Published online May 15, 2020. doi: 10.4251/wjgo.v12.i5.559
Peer-review started: November 27, 2019
First decision: December 26, 2019
Revised: March 18, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: May 15, 2020
Processing time: 168 Days and 11.8 Hours
Neoadjuvant/perioperative chemotherapy is the recommended treatment for advanced stages of gastric cancer (> T2, N+) before tumour resection in many European guidelines. However, there is no consensus as to whether perioperative chemotherapy is as effective in distal as in proximal tumours, in addition to a relevant uncertainty concerning appropriate treatment modalities for elderly patients.
To investigate the role of perioperative chemotherapy in advanced gastric cancer in patients from a German tertiary clinic with respect to efficacy, localisation, and age.
We performed a retrospective analysis of 158 patients from our clinic with adenocarcinoma of the stomach or the gastroesophageal junction who underwent resection between 2008 and 2016. The data were evaluated particularly in relation to patient age, tumour site, and perioperative therapy.
Administration of perioperative chemotherapy did not lead to a significant survival advantage in our study population. The 5-year survival rates were 40% for patients who received perioperative chemotherapy and 29% for the group without perioperative chemotherapy (P = 0.125). Our patients were on average distinctly older than patients in most of the published randomised controlled trials. Patients elder than 75 years received perioperative chemotherapy far less frequently. Patients with a proximal tumour received perioperative chemotherapy much more often.
This analysis reconfirms our previous data concerning the effectiveness of perioperative chemotherapy for advanced gastric cancer. There is reasonable doubt that the quality of the existing randomized controlled trials is sufficient to generally justify perioperative chemotherapy in patients with advanced gastric cancer independent of tumour localization or age.
Core tip: Due to the unfavourable prognosis of locally advanced gastric carcinoma, multimodal therapy has been propagated worldwide in the last decade. European guidelines recommend perioperative chemotherapy on the basis of a few randomized trials, which are of limited validity for certain reasons. These studies had shown a better 5-year overall survival of approximately 15%, but neither the studies nor guidelines focused on the age and tumour localization of the patients. The goal of our study was to compare the observed effects of randomized controlled studies with real life data from a German community hospital with a focus on patient age and tumour localization.
- Citation: Bauer K, Manzini G, Henne-Bruns D, Buechler P. Perioperative chemotherapy for advanced gastric cancer - results from a tertiary-care hospital in Germany. World J Gastrointest Oncol 2020; 12(5): 559-568
- URL: https://www.wjgnet.com/1948-5204/full/v12/i5/559.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i5.559
In contrast to Asian countries, where prophylactic gastroscopy is implemented in the national healthcare system due to the high incidence of gastric cancer, gastric cancer in European countries is often only detected in an advanced stage due to late-appearing symptoms. Administration of perioperative chemotherapy has been recommended in the European guidelines to improve the prognosis of adenocarcinomas of the stomach and the gastro-oesophageal junction from stage > T2/N+ for many years[1-3]. Mainly patients younger than 75 years were included in the randomized controlled trials (RCTs), which build the basis for these guideline recommendations (in Germany, Great Britain, and Europe). Therefore, there is no convincing evidence concerning the benefit of perioperative chemotherapy for elderly patients. The guidelines also do not mention the effectiveness of perioperative chemotherapy in relation to the tumour site (proximal or distal stomach). At congresses and in tumour conferences, the question of whether perioperative chemotherapy of antrum and pyloric carcinomas is just as effective as in the proximal sections of the stomach is much debated. Another unclear question is whether elderly individuals (> 75 years), who make up the majority of patients in the everyday European hospital routine, profit just as much from the recommended therapy as younger patients who are regularly included in the RCTs. These questions are of growing importance because the incidence of distal gastric tumours has decreased in the last decades, whereby proximal tumours are increasingly diagnosed.
Considering our retrospective data and the resulting survival times, it should be investigated whether the patient age and tumour location should influence the decision for a perioperative chemotherapy, and what prognostic differences exist for the treated groups.
One hundred and fifty-eight patients who underwent resection of adenocarcinomas of the stomach or the gastro-oesophageal junction in our clinic between 2008 and 2016 were analysed. One hundred and twenty-nine of these patients presented with an advanced tumour stage [Union internationale contre le cancer (UICC) > Stage II].
As a tertiary-care hospital, the clinic of Kempten is certified by the German Cancer Society for the treatment of carcinomas of the stomach, colorectum, and pancreas.
The date of birth, gender, month of diagnosis, extent of the operation, application of perioperative chemotherapy, TNM-classification, and UICC-stage, including all relevant histologic criteria and eventual date of death, were recorded for each patient.
An endoscopic examination with histological confirmation of diagnosis, as well as a computed tomography of the abdomen and the thorax for staging, was performed to rule out distant metastases and to assess the preoperative tumour stage. Endosonography was not routinely performed in all patients.
Application of perioperative chemotherapy was recommended by the interdisciplinary tumour conference depending on the preoperative suspected TNM stage, the patient’s general condition, and the urgency of the tumour operation (e.g., bleeding of the tumour). According to the German guidelines, perioperative chemotherapy was usually recommended for tumours > T2 and/or N+. Upfront surgery was preferred in patients with poor performance status and/or severe comorbidity.
Subtotal gastric resection, gastrectomy, expanded gastrectomy, transhiatal distal oesophageal resection (Merendino), or abdomino-thoracal oesophageal resection with gastric endo-sleeve was performed, depending on the tumour site and the Lauren classification. A D2-lymphadenectomy was the standard procedure.
In order to analyse comparable groups of patients regarding the TNM-Status, we excluded the patients of the surgery-only group with the postoperative stadium of pT1/N0 and pT2/N0. Therefore, from an initial 158 patients with curatively-resected gastric cancer, 129 patients with advanced tumour stages remained.
Two young patients of the surgery-only group, in whom the preoperative diagnostic tools had led to an under-staging, received adjuvant chemotherapy.
Perioperative chemotherapy was administered according to the epirubicin, oxaliplatin, and capecitabine schema from 2008 to 2014: Each 3-wk cycle consisted of epirubicin (50 mg/m2) intravenously on d 1, oxaliplatin (130 mg/m2) intravenously on d 1, and capecitabine (625 mg/m2, twice/d) orally administered from d 1 to d 21.
From 2014, the 5-FU, oxaliplatin, und docetaxel (FLOT) schema was used: 5-floururacil (2600 mg/m2) + natriumfolinat (200 mg/m2) intravenously on d 1, oxaliplatin (85 mg/m2) intravenously on d 1, and docetaxel (50 mg/m2) intravenously on d 1.
Both the epirubicin, oxaliplatin, and capecitabine schema, and the FLOT schema consisted of three preoperative cycles and three postoperative cycles of chemotherapy, so for this study the term perioperative chemotherapy is used.
It was not necessary to obtain a decision by the Ethics Commission for this retrospective analysis of our internal hospital data according to a consultation with the Federal Medical Association.
Values are presented as the mean ± SD and median (range) for continuous variables. Dichotomic variables are presented as absolute number as well as percent. A two-sided P value < 0.05 was considered statistically significant. Survival curves were obtained using the Kaplan-Meier method according to chemotherapy (yes or no), localisation of the gastric tumour (proximal or distal), and age (more or less 75 years). Additionally, a subgroup survival analysis was performed in order to investigate the role of chemotherapy selectively on both distal and proximal gastric cancer. Missing values were < 5% in the dataset, and no imputation strategies were used. All calculations were conducted using R Project for Statistical Computing (The R Foundation, Version 3.1.0, Vienna, Austria). All patients have been followed up for at least 24 mo.
Fifty-three of the 129 above-mentioned patients (41%) were 75 years of age or older when diagnosed. Forty-five of all patients (35%) received perioperative chemotherapy. Patient characteristics are listed in Table 1. A tumour located in the cardia, fundus, or the gastro-oesophageal junction (AEG) was defined as proximal, and a tumour in the pylorus, antrum, or corpus was defined as distal.
| Characteristic | 129 patients | % |
| Ages at diagnosis | ||
| Average | 69.8 yr (SD: ± 11.4) | |
| Median | 71 yr (range: 38-91) | |
| Ages at diagnose | ||
| < 75 yr | 76 | 59% of 129 |
| ≥ 75 yr | 53 | 41% of 129 |
| ≥ 80 yr | 24 | 19% of 129 |
| Gender | ||
| Male | 93 | 72% of 129 |
| Female | 36 | 28% of 129 |
| Tumour sites | ||
| Proximal | 47 | 36% of 129 |
| Distal | 82 | 64% of 129 |
| Proximal < 75 yr | 34 | 72% of 47 |
| Proximal ≥ 75 yr | 13 | 28% of 47 |
| Among whom proximal ≥ 80 yr | 1 | 2% of 47 |
| Distal < 75 yr | 42 | 51% of 82 |
| Distal ≥ 75 yr | 40 | 49% of 82 |
| Among whom distal ≥ 80 yr | 23 | 28% of 82 |
| Perioperative chemotherapy | ||
| Yes | 45 | 35% of 129 |
| No | 84 | 65% of 129 |
| Ages at perioperative chemotherapy | ||
| Median of all 45 patients | 66 yr (range: 38-82) | |
| Average of all 45 patients | 65.7 yr (SD: ± 8.6) | |
| < 75 yr | 37 | 82% of 45 |
| ≥ 75 yr | 8 | 18% of 45 |
| Among whom ≥ 80 yr | 1 | 1% of 45 |
| Ages of patients without perioperative chemotherapy | ||
| Median of all 84 patients | 75 yr (range: 44-91) | |
| Average of all 84 patients | 72 yr (SD: ± 11.9) | |
| < 75 yr | 39 | 46% of 84 |
| ≥ 75 yr | 45 | 54% of 84 |
| Among whom ≥ 80 yr | 23 | 27% of 84 |
Table 2 shows the operative procedures undertaken on the 129 patients with gastric adenocarcinoma and the histological results. Seventeen patients with intraoperative-detected liver metastasis and/or peritoneal tumour spread (UICC IV) and macroscopic R0-resection were included in the analysis.
| Kind of operation | 129 patients | % of the 129 | Number/percent with neoadjuvant chemotherapy |
| Subtotal resection | 40 | 31% | 4 of 40/10% |
| Gastrectomy | 57 | 44% | 21 of 57/37% |
| Expanded gastrectomy | 11 | 9% | 6 of 11/55% |
| Transhiatal oesophagus resection (Merendino) | 3 | 2% | 1 of 3/33% |
| Transthoracal oesophagus resection (with gastric endo-sleeve) | 18 | 14% | 14 of 18/78% |
| LAD | |||
| D1 (intraoperative M+, PC or no R0 possible) | 11 | 8.5% | |
| D2 | 118 | 91.5% | |
| T | |||
| T0 | 7 | 5.4% | (ypT0 = 7) |
| T1 | 8 | 6.2% | (ypT1N0 = 5, pT1/N1 = 3) |
| T2 | 25 | 19.4% | (ypT2 = 12, pT2/N+ = 13) |
| T3 | 56 | 43.4% | (ypT3 = 18, pT3 = 37) |
| T4 | 33 | 25.6% | (ypT4 = 3, pT4 = 30) |
| N | |||
| N0 | 37 | 29% | |
| N+ | 92 | 71% | |
| M | |||
| M0 | 112 | 87% | |
| M1 | 17 | 13% | |
| Signet-ring cells | |||
| Yes | 28 | 22% | |
| No | 101 | 78% | |
| UICC | |||
| UICC IA | 12 | 9.3% | |
| UICC IB | 7 | 5.4% | |
| UICC IIA | 21 | 16.3% | |
| UICC IIB | 26 | 20.2% | |
| UICC IIIA | 17 | 13.2% | |
| UICC IIIB | 14 | 10.9% | |
| UICC IIIC | 16 | 12.4% | |
| UICC IV | 16 | 12.4% | |
The part of patients who received perioperative chemotherapy demonstrates a clear dependence on tumour location. The percentage of pre-treated patients increased from 10% for distal tumours (pylorus, antrum, and distal corpus) to 78% for tumours located in the gastro-oesophageal junction (AEG I/II), which required a two-cavity intervention. Merendino’s procedure constituted an exception, and was usually only offered to patients who, due to their physical constitution, did not qualify for a two-cavity procedure.
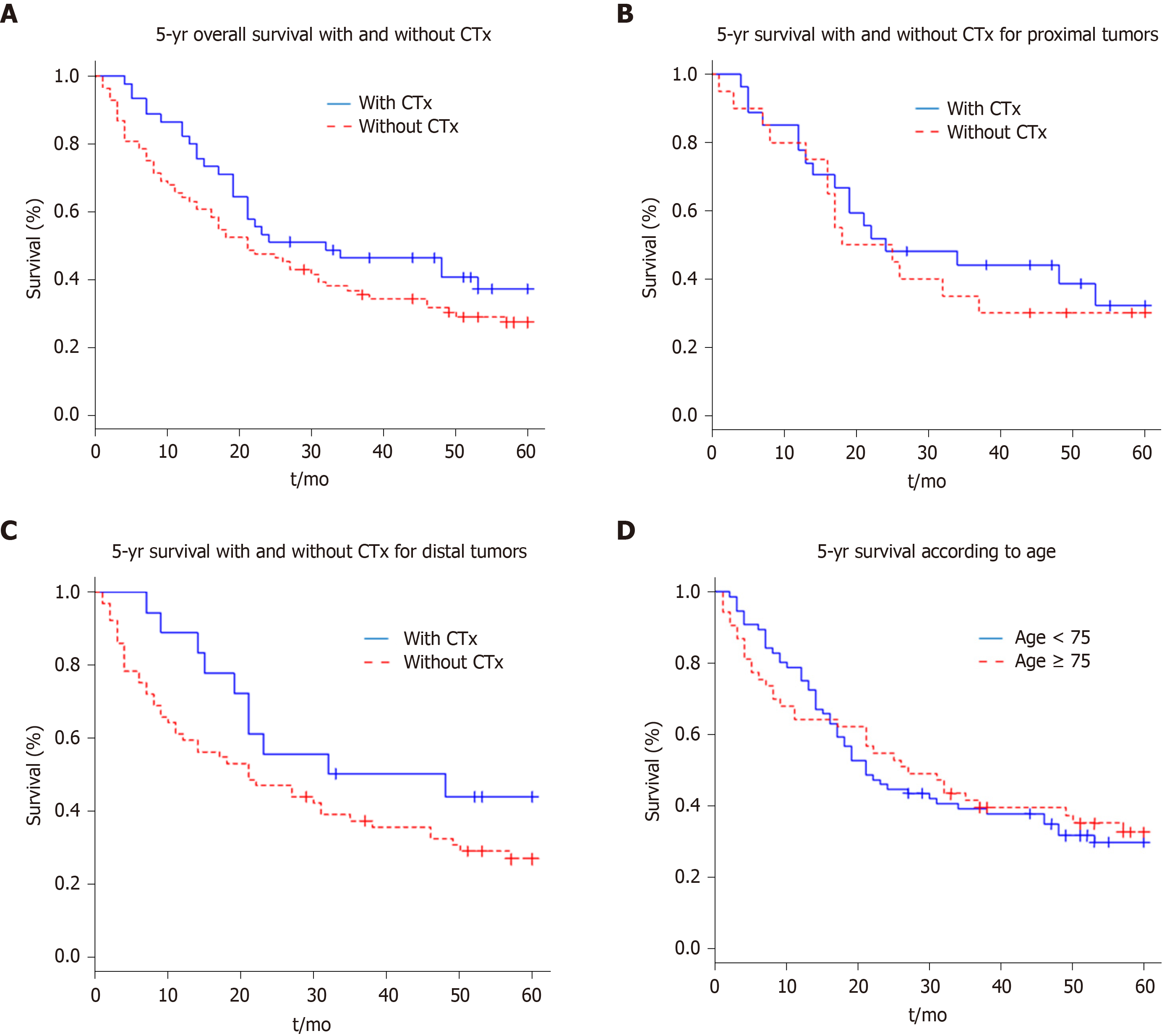
On the basis of the recorded data, survival curves were obtained with the Kaplan-Meier method. Figure 1A shows the effects of perioperative chemotherapy, regardless of tumour localisation. Although the survival interval seemed to be prolonged for the whole group of patients receiving preoperative chemotherapy, a statistically significant difference (P = 0.125) could not be detected. The 5-year survival rate was 33% for all patients (42 of 129); for the group of patients who received perioperative chemotherapy, the 5-year survival rate was 40% (18 of 45 patients) and 29% for the group without perioperative chemotherapy (24 of 84 patients).
Further, we evaluated whether the tumour localization, independently of perioperative chemotherapy, determined the prognosis. Survival did not differ significantly between the patient group with a proximal vs that with a distal carcinoma (P = 0.782).
In subgroups, we examined whether there were differences in outcomes between the patient groups with distal and with proximal tumours depending on the administration of perioperative chemotherapy. There was no significant difference in median survival between the patients (Figure 1B) with proximal tumours who had received perioperative chemotherapy and those who had not (P = 0.614).
The subgroup analysis also showed no significant difference in survival times in the patients with distal tumours (Figure 1C) who received perioperative chemotherapy and those who did not (P = 0.134).
Finally, we examined if there was a difference in survival time between the elderly patients subgroup (≥ 75 years) and the younger ones (< 75 years). Figure 1D shows no significant difference in survival (P = 0.855) regarding the age of the patient.
Summarizing the results from our retrospective analysis, we found that perioperative chemotherapy does not significantly improve survival. In detail, we could observe that: (1) The patients with gastric carcinoma in our hospital in Kempten (Germany) are on average (70 years) older than the patients in the RCTs (4, 5, 6) that analysed the effectiveness of perioperative chemotherapy for adenocarcinoma of the stomach; (2) Elderly patients (≥ 75 years) receive perioperative chemotherapy far less often than younger patients with the same preoperatively-determined tumour stage; (3) The group with distal, non-pre-treated gastric carcinoma contained an above-average number of elderly patients (≥ 75 years of age); (4) The incidence of proximal gastric carcinomas decreases with increasing age of the patient from 72% in the patients group younger than 75 years to 28% in the patients group older than 75 years; (5) Our hospital-intern tumour conference recommends perioperative chemotherapy significantly more often for patients with proximal tumours (e.g., from 10% for carcinomas of the pylorus up to 78% for carcinomas of the gastroesophageal junction); (6) The 5-year survival time of patients with distal tumours (36%) did not significantly differ from that of patients with proximal tumours (33%), regardless of whether they had received perioperative chemotherapy or not; and (7) The subgroup analysis (distal or proximal tumours) also showed no significant difference in survival times for patients with or without perioperative chemotherapy and for elder and younger patients.
Based on the results of some RCTs[4-6], perioperative/neoadjuvant chemotherapy has been recommended in the German guidelines for advanced adenocarcinoma of the stomach and the gastro-oesophageal junction zone[1] since 2010. Perioperative chemo-radiotherapy (AEG I) is also recommended in the revised S3 guidelines for tumours > T2 and/or N+. The guideline does not comment on the patient’s age or on tumour localisation.
Our former analysis[7-9] showed that the few RCTs that examined the effectiveness of perioperative chemotherapy for advanced gastric cancer had some grave shortcomings in their validity. We concluded that perioperative chemotherapy cannot be generally recommended for advanced gastric cancer based on these RTCs. In addition, part of our working group analysed the validity of RCTs on the subject of adjuvant chemotherapy after resecting gastric cancer[10]. Several of the RCTs also showed substantial deficits in their validity.
We therefore wanted to examine the effectiveness of perioperative chemotherapy in a tertiary-care hospital, which is certified by the German Cancer Society as an oncosurgical centre for the treatment of carcinomas of the stomach, the colon/rectum, and the pancreas. Patients with advanced gastric cancer have been treated with perioperative chemotherapy in Kempten, Germany since 2007 according to the national guidelines. The analysis of our patients, which was conducted and evaluated over 9 years (2008-2016), showed that perioperative chemotherapy produced no significant advantage in the 5-year survival time: 40% for the perioperative chemotherapy group and 29% for the surgery-only group (P = 0.125). Cunningham et al[4] report a 5-year survival rate of 36% for the perioperative chemotherapy group vs 23% for the surgery-only group; Ychou et al[6] could detect a 5-year survival rate of 38% for the perioperative chemotherapy group vs 24% for the surgery-only group. Compared to the two RCTs of Cunningham et al[4] and Ychou et al[6], which had built the basis of the guideline recommendations, the patients of our analysis had a slightly better 5-year survival time. This might be caused by the high D2-lymphadenectomy rate of about 91.5% compared with 41% in the study of Cunningham et al[4]. In our analysis, we excluded the postoperative staged pT1/N0 and pT2/N0-tumours of the surgery-only group to avoid a positive effect on the 5-year survival rate of these patients. Cunningham et al[4] included 16 patients (8.3% of 193 surgery-only patients) with a pT1 stadium, and 55 patients of the same group with pT2 (28.5% of 193). Unfortunately, the N-stage is reported for only 291 of the 503 patients (57.8%) and the TNM stage is not mentioned.
A recent Asian study from Eom et al[11] matched 43 patients who were treated with perioperative chemotherapy (S-1 and docetaxel) and 86 patients who received surgery only. The neoadjuvant group had a significantly higher 5-year overall survival rate (73.3% vs 51.1%, P = 0.005) and a trend towards a higher 5-year progression-free survival rate (62.8% vs 49.9%, P = 0.145). The authors concluded that perioperative chemotherapy was associated with better long-term survival without increasing postoperative complications in the setting of D2 surgery, suggesting that perioperative chemotherapy can also be a therapeutic option in East Asian countries.
In 2018, Kanaji et al[12] published a review that summarizes recent evidence of the benefits of (neo-)adjuvant therapy for locally advanced gastric cancer according to the tumour stage and the histological subtype in Asia, the United States and Europe. They concluded that FLOT can be considered the new standard care in perioperative treatment for European patients with resectable gastric and gastroesophageal junction adenocarcinoma. In Asia, the perioperative chemotherapy combination S1 and oxaliplatin is considered to reduce both hematogenous and peritoneal recurrence for Stage III adenocarcinoma, while for bulky lymph node metastasis and scirrhous carcinomas, additional neoadjuvant chemotherapy with S‐1/cisplatin followed by postadjuvant treatment with S‐1 is not recommended.
The subgroup analysis, in which patients with distal and proximal tumours were separately evaluated, also showed no significant difference in survival between the preoperatively-treated patients and those who only underwent resection.
Nevertheless, in our hospital, perioperative chemotherapy is offered to patients with proximal tumours far more often by our tumour conference. This may be explained on one side by the persisting opinion that chemotherapy is less effective for distal carcinomas, and by the fact that the patient group with proximal tumours is the younger one. Indeed, Ychou et al[6] were able to detect a significant treatment effect of perioperative chemotherapy exclusively for the patient group with tumours of the gastroesophageal junction.
Fifty-three of 129 patients (41%) with gastric cancer in our hospital were older than 75 years at the time of diagnosis. These patients are not represented in most of the RCTs in relation to the effectiveness and tolerance of perioperative chemotherapy for advanced gastric cancer. The MAGIC trial by Cunningham et al[4] had no age limit in its inclusion criteria, while the two other large studies on perioperative therapy for gastric cancer by Schuhmacher et al[5] and Ychou et al[6] were limited to patients younger than 75 years. The median age of patients in our hospital was 71 years, and thus consequently our population was distinctly older that the ones that were included in the large RCTs on which guidelines are based. Patients included in the MAGIC trial by Cunningham et al[4] had a median age of 57 years (range: 23 to 85); patients in the EORTC study by Schuhmacher et al[5] had a median age of 57 years (range: 26 to 70); patients in the ACCORD study by Ychou et al[6] had a median age of 63 years (range: 36 to 75).
Since our group of patients with gastric cancer contained an above-average number of patients older than 75 with distal carcinomas and without application of perioperative chemotherapy, we expected a worse prognosis for this patient group. Only 8 patients (18%) who received perioperative chemotherapy were older than 75 years and only one patient (1%) was over 80 years. These were of course biologically fit patients. Surprisingly, the age of the patient had no influence on the median survival. In contrast to our data, Ciesielski et al[13] could detect an extremely high mortality rate after a successful gastrectomy for cancer in elderly patients.
Our data show that treatment of advanced gastric cancer still needs further improvement and reflects the aggressive biology of gastric cancer. All patients seem to have a similar bad prognosis regardless of age, tumour localisation, and tentative donation of perioperative chemotherapy. It is still necessary to continue research on the detection of tools that help to identify the patients that really benefit from perioperative chemotherapy and those who do not.
A limitation of our study is the small sample size, consequently a Beta error could be present, and power could not be enough to detect the effect of chemotherapy on survival. For subgroup analysis concerning the effectiveness of the selective application of chemotherapy in distal and proximal carcinomas depending on patient age, the different groups would have been too small to receive a significant result. However, the data reflect the reality in German hospitals. In the last three years, between 30 and 45 patients were annually treated operatively in our department by two surgeons. This provides much more homogenous treatment for our patients, compared to the 503 patients of the MAGIC study[4] who were treated by 129 surgeons from four continents over a period of 8 years without a standardized operative procedure and a D2-lymphadenectomy rate of only 41%. There are studies, such as pilot studies and RCTs, that offer a smaller sample size than our analysis[5,14-16]. Our study should be considered a basis to investigate the role of chemotherapy in a setting of RCT in the future.
Another limitation is the acquisition of retrospective data. In contrast to a randomized controlled study where patient characteristics should be well balanced in the different groups, in a retrospective study a selection bias concerning the decision whether to recommend perioperative chemotherapy or not cannot be excluded. It is likely that an interdisciplinary tumour conference prefers upfront surgery in the case of patients with poor performance status or comorbidity. This would signify that the surgery-only group probably had a poorer prognosis per se, and would support the result that even on the basis of two slightly different groups of patients, there was no statistically significant difference in the five-year survival.
On the basis of the present analysis of patients from a German tertiary-care hospital certified for gastric cancer treatment by the German Cancer Society, we have to conclude that the effectiveness of perioperative chemotherapy in advanced gastric cancer referring to the prolongation of survival has to be challenged again.
Consequently, our previous statements demonstrating that the quality of the existing RCTs is not sufficient to justify perioperative chemotherapy in patients with advanced gastric cancer could be confirmed by this study.
For ten years, European guidelines have recommended perioperative chemotherapy for advanced gastric cancer. The recommendation is based on a few randomized controlled trials (RCTs) of poor validity. Decisions regarding therapy often differ between selected patients included in an RCT and elderly patients with comorbidities in regular healthcare. Oftentimes, latter patients have to break up perioperative chemotherapy because of adverse effects. Considering the increasing incidence of proximal gastric cancer and the fact that gastric cancer is one of the most frequent reasons of tumour-associated deaths worldwide, it is particularly important to study this topic.
Guidelines should be applicable for daily patient treatment, but European guidelines do not mention tumour localization nor the age of the patient. In order to find out which patients will have a real benefit from perioperative chemotherapy, we wanted to analyse the effect of perioperative chemotherapy in patients from our clinic with respect to tumour localization and age. This is important to save resources and to protect patients who will not benefit from perioperative chemotherapy from experiencing adverse effects.
The aim of this study was to analyse the efficacy of perioperative chemotherapy in our total patient population as well as in subgroups with respect to tumour localization and age.
Patient characteristics before and after therapy of every resected patient with advanced gastric adenocarcinoma between 2008 and 2016 were added to our database. Survival curves were obtained using the Kaplan-Meier method according to chemotherapy (yes or no), localisation of the gastric tumour (proximal or distal) as well as age (more or less than 75 years).
Administration of perioperative chemotherapy did not lead to a significant survival advantage in our study population. Thus, our research could not confirm the data of RCTs, which are the basis of the European guidelines.
Fifty-three patients of the above-mentioned 129 (41%) were 75 years of age or older when diagnosed. The lack of a significant survival benefit due to perioperative chemotherapy was independent of tumour localization and age. Gastric cancer is not very sensitive to chemotherapy. Therefore, all efforts have to be done to detect it earlier or to identify tumour characteristics whose treatment offers more personalized medicine. The treatment of advanced gastric cancer differs substantially in different parts of the world. Individual tumour stage depends on genetic diversity, prophylactic gastroscopy, quality of surgical treatment (e.g., D2 lymphadenectomy or not), and other differences in particular healthcare systems. The few RCTs that analysed perioperative chemotherapy in gastric cancer are known all over the world but led to different guideline recommendations on different continents. The study wanted to prove the hypothesis that perioperative chemotherapy is effective and that this efficacy is dependent on tumour localization or patient age. The retrospective analysis of our database does not provide any new methods. We found that patients treated due to advanced gastric cancer are on average much older than cohorts of RCTs on this topic. Elder patients (> 75 years) do not have worse prognoses compared to younger ones. The incidence of proximal gastric carcinomas decreases with increasing patient age. Elderly patients (≥ 75 years) receive perioperative chemotherapy far less often than younger. The 5-year survival time of patients with distal tumours did not significantly differ from that of patients with proximal tumours, regardless of whether they had received perioperative chemotherapy or not. The study could refute the hypotheses that perioperative chemotherapy is more effective in patients with proximal gastric cancer. The decision of whether or not to apply perioperative chemotherapy in future research is necessary. Until new insights arise, tumour conferences concerning the decision of perioperative chemotherapy should not be influenced by tumour localization but only by tumour stage.
It is necessary to prove the applicability of guidelines to daily patient treatment, particularly if the guideline recommendations are based on few RCTs with poor validity. Stratification according to defined risk factors, such as tumour characteristics, should be introduced to identify possible responders to therapy and thereby reduce the number of unnecessary treatments, particularly because the clinical approach to oncological patients has switched from standardized to personalized medicine. For example, MSI status should be evaluated, as in recent studies it was shown that patients with mismatch repair deficiency should not be treated with perioperative chemotherapy because of severe adverse effects and missing survival benefits. Instead of new RCTs, which often fail due to the difficult recruitment of highly selected patients, we recommend analysing big cohorts of registered patients in order to better understand the real situation in a particular country.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Deutsche Gesellschaft für Chirurgie e.V. (18162).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, Lin Q, Nishida T, Tarnawski AS S-Editor: Dou Y L-Editor: Filipodia E-Editor: Qi LL
| 1. | Moehler M, Al-Batran SE, Andus T, Anthuber M, Arends J, Arnold D, Aust D, Baier P, Baretton G, Bernhardt J, Boeing H, Böhle E, Bokemeyer C, Bornschein J, Budach W, Burmester E, Caca K, Diemer WA, Dietrich CF, Ebert M, Eickhoff A, Ell C, Fahlke J, Feussner H, Fietkau R, Fischbach W, Fleig W, Flentje M, Gabbert HE, Galle PR, Geissler M, Gockel I, Graeven U, Grenacher L, Gross S, Hartmann JT, Heike M, Heinemann V, Herbst B, Herrmann T, Höcht S, Hofheinz RD, Höfler H, Höhler T, Hölscher AH, Horneber M, Hübner J, Izbicki JR, Jakobs R, Jenssen C, Kanzler S, Keller M, Kiesslich R, Klautke G, Körber J, Krause BJ, Kuhn C, Kullmann F, Lang H, Link H, Lordick F, Ludwig K, Lutz M, Mahlberg R, Malfertheiner P, Merkel S, Messmann H, Meyer HJ, Mönig S, Piso P, Pistorius S, Porschen R, Rabenstein T, Reichardt P, Ridwelski K, Röcken C, Roetzer I, Rohr P, Schepp W, Schlag PM, Schmid RM, Schmidberger H, Schmiegel WH, Schmoll HJ, Schuch G, Schuhmacher C, Schütte K, Schwenk W, Selgrad M, Sendler A, Seraphin J, Seufferlein T, Stahl M, Stein H, Stoll C, Stuschke M, Tannapfel A, Tholen R, Thuss-Patience P, Treml K, Vanhoefer U, Vieth M, Vogelsang H, Wagner D, Wedding U, Weimann A, Wilke H, Wittekind C; AWMF; AWMF. [German S3-guideline "Diagnosis and treatment of esophagogastric cancer"]. Z Gastroenterol. 2011;49:461-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 3. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1117] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 4. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4606] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 5. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 6. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 7. | Bauer K, Porzsolt F, Henne-Bruns D. Validity of the MAGIC study: sufficient for recommendations? Hepatogastroenterology. 2013;60:1822-1824. [PubMed] |
| 8. | Bauer K, Porzsolt F, Henne-Bruns D. Can perioperative chemotherapy for advanced gastric cancer be recommended on the basis of current research? A critical analysis. J Gastric Cancer. 2014;14:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Bauer K, Schroeder M, Porzsolt F, Henne-Bruns D. Comparison of international guidelines on the accompanying therapy for advanced gastric cancer: reasons for the differences. J Gastric Cancer. 2015;15:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Manzini G, Henne-Bruns D, Kremer M. Validity of studies suggesting postsurgical chemotherapy for resectable gastric cancer: critical appraisal of randomised trials. BMJ Open Gastroenterol. 2017;4:e000138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Eom BW, Kim S, Kim JY, Yoon HM, Kim MJ, Nam BH, Kim YW, Park YI, Park SR, Ryu KW. Survival Benefit of Perioperative Chemotherapy in Patients with Locally Advanced Gastric Cancer: a Propensity Score Matched Analysis. J Gastric Cancer. 2018;18:69-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kanaji S, Suzuki S, Matsuda Y, Hasegawa H, Yamamoto M, Yamashita K, Oshikiri T, Matsuda T, Nakamura T, Sumi Y, Kakeji Y. Recent updates in perioperative chemotherapy and recurrence pattern of gastric cancer. Ann Gastroenterol Surg. 2018;2:400-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Ciesielski M, Kruszewski WJ, Szajewski M, Walczak J, Spychalska N, Szefel J, Zieliński J. Extremely High Mortality Rate after a Successful Gastrectomy for Cancer in Older Adults. J Gastric Cancer. 2019;19:202-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, Bonomo G, Crosta C, Huber O. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 15. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH; Cooperating Investigators of The Dutch Gastric Cancer Group. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Yonemura Y, Sawa T, Kinoshita K, Matsuki N, Fushida S, Tanaka S, Ohoyama S, Takashima T, Kimura H, Kamata T. Neoadjuvant chemotherapy for high-grade advanced gastric cancer. World J Surg. 1993;17:256-261; discussion 261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |