 95%CI: 0.52-0.84)[119]. However, there was no significant incremental benefit of oxaliplatin in patients older than 75 years (HR = 0.84; 95%CI: 0.69-1.04)[120].
While there is no clear consensus regarding the impact of combination chemotherapy in older adults, data on its safety are reassuring in that there were no increases in emergency department use, hospitalizations, and early deaths[121]. In patients older than 75 years, the odds of developing chemotherapy induced nausea and vomiting [odds ratio (OR) = 2.14; 95%CI: 1.73-2.65), as well as neutropenia (OR = 17.3; 95%CI: 9.80-30.42) were higher in patients receiving FOLFOX as compared to 5FU alone. However, no differences were observed in rates of diarrhea, dehydration, infection, or acute coronary events.
The benefit of adjuvant chemotherapy in resected stage II colon cancer is debatable, with most guidelines recommending that it be considered only in patients with high risk features. Moreover, data of older patients with stage II colon cancer are scarce, with the majority of current treatment recommendations extrapolated from those in younger patients. A SEER/Medicare database study identified 24847 patients aged more than 65 years with stage II colon cancer, of whom 75% had at least one high risk feature, and 20% of these received adjuvant chemotherapy. There was no difference in 5-year OS in treated and untreated patients (HR = 1.03; 95%CI: 0.94-1.13; P = 0.47)[122]. This raises the question of benefit of adjuvant chemotherapy in older adults with stage II colon cancer. The SIOG recommendations on treatment of older adults with stage II colon cancer acknowledge the limited data in this clinical situation[123]. However, older age by itself should not be a sole exclusion criterion to offer adjuvant chemotherapy in stage II colon cancer with high-risk features. Thus, a discussion with older patients with high-risk stage II colon cancer regarding a small potential benefit and possible toxicities must be conducted while considering the patients’ preferences.
Underrepresentation of older patients in clinical trials of adjuvant chemotherapy for stage II and III colon cancer has contributed to significant gaps in our understanding of the true benefit of such therapy. However, real-world evidence and pooled analysis from these clinical trials have allowed oncologists to arrive at some agreement. In older patients with stage II colon cancer, there are limited data to suggest efficacy of adjuvant chemotherapy, even among those with high-risk features. In contrast, single agent fluoropyrimidine appears to provide similar benefit in older patients as younger patients. There are contradicting data on the incremental value of adding oxaliplatin to fluoropyrimidines in stage III colon cancer. This is largely because most of the analyses were either post hoc (exploratory) in design or underpowered to reach definitive conclusions. Likewise, available data from real-world evidence are limited by inherent selection bias and confounding by indication. However, further real-world evidence using novel statistical methods eliminating such confounding and biases is likely to shine further light on controversies which are unlikely to be resolved by future clinical trials. As we move forward, the integration of CGAs of older adults may represent a more useful and reliable method to select appropriate older patients for adjuvant chemotherapy. This is due to the observation that chronological age is a poor proxy for functional status and should not be used as a standalone factor for treatment decision-making since it may unintentionally deny older patients the potential benefits of adjuvant chemotherapy.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Clinical Oncology; European Society for Medical Oncology; Canadian Association of Medical Oncologists.
Specialty type: Oncology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouvier AM, Hidaka E S-Editor: Gong ZM L-Editor: A E-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | SEER. Cancer of the Colon and Rectum - Cancer Stat Facts [Internet]. [accessed 14 February 2020]. Available from: URL: https://seer.cancer.gov/statfacts/html/colorect.html. |
| 3. | Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am. 2008;37:1-24, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64-vi72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 650] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 5. | Costas-Chavarri A, Nandakumar G, Temin S, Lopes G, Cervantes A, Cruz Correa M, Engineer R, Hamashima C, Ho GF, Huitzil FD, Malekzadeh Moghani M, Sharara AI, Stern MC, Teh C, Vázquez Manjarrez SE, Verjee A, Yantiss R, Shah MA. Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J Glob Oncol. 2019;5:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | National Comprehensive Cancer Network. Colon Cancer (Version 1.2020). [accessed 14 February 2020]. Available from: URL: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 7. | André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T, Tabernero J, Van Laethem JL, Banzi M, Maartense E, Shmueli E, Carlsson GU, Scheithauer W, Papamichael D, Möehler M, Landolfi S, Demetter P, Colote S, Tournigand C, Louvet C, Duval A, Fléjou JF, de Gramont A. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol. 2015;33:4176-4187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 395] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 8. | Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 9. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kröning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schüller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 860] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 10. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 11. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 686] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 12. | McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, O'Connell M, Twelves CJ, Saltz LB, Haller DG, Sargent DJ. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, Yates JW. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123-2134. [PubMed] |
| 14. | Yancik R, Ries LA. Cancer in older persons. Magnitude of the problem--how do we apply what we know? Cancer. 1994;74:1995-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Kenis C, Bron D, Libert Y, Decoster L, Van Puyvelde K, Scalliet P, Cornette P, Pepersack T, Luce S, Langenaeken C, Rasschaert M, Allepaerts S, Van Rijswijk R, Milisen K, Flamaing J, Lobelle JP, Wildiers H. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24:1306-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Khozin S, Blumenthal GM, Pazdur R. Real-world Data for Clinical Evidence Generation in Oncology. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 378] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Kahn KL, Adams JL, Weeks JC, Chrischilles EA, Schrag D, Ayanian JZ, Kiefe CI, Ganz PA, Bhoopalam N, Potosky AL, Harrington DP, Fletcher RH. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002;20:3992-3998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Fluorouracil as an adjuvant to colorectal cancer surgery; the breakthrough that never was. JAMA. 1976;236:1935-1936. [PubMed] |
| 22. | Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, McCormack GW, Gerstner JB, Krook JE, Malliard J. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 481] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, Jones J, Glass A, Lerner H, Lawrence W. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 251] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA. 1988;259:3571-3578. [PubMed] |
| 25. | Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1678] [Cited by in RCA: 1625] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 26. | Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, Bear HD, Atkins JN, Dimitrov NV, Glass AG, Fisher ER, Fisher B. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671-8678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, Colbert N, Boaziz C, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Buyse M, de Gramont A. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003;21:2896-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | André T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, Achille E, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Lledo G, Raoul Y, Debrix I, Buyse M, de Gramont A. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: final results of GERCOR C96.1. J Clin Oncol. 2007;25:3732-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Lembersky BC, Wieand HS, Petrelli NJ, O'Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall ME, Jacobs AD, Colman LK, Soran A, Yothers G, Wolmark N. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2733] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 32. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1646] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 33. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2200] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 34. | Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, Fields AL, Mayer RJ. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456-3461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, Shacham-Shmueli E, Rivera F, Kwok-Keung Choi C, Santoro A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, André T, Sobrero AF, Mini E, Greil R, Di Costanzo F, Collette L, Cisar L, Zhang X, Khayat D, Bokemeyer C, Roth AD, Cunningham D. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 37. | Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, O'Reilly S, Chu L, Azar CA, Lopa S, Wolmark N. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 441] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 38. | de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, André T, Hoff PM. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 39. | Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, Kahlenberg MS, Shields AF, Quesenberry JT, Webb TA, Farr GH Jr, Pockaj BA, Grothey A, Goldberg RM. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 40. | Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN, Blons H, Collette L, Van Cutsem E, Rougier P, Salazar R, Bedenne L, Emile JF, Laurent-Puig P, Lepage C; PETACC-8 Study Investigators. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 41. | Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939-944. [PubMed] |
| 42. | Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol. 1995;13:2936-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 252] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Matsuda C, Ishiguro M, Teramukai S, Kajiwara Y, Fujii S, Kinugasa Y, Nakamoto Y, Kotake M, Sakamoto Y, Kurachi K, Maeda A, Komori K, Tomita N, Shimada Y, Takahashi K, Kotake K, Watanabe M, Mochizuki H, Nakagawa Y, Sugihara K; SACURA Study Group. A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage II colon cancer: SACURA trial. Eur J Cancer. 2018;96:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Schippinger W, Samonigg H, Schaberl-Moser R, Greil R, Thödtmann R, Tschmelitsch J, Jagoditsch M, Steger GG, Jakesz R, Herbst F, Hofbauer F, Rabl H, Wohlmuth P, Gnant M, Thaler J; Austrian Breast and Colorectal Cancer Study Group. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. Br J Cancer. 2007;97:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 973] [Article Influence: 54.1] [Reference Citation Analysis (1)] |
| 46. | Allegra CJ, Kim G, Kirsch IR. Microsatellite instability in colon cancer. N Engl J Med. 2003;349:1774-1776; author reply 1774-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, Allegra CJ; National Cancer Institute. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, Kim GP, Yothers G, Allegra C, Moore MJ, Gallinger S, Sargent DJ. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 49. | Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30:2036-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 50. | Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626-4631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 51. | Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 814] [Article Influence: 37.0] [Reference Citation Analysis (1)] |
| 52. | Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1648] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 53. | Canouï-Poitrine F, Lièvre A, Dayde F, Lopez-Trabada-Ataz D, Baumgaertner I, Dubreuil O, Brunetti F, Coriat R, Maley K, Pernot S, Tournigand C, Hagege M, Aparicio T, Paillaud E, Bastuji-Garin S. Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey. Oncologist. 2019;24:e1351-e1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Javid SH, Unger JM, Gralow JR, Moinpour CM, Wozniak AJ, Goodwin JW, Lara PN Jr, Williams PA, Hutchins LF, Gotay CC, Albain KS. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1694] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 56. | Unger JM, Coltman CA, Crowley JJ, Hutchins LF, Martino S, Livingston RB, Macdonald JS, Blanke CD, Gandara DR, Crawford ED, Albain KS. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Freedman RA, Foster JC, Seisler DK, Lafky JM, Muss HB, Cohen HJ, Mandelblatt J, Winer EP, Hudis CA, Partridge AH, Carey LA, Cirrincione C, Moreno-Aspitia A, Kimmick G, Jatoi A, Hurria A. Accrual of Older Patients With Breast Cancer to Alliance Systemic Therapy Trials Over Time: Protocol A151527. J Clin Oncol. 2017;35:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 58. | Gouverneur A, Salvo F, Berdaï D, Moore N, Fourrier-Réglat A, Noize P. Inclusion of elderly or frail patients in randomized controlled trials of targeted therapies for the treatment of metastatic colorectal cancer: A systematic review. J Geriatr Oncol. 2018;9:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Papamichael D, Audisio R, Horiot JC, Glimelius B, Sastre J, Mitry E, Van Cutsem E, Gosney M, Köhne CH, Aapro M; SIOG. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 60. | Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011;26:783-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 61. | Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part II. Cancer J. 2005;11:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Launay-Vacher V, Chatelut E, Lichtman SM, Wildiers H, Steer C, Aapro M; International Society of Geriatric Oncology. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol. 2007;18:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 64. | Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Hines RB, Chatla C, Bumpers HL, Waterbor JW, McGwin G Jr, Funkhouser E, Coffey CS, Posey J, Manne U. Predictive capacity of three comorbidity indices in estimating mortality after surgery for colon cancer. J Clin Oncol. 2009;27:4339-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Erichsen R, Horváth-Puhó E, Iversen LH, Lash TL, Sørensen HT. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer. 2013;109:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109:2410-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 471] [Article Influence: 23.6] [Reference Citation Analysis (2)] |
| 69. | Van Cutsem E, Hoff PM, Blum JL, Abt M, Osterwalder B. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol. 2002;13:484-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, Karabelis A, Tsavaris N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 71. | Baker SD, Grochow LB. Pharmacology of cancer chemotherapy in the older person. Clin Geriatr Med. 1997;13:169-183. [PubMed] |
| 72. | Egorin MJ. Cancer pharmacology in the elderly. Semin Oncol. 1993;20:43-49. [PubMed] |
| 73. | Campisi J. Aging and cancer: the double-edged sword of replicative senescence. J Am Geriatr Soc. 1997;45:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Rockwell S, Hughes CS, Kennedy KA. Effect of host age on microenvironmental heterogeneity and efficacy of combined modality therapy in solid tumors. Int J Radiat Oncol Biol Phys. 1991;20:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Stein BN, Petrelli NJ, Douglass HO, Driscoll DL, Arcangeli G, Meropol NJ. Age and sex are independent predictors of 5-fluorouracil toxicity. Analysis of a large scale phase III trial. Cancer. 1995;75:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Vestal RE. Aging and pharmacology. Cancer. 1997;80:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 415] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 78. | Tam-McDevitt J. Polypharmacy, aging, and cancer. Oncology (Williston Park). 2008;22:1052-1055, discussion 1055, 1058, 1060. [PubMed] |
| 79. | Sanoff HK, Goldberg RM, Pignone MP. A systematic review of the use of quality of life measures in colorectal cancer research with attention to outcomes in elderly patients. Clin Colorectal Cancer. 2007;6:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;86:1766-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 256] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Given B, Given CW. Older adults and cancer treatment. Cancer. 2008;113:3505-3511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Estapé T. Cancer in the Elderly: Challenges and Barriers. Asia Pac J Oncol Nurs. 2018;5:40-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Hurria A. Geriatric assessment in oncology practice. J Am Geriatr Soc. 2009;57 Suppl 2:S246-S249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 85. | Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation of chemotherapeutic agents. New York, NY: Columbia University Press; 1949: 191-205. |
| 86. | Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1200] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 88. | Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, Parodi S, Dal Lago D, Gioia F, Monfardini S, Aapro MS, Serraino D, Zagonel V. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 89. | Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 637] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 90. | Chaïbi P, Magné N, Breton S, Chebib A, Watson S, Duron JJ, Hannoun L, Lefranc JP, Piette F, Menegaux F, Spano JP. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol. 2011;79:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 91. | Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ, Balducci L. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 783] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 92. | Extermann M, Bonetti M, Sledge GW, O'Dwyer PJ, Bonomi P, Benson AB. MAX2--a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer. 2004;40:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1336] [Article Influence: 95.4] [Reference Citation Analysis (2)] |
| 94. | Ramjaun A, Nassif MO, Krotneva S, Huang AR, Meguerditchian AN. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Rubenstein LZ. Evolving models of comprehensive geriatric assessment. J Am Med Dir Assoc. 2015;16:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol. 2005;54:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, Stadler WM, Rodin M. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 99. | Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, Soubeyran PL. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 706] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 100. | Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437-e444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 101. | Mohile SG, Dale W, Somerfield MR, Hurria A. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J Oncol Pract. 2018;14:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 102. | Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, Rostoft S, Overcash J, Wildiers H, Steer C, Kimmick G, Kanesvaran R, Luciani A, Terret C, Hurria A, Kenis C, Audisio R, Extermann M. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015;26:288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 526] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 103. | Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 693] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 104. | O'Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ Jr, Erlichman C, Shepherd L, Moertel CG, Kocha WI, Pazdur R, Wieand HS, Rubin J, Vukov AM, Donohue JH, Krook JE, Figueredo A. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 286] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 105. | Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G, Civitelli S. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 106. | Zuckerman IH, Rapp T, Onukwugha E, Davidoff A, Choti MA, Gardner J, Seal B, Mullins CD. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with Stage III colon cancer. J Am Geriatr Soc. 2009;57:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 108. | Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, Tsai WY, Grann VR, Hershman DL. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 110. | Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL; Capecitabine Colorectal Cancer Study Group. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 393] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 111. | Yoshitani S, Takashima S. Efficacy of postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly patients with resected colorectal cancer. Cancer Biother Radiopharm. 2009;24:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L, de Gramont A. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 113. | Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 486] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 114. | Meyers BM, Cosby R, Quereshy F, Jonker D. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin Oncol (R Coll Radiol). 2017;29:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (2)] |
| 115. | Haller DG, O'Connell MJ, Cartwright TH, Twelves CJ, McKenna EF, Sun W, Saif MW, Lee S, Yothers G, Schmoll HJ. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol. 2015;26:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Batra A, Cheung WY. Role of real-world evidence in informing cancer care: lessons from colorectal cancer. Curr Oncol. 2019;26:S53-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Green SL, Dawe DE, Nugent Z, Cheung WY, Czaykowski PM. The use of chemotherapy in older patients with stage II and III colon cancer: Variation by age and era of diagnosis. J Geriatr Oncol. 2019;10:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Lund JL, Stürmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, Warren JL. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care. 2013;51:e27-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 119. | Sanoff HK, Carpenter WR, Martin CF, Sargent DJ, Meyerhardt JA, Stürmer T, Fine JP, Weeks J, Niland J, Kahn KL, Schymura MJ, Schrag D. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst. 2012;104:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Sanoff HK, Carpenter WR, Stürmer T, Goldberg RM, Martin CF, Fine JP, McCleary NJ, Meyerhardt JA, Niland J, Kahn KL, Schymura MJ, Schrag D. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 121. | Sanoff HK, Carpenter WR, Freburger J, Li L, Chen K, Zullig LL, Goldberg RM, Schymura MJ, Schrag D. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118:4309-4320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381-3388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 123. | Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, Köhne CH, Rostoft S, Lemmens V, Mitry E, Rutten H, Sargent D, Sastre J, Seymour M, Starling N, Van Cutsem E, Aapro M. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |