Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1216
Peer-review started: August 9, 2020
First decision: August 22, 2020
Revised: September 6, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 15, 2020
Processing time: 94 Days and 12 Hours
Programmed death ligand 1 (PD-L1) immunotherapy remains poorly efficacious in colorectal cancer (CRC). The recepteur d’origine nantais (RON) receptor tyrosine kinase plays an important role in regulating tumor immunity.
To identify the patterns of RON and PD-L1 expression and explore their clinical significance in CRC.
Gene expression data from the Gene Expression Omnibus database (GEO; n = 290) and patients at the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZUSM; n = 381) were analyzed to determine the prognostic value of RON and PD-L1 expression within the tumor microenvironment of CRC. HT29 cell line was treated with BMS-777607 to explore the relationship between RON activity and PD-L1 expression. Signaling pathways and protein expression perturbed by RON inhibition were evaluated by cellular immunofluorescence and Western blot.
In the GEO patient cohort, cut-off values for RON and PD-L1 expression were determined to be 7.70 and 4.3, respectively. Stratification of patients based on these cutoffs demonstrated that high expression of RON and PD-L1 was associated with a poor prognosis. In the FAHZUSM cohort, rates of high expression of RON in tumor cells, high PD-L1 expression in tumor cells and tumor infiltrating monocytes, and both high RON and high PD-L1 expression in the tumor microenvironment were 121 (32%), 43 (11%), 91 (24%), and 51 (13.4%), respectively. High expression of RON was significantly correlated with high expression of PD-L1 in the tumor cell compartment (P < 0.001). High expression of RON and that of PD-L1 were independent prognostic factors for poorer overall survival. Concurrent high expression of both RON and PD-L1 in the tumor microenvironment was significantly associated with a poor prognosis. In vitro, BMS-777607 inhibited the phosphorylation of RON, inhibited PD-L1 expression, and attenuated activation of the ERK1/2 and AKT signaling pathways in CRC cells.
RON, PD-L1, and their crosstalk are significant in predicting the prognostic value of CRC. Moreover, phosphorylation of RON upregulates PD-L1 expression, which provides a novel approach to immunotherapy in CRC.
Core Tip: Recepteur d’origine nantais (RON) expression plays an important role in regulating tumor immunity, and the efficacy of programmed death ligand 1 (PD-L1) immunotherapy in colorectal cancer (CRC) is still not satisfactory. This inspired us to study the clinical significance and correlation of RON and PD-L1 in CRC. The major discoveries and findings in this study are: (1) The abnormal expression of RON and PD-L1 was not only correlated, but was also significant biomarkers for poor prognosis of CRC. This finding was identified in 389 primary CRC clinical samples via multiplex immunofluorescence staining and immunohistochemistry staining; and (2) In in vitro experiments, activation of RON phosphorylation up-regulated the expression of PD-L1 in CRC cells.
- Citation: Liu YZ, Han DT, Shi DR, Hong B, Qian Y, Wu ZG, Yao SH, Tang TM, Wang MH, Xu XM, Yao HP. Pathological significance of abnormal recepteur d’origine nantais and programmed death ligand 1 expression in colorectal cancer. World J Gastrointest Oncol 2020; 12(11): 1216-1236
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1216.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1216
Colorectal cancer (CRC) is the fourth most common malignancy worldwide in 2018[1]. Despite advances in CRC treatment methods, it still has a poor prognosis, and many patients will have local recurrence and/or distant metastasis within 5 years after treatment[2].
Immunotherapy has become a promising strategy for treating a wide variety of malignancies[3]. Therapeutic antibodies that block the activity of programmed death ligand 1 (PD-L1, also known as CD274) protein are effective against many cancer types[4]. PD-L1 is a transmembrane immune checkpoint protein that can be expressed on both immune cells and cancer cells, including CRC cells[5-7]. When expressed on immune cells, PD-L1 can be regulated by a variety of inflammatory mediators and cytokines. When expressed on the surface of tumor cells (TCs), PD-L1 enables TCs to evade the immune system[8,9]. Cancer cells can induce the expression of PD-L1 through activation of oncogenic pathways, such as the RAS-ERK or PI3K-AKT pathways, to facilitate immune escape[10-12].
However, the efficacy of PD-L1 immunosuppressive agents in CRC is poor[13,14]. The overall poor response of CRC to PD-L1 blockade may be due to the activity of multiple oncogenes[15,16]. For example, KRAS mutations can up-regulate the expression of PD-L1 in CRC cells, thereby reducing the efficacy of PD-L1 immunosuppressive agents[17]. RON (macrophage-stimulating 1 receptor, MST1R), a member of the MET proto-oncogene family and a receptor for MSP, is an effector of KRAS signaling, and exhibits “KRAS addiction”, a phenomenon that plays an important role in the initiation and development of tumors[18-20]. RON is overexpressed pathologically in various types of cancer, including CRC, and many small-molecule inhibitors are being developed to target RON and its associated compounds[20-22]. BMS-777607 is a highly selective small molecule inhibitor of RON (IC50 1.8nM for RON)[23]. Activation of RON leads to RON phosphorylation, which then activates different signaling pathways in cancer cells, including the RAS-ERK and PI3K-AKT pathways[24-26]. Interactions between RON and other membrane receptors further promote pathway activation, enhancing the migration and invasion of CRC cells[20,22]. In addition, overexpression of RON can damage immune cells and inhibit anti-tumor immunity[27,28]. Thus, abnormal expression of RON may affect the efficacy of PD-L1 immunotherapy in CRC.
In this study, we investigated the clinical significance of expression patterns of RON and PD-L1 in primary CRC samples, and evaluated the potential for RON and PD-L1 as prognostic biomarkers in CRC. We treated CRC cells with BMS777607 in vitro to explore if RON inhibition affects the expression of PD-L1 and results in changes in related signaling pathways in CRC cells. Our data suggested that RON inhibition may be a novel approach to augment immunotherapy in CRC.
This study involved a GEO (Gene Expression Omnibus) cohort and a FAHZUSM (The First Affiliated Hospital, Zhejiang University School of Medicine) cohort. In the GEO cohort, the median age of all 287 CRC patients was 70 years (range: 24-97 years). There were 158 (55.1%) male patients and 129 (44.9%) female patients. The median survival time was 58 months, and 94 patients died during follow-up. In the FAHZUSM cohort, the median age of the CRC patients undergoing tumor resection was 61 years (range: 29-94 years). In this cohort, 215 (56.4%) patients were male and 166 (43.6%) were female. The median survival time was 100 months, and 91 patients died during follow-up. The primary site of a tumor, pathological grade, TNM stage, disease stage, histological type, and treatment status of CRC patients are shown in Table 1.
| FAHZUSM cohort (n = 381) | GEO cohort (n = 287) | ||||||||||||||||
| Characteristic | Cases | RON expression | PD-L1 expression | Cases No (%) | RON expression | PD-L1 expression | |||||||||||
| Low | High | 1P value | Low | High (TIMC) | 2P value | Low | High (TC) | 3P value | Low | High | 1P value | Low | High (TC) | 3P value | |||
| No. of cases number | 381 | 260 | 121 | 247 | 91 | 247 | 43 | 287 | 193 | 94 | 48 | 239 | |||||
| Age (mean ± SD) | 61.16 ± 12.419 | 60.40 ± 12.560 | 62.81 ± 11.997 | 0.859 | 60.94 ± 12.37 | 62.04 ± 13.080 | 0.771 | 60.94 ± 12.37 | 61.16 ± 12.419 | 0.938 | 68.44 ± 14.00 | 66.87 ± 13.18 | 71.65 ± 15.12 | 0.006 | 66.94 ± 14.33 | 68.74 ± 13.95 | 0.417 |
| Sex | |||||||||||||||||
| Man | 215 (56.4) | 157 (60.4) | 58 (47.9) | 0.023 | 143 (57.9) | 54 (59.3) | 0.811 | 143 (57.9) | 18 (41.9) | 0.051 | 158 (55.1) | 111 (57.5) | 47 (50) | 0.230 | 30 (62.5) | 128 (53.6) | 0.256 |
| Women | 166 (43.6) | 103 (39.6) | 63 (52.1) | 104 (42.1) | 37 (40.7) | 104 (42.1) | 25 (58.1) | 129 (44.9) | 82 (42.5) | 47 (50) | 18 (37.5) | 111 (46.4) | |||||
| Principal diagnosis | |||||||||||||||||
| Rectum | 229 (60.1) | 158 (61.1) | 71 (58.7) | 0.698 | 153 (61.9) | 47 (51.6) | 0.088 | 153 (61.9) | 29 (67.4) | 0.491 | 165 (57.5) | 111 (57.5) | 54 (57.4) | 0.920 | 34 (70.8) | 131 (54.8) | 0.040 |
| Colon | 152 (39.9) | 102 (38.9) | 50 (41.3) | 94 (38.1) | 44 (48.4) | 94 (38.1) | 14 (32.6) | 122 (42.5) | 82 (42.5) | 40 (42.6) | 14 (29.2) | 108 (45.2) | |||||
| Pathological grade | |||||||||||||||||
| Well/moderate | 9 (2.4) | 6 (2.3) | 3 (2.5) | 0.177 | 5 (2) | 3 (3.3) | 0.778 | 5 (2) | 1 (2.3) | 0.898 | NA | ||||||
| Poor | 346 (90.8) | 232 (89.2) | 114 (94.2) | 224 (90.7) | 82 (90.1) | 224 (90.7) | 40 (93) | ||||||||||
| Unknown | 26 (6.8) | 22 (8.5) | 4 (3.3) | 18 (7.1) | 6 (6.3) | 18 (7.3) | 2 (4.7) | ||||||||||
| T stage | |||||||||||||||||
| Tis-T2 | 107 (28.1) | 85 (32.7) | 22 (18.2) | 0.001 | 73 (29.6) | 23 (25.3) | 0.105 | 73 (29.6) | 11 (25.6) | 0.864 | 28 (9.7) | 18 (9.3) | 10 (10.6) | 0.459 | 3 (6.3) | 25 (10.5) | 0.437 |
| T3 | 177 (46.5) | 121 (46.5) | 56 (46.3) | 118 (47.8) (46.8%) | 37 (40.7) | 118 (47.8) | 22 (51.2) | 208 (72.5) | 144 (74.6) | 64 (68.1)20 | 39 (81.3) | 169 (70.7) | |||||
| T4 | 97 (25.5) | 54 (20.8) | 43 (35.5) | 56 (22.7) | 31 (34.1) | 56 (22.7) | 10 (23.3) | 51 (17.8) | 31 (16.1) | 20 (21.3) | 6 (12.5) | 45 (18.8) | |||||
| N stage | |||||||||||||||||
| N0 | 251 (65.9) | 184 (70.8) | 67 (55.4) | 0.003 | 164 (66.4) | 62 (68.1) | 0.764 | 164 (66.4) | 25 (58.1) | 0.294 | 141 (49.1) | 97 (50.3) | 44 (46.8) | 0.583 | 27 (56.3) | 114 (44.7) | 0.279 |
| N (1-2) | 91 (23.9) | 76 (29.2) | 54 (44.6) | 83 (33.6) | 29 (31.9) | 83 (33.6) | 12 (27.9) | 146 (50.9) | 96 (49.7) | 50 (53.2) | 21 (43.7) | 125 (52.3) | |||||
| M stage | |||||||||||||||||
| M0 | 368 (96.6) | 252 (96.9) | 116 (95.9) (96.3%) | 0.822 | 241 (97.6) | 85 (93.4) | 0.133 | 241 (97.6) | 42 (97.7) | 1.000 | 268 (93.4) | 183 (94.8) | 85 (90.4) | 0.160 | 47 (97.9) | 221 (92.5) | 0.216 |
| M1 | 13 (3.4) | 8 (3.1) | 5 (4.1) | 6 (2.4) | 6 (6.6) | 6 (2.4) | 1 (2.3) | 19 (6.6) | 10 (5.2) | 9 (9.6) | 1 (2.1) | 18 (7.5) | |||||
| Disease stage | |||||||||||||||||
| 0-II | 246 (64.6) | 180 (69.2) | 66 (54.5) | 0.016 | 162 (65.6) | 59 (64.8) | 0.897 | 162 (65.6) | 25 (58.1) | 0.346 | 139 (48.4) | 95 (49.2) | 44 (46.8) | 0.701 | 27 (56.3) | 112 (46.9) | 0.235 |
| III-IV | 135 (35.4) | 80 (30.8) | 55 (45.5) | 85 (34.4) | 32 (35.2) | 85 (34.4) | 18 (41.9) | 148 (51.6) | 98 (50.8) | 50 (53.2) | 21 (43.8) | 127 (53.1) | |||||
| Histological type | |||||||||||||||||
| Adenocarcinoma | 335 (87.9) | 227 (87.3) | 108 (89.3) | 0.706 | 214 (86.6) | 84 (92.3) | 0.130 | 214 (86.6) | 37 (86) | 0.488 | 287 (100) | 193 (100) | 94 (100) | 48 (100) | 239 (100) | ||
| Mucinous/SRCC | 36 (9.4) | 25 (9.6) | 11 (9.1) | 28 (11.3) | 4 (4.4) | 28 (11.3) | 4 (9.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Other | 10 (2.6) | 8 (3.1) | 2 (1.7) | 5 (2.0) | 3 (3.3) | 5 (2.0) | 2 (4.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Treatment | |||||||||||||||||
| Yes | 173 (45.4) | 110 (42.3) | 63 (52.1) | 0.043 | 112 (45.3) | 38 (41.8) | 0.694 | 112 (45.3) | 23 (32.6) | 0.472 | 115 (40.1) | 89 (46.1) | 26 (27.7) | 0.003 | 22 (45.8) | 93 (38.9) | 0.372 |
| No | 158 (41.5) | 119 (45.8) | 39 (32.2) | 105 (42.5) | 39 (42.9) | 105 (42.5) | 14 (53.5) | 172 (59.9) | 104 (53.9) | 68 (72.3) | 26 (54.2) | 146 (61.1) | |||||
| Unknown | 50 (13.1) | 31 (11.9) | 19 (15.7) | 30 (12.1) | 14 (15.4) | 30 (12.1) | 6 (14) | 0 (0) | 0 (0) | ||||||||
| PD-L1 (protein) | |||||||||||||||||
| Low | 247 (64.8) | 177 (73.1) | 70 (26.9) | 0.9764 | NA | ||||||||||||
| High (TIMCs) | 91 (23.9) | 65 (26.9) | 26 (27.1) | ||||||||||||||
| High (TCs) | 43 (11.3) | 18 (9.2) | 25 (26.3) | < 0.0015 | |||||||||||||
| PD-L1 (mRNA) | |||||||||||||||||
| Low | NA | 48 (16.7) | 31 (16.1) | 17 (18.1) | 0.666 | ||||||||||||
| High | 239 (83.3) | 162 (83.9) | 77 (81.9) | ||||||||||||||
The CRC cell line HT29 was obtained from the American Type Cell Culture (ATCC, Manassas, VA, United States). Flow cytometric analysis revealed that RON and PD-L1 were strongly expressed in HT29 cells (Supplementary Figure 1). Cells were cultured in growth medium supplemented with 10% fetal bovine serum and incubated under standard cell culture conditions at 37 °C and 5% CO2. Rabbit anti-RON antibody (5029) and mouse anti-RON monoclonal antibodies (Zt/f2 and Zt/g4) were used as previously described[28]. Macrophage-stimulating protein (MSP) is an activation factor for RON receptor tyrosine kinase phosphorylation and catalytic activity of a number of signal transduction proteins[29]. Human mature MSP was obtained from R&D Systems (Minneapolis, MN, United States). Antibodies against phospho-tyrosine (P-Tyr-100, Cat # 9411), AKT (Cat # 4685), phospho-AKT (Cat # 4060), extracellular signaling regulated kinase (ERK) 1/2 (Cat # 4695), and phospho-ERK1/2 (p44/42) (Cat # 4376) were obtained from Cell Signaling Technology (Beverly, MA, United States). CK antibody was from ZSGB-BIO (Cat # ZM-0069, Beijing, China). The BMS-777607 RON inhibitor was from Medchem Express (Monmouth Junction, NJ, United States). BMS-777607 was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 mmol/L and stored at -20 °C. The PD-L1 antibody (Cat # 13864) for immunohistochemistry and multiplex immunofluorescence was obtained from Cell Signaling Technology (Beverly, MA, United States). The PD-L1 antibody (Cat # 205921) used for cell experiments was from Abcam (Burlingame, CA, United States). Opal tyramine signal amplification (TSA) staining kit was from PerkinElmer (Hopkinton, MA, United States).
Gene expression data for RON and PD-L1 and clinical data for CRC patients are available from the GEO database and accessed through the browser web site (https://geonames.org/)[30]. A total of 290 primary CRC tumors from patients with detailed RON and PD-L1 expression data were selected from the GEO database, based on the completeness of patient clinical data. Only patients with complete tumor characteristics, overall survival (OS), and RNA-seq information, and patients whose tumor samples were obtained prior to treatment were included. The clinicopathologic features of the patients included age, sex, tumor location, histological type, tumor-lymph node-metastasis (TNM) stage, treatment regimen, and OS.
The CRC specimens used for this study were obtained from 381 CRC patients who were pathologically diagnosed and treated between January 2006 and November 2009 at the FAHZUSM. Clinical data for patients included age, sex, tumor location, histological type, TNM stage, tumor differentiation, treatment pattern, and OS rate (Table 1). CRC tissues were fixed in 10% buffered formalin, embedded in paraffin, and were available for immunohistochemical (IHC) evaluation. Detailed demographic data and clinical features of the FAHZUSM cohort are provided in Table 1. This study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (reference number: 2017427-1).
Multiplex immunofluorescence staining of paraffin-embedded sections was performed using Zt/f2 (5 μg/mL) as the primary antibody for RON, rabbit anti-PD-L1 antibody (1:150, 13864, CST) for PD-L1, and mouse anti-CK mAb (1:200, ZM-0069, ZSGB-BIO) for CK, and visualized using the Opal tyramine signal amplification (TSA) staining kit. First, the concentration and sequence of the RON, PD-L1, and CK antibodies were optimized. The primary antibodies/fluorescent dyes for PD-L1/Opal 530, RON/Opal 690, and CK /Opal 520 were applied to the CRC tissues in sequence. Finally, sections were stained with DAPI and mounted with an anti-fading mounting agent. PerkinElmer software version 2.1 was used to analyze multispectral images.
IHC staining was performed according to standard protocols. Primary antibodies were incubated overnight using a dilution of 1:150 for PD-L1 (13684, CST, United States) and 1:800 for RON. Mouse anti-RON mAb (Zt/f2) was used as previously described[31]. The primary antibody was visualized with diaminobenzidine until a brown precipitate appeared at the antigenic site using EnVision + System-HRP (Dako, Carpentaria, CA, United States). Then, sections were counterstained with hematoxylin.
The IHC-stained sections were independently scored by two pathologists who were blinded to the clinicopathological data. RON expression was determined using a semi-quantitative system. According to the percentage of positive staining area in the whole cancer area, the scoring was as follows: 0 (< 5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (> 75%). The staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), or 3 (strong). The total score was obtained by adding the positive proportion score and the staining level score. Samples were categorized as follows: Samples with a final staining score ≤ 4 were considered to be low and those with a score of > 4 were considered to be high for RON expression.
PD-L1 expression was evaluated using an anti-PD-L1 rabbit antibody (13684, CST, United States). A cutoff of 5% for the total proportion of cells that were positive for PD-L1 was used, such that samples with < 5% PD-L1 positive cells were considered to be low and samples with ≥ 5% PD-L1 positive cells were considered to be high for PD-L1 expression. This standard has been established in various types of cancer[32-34].
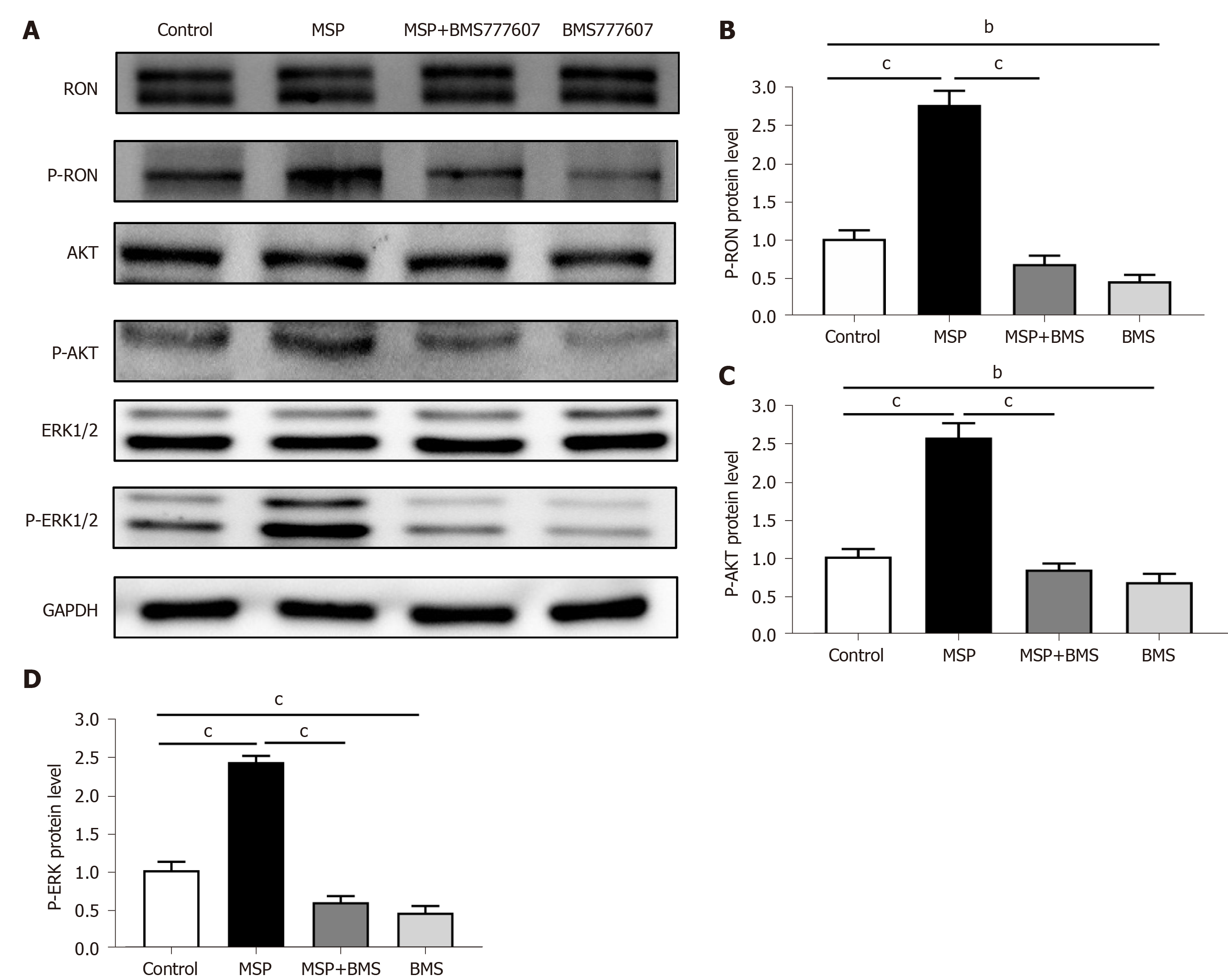
The result of Cell Counting Kit 8 illustrated that the half-maximal inhibitory concentration (IC50) of BMS-777607 in HT29 cells at 72 h was 2 μmol/L (Supplementary Figure 2). HT29 cells were treated with 2 nmol/L MSP, 2 μmol/L BMS-777607, or 2 nmol/L MSP + 2 μmol/L BMS-777607 for 24 h. The culture medium was removed and the cells were washed with ice-cold phosphate buffered saline (PBS). Cells were digested with trypsin, collected, and washed with PBS to remove residual trypsin. Cells were lysed in radioimmunoprecipitation (RIPA) buffer, and 25 μg of total protein per sample was separated by 8% SDS-PAGE. Western blot was performed using antibodies against RON, phospho-RON, PD-L1, ERK1/2, phospho-ERK1/2, AKT, and phospho-AKT; GAPDH protein was evaluated as an internal control to ensure equal sample loading. Immunoreactive protein bands were visually detected using enhanced chemiluminescence reagents.
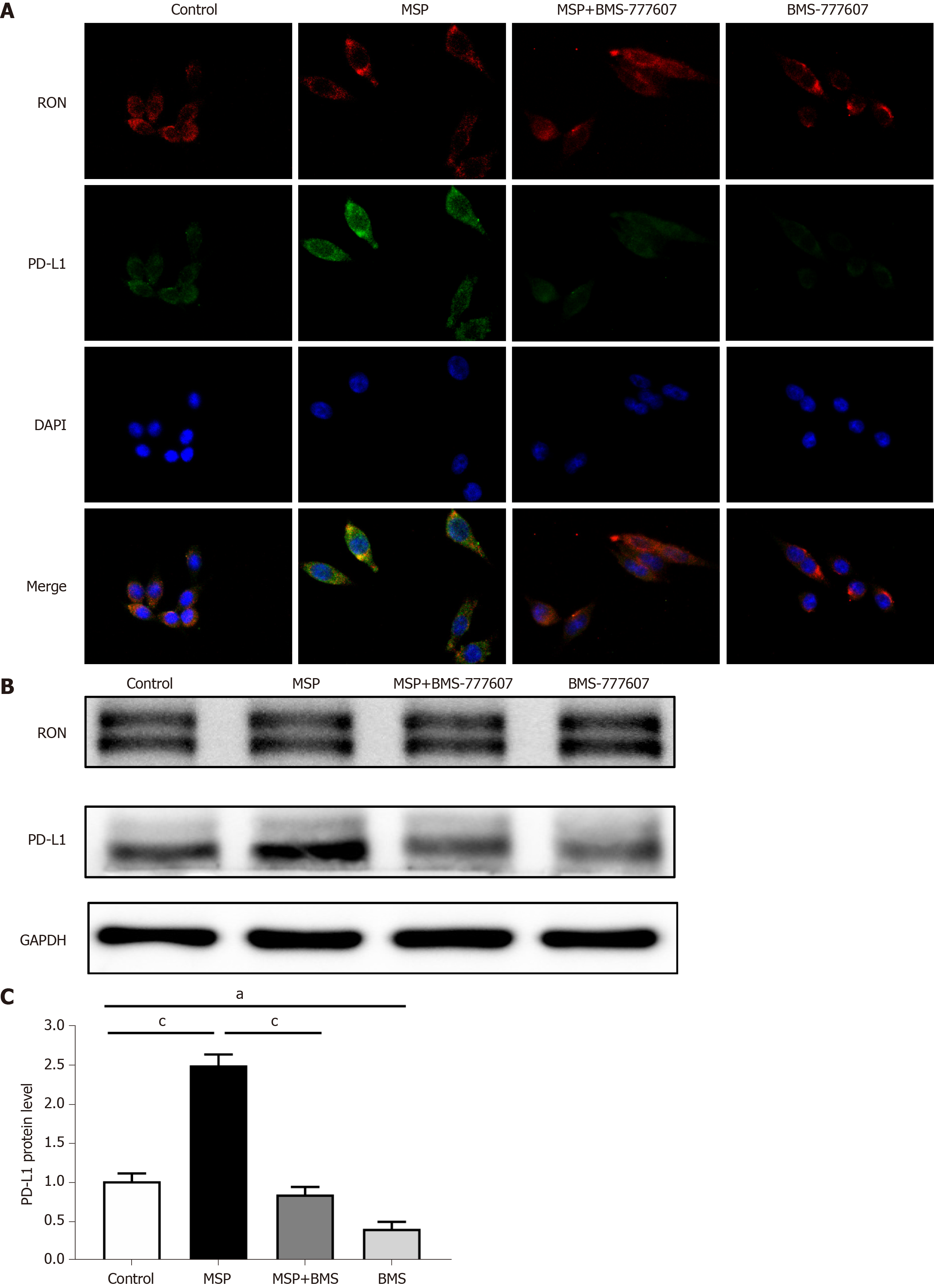
HT29 cells were cultured overnight in a 6-well plate on a cover slide. After treatment with 2 nmol/L MSP, 2 μmol/L BMS-777607, or 2 nmol/L MSP + 2 μmol/L BMS-777607 for 24 h, cells were fixed with 4% paraformaldehyde at 37 °C for 20 min, then washed with PBS five times for 3 min. Cover slides were then blocked with 5% BSA in PBS at room temperature for 30 min. After that, the cells were incubated with primary antibody against PD-L1 (205921, Abcam, United States) and mouse anti-RON mAb (Zt/f2) overnight at 4 °C. Primary antibodies were then removed by washing with PBS five times for 3 min. The cells were then incubated with a fluorescently labeled secondary antibody in the dark at room temperature for 1 h. Excess secondary antibody was removed and the cells were counterstained with DAPI. Slides were mounted with an anti-quenching agent and sealed. The fluorescently labeled cells were observed using a LEICA DMi8 microscope (Leica). Representative images were selected and photographed.
Clinical quantitative data are expressed as the mean ± standard deviation (SD) and count data are expressed as constituent ratio (%) or ratio (%). Statistical evaluation was conducted with SPSS 25.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism v.8 (La Jolla, CA, United States). X-tile 3.6.1 software (Yale University, New Haven, CT, United States) was used to determine the optimal cut-off values for RON and PD-L1 expression in the GEO cohort[35]. χ2 tests were used to analyze relationships between clinicopathological parameters and RON and PD-L1 expression. Differences between quantitative data with a normal distribution were compared using two independent samples t-tests between two groups and one-way analysis of variance among three groups and SNK-q test between groups. Kaplan-Meier method and log-rank test were used for OS analysis. Univariate and multivariate Cox regression analyses were used for identifying the risk factors for OS. A P value < 0.05 was considered statistically significant. All confidence intervals (CIs) are stated at the 95% confidence level.
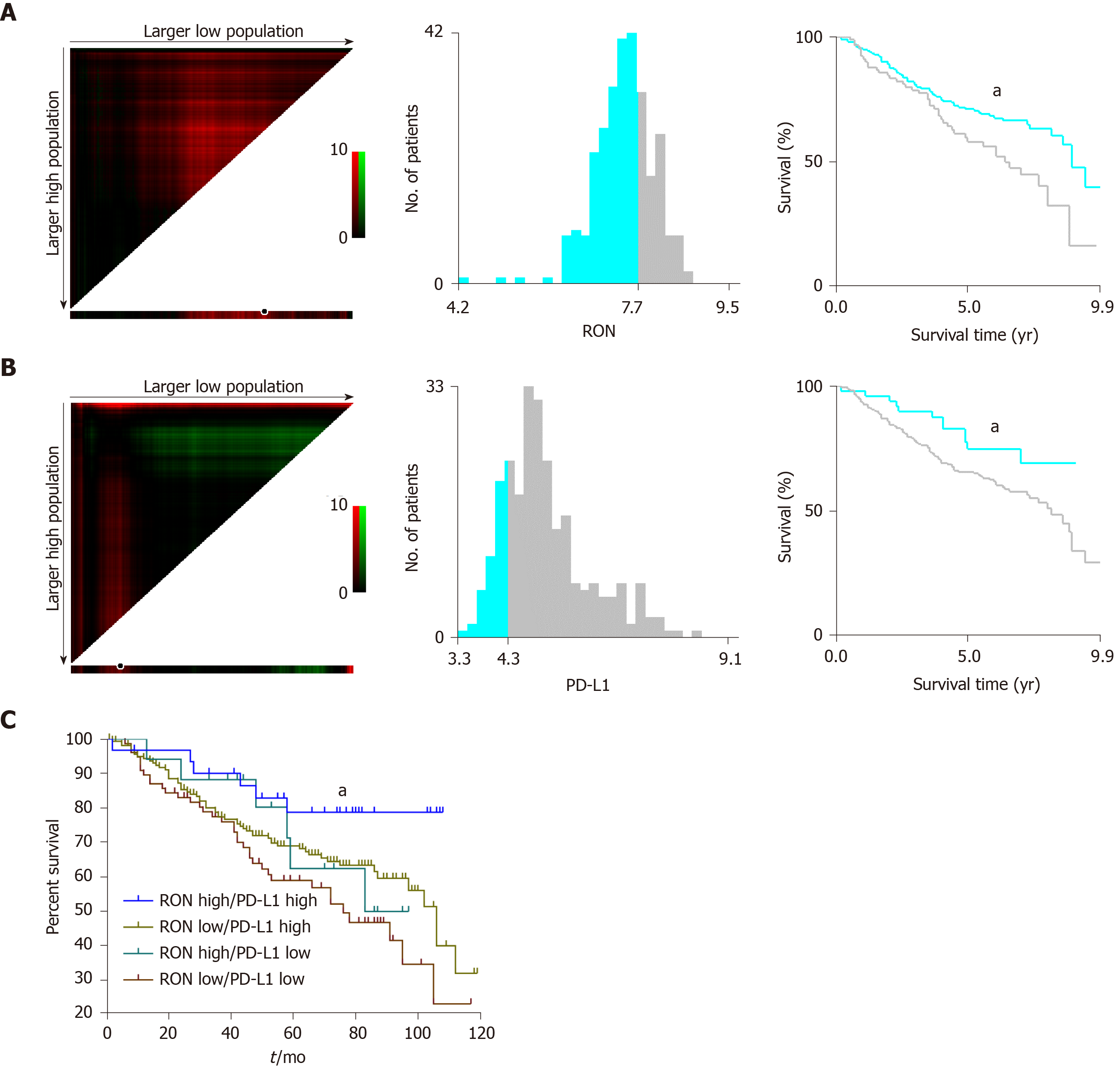
In the GEO cohort, X-tile was used to find the optimal cutoff values to divide the data of RON (the upper panel) and PD-L1 (the lower panel) mRNA expression into either low or high expression by selecting the highest χ2 value and the smallest P value of a standard log-rank test. The cut-off values for RON and PD-L1 mRNA in TCs were determined as 7.70 and 4.30, respectively (Figure 1A and B), and the log-rank χ2 values for RON and PD-L1 were 4.544 and 4.078, respectively. Patients in the GEO cohort were stratified according to RON and PD-L1 status: RON ≤ 7.70 and RON > 7.70, and PD-L1 ≤ 4.30 and PD-L1 > 4.30 (Figure 1A and B). We found that the high expression of RON and PD-L1 was associated with a poor prognosis.
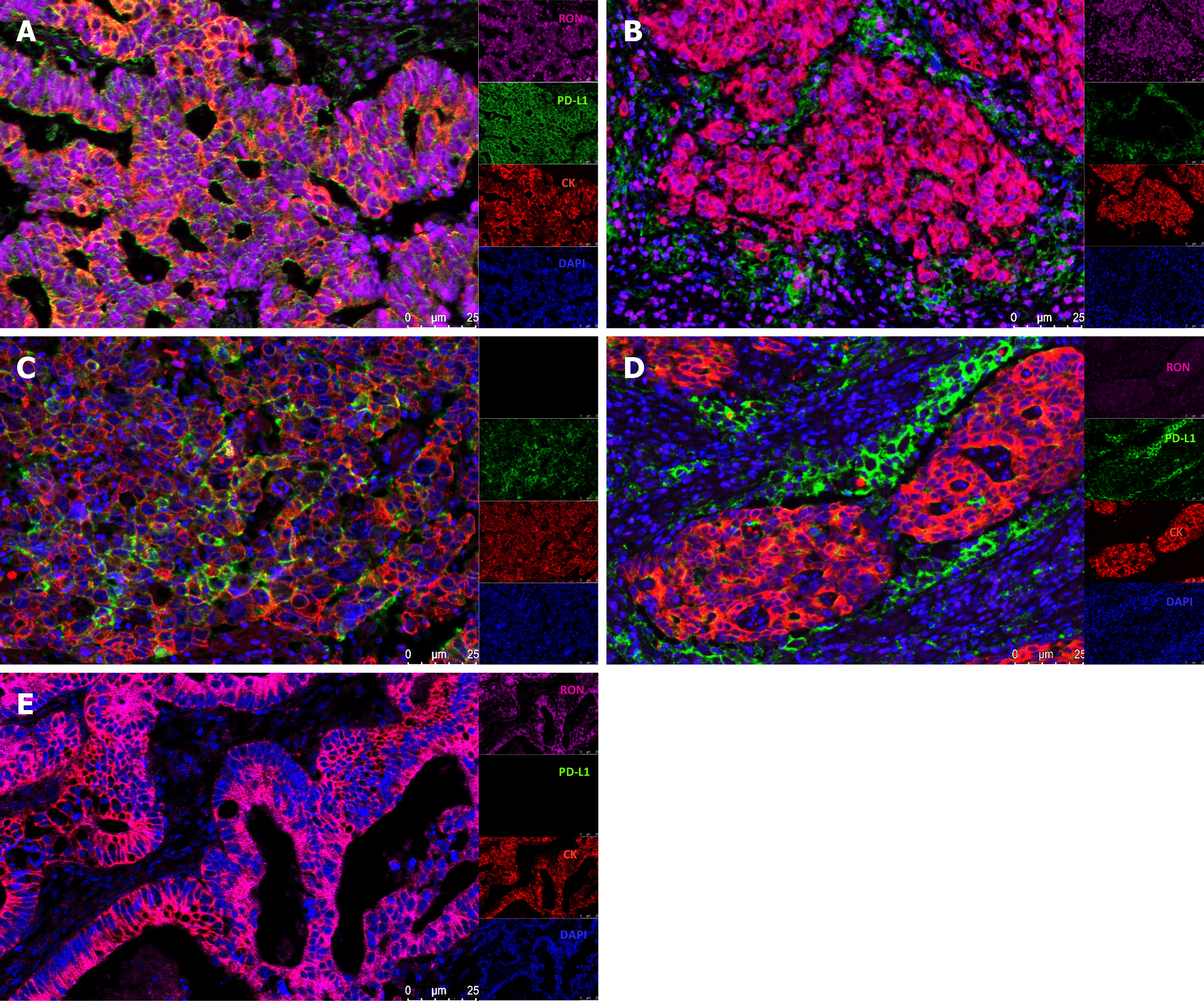
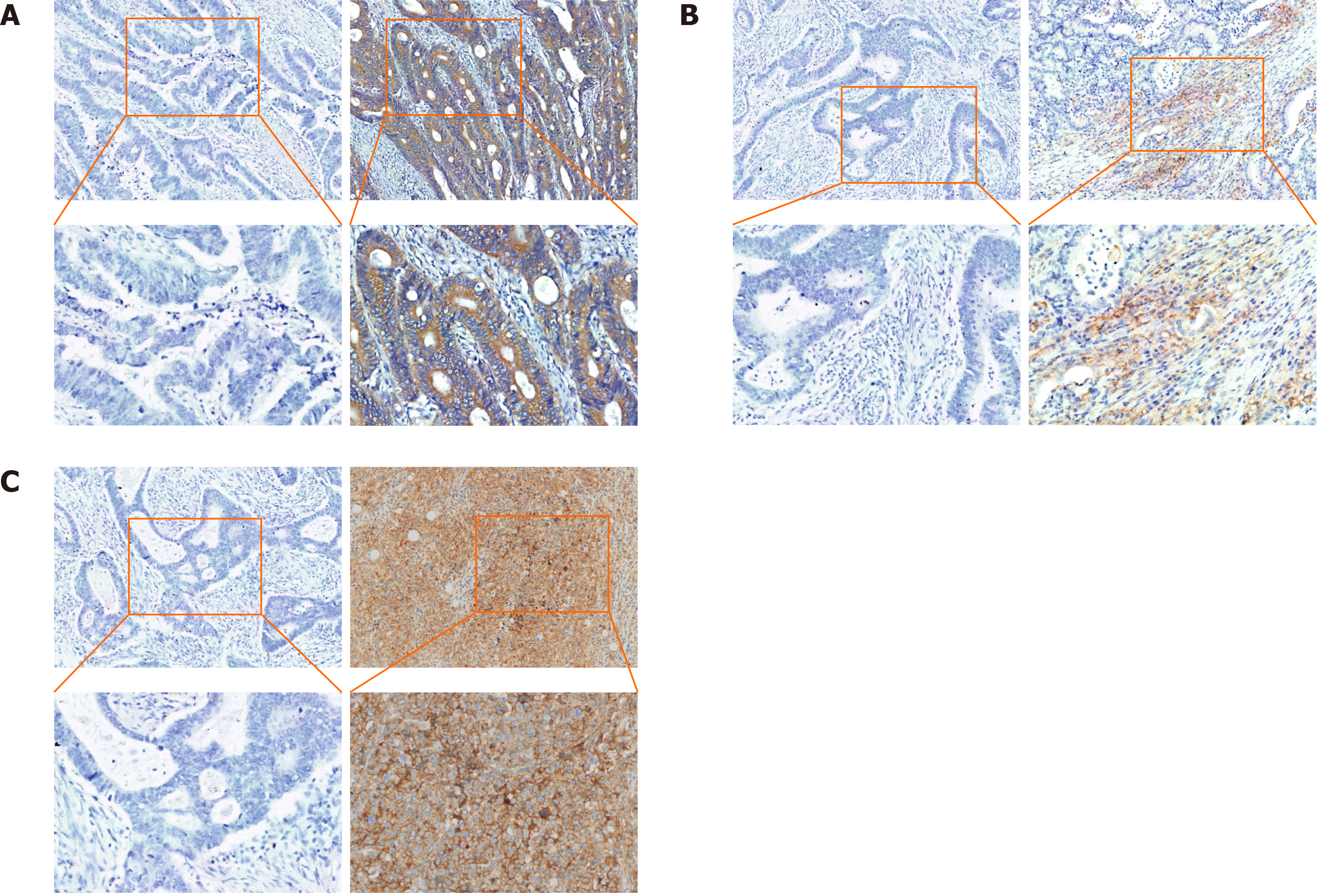
In CRC tissue samples, multiple immunofluorescence staining was used to show the expression patterns of RON in TCs and PD-L1 in the tumor microenvironment. We found that both RON and PD-L1 exhibited membrane-enhanced expression in TCs (Figure 2A); elevated RON expression on the tumor cell membrane was accompanied by enhanced PD-L1 expression in tumor-infiltrating mononuclear cells (TIMCs) (Figure 2B). We also observed cases where PD-L1 expression was enhanced on the tumor cell membrane (Figure 2C) or in TIMCs (Figure 2D), but RON expression was low. Additionally, there were cases where RON expression was enhanced on the TC membrane, but PD-L1 was not expressed in the tumor microenvironment (Figure 2E). In addition, RON and PD-L1 were often accompanied by a cytoplasmic expression of cancer cells. In general, when RON and PD-L1 were enhanced in the tumor cell membrane and cytoplasm, PD-L1 was also expressed in TIMCs. The results of multiplex immunofluorescence are consistent with the evaluation of RON and PD-L1 by immunohistochemical staining (Figure 3A-C).
In the FAHZUSM cohort, CRC patients were divided into two groups based on high or low expression of RON and PD-L1 detected by IHC staining. In 381 CRC patients, high and low RON expression in TCs was observed in 121 (31.8%) and 260 (68.2%), respectively. High and low expression of PD-L1 in TCs and TIMCs was noted in 43 (11.3%) and 91 (23.9%) patients, respectively, and PD-L1 was lowly expressed in TIMCs in 247 (64.8%) cases. None of the patients had high PD-L1 expression in both TCs and TIMCs. Among all samples expressing RON and PD-L1 in TCs, the proportion with both high RON and high PD-L1 expression was 8.6% (25/290), while the proportion of samples expressing high PD-L1 in TIMCs and high RON in TCs was 7.7% (26/338). Chi-square tests were used to determine the correlation between the expression of RON and PD-L1 in tumor tissues. Compared to samples with low expression of RON, samples with high RON expression levels were more likely to have high levels of PD-L1 in TCs, demonstrating a significant correlation between high RON expression and high PD-L1 expression in TCs (P < 0.001, Table 1). The expression of PD-L1 in TIMCs was not significantly correlated with RON expression in TCs (Table 1).
We compared the clinical characteristics of patient groups categorized based on the expression of RON and PD-L1. High expression of RON was associated with gender, T stage, N stage, disease stage, and treatment. High RON and high PD-L1 expression in TCs was related to T stage (Table 2). High expression of RON and PD-L1 (TIMCs) was associated with T stage, principal diagnosis, histological type, and treatment (P < 0.05; Table 3).
| Characteristic | Cases | RON and PD-L1 (TCs) expression in the tumor microenvironment | ||||
| RON low/PD-L1 low | RON high/PD-L1 low | RON low/PD-L1 high | RON high/PD-L1 high | P value | ||
| Number of cases | 290 | 177 | 70 | 18 | 25 | |
| Age (mean ± SD) | 60.89 ± 12.215 | 60.37 ± 12.299 | 62.40 ± 12.518 | 58.39 ± 12.930 | 62.12 ± 10.179 | 0.495 |
| Sex | ||||||
| Man | 161 (55.5) | 108 (61) | 35 (50.0) | 9 (50.0) | 9 (36.0) | 0.061 |
| Women | 129 (44.5) | 68 (39) | 35 (50.0) | 9 (50.0) | 16 (64.0) | |
| Principal diagnosis | s | |||||
| Rectum | 182 (62.8) | 116 (65.5) | 37 (52.9) | 14 (77.8) | 15 (60.0) | 0.149 |
| Colon | 108 (37.2) | 61 (34.5) | 33 (47.1) | 4 (22.2) | 10 (40.0) | |
| Pathological grade | ||||||
| Well/moderate | 6 (2.1) | 3 (1.7) | 2 (2.9) | 0 (0.0) | 1 (4.0) | 0.264 |
| Poor | 264 (9.1) | 158 (89.3) | 66 (94.3) | 16 (88.9) | 24 (96.0) | |
| Unknown | 20 (6.9) | 16 (9.0) | 2 (2.9) | 2 (11.1) | 0 (0.0) | |
| T stage | ||||||
| Tis-T2 | 84 (29) | 64 (36.2) | 9 (12.9) | 5 (27.8) | 6 (24.0) | 0.003 |
| T3 | 140 (48.3) | 82 (46.3) | 36 (51.4) | 11 (61.1) | 11 (44.0) | |
| T4 | 66 (22.8) | 31 (17.5) | 25 (35.7) | 2 (11.1) | 8 (32.0) | |
| N stage | ||||||
| N0 | 238 (66.9) | 125 (70.6) | 39 (55.7) | 12 (66.7) | 13 (66.4) | 0.072 |
| N (1-2) | 118 (33.1) | 52 (29.4) | 31 (44.3) | 6 (33.3) | 12 (33.6) | |
| M stage | ||||||
| M0 | 283 (97.6) | 173 (97.7) | 68 (97.1) | 18 (100) | 24 (96.0) | 0.735 |
| M1 | 7 (2.4) | 4 (2.3) | 2 (2.9) | 0 (0.0) | 1 (4.0) | |
| Stage | ||||||
| 0-II | 187 (64.5) | 123 (69.5) | 39 (55.7) | 12 (66.7) | 13 (52.0) | 0.110 |
| III-IV | 103 (35.5) | 54 (30.5) | 31 (33.3) | 6 (33.3) | 12 (48.0) | |
| Histological type | ||||||
| Adenocarcinoma | 251 (86.6) | 152 (85.9) | 62 (88.6) | 15 (83.3) | 22 (88.0) | 0.493 |
| Mucinous/SRCC | 32 (11) | 21 (11.9) | 7 (10.0) | 1 (5.6) | 3 (12.0) | |
| Other | 7 (2.4) | 4 (2.3) | 1 (1.4) | 2 (11.1) | 0 (0.0) | |
| Treatment | ||||||
| Yes | 135 (46.6) | 83 (46.9) | 22 (31.4) | 6 (33.3) | 12 (48.0) | 0.158 |
| No | 119 (41.0) | 74 (41.8) | 38 (54.3) | 11 (61.1) | 8 (32.0) | |
| Unknown | 36 (12.4) | 20 (11.3) | 10 (14.3) | 1 (5.6) | 5 (20.0) | |
| Characteristic | Cases | RON and PD-L1 (TIMCs) expression in the tumor microenvironment | ||||
| RON low/PD-L1 low | RON high/PD-L1 low | RON low/PD-L1 high | RON high/PD-L1 high | P value | ||
| Number of cases | 338 | 177 | 70 | 65 | 26 | |
| Age (mean ± SD) | 61.24 ± 12.555 | 60.37 ± 12.299 | 62.40 ± 12.518 | 61.03 ± 13.286 | 64.58 ± 12.436 | 0.348 |
| Sex | ||||||
| Man | 197 (58.3) | 108 (61) | 35 (50.0) | 40 (61.5) | 14 (52.8) | 0.368 |
| Women | 141 (41.7) | 68 (39) | 35 (50.0) | 25 (38.5) | 12 (46.2) | |
| Principal diagnosis | ||||||
| Rectum | 200 (59.2) | 116 (65.5) | 37 (52.9) | 28 (43.1) | 19 (73.1) | 0.004 |
| Colon | 138 (40.8) | 61 (34.5) | 33 (47.1) | 37 (56.9) | 7 (26.9) | |
| Pathological grade | ||||||
| Well/moderate | 8 (2.4) | 3 (1.7) | 2 (2.9) | 3 (4.6) | 0 (0) | 0.492 |
| Poor | 306 (90.5) | 158 (89.3) | 66 (94.3) | 58 (89.2) | 24 (92.3) | |
| Unknown | 24 (7.1) | 16 (9.0) | 2 (2.9) | 4 (6.2) | 2 (7.7) | |
| T stage | ||||||
| Tis-T2 | 96 (28.4) | 64 (36.2) | 9 (12.9) | 16 (2.6) | 7 (26.9) | 0.002 |
| T3 | 155 (45.9) | 82 (46.3) | 36 (51.4) | 28 (43.1) | 9 (34.6) | |
| T4 | 87 (25.7) | 31 (17.5) | 25 (35.7) | 21 (32.3) | 10 (38.5) | |
| N stage | ||||||
| N0 | 226 (66.9) | 125 (70.6) | 39 (55.7) | 47 (72.3) | 15 (57.7) | 0.075 |
| N (1-2) | 112 (33.1) | 52 (29.4) | 31 (44.3) | 18 (27.7) | 11 (42.3) | |
| M stage | ||||||
| M0 | 326 (96.4) | 173 (97.7) | 68 (97.1) | 61 (93.8) | 24 (92.3) | 0.235 |
| M1 | 12 (3.6) | 4 (2.3) | 2 (2.9) | 4 (2.3) | 2 (7.7) | |
| Stage | ||||||
| 0-II | 221 (65.4) | 123 (69.5) | 39 (55.7) | 45 (69.2) | 14 (53.8) | 0.104 |
| III-IV | 117 (34.6) | 54 (30.5) | 31 (33.3) | 20 (30.8) | 12 (46.2) | |
| Histological type | ||||||
| Adenocarcinoma | 287 (84.9) | 152 (85.9) | 62 (88.6) | 60 (92.3) | 13 (50) | < 0.001 |
| Mucinous/SRCC | 40 (11.8) | 21 (11.9) | 7 (10.0) | 3 (4.6) | 9 (34.6) | |
| Other | 11 (3.3) | 4 (2.3) | 1 (1.4) | 2 (3.1) | 4 (15.4) | |
| Treatment | ||||||
| Yes | 298 (88.2) | 83 (46.9) | 22 (31.4) | 30 (46.2) | 24 (92.3) | < 0.001 |
| No | 32 (9.5) | 74 (41.8) | 38 (54.3) | 25 (38.5) | 1 (3.8) | |
| Unknown | 8 (2.4) | 20 (11.3) | 10 (14.3) | 10 (15.4) | 1 (3.8) | |
In the GEO cohort, univariate Cox regression analysis showed that age, T stage, M stage, disease stage, RON, and PD-L1 (TCs) expression were significantly correlated with a poor prognosis in CRC patients (P < 0.05). Multivariate analysis after adjustment showed that only age, T stage, and M stage were independent prognostic factors for OS in CRC patients (P < 0.05); disease stage, RON expression, and PD-L1 expression were not significant factors in the multivariate analysis (P > 0.05; Table 4). Kaplan-Meier analysis showed that high expression of RON or PD-L1 (TCs), and both high RON and PD-L1 expression were all associated with a poor OS (P < 0.05; Figure 1A-C). High expression of RON was associated with patient age and treatment type, and high expression of PD-L1 (TCs) was associated with the primary site of the tumor (P < 0.05; Table 1).
| Variable | FAHZUSM cohort | GEO cohort | ||||||
| Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value | Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value | |
| Age (mean ± SD) | 1.029 (1.011-1.048) | 0.002 | 1.031 (1.011-1.050) | 0.002 | 1.025 (1.009-1.041) | 0.002 | 1.028 (1.012-1.045) | 0.001 |
| Gender | ||||||||
| Male | 1.000 | 0.011 | 1.000 | 0.008 | 1.000 | 0.293 | 1.000 | 0.076 |
| Female | 0.562 (0.362-0.874) | 0.527 (0.328-0.847) | 0.814 (0.555-1.194) | 0.698 (0.469-1.039) | ||||
| Principal diagnosis | ||||||||
| Rectum | 1.000 | 0.298 | 1.000 | 0.318 | 1.000 | 0.688 | 1.000 | 0.544 |
| Colon | 0.796 (0.518-1.224) | 0.790 (0.497-1.255) | 1.081 (0.738-1.585) | 0.882 (0.589-1.322) | ||||
| Histological type | ||||||||
| Adenocarcinoma | 1.000 | 0.408 | 1.000 | 0.387 | NA | |||
| Mucinous/SRCC | 2.464 (0.343-17.710) | 2.720 (0.263-28.122) | ||||||
| Unknown | 3.355 (0.433-25.986) | 1.232 (0.129-11.795) | ||||||
| T stage | ||||||||
| Tis-T2 | 1.000 | < 0.001 | 1.000 | 0.003 | 1.000 | 0.001 | 1.000 | 0.007 |
| T3 | 0.211 (0.108-0.413) | 0.298 (0.141-0.632) | 0.333 (0.145-0.766) | 0.381 (0.159-0.914) | ||||
| T4 | 0.503 (0.325-0.781) | 0.596 (0.361-0.897) | 0.455 (0.292-0.709) | 0.476 (0.295-0.769) | ||||
| N stage | ||||||||
| N0 | 1.000 | < 0.001 | 1.000 | 0.111 | 1.000 | 0.062 | 1.000 | 0.141 |
| N (1-2) | 3.156 (2.083-4.783) | 5.376 (0.678-42.660) | 1.435 (0.982-2.097) | 0.306 (0.063-1.479) | ||||
| M stage | ||||||||
| M0 | 1.000 | < 0.001 | 1.000 | 0.039 | 1.000 | < 0.001 | 1.000 | 0.008 |
| M1 | 4.338 (2.094-8.985) | 2.445 (1.048-5.701) | 3.546 (2.013-6.247) | 2.481 (1.269-4.852) | ||||
| Stage | ||||||||
| 0-II | 1.000 | < 0.001 | 1.000 | 0.406 | 1.000 | 0.029 | 1.000 | 0.099 |
| III-IV | 3.102 (2.044-4.708) | 0.409 (0.050-3.366) | 1.530 (1.045-2.242) | 3.924 (0.774-19.888) | ||||
| Pathological grade | ||||||||
| Well/moderate | 1.000 | < 0.001 | 1.000 | 0.003 | NA | |||
| Poor | 7.689 (2.434-24.290) | 4.044 (1.103-14.830) | ||||||
| Unknown | 1.266 (0.513-3.126) | 0.375 (0.104-1.357) | ||||||
| RON (protein) | ||||||||
| Low | 1.000 | 0.001 | 1.000 | 0.012 | 1.000 | 0.035 | 1.000 | 0.466 |
| High | 2.060 (1.364-3.112) | 1.788 (1.138-2.808) | 1.515 (1.030-2.228) | 1.170 (0.767-1.785) | ||||
| RON (mRNA) | ||||||||
| Low | NA | 1.000 | 0.035 | 1.000 | 0.466 | |||
| High | 1.515 (1.030-2.228) | 1.170 (0.767-1.785) | ||||||
| PD-L1 (protein) | ||||||||
| Low | 1.000 | < 0.001 | 1.000 | 0.001 | NA | |||
| High (TIMCs) | 0.544 (0.293-1.011) | 0.676 (0.350-1.304) | ||||||
| High (TCs) | 1.325 (0.669-2.511) | 1.654 (0.819-3.338) | ||||||
| PD-L1 (mRNA) | ||||||||
| Low | NA | 1.000 | 0.047 | 1.000 | 0.083 | |||
| High | 1.835 (1.007-3.345) | 1.719 (0.931-3.173) | ||||||
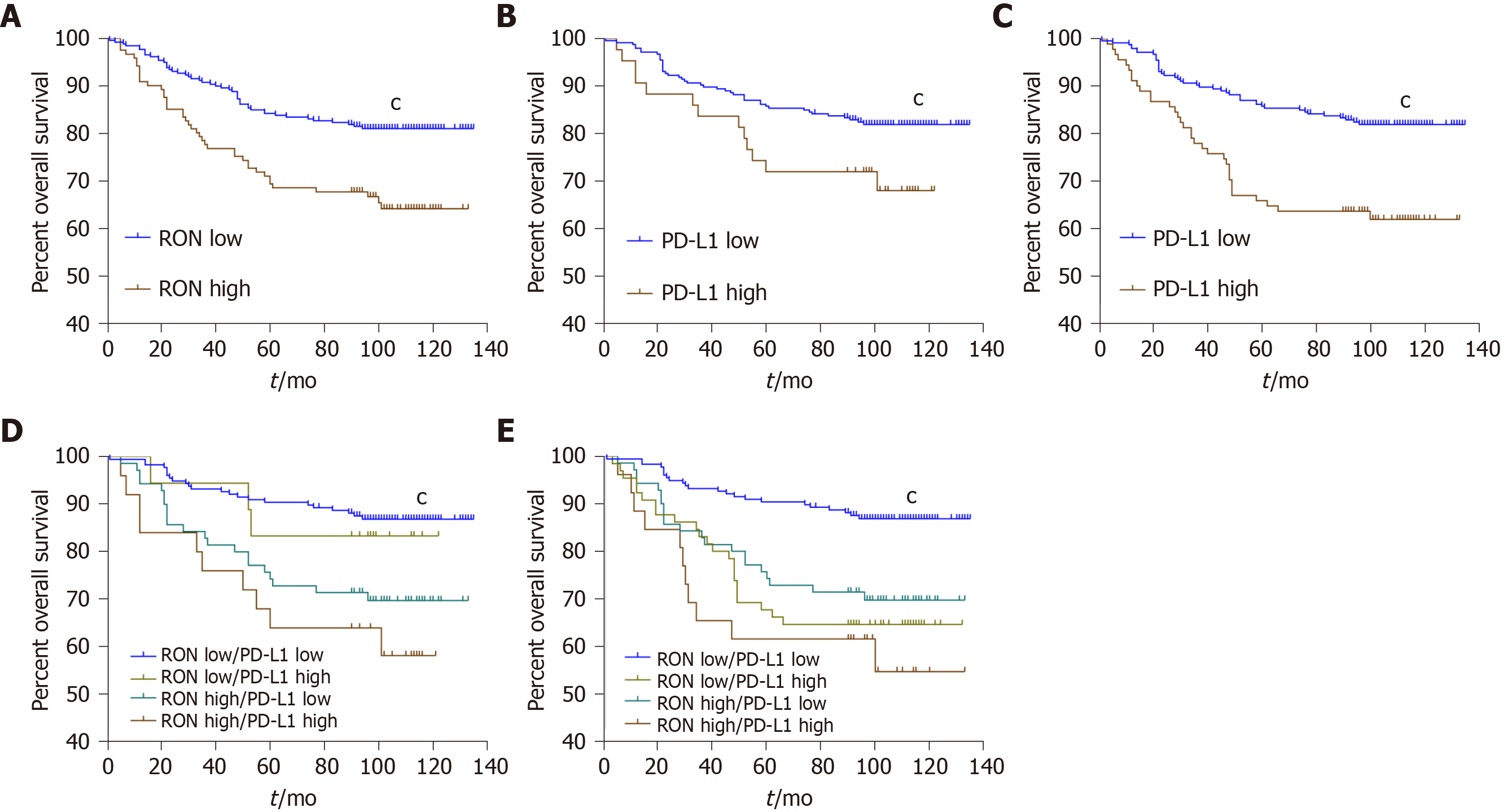
In the FAHZUSM cohort, Kaplan-Meier analysis showed that high expression of RON in TCs, high PD-L1 expression in TCs, or high PD-L1 expression in TIMCs in CRC tissue samples was correlated with a lower OS (P < 0.05) (Figure 4A-C). Univariate Cox regression analysis showed that age, gender, TNM stage, disease stage, pathological grade, RON expression, and PD-L1 expression were associated with a poor OS in patients with CRC (P < 0.05). Multivariate analysis after adjustment showed that age, gender, T stage, M stage, pathological grade, RON expression, and PD-L1 expression remained independent prognostic factors for OS in CRC patients (P < 0.05), while N and disease stage were not significant (P > 0.05; Table 4). We further analyzed the relationship between OS and RON and PD-L1 expression in the tumor microenvironment. High expression of both RON and PD-L1 in TCs predicted a significantly worse OS, and high RON expression in TCs and high PD-L1 expression in TIMCs were associated with a significantly worse prognosis (P < 0.001; Figure 4D and E).
In order to study the relationship between RON and PD-L1 in CRC cells, HT29 cells (following serum starvation) were treated with MSP, BMS-777607, or MSP + BMS-777607 for 24 h. The effects of treatment on RON and PD-L1 expression were analyzed by cellular immunofluorescence. Activating RON phosphorylation promoted PD-L1 expression, while inhibiting RON phosphorylation down-regulated PD-L1 expression (Figure 5A). This result was confirmed by analysis of protein expression by Western blot (P < 0.05; Figure 5B and C). In addition, we found that RON inhibition significantly reduced the phosphorylation of AKT and ERK1/2, which signal downstream of RON (Figure 6A-D).
The application of PD-L1 immunotherapy in cancer treatment is becoming more and more important, but its therapeutic effect has been limited in CRC. This may be due in part to the prevalence of the mismatch repair (MMR) proficient or microsatellite stabilization (MSS) subtypes of CRC[36]. In addition, c-MET plays an important role in regulating PD-L1 expression[37,38]. Therefore, RON may promote the expression of PD-L1 in tumor tissue to affect the efficacy of PD-L1 immunosuppressants.
RON and PD-L1 play important roles in cancer initiation and development and are important targets for tumor therapy. However, few studies have explored the relationship between RON and PD-L1 expression in CRC and the impact that RON and PD-L1 expression patterns may have on pathological characteristics and disease outcomes. It was our hypothesis that activation of the RON receptor tyrosine kinase may promote the expression of PD-L1 in CRC tissue and impair the efficacy of PD-L1 immunotherapy. The purpose of our study was to analyze the expression and clinical significance of RON and PD-L1 in the tumor microenvironment in CRC samples, and to determine whether RON can regulate the expression of PD-L1 in CRC. We discovered that high expression of both RON and PD-L1 in the tumor microenvironment was associated with the poorest OS rate compared to CRC tumors with other RON and PD-L1 expression states. RON and PD-L1 were independent prognostic factors for OS in CRC patients. In addition, we showed that RON phosphorylation led to increased expression of PD-L1 in CRC cells, and that inhibiting RON phosphorylation can reduce PD-L1 expression.
We used publicly available data from the GEO database to investigate the relationship between RON and PD-L1 expression and clinical outcomes in CRC. We used X-tile to determine the optimal cut-off values for RON and PD-L1 levels in the GEO cohort. Kaplan-Meier survival analysis revealed a positive correlation of high RON or PD-L1 expression with worse OS, and high expression of RON and PD-L1 together was associated with the worst OS for CRC patients. However, RON and PD-L1 levels in the GEO dataset were determined by RNA sequencing using RNA extracted from tumor tissue to quantify the expression level in TCs, and it is difficult to determine the interactions between RON and PD-L1 that may arise within the tumor microenvironment from the GEO dataset. Therefore, we used the FAHZUSM cohort, a larger patient cohort with pathological tumor samples, to further study the expression of RON and PD-L1 in CRC tissues and to determine the pathological significance of elevated RON and PD-L1 expression in the tumor microenvironment.
Dysregulation of RON and PD-L1 signal transduction is a key feature of many tumors. Our results and previous studies demonstrated that high expression of RON or PD-L1 in CRC tumor tissues correlated with the extent of primary tumor, lymph node metastasis, and distant metastasis[39,40]. Furthermore, high expression of RON or PD-L1 in the tumor microenvironment was associated with a poor survival in CRC patients[22,39-42].
We demonstrated that high expression of both RON and PD-L1 in TCs was associated with a poor OS in CRC patients. Interestingly, the expression levels of RON were positively correlated with those of PD-L1 in TCs, which has not been previously reported. This may be related to the fact that RON can regulate both the production of and response to IFN-γ[43]. In TCs, long-term continuous activation of IFN-γ can mediate adaptive resistance to PD-L1 tumor immunosuppression; high expression of RON may attenuate resistance, restore the efficacy of PD-L1 targeted therapy, and may even have a promoting effect on the expression of PD-L1 by inhibiting the production of IFN-γ[10,43-45].
MEK/ERK and PI3K/AKT signaling pathways play a central role towards understanding the mechanisms underlying tumorigenesis and tumor development, prevention, and treatment. For example, autophagy caused by the activation of PI3K/AKT pathway plays a significant role in tumors[46,47]. In our study, we demonstrated that RON phosphorylation increased the expression of PD-L1 in CRC cells, proving that RON activation promotes the expression of PD-L1, likely acting through the downstream AKT and ERK1/2 signaling pathways. Previous studies have shown that there is crosstalk between RON phosphorylation and other receptors, which can affect the surface expression of other receptors[22]. Phosphorylation of RON activates downstream oncogenic signaling pathways, such as the RAS-ERK and PI3K-AKT pathways, thereby promoting tumor initiation, growth, invasion, and metastasis[20]. Treatment with BRAF inhibitors or MEK inhibitors, or down-regulation of ERK1/2 can lead to down-regulation of PD-L1 expression in TCs[48]. Thus, RON phosphorylation may promote the expression of PD-L1 in TCs through the RAS-ERK signaling pathway. In addition, PTEN interacts with RTK-dependent signals at multiple levels[48], and has an antagonistic effect on PI3K, which plays an important role in mediating RTK-dependent cell signaling and tumor immune escape[10,48,49]. Therefore, RON phosphorylation may interact with PTEN to activate the PI3K-AKT signaling pathway, thereby promoting PD-L1 expression in TCs.
TIMCs are regarded as an indicator of host immune response to a tumor, and have long been considered an unfavorable prognostic marker in CRC[41,42]. Moreover, RON expression plays an important role in anti-tumor immune responses[27,50]. Previous studies also have shown that RON activation reduced the polarization of inflammatory M1 macrophages and induced the differentiation of immunosuppressive M2 macrophages. M2 macrophages promote PD-L1 expression through autocrine VEGF signaling[51]. In our study, we demonstrated that high expression of both RON and PD-L1 (TIMCs) was associated with a poor OS in patients with CRC. Thus, high expression of RON may impair the anti-tumor immune response by promoting the expression of PD-L1 in TIMCs, leading to tumor growth, invasion, and metastasis.
There were some limitations in this study. First, although we have analyzed a large patient cohort, this was a retrospective analysis and there is the potential for selection bias. Second, we did not evaluate which specific immune cell components in TIMCs expressed PD-L1. Third, although we have preliminarily studied that RON phosphorylation promotes the expression of PD-L1 in CRC cells, the mechanism by which RON promotes PD-L1 expression still needs to be further studied.
In summary, high expression of RON and/or PD-L1 in CRC samples is associated with a poor prognosis in CRC patients. The expression status of RON and PD-L1 may be helpful as biomarkers to evaluate the prognosis of patients with CRC. In addition, RON phosphorylation can promote the expression of PD-L1 in CRC cells, possibly through activation of the AKT and ERK1/2 signaling pathways, which provides a new idea for the immunotherapy of CRC.
Programmed death ligand 1 (PD-L1) protein expression on immune cells enables tumor cells to evade the immune system in a wide variety of malignancies. However, the efficacy of PD-L1 immunosuppressive agents in colorectal cancer (CRC) is poor. The recepteur d’origine nantais (RON) receptor tyrosine kinase plays an important role in regulating tumor immunity.
The poor anti-tumor effect of PD-1 inhibitors in CRC promoted the research on the mechanism of PD-1 expression, so as to improve the therapeutic effect of PD-1 inhibitors on CRC.
The present study aimed to identify patterns of RON and PD-L1 expression and explore the clinical significance of these patterns in CRC.
The gene expression data from the Gene Expression Omnibus database (GEO; n = 290) and patients at the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZUSM; n = 381) were analyzed to determine the prognostic value of RON and PD-L1 expression in the tumor microenvironment of CRC. HT29 cells were treated with BMS-777607 to explore the relationship between RON activity and PD-L1 expression. Signaling pathways and protein expression perturbed by RON inhibition were evaluated by cellular immunofluorescence and Western blot.
In the GEO patient cohort, the cut-off values for RON and PD-L1 expression were determined to be 7.70 and 4.30, respectively. Stratification of patients based on these cutoffs demonstrated that high expression of RON and PD-L1 was associated with a poor prognosis. In the FAHZUSM cohort, rates of high expression of RON in tumor cells, high PD-L1 expression in tumor cells and tumor infiltrating monocytes, and both high RON and high PD-L1 expression in the tumor microenvironment were 121 (32%), 43 (11%), 91 (24%), and 51 (13.4%), respectively. High expression of RON was significantly correlated with high expression of PD-L1 in the tumor cell compartment (P < 0.001). High expression of RON and PD-L1 were independent prognostic factors for poorer overall survival. Concurrent high expression of both RON and PD-L1 in the tumor microenvironment was significantly associated with a poor prognosis. In vitro, BMS-777607 inhibited the phosphorylation of RON and PD-L1 expression, and attenuated the activation of the ERK1/2 and AKT signaling pathways in CRC cells.
RON, PD-L1, and their crosstalk are significant in predicting the prognostic value of CRC. Moreover, phosphorylation of RON upregulates PD-L1 expression, which provides a novel approach to immunotherapy in CRC.
The study of RON and PD-L1 expression will help improve the efficacy of PD-L1 immunosuppressive agents for CRC.
Manuscript source: Invited manuscript
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koustas E, Zhu Y S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Arshad U, Sutton PA, Ashford MB, Treacher KE, Liptrott NJ, Rannard SP, Goldring CE, Owen A. Critical considerations for targeting colorectal liver metastases with nanotechnology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 3. | Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 3211] [Article Influence: 321.1] [Reference Citation Analysis (0)] |
| 4. | Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, Inamura K, Cao Y, Song M, Nowak JA, Liao X, Nosho K, Chan AT, Giannakis M, Bass AJ, Hodi FS, Freeman GJ, Rodig S, Fuchs CS, Qian ZR, Ogino S. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 6. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1966] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 7. | Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S; MTN-020–ASPIRE Study Team. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375:2121-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 633] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 8. | Prall F, Hühns M. The PD-1 expressing immune phenotype of T cell exhaustion is prominent in the 'immunoreactive' microenvironment of colorectal carcinoma. Histopathology. 2017;71:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Ji S, Xu Y, Han D, Peng X, Lu X, Brockmeyer NH, Wu N. Changes in Lipid Indices in HIV+ Cases on HAART. Biomed Res Int. 2019;2019:2870647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 1018] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 11. |
Feng D, Qin B, Pal K, Sun L, Dutta S, Dong H, Liu X, Mukhopadhyay D, Huang S, Sinicrope FA. |
| 12. | Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, Nishiyama A, Arai S, Yano S, Wang W. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. 2019;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 13. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9912] [Article Influence: 762.5] [Reference Citation Analysis (0)] |
| 14. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6290] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 15. | Ng C, Li H, Wu WKK, Wong SH, Yu J. Genomics and metagenomics of colorectal cancer. J Gastrointest Oncol. 2019;10:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Arai H, Battaglin F, Wang J, Lo JH, Soni S, Zhang W, Lenz HJ. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev. 2019;81:101912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Ledys F, Klopfenstein Q, Truntzer C, Arnould L, Vincent J, Bengrine L, Remark R, Boidot R, Ladoire S, Ghiringhelli F, Derangere V. RAS status and neoadjuvant chemotherapy impact CD8+ cells and tumor HLA class I expression in liver metastatic colorectal cancer. J Immunother Cancer. 2018;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with "K-Ras addiction" reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 705] [Cited by in RCA: 676] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 19. | Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195-1202. [PubMed] |
| 20. | Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013;13:466-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Graves-Deal R, Bogatcheva G, Rehman S, Lu Y, Higginbotham JN, Singh B. Broad-spectrum receptor tyrosine kinase inhibitors overcome de novo and acquired modes of resistance to EGFR-targeted therapies in colorectal cancer. Oncotarget. 2019;10:1320-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Lee CT, Chow NH, Su PF, Lin SC, Lin PC, Lee JC. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis Colon Rectum. 2008;51:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Schroeder GM, An Y, Cai ZW, Chen XT, Clark C, Cornelius LA, Dai J, Gullo-Brown J, Gupta A, Henley B, Hunt JT, Jeyaseelan R, Kamath A, Kim K, Lippy J, Lombardo LJ, Manne V, Oppenheimer S, Sack JS, Schmidt RJ, Shen G, Stefanski K, Tokarski JS, Trainor GL, Wautlet BS, Wei D, Williams DK, Zhang Y, Zhang Y, Fargnoli J, Borzilleri RM. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem. 2009;52:1251-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Wang J, Rajput A, Kan JL, Rose R, Liu XQ, Kuropatwinski K, Hauser J, Beko A, Dominquez I, Sharratt EA, Brattain L, Levea C, Sun FL, Keane DM, Gibson NW, Brattain MG. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J Biol Chem. 2009;284:10912-10922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Xu XM, Wang D, Shen Q, Chen YQ, Wang MH. RNA-mediated gene silencing of the RON receptor tyrosine kinase alters oncogenic phenotypes of human colorectal carcinoma cells. Oncogene. 2004;23:8464-8474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Yao HP, Zhou YQ, Ma Q, Guin S, Padhye SS, Zhang RW, Wang MH. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. Mol Cancer. 2011;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Eyob H, Ekiz HA, Welm AL. RON promotes the metastatic spread of breast carcinomas by subverting antitumor immune responses. Oncoimmunology. 2013;2:e25670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Eyob H, Ekiz HA, Derose YS, Waltz SE, Williams MA, Welm AL. Inhibition of ron kinase blocks conversion of micrometastases to overt metastases by boosting antitumor immunity. Cancer Discov. 2013;3:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Danilkovitch A, Donley S, Skeel A, Leonard EJ. Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells. Mol Cell Biol. 2000;20:2218-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Clough E, Barrett T. The Gene Expression Omnibus Database. Methods Mol Biol. 2016;1418:93-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1445] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 31. | Feng L, Yao HP, Wang W, Zhou YQ, Zhou J, Zhang R, Wang MH. Efficacy of anti-RON antibody Zt/g4-drug maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for targeted colorectal cancer therapy. Clin Cancer Res. 2014;20:6045-6058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4187] [Article Influence: 380.6] [Reference Citation Analysis (0)] |
| 33. | Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 2204] [Article Influence: 244.9] [Reference Citation Analysis (0)] |
| 34. | Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2517] [Cited by in RCA: 2894] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 35. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2940] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 36. | Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 37. | Chun HW, Hong R. Significance of PD-L1 clones and C-MET expression in hepatocellular carcinoma. Oncol Lett. 2019;17:5487-5498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Kammerer-Jacquet SF, Medane S, Bensalah K, Bernhard JC, Yacoub M, Dupuis F, Ravaud A, Verhoest G, Mathieu R, Peyronnet B, Brunot A, Laguerre B, Lespagnol A, Mosser J, Dugay F, Belaud-Rotureau MA, Rioux-Leclercq N. Correlation of c-MET Expression with PD-L1 Expression in Metastatic Clear Cell Renal Cell Carcinoma Treated by Sunitinib First-Line Therapy. Target Oncol. 2017;12:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Park YL, Lee GH, Kim KY, Myung E, Kim JS, Myung DS, Park KJ, Cho SB, Lee WS, Jung YD, Kim HS, Joo YE. Expression of RON in colorectal cancer and its relationships with tumor cell behavior and prognosis. Tumori. 2012;98:652-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, Tanabe Y, Moriyama J, Fujii T, Matoba S, Kuroyanagi H, Takano T. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108:853-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Wang L, Ren F, Wang Q, Baldridge LA, Monn MF, Fisher KW, Sheng W, Zhou X, Du X, Cheng L. Significance of Programmed Death Ligand 1 (PD-L1) Immunohistochemical Expression in Colorectal Cancer. Mol Diagn Ther. 2016;20:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ, Shike M, Stadler ZK, Vakiani E, Klimstra DS, Shia J. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Wilson CB, Ray M, Lutz M, Sharda D, Xu J, Hankey PA. The RON receptor tyrosine kinase regulates IFN-gamma production and responses in innate immunity. J Immunol. 2008;181:2303-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 45. | Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 46. | Koustas E, Papavassiliou AG, Karamouzis MV. The role of autophagy in the treatment of BRAF mutant colorectal carcinomas differs based on microsatellite instability status. PLoS One. 2018;13:e0207227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Koustas E, Sarantis P, Kyriakopoulou G, Papavassiliou AG, Karamouzis MV. The Interplay of Autophagy and Tumor Microenvironment in Colorectal Cancer-Ways of Enhancing Immunotherapy Action. Cancers (Basel). 2019;11:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 400] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 49. | Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, Comin-Anduix B, Ribas A. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 50. | Babicky ML, Harper MM, Chakedis J, Cazes A, Mose ES, Jaquish DV, French RP, Childers B, Alakus H, Schmid MC, Foubert P, Miyamoto J, Holman PJ, Walterscheid ZJ, Tang CM, Varki N, Sicklick JK, Messer K, Varner JA, Waltz SE, Lowy AM. MST1R kinase accelerates pancreatic cancer progression via effects on both epithelial cells and macrophages. Oncogene. 2019;38:5599-5611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Lai YS, Wahyuningtyas R, Aui SP, Chang KT. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J Cell Mol Med. 2019;23:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |