Published online Jan 15, 2020. doi: 10.4251/wjgo.v12.i1.66
Peer-review started: April 15, 2019
First decision: May 16, 2019
Revised: July 26, 2019
Accepted: October 1, 2019
Article in press: October 1, 2019
Published online: January 15, 2020
Processing time: 260 Days and 23.7 Hours
Prevalence of nonalcoholic fatty liver disease (NAFLD) is rapidly increasing, and NAFLD has become one of the most common chronic liver diseases worldwide. With abnormal CD44 activation, the severe form of NAFLD can progress to liver cirrhosis and hepatocellular carcinoma (HCC). Thus, the molecular mechanism of CD44 in NAFLD needs to be identified.
To investigate the relationship between CD44 activation and malignant transformation of rat hepatocytes under nonalcoholic lipid accumulation.
Sprague-Dawley rats were fed a high-fat (HF) for 12 wk to entice NAFLD and then with HF plus 2-fluorenylacetamide (0.05%) to induce HCC. Rats were sacrificed every 2 wk, and subsequently divided into the groups based on liver pathological examination (hematoxylin and eosin staining): NAFLD, denaturation, precancerosis, HCC, and control. Liver CD44 mRNA was detected by OneArray. Liver fat as assessed by Oil red O staining or CD44 by immunohistochemical assay was compared with their integral optic density. Serum CD44, alanine aminotransferase, aspartate aminotransferase, triglyceride, total cholesterol, and AFP levels were quantitatively tested.
Elevated CD44 was first reported in hepatocarcinogenesis, with increasing expression from NAFLD to HCC at the protein or mRNA level. The CD44 integral optic density values were significantly different between the control group and the NAFLD (t = 25.433, P < 0.001), denaturation (t = 48.822, P < 0.001), precancerosis (t = 27.751, P < 0.001), and HCC (t = 16.239, P < 0.001) groups, respectively. Hepatic CD44 can be secreted into the blood, and serum CD44 levels in HCC or precancerous rats were significantly higher (P < 0.001) than those in any of the other rats. Positive correlations were found between liver CD44 and CD44 mRNA (rs = 0.373, P = 0.043) and serum CD44 (rs = 0.541, P = 0.002) and between liver CD44 mRNA and serum CD44 (rs = 0.507, P = 0.004). Moreover, significant correlations were found between liver CD44 and liver AFP (rs = 0.572, P = 0.001), between serum CD44 and serum AFP (rs = 0.608, P < 0.001), and between CD44 mRNA and AFP mRNA (rs = 0.370, P = 0.044).
The data suggested that increasing CD44 expression is associated with the malignant transformation of hepatocytes in NAFLD.
Core tip: CD44, which belongs to a family of adhesion molecules, is a marker of cancer stem cells and is related to the transformation of nonalcoholic fatty liver disease to nonalcoholic steatohepatitis and hepatocellular carcinoma. Dynamic expression of CD44 in livers or blood at protein or mRNA level was first investigated at different stages of the progression of fat accumulating fatty liver. Increasing CD44 expression could be one of the most important progenitors and was associated with the malignant transformation of hepatocytes with lipid accumulation.
- Citation: Fang M, Yao M, Yang J, Zheng WJ, Wang L, Yao DF. Abnormal CD44 activation of hepatocytes with nonalcoholic fatty accumulation in rat hepatocarcinogenesis. World J Gastrointest Oncol 2020; 12(1): 66-76
- URL: https://www.wjgnet.com/1948-5204/full/v12/i1/66.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i1.66
Hepatocellular carcinoma (HCC) is the main form of primary liver cancer characterized by high malignancy, easy recurrence and metastasis, and geographical diversity, and both its incidence and mortality are increasing in the world[1,2]. Despite improved treatment modalities, the prognosis of HCC patients is still rather poor because of frequent metastasis and recurrence[3,4]. Major risk factors, except for infection with hepatitis B virus or hepatitis C virus, are nonalcoholic fatty liver disease (NAFLD) and metabolic-related disorders[5-7]. The incidence of NAFLD has significantly increased, and the proportion of HCC due to malignant transformation of NAFLD shows an increasing trend. Lipid accumulation is strictly linked to chronic hepatocyte damage, resulting in the generation of an inflammation microenvironment and creation of a pro-oncogenic milieu, thus promoting malignant transformation of hepatocytes with no mitochondrial carnitine palmitoyl transferase-II activity[8]. Recently, accumulating evidence supports that HCC contains a small subpopulation of cancer stem-like cells (CSC)[9] with potential biomarkers (CD44, CD133, and aldehyde dehydrogenase 1) that might be important factors in HCC occurrence[10], of which CD44 could be a key player in non-alcoholic steatohepatitis[11].
Transmembrane glycoprotein CD44 is closely associated with aggressive behavior and poor prognosis in a variety of human malignancies[12,13]. It can bind to hyaluronic acid (the most important ligand), collagen, fibrin, and laminin, mediate specific adhesion between cells as well as between cells and the extracellular matrix, and be involved in many biological processes such as transmitting intracellular signals and regulating the growth, invasion, and metastasis of HCC[14,15]. CD44 is one of the most frequently reported CSC markers in NAFLD, and CD44 positive cells have CSC properties, such as self-renewal and tumorigenicity. Recently, high CD44 expression has been closely linked to NAFLD progression to HCC[16]. However, the relationship between CD44 expression and hepatocarcinogenesis is still controversial, with unclear particular mechanisms. The objective of this study was to highlight correlations between the alterations of CD44 expression and malignant transformation of lipid-accumulating hepatocytes.
In total, 78 4-wk-old male Sprague-Dawley rats, weighing 100-120 g, were randomly divided into either a control group (n = 12) or an NAFLD model group (n = 66). All animals were raised at 22 ± 2 °C, with a light/dark period of 12 h, and a humidity of 55%. According to a previous method[8], the rats of the control group were fed a routine diet, whereas those of the NAFLD model group were fed a high fat diet (10% egg yolk powder, 10% lard, 4% cholesterol, 1% cholic acid, and 75% common feed) for 2 wk. Then, the NAFLD rats (n = 42) were given a high fat diet plus 0.05% of 2-fluorenylacetamide (2-FAA, Sigma, St Louis, MO, United States) to induce HCC formation. Two control rats, four NAFLD rats, and one HCC rat were sacrificed by ether anesthetization every 2 wk. Blood samples were collected from the heart and stored at -20 °C, and liver tissues were taken after operation, frozen quickly in liquid nitrogen, and stored at -80 °C. Liver tissues were used for Oil red O, hematoxylin and eosin, and immunohistochemical (IHC) staining. All in vivo procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Nantong University, China.
Dried paraffin-embedded sections were deparaffinized in xylene, rehydrated with a graded series of ethanol, and stained with hematoxylin for 5 min. Subsequently, the sections were immerged in hydrochloric acid and ammonia for seconds, rinsed for 1 h, placed in distilled water for a moment, decolorized with 70% and 90% alcohol for 10 min each, and stained with eosin for 3 min. After dyeing, the sections were dehydrated with 100% alcohol, cleared with xylene, and sealed with resin. Based on the alterations of histopathological characteristics under a microscope, the livers were divided into control, NAFLD, denaturation, precancerosis, and HCC groups.
We prepared the application fluid and filtered it according to the kit manufacturer’s instructions. The frozen slices stored in the refrigerator at -80 °C in advance were placed at room temperature for 10 min, then stained with reagent one for 15 min and washed with distilled water at 37 °C for 20 s. After that, they were stained again with reagent two for 3-5 min and washed with distilled water at 37 °C for 30 s. Subsequently, we added the water-based sealant to the surface before drying. The slices were observed and photographed under microscope and analyzed by Image-Pro Plus v6.0 software with integral optic density (IOD) value[17]. For measuring IOD, the image system comprised a Leica CCD camera DFC420 connected to a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions Ltd, Cambridge, United Kingdom). Photographs of representative fields were captured under high-power magnification (× 200) with Leica QWin Plus v3 software. The IOD value of each image was measured with Image-Pro Plus v6.0 software (Media Cybernetics Inc, Bethesda, MD, United States).
Serum total cholesterol (Tch) and triglyceride (TG) levels were measured with a kit from Nanjing Jiancheng Biotechnology Company (Nanjing, China). Briefly, the blank, calibration, and sample wells were set up. We added 10 µL distilled water as well as standards and samples with 1000 µL working liquid into corresponding wells. After being incubated at 37 °C for 10 min, absorbance of each well was read on a spectrophotometer, with 510 nm as the primary wavelength, and the average concentration was calculated according to the formula.
The liver sections were put in 80 °C drying box for 2 h. Then, after being dewaxed, dehydrated, and washed by flowing water, the slices were soaked in the citrate antigen recovery buffer and heated in the microwave oven until boiling for 5 min. Each slice was exposed to 100 µL 3% H2O2, incubated, and washed with phosphate buffered saline with primary rabbit anti-human CD44 antibody (ab157107, Abcam, Cambridge, United Kingdom) at 1:100 dilution. After that, polymer reinforcements and horseradish peroxidase-conjugated goat anti-rabbit IgG (ab97051, Abcam) at 1:500 dilutions were followed and repeatedly washed. Finally, the slices were added with 3,3’-diaminobenzidine dye liquor, counterstained with Hematoxylin, and soaked in 0.1% HCl. After rinsing, blueness, dehydration in ethanol, clearness with xylene, and sealing with neutral balsam were observed by optical microscope (MX53 Olympus, Tokyo, Japan) and analyzed with the Image Pro plus v6.0 software with IOD value[17].
According to the protocol, every 100 mg tissue was homogenized in a glass grinder with 1 mL TRI reagent and then transferred into Eppendorf (EP) tube, in which the reagent was mixed up and down 10 times, and rested for 5 min at room temperature. Next, 0.2 mL chloroform was added, mixed, rested, and then centrifuged (12000 rpm, 4 °C, 15 min). The upper water was transferred into a new aseptic EP tube, mixed with 0.5 mL isopropanol, and centrifuged (12000 rpm, 4 °C, 10 min). Afterwards, the supernatant was removed, sRNA precipitation was hacked and washed with 80% ethanol, and the centrifugation (7500 rpm, 4 °C, 5 min) was repeated. Finally, the supernatant was carefully poured out, the precipitation was dried (30 min, until RNA precipitation became transparent, not completely dry), and then the pellet dissolved with 30 µL of DEPC water. The quantity and quality of the RNA samples were determined with the use of the NanoDrop ND-1000 spectrophotometer.
The strand of cDNA and antisense RNA was synthesized by using OneArray plus RNA amplification kit developed by the Hualian Company (Beijing, China). In the process, aa-UTP and NHS-CyeDye were added to make aRNA become CyeDye-aRNA to complete calibration. After purification, we made the hybridization between the product and Phalanx OneArrayTM and, furthermore, entered the analysis process after cleaning and signal detection. The scanner was the Agilent Microarray Scanner (G2505C, Santa Clara, CA, United States). Finally, transcriptional levels of AFP and CD44 in five groups of rats were.
Five groups of rats were created based on pathological hematoxylin and eosin (H and E) staining. Liver tissue (20 mg) was mixed with 200 µL of mixed radioimmuno-precipitation assay buffer (UNOCI Biological Company, WB020) in 1.5 mL EP tubes and homogenized. The tissue was preserved with ice for 4 h and centrifuged for 5 min at 12000 g. The supernatant was divided into two parts: one was stored at -80 °C after measuring the concentration and the other was denatured in boiling water with 5 × protein loading buffer for 5 min and stored at -80 °C.
About 5 mL of blood was taken from the rat heart and incubated at 4 °C overnight. After centrifugation (2000 rpm, 20 min), we removed sera into the EP tube. Based on H and E staining, we divided all sera into five groups and stored them at -80 °C and avoided repeated freeze-thaw cycles.
The concentration of AFP and CD44 in the liver homogenate and in the sera of rats was detected according to the manufacturer’s instructions of the ELISA kit (Cloud-Clone Corp, Katy, TX, United States). We set the blank and added 100 µL standards, liver homogenates, and serum to the microplate, where the reagents were incubated 1 h at 37 °C. Then, we removed the liquid, added prepared biotinylated labeled detector antibody, and incubated the samples at 37 °C. Subsequently, we aspirated and washed each tube, added prepared streptavidin-horseradish peroxidase mixture to each tube, incubated the mixture again, aspirated and washed each well, added the TMB solution to each well until color developed, and then added the Stop solution. The optical density values were measured at 450 nm on a microplate reader (Biotek Synergy, Winooski, VT, United States), and the corresponding protein concentration for each sample was obtained by a standard curve.
Image pro plus 6.0, GraphPad prism 5.0 (La Jolla, CA, United States), and Photoshop software were used to analyze data and generate figures. Microsoft Excel and IBM SPSS statistics 23 software (Armonk, NY, United States) were applied to analyze data and calculate the mean ± SD. The Student’s t test was used to compare CD44 and AFP levels in liver homogenates and sera of rats. A P < 0.05 was considered significant.
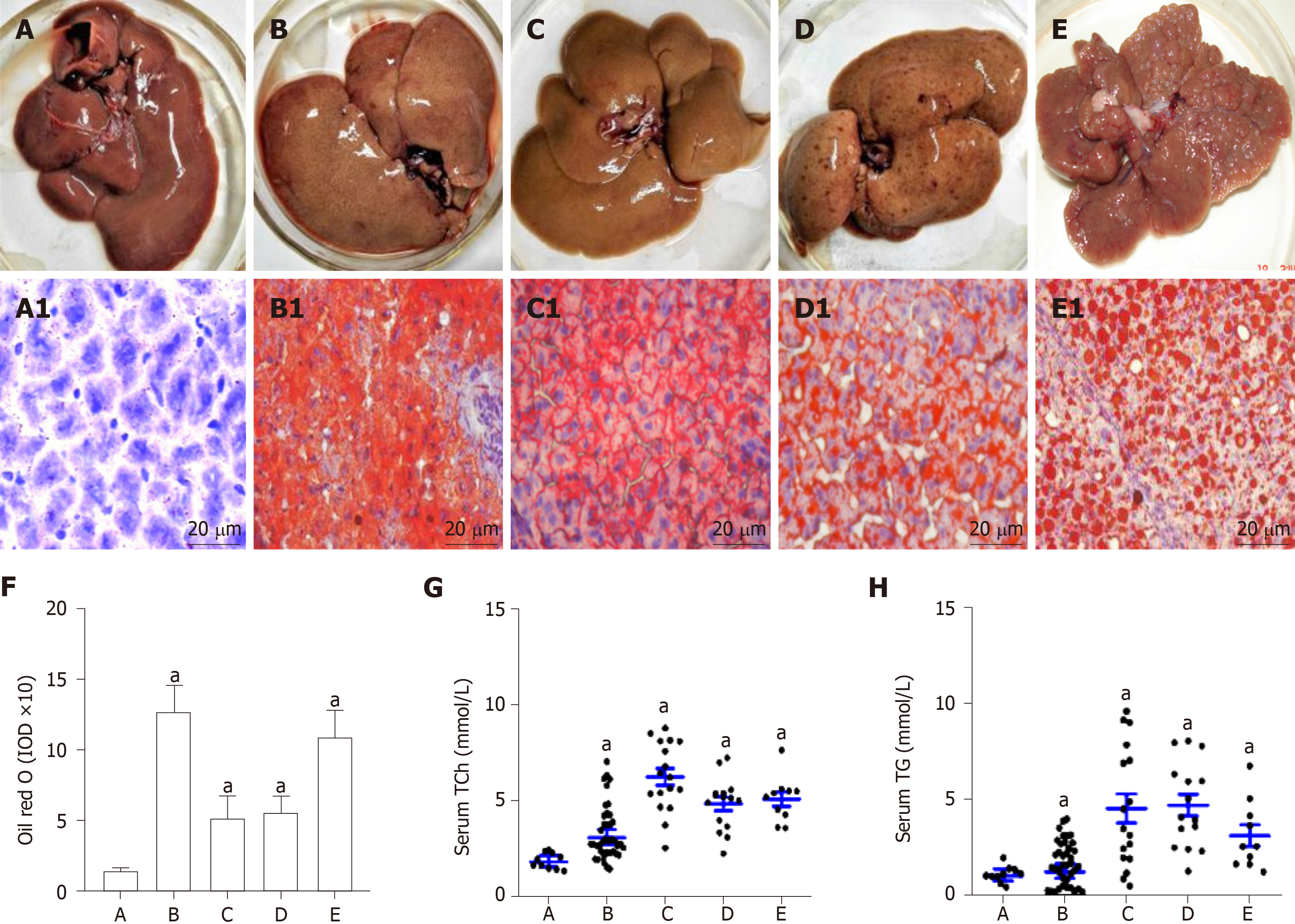
Rat livers with lipid accumulation and circulating lipid levels are shown in Figure 1. Compared with the normal control (Figure 1A and A1), the rat NAFLD models have been successfully made with lipid accumulation (Figure 1B and B1). After the rats were fed with a high fat plus 2-FAA diet, the rat livers were collected at the early (Figure 1C and C1), middle (Figure 1D and D1), and last (Figure 1E and E1) stage. The corresponding liver sections by the Oil red O staining were confirmed with over fatty accumulation in hepatocytes, except for control rats, whose levels of hepatic lipid were relatively quantified by the IOD (Figure 1F). Compared with the control group, hepatocyte lipid contents were significantly higher in the NAFLD (t = 12.461, P < 0.001), hepatocytes denaturation (t = 6.541, P = 0.02), precancerosis (t = 14.133, P = 0.005), and HCC (t = 9.797, P = 0.009) groups, respectively. Furthermore, the circulating total cholesterol (Figure 1G) levels with 2-3 times or triglycerides (Figure 1H) levels with 1.50-4.53 times in any group of all rats with high fat diet were significantly higher (P < 0.05) than those in the control rats.
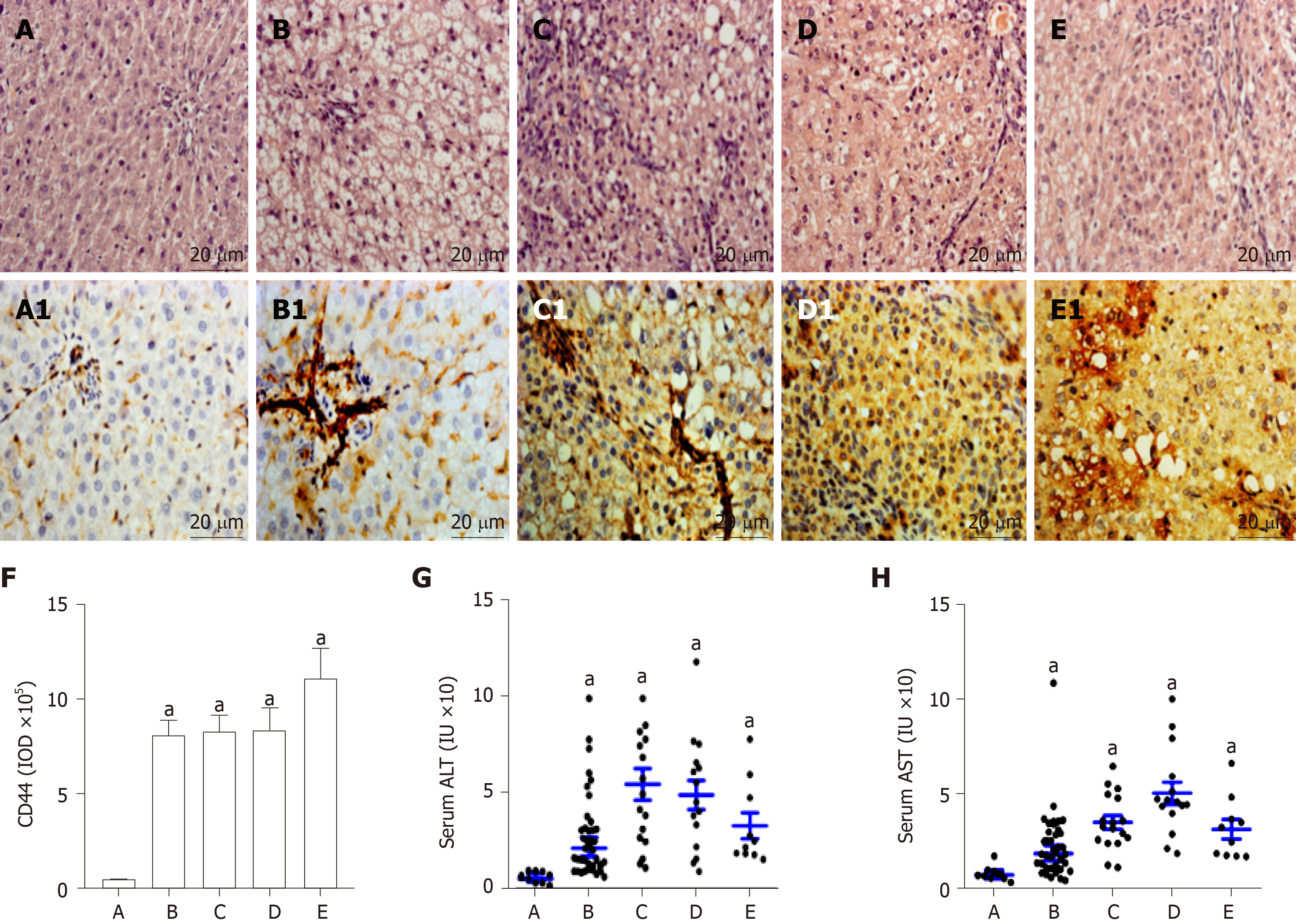
The alterations of liver histopathological examination and the IHC analysis of liver CD44 expression in rat hepatocarcinogenesis are shown in Figure 2. According to pathological results with H and E staining, the rat livers were divided into five groups: the controls (n = 12, Figure 2A) with normal diet only, the NAFLD formation (n = 24, Figure 2B) with high fat diet, the hepatocytes damage (denaturation, n = 17, Figure 2C) at early stage, the precancerosis (n = 15, Figure 2D) at middle stage, and the HCC formation (n = 10, Figure 2E) at last stage after high fat diet plus 2-FAA. The hepatic CD44 levels of the corresponding sections were analyzed by immunohistochemistry with anti-rat CD44 antibodies. Liver CD44 was overexpressed in rat hepatocytes (Figure 2B1, C1, D1 and E1) except for normal controls (Figure 2A1). The IOD values of CD44 expression (Figure 2F) were significantly different between the control group and the NAFLD (t = 25.433, P < 0.001), hepatocytes denaturation (t = 48.822, P < 0.001), precancerosis (t = 27.751, P < 0.001), and HCC (t = 16.239, P < 0.001) groups, respectively. Also, the liver damage with abnormal liver alanine aminotransferase (Figure 2G) or aspartate aminotransferase (Figure 2H) activity was higher in any group of the rats with high fat diet (P < 0.05) than in the control rats during malignant transformation of NAFLD.
The dynamic alterations of liver or circulating CD44 expression at protein level and comparative analysis with AFP expression in rat hepatocarcinogenesis are shown in Table 1. In the rat liver tissues, CD44 expression was lower in the control group and was significantly increasing in the NAFLD group; no significant difference of liver AFP was found between the control and NAFLD groups. After the NAFLD rats were fed with 2-FAA in hepatocarcinogenesis, the increasing liver CD44 expression was significantly higher in the precancerosis and HCC groups than in the control, NAFLD, and denaturation groups; the increased liver AFP expression was significantly higher in the denaturation, precancerosis, and HCC groups than in the control or NAFLD group. In the circulating blood of rats, CD44 expression was lower in the control group. No significant difference of serum CD44 or AFP was found between the control group and the NAFLD group. However, the serum CD44 or AFP level in the denaturation, precancerosis, or HCC group of the NAFLD rats with 2-FAA in hepatocarcinogenesis was significantly higher than that in the control or NAFLD group. Significantly close correlations were found between liver CD44 and serum CD44 (rs = 0.541, P = 0.002) and liver AFP (rs = 0.572, P = 0.001) and between serum CD44 and serum AFP (rs = 0.608, P < 0.001).
| Group | n | Liver CD44, ng/per mg liver | Serum CD44, ng/mL | Liver AFP, ng/per mg liver | Serum AFP, ng/mL |
| Control | 12 | 1.465 ± 0.341 | 9.193 ± 1.176 | 1.757 ± 0.452 | 0.881 ± 0.092 |
| NAFLD | 24 | 1.920 ± 0.311a | 10.432 ± 2.288 | 2.185 ± 0.553 | 0.958 ± 0.131 |
| Denaturation | 17 | 1.830 ± 0.460a | 19.913 ± 7.277a | 3.023 ± 0.797a | 1.460 ± 0.394a |
| Precancerosis | 15 | 2.203 ± 0.303a | 20.628 ± 2.756a | 3.282 ± 0.683a | 1.622 ± 0.418a |
| HCC | 10 | 2.577 ± 0.425a | 29.597 ± 6.907a | 3.877 ± 0.625a | 1.830 ± 0.537a |
The dynamic expression of liver CD44 mRNA and the comparative analysis with AFP mRNA in rat hepatocarcinogenesis are shown in Table 2. The level of liver CD44 mRNA or AFP mRNA expression in the control group was low. Moreover, a similar observation was found in the NAFLD group, and there was no significant difference of liver CD44 mRNA or AFP mRNA found between the control and NAFLD groups. After the NAFLD rats were fed with 2-FAA in hepatocarcinogenesis, the expression of liver CD44 mRNA in the denaturation, precancerosis, or HCC group was significantly higher than that in the control or NAFLD group; liver AFP mRNA expression in the precancerosis or HCC group was significantly higher than that in any of the control, NAFLD, or denaturation group. Significantly close correlations were found between liver CD44 (Table 1) and CD44 mRNA (rs = 0.373, P = 0.043) and between liver CD44 mRNA and serum CD44 (Table 1, rs = 0.507, P = 0.004) or AFP mRNA (rs = 0.370, P = 0.044).
Alterations of hepatic metabolism are critical to the malignant transformation of hepatocytes[18,19]. The incidence of NAFLD among healthy populations is increasing and has become one of the most common causes of HCC worldwide[20-22]. An accumulation of ectopic fat, including visceral obesity and fatty liver, leads to dysfunction of the adipose tissue, with impaired production of adipocytokines and inactivity of mitochondrial inner membrane (carnitine palmitoyl transferase-II)[8,23]; abnormal CD44 expressions in NAFLD lead to the emergence of a microenvironment favorable to HCC development. Human HCC follows a pattern of pathologic evolution involving multistep processes, starting from hepatocyte injury and cirrhosis to low-grade dysplastic nodules, high-grade dysplastic nodules, early liver cancer, and progressed HCC[24,25]. However, the correlation between CD44 and hepatocarcinogenesis is still controversial. In this study, the increasing features of CD44 activation at different stages were first investigated in the cascade of NAFLD to HCC progression.
Hepatocarcinogenesis is of fundamental importance to analyze the dynamic alteration of HCC-related biomarkers and to understand the molecular mechanisms of cancer development[26-28]. NAFLD models with lipid accumulation were confirmed with Oil red O staining, and then the malignant transformation of rat hepatocytes induced with 2-FAA was identified by histopathological H and E examination. The lipid IOD value of the rat liver sections in the NAFLD group was significantly higher than that in the control group, with increasing serum triglyceride or total cholesterol levels and higher hepatic enzymatic alanine aminotransferase or aspartate aminotransferase activity. After the NAFLD rats were fed 2-FAA, rat hepatocytes were malignantly transformed from normal liver cells to denaturation at the early-, to precancerosis at the middle-, and to HCC formation at last-stage. The data indicated that the rat models with lipid accumulation were suitable to observe the CD44 activation from NAFLD involving inflammatory with abnormal metabolism to HCC progression[29,30].
The fastest growing cause of cancer-related death is HCC, which is at least partly attributable to the rising incidence of NAFLD that encompasses a broad spectrum of conditions, ranging from non-progressive bland steatosis to hepatocarcinogenesis[31,32]. In line with these clinical risk factors, high-fat administration over a prolonged period results in spontaneous HCC development. Liver CD44 was overexpressed in all rat livers except for normal controls. Significant difference of the CD44 IOD values was found between control rats and NAFLD, denaturation, precancerosis, or HCC rats, suggesting that elevated CD44 level could contribute to malignant transformation of hepatocytes and HCC development[33,34]. As a hyaluronic acid receptor, CD44, whose expression could be rapidly induced in a STAT3-dependent manner, potentiates AKT activation to escape p53-induced death and responds to proliferative signals that become HCC progenitors[13].
CD44 as a major adhesion molecule of the extracellular matrix has been implicated in a wide range of biological processes, such as transmitting intracellular signals and regulating the growth, invasion, and metastasis of tumors[12,35]. The binding of CD44 with active hyaluronic acid in rat nonalcoholic steatohepatitis (NASH) could induce the accumulation of leukocytes around hepatic sinusoid, and its deficiency could not completely prevent inflammation. CD44 expression in NASH patients is significantly decreased while a fatty disappears after the liver operation. Both CD44 gene knockout and wild type mice with methionine and choline deficient diet were fed to induce NAFLD[36]. In this study, abnormal CD44 expression had a relationship between NAFLD with liver ballooning and malignant transformation of hepatocytes. Although the complex molecular mechanisms of CD44 in rat hepatocarcinogenesis needs to be explored further, the molecular profiling of NAFLD related to increasing CD44 expression during HCC development holds great translational potential for individualized surveillance, prevention, and therapy[16].
Recent evidence indicated that HCC contains a small subpopulation of cells called CSCs that were key drivers of HCC formation and progression, especially relating to invasion and metastasis[33,37]. Among potential CSCs markers, such as CD44, CD133, and aldehyde dehydrogenase 1, several studies similarly utilized CD44 positivity to isolate cells with stem cell-like and cancer-initiating properties from other cancer cells. Interestingly, some CSCs biomarkers have been used to identify by immunohistochemistry CSCs in HCC[10,38]. In this study, both CD44 and AFP were involved in HCC progression, with abnormal expression at the protein or mRNA level and provided a concise overview on the molecular pathogenesis of the NAFLD-NASH-HCC sequence, suggesting that CD44 as a hepatic progenitor might be an important factor in hepatocyte malignant transformation.
In conclusion, to the best of our knowledge, this is the first report to investigate the relationship between increasing CD44 activation and malignant transformation of hepatocytes. The findings are promising, and the initial evidence confirmed that hepatic CD44 was one of the early molecules from NAFLD to HCC progression. However, the investigation of liver histology had not analyzed the relationship between CD44 level and liver fibrosis. Future studies should evaluate liver tissues concerning the degree of fibrosis and CD44 activation, clarify the molecular mechanisms or HCC-related signal pathways of the upregulation of CD44 expression, and elucidate the role of CD44 as a hepatic progenitor in hepatocyte malignant transformation[39,40].
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, and its prevalence is rapidly increasing worldwide. The severe form of NAFLD can progress to liver cirrhosis and hepatocellular carcinoma (HCC). Recently, several related papers expounded that CD44 played an important role in NAFLD and that there was rather little known knowledge about CD44 expression in different stages of hepatocyte malignant transformation correlated with fatty accumulation.
Although CD44 is initially regarded as an adhesion molecule, which has a close relationship with tumor growth, invasion, and metastasis of HCC, the abnormal activation of CD44 in NAFLD has yet to be discovered, and the fact that CD44 is overexpressed in hepatocytes with fatty accumulation needs to be investigated.
CD44 is a non-kinase transmembrane glycoprotein, and its expression is high in malignant tumors and low in benign and low-metastatic tumors. This new mechanism of CD44 expression with fatty metabolism was worthy to be explored. The objective of this study was to initiate the investigation of the relationship between CD44 activation and hepatocyte malignant transformation under nonalcoholic lipid accumulation
In order to clarify the mechanism of CD44 high expression and NAFLD, the models with lipid accumulation were constructed and then the malignant transformation of rat hepatocytes was induced with 2-fluorenylacetamide. Histopathological alterations were identified from normal liver cells to denaturation at the early-, to precancerosis at the middle-, and to HCC formation at last-stage by hematoxylin and eosin examination, with increasing CD44 activation from NAFLD involving inflammation with abnormal metabolism to HCC progression.
CD44 in hepatocarcinogenesis of rat liver cells was increased from NAFLD to HCC at the protein or mRNA level. Significant difference of CD44 was found between the control group and the NAFLD, denaturation, precancerosis, or HCC group, respectively. Serum CD44 levels in HCC or precancerous rats were significantly higher than those in any of the other rats. Positive correlations were found between liver CD44 mRNA and circulating CD44 or alpha-fetoprotein.
To the best of our knowledge, this is the first report to investigate the relationship between increasing CD44 activation and malignant transformation of hepatocytes. Hepatic CD44 mRNA and circulating CD44 expression are early molecules contributing to the progression from NAFLD to HCC. The new findings are promising, and the initial evidence confirmed that hepatic CD44 is one of the early molecules leading to the progression from NAFLD to HCC.
CD44 represents a continuous increasing expression during the entire process of hepatocyte malignant transformation associated with fatty accumulation. Targeting CD44 might prevent NAFLD from turning into HCC and might become a potential therapeutic strategy for HCC. Moreover, further experiments should be conducted to collect the data of CD44 in normal people and of NAFLD, hepatitis, cirrhosis, and HCC and to clarify the molecule mechanism of high expression and carcinogenesis of CD44.
The authors thank Dr. FitzGibbon T for comments on earlier drafts of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gassler N, Ho HK, Ozaki I, Perse M S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 2. | Lee WY, Bachtiar M, Choo CCS, Lee CG. Comprehensive review of Hepatitis B Virus-associated hepatocellular carcinoma research through text mining and big data analytics. Biol Rev Camb Philos Soc. 2019;94:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Calandri M, Mauri G, Yevich S, Gazzera C, Basile D, Gatti M, Veltri A, Fonio P. Fusion Imaging and Virtual Navigation to Guide Percutaneous Thermal Ablation of Hepatocellular Carcinoma: A Review of the Literature. Cardiovasc Intervent Radiol. 2019;42:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Fetzer DT, Rodgers SK, Seow JH, Dawkins AA, Joshi G, Gabriel H, Kamaya A. Ultrasound Evaluation in Patients at Risk for Hepatocellular Carcinoma. Radiol Clin North Am. 2019;57:563-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Athuluri-Divakar SK, Hoshida Y. Generic chemoprevention of hepatocellular carcinoma. Ann N Y Acad Sci. 2019;1440:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Wong CR, Nguyen MH, Lim JK. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8294-8303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Sumida Y, Seko Y, Yoneda M; Japan Study Group of NAFLD (JSG-NAFLD). Novel antidiabetic medications for non-alcoholic fatty liver disease with type 2 diabetes mellitus. Hepatol Res. 2017;47:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Gu JJ, Yao M, Yang J, Cai Y, Zheng WJ, Wang L, Yao DB, Yao DF. Mitochondrial carnitine palmitoyl transferase-II inactivity aggravates lipid accumulation in rat hepatocarcinogenesis. World J Gastroenterol. 2017;23:256-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Castelli G, Pelosi E, Testa U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 10. | Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism. 2016;65:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Patouraux S, Rousseau D, Bonnafous S, Lebeaupin C, Luci C, Canivet CM, Schneck AS, Bertola A, Saint-Paul MC, Iannelli A, Gugenheim J, Anty R, Tran A, Bailly-Maitre B, Gual P. CD44 is a key player in non-alcoholic steatohepatitis. J Hepatol. 2017;67:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Iqbal J, Sarkar-Dutta M, McRae S, Ramachandran A, Kumar B, Waris G. Osteopontin Regulates Hepatitis C Virus (HCV) Replication and Assembly by Interacting with HCV Proteins and Lipid Droplets and by Binding to Receptors αVβ3 and CD44. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Dhar D, Antonucci L, Nakagawa H, Kim JY, Glitzner E, Caruso S, Shalapour S, Yang L, Valasek MA, Lee S, Minnich K, Seki E, Tuckermann J, Sibilia M, Zucman-Rossi J, Karin M. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell. 2018;33:1061-1077.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 14. | Luo Y, Tan Y. Prognostic value of CD44 expression in patients with hepatocellular carcinoma: meta-analysis. Cancer Cell Int. 2016;16:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Park NR, Cha JH, Jang JW, Bae SH, Jang B, Kim JH, Hur W, Choi JY, Yoon SK. Synergistic effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem Biophys Res Commun. 2016;477:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Gu J, Yao M, Yao D, Wang L, Yang X, Yao D. Nonalcoholic Lipid Accumulation and Hepatocyte Malignant Transformation. J Clin Transl Hepatol. 2016;4:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Chen KJ, Jin RM, Shi CC, Ge RL, Hu L, Zou QF, Cai QY, Jin GZ, Wang K. The prognostic value of Niemann-Pick C1-like protein 1 and Niemann-Pick disease type C2 in hepatocellular carcinoma. J Cancer. 2018;9:556-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Brar G, Tsukamoto H. Alcoholic and non-alcoholic steatohepatitis: global perspective and emerging science. J Gastroenterol. 2019;54:218-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Safaei A, Arefi Oskouie A, Mohebbi SR, Rezaei-Tavirani M, Mahboubi M, Peyvandi M, Okhovatian F, Zamanian-Azodi M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 2016;9:158-173. [PubMed] |
| 20. | Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 497] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 21. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 22. | Livadariu R, Timofte D, Danilă R, Ionescu L, Diaconu C, Soroceanu P, Sângeap AM, Drug VL, Trifan A. Nonalcoholic fatty liver disease and its complications--assessing the population at risk. a small series report and literature review. Rev Med Chir Soc Med Nat Iasi. 2015;119:346-352. [PubMed] |
| 23. | Ye Q, Qian BX, Yin WL, Wang FM, Han T. Association between the HFE C282Y, H63D Polymorphisms and the Risks of Non-Alcoholic Fatty Liver Disease, Liver Cirrhosis and Hepatocellular Carcinoma: An Updated Systematic Review and Meta-Analysis of 5,758 Cases and 14,741 Controls. PLoS One. 2016;11:e0163423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Piccinin E, Villani G, Moschetta A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators. Nat Rev Gastroenterol Hepatol. 2019;16:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 25. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 26. | Chen K, Ma J, Jia X, Ai W, Ma Z, Pan Q. Advancing the understanding of NAFLD to hepatocellular carcinoma development: From experimental models to humans. Biochim Biophys Acta Rev Cancer. 2019;1871:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Lau JKC, Zhang X, Yu J. Animal Models of Non-alcoholic Fatty Liver Diseases and Its Associated Liver Cancer. Adv Exp Med Biol. 2018;1061:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1704] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 29. | Wu J. Utilization of animal models to investigate nonalcoholic steatohepatitis -associated hepatocellular carcinoma. Oncotarget. 2016;7:42762-42776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Jacobs A, Warda AS, Verbeek J, Cassiman D, Spincemaille P. An Overview of Mouse Models of Nonalcoholic Steatohepatitis: From Past to Present. Curr Protoc Mouse Biol. 2016;6:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Sun LM, Lin MC, Lin CL, Liang JA, Jeng LB, Kao CH, Lu CY. Nonalcoholic Cirrhosis Increased Risk of Digestive Tract Malignancies: A Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Reid DT, Eksteen B. Murine models provide insight to the development of non-alcoholic fatty liver disease. Nutr Res Rev. 2015;28:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Asai R, Tsuchiya H, Amisaki M, Makimoto K, Takenaga A, Sakabe T, Hoi S, Koyama S, Shiota G. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 2019;8:773-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Fan Z, Xia H, Xu H, Ma J, Zhou S, Hou W, Tang Q, Gong Q, Nie Y, Bi F. Standard CD44 modulates YAP1 through a positive feedback loop in hepato- cellular carcinoma. Biomed Pharmacother. 2018;103:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Yang Z, Qin W, Chen Y, Yuan B, Song X, Wang B, Shen F, Fu J, Wang H. Cholesterol inhibits hepatocellular carcinoma invasion and metastasis by promoting CD44 localization in lipid rafts. Cancer Lett. 2018;429:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Gao Y, Ruan B, Liu W, Wang J, Yang X, Zhang Z, Li X, Duan J, Zhang F, Ding R, Tao K, Dou K. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial- mesenchymal transition. Oncotarget. 2015;6:7828-7837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Gu Y, Wei X, Sun Y, Gao H, Zheng X, Wong LL, Jin L, Liu N, Hernandez B, Peplowska K, Zhao X, Zhan QM, Feng XH, Tang ZY, Ji J. miR-192-5p Silencing by Genetic Aberrations Is a Key Event in Hepatocellular Carcinomas with Cancer Stem Cell Features. Cancer Res. 2019;79:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Kim BH, Park JW, Kim JS, Lee SK, Hong EK. Stem Cell Markers Predict the Response to Sorafenib in Patients with Hepatocellular Carcinoma. Gut Liver. 2019;13:342-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Tian Y, Mok MT, Yang P, Cheng AS. Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |