Published online Sep 15, 2019. doi: 10.4251/wjgo.v11.i9.741
Peer-review started: February 22, 2019
First decision: June 5, 2019
Revised: June 25, 2019
Accepted: July 29, 2019
Article in press: July 29, 2019
Published online: September 15, 2019
Processing time: 204 Days and 17.4 Hours
Oesophageal cancer is the eighth most common cancer worldwide. The prognosis of oesophageal cancer patients still remains poor. The 5-year survival rate rarely exceeds 5% in case of metastatic disease. Some patients may however present with oligometastasis which can be treated with loco-regional therapy.
To assess the current practice regarding the management of patients with oligometastatic oesophageal cancer and identify prognostic factors affecting survival following treatment for oligometastasis.
A systematic search of the literature was performed in Cochrance Library, MEDLINE and EMBASE databases from September 1950 to January 2019. Relevant electronic databases were searched for studies assessing the clinical outcome of oligometastasis.
A total of 14 publications were included, of which 12 studies assessing metachronous oligometastasis and 2 on synchronous oligometastasis. All included articles evaluated the specific outcomes of metastasis, management modality and survival outcomes. The majority of the patients presented with oesophageal squamous cell carcinoma. The median disease free interval (time to recurrence) in patients was 19.6 mo and the overall survival reached 30.8 months. Unfavourable prognostic factors were assessed in eight studies and included time to recurrence < 12 mo, large diameter pulmonary lesions (> 20 mm), disease free interval (DFI) < 12 mo, extra-pulmonary metastasis, primary tumour pathological stage III/IV.
Oligometastatic oesophageal cancer in selected patients is amenable to loco-regional treatment, and the overall survival of this patient cohort may be improved with patient and tumour-specific treatments.
Core tip: Oesophageal cancer often presents with early metastatic spread, which carries a poor prognosis. Some patients may have limited metastatic disease that can be treated with loco-regional therapy. The guidelines for the management of oligometastasis in oesophageal cancer are however not clearly established and survival outcomes remain unclear. The aims of this review were to assess the current practice for the treatment of oligometastatic oesophageal cancer and factors affecting survival following treatment of oligometastasis. A total of 14 publications were included assessing the management and survival outcomes and the majority of these studies opted for aggressive treatment in appropriate patient selection.
- Citation: Jamel S, Tukanova K, Markar S. Detection and management of oligometastatic disease in oesophageal cancer and identification of prognostic factors: A systematic review. World J Gastrointest Oncol 2019; 11(9): 741-749
- URL: https://www.wjgnet.com/1948-5204/full/v11/i9/741.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i9.741
Oesophageal cancer is the eight most common cancer and the sixth leading cause of death from cancer with 400200 deaths[1-3]. Metastatic spread can occur early, and symptoms often only become apparent in the later stage of the disease. Hence, the prognosis of oesophageal cancer still remains poor with an overall 5-year survival rate of 18%[4]. Surgical resection is the standard treatment for patients presenting with early stage cancer. However, the survival is still low due to high incidence of either loco-regional or distant recurrence, ranging from 29% to 59%[5-11]. Moreover, approximately half of the patients present primarily with distant metastasis at the time of diagnosis with a 5-year survival of less than 5%[6]. Combined treatment modalities are used for the management of locally advanced disease, consisting of neoadjuvant chemotherapy with or without radiotherapy followed by surgery[12]. Meanwhile, patients with recurrent or metastatic disease most commonly undergo systemic palliative therapy[13-15].
Several factors are believed to influence the long-term survival in patients undergoing curative treatment. One study found that presence of regional lymph node metastasis and chemoradiation, compared to surgery, were associated with poor 5-year survival, whilst female gender and patients receiving neoadjuvant therapy had better outcomes[16]. Nevertheless, the specific prognostic factors in patients treated loco-regionally for oligometastatic disease in oesophageal cancer remain unclear. Type and extent of recurrence may also affect survival as distant recurrence and more than three recurrent locations were associated with worse post-recurrence survival compared to loco-regional and solitary recurrence respectively[17]. Most recurrences occur in the first postoperative year, and approximately 90% develop by the end of the third year[9]. Metastatic oesophageal cancer has been regarded as end-stage disease with the most commonly affected sites for metastasis being the distant lymph nodes, liver, brain, lung and bone[18]. However, some of these patients may present with oligometastatic cancer. Oligometastasis is defined as a state of limited metastatic disease characterised by fewer than 5 metastasis[19]. Oligometastasis can be synchronous oligometastasis, which are detected at the time of primary cancer diagnosis or metachronous, which occur following treated primary cancer site [20]. The clinical implication of oligometastasis lies largely in the possible improvement in disease control and survival when patients with oligometastatic disease are treated with definitive loco-regional therapy. Early detection of oligometastasis enables early intervention and may thus potentially improve survival. Careful surveillance is therefore one of the key components in the management of oesophageal cancer. However, to date there is a lack in specific guidelines regarding optimal management of patients presenting with oligometastatic oesophageal cancer.
For the purposes of our study, we defined oligometastasis as a single solid organ recurrence. This systematic review focuses on the current practice regarding treatment of oligometastatic oesophageal cancer and factors affecting survival following treatment of oligometastasis.
A systematic literature search of MEDLINE (January 1950 to September 2018), EMBASE (January 1974 to September 2018), Web of Science (January 1990 to September 2018), and the Cochrane Library databases was performed. The following search terms were used “(o)esophageal cancer”, “oligometastasis” and “oligo-recurrence” and the Medical Subject Headings (MeSH) term “esophageal neoplasms”. The search was expanded by identifying synonyms or closely related terms and a manual search of the references of included studies was performed to identify any missing articles. The full search strategy is shown in Supplementary Table 1. Two reviewers (SJ and KT) independently assessed titles and abstracts for inclusion of relevant references, followed by screening of the full text. Articles were included if the following elements were evaluated: (1) Assessment of survival outcomes and/or prognostic factors in patients presenting with solid organ metastasis following treatment for oesophageal cancer; and (2) Synchronous or metachronous oligometastasis. Only articles published in English were included. Review articles were excluded. Articles focusing on solely lymph node recurrence without solid organ metastasis were excluded. The following data was extracted: study design, sample size, mean age, diagnostic tool, type of treatment modality, histological subtype, site of metastatic lesion, disease free survival and overall survival for synchronous oligometastasis and metachronous oligometastasis.
The methodological quality of included studies was assessed by means of the Newcastle-Ottawa scales for cohort and case-control studies. The quality is rated by awarding stars in each domain with three domains in total (selection, comparability and exposure)[21]. Articles are graded as “good quality” if 3 or 4 stars in the selection domain and 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome/exposure domain, “fair quality” if 2 stars in the selection domain and 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome/exposure domain or “poor quality” if 0 or 1 star in the selection domain OR 0 stars in comparability domain OR 0 or 1 stars in the outcome/exposure domain. The methodological quality assessment of the case series was reported using a novel tool based on modifications of the Newcastle-Ottawa, Pierson and Bradford Hills scales[22]. Eight items are categorised into four domains (selection, ascertainment, causality and reporting). A total score on these 8 items can be created by adding up the binary response to a sum score.
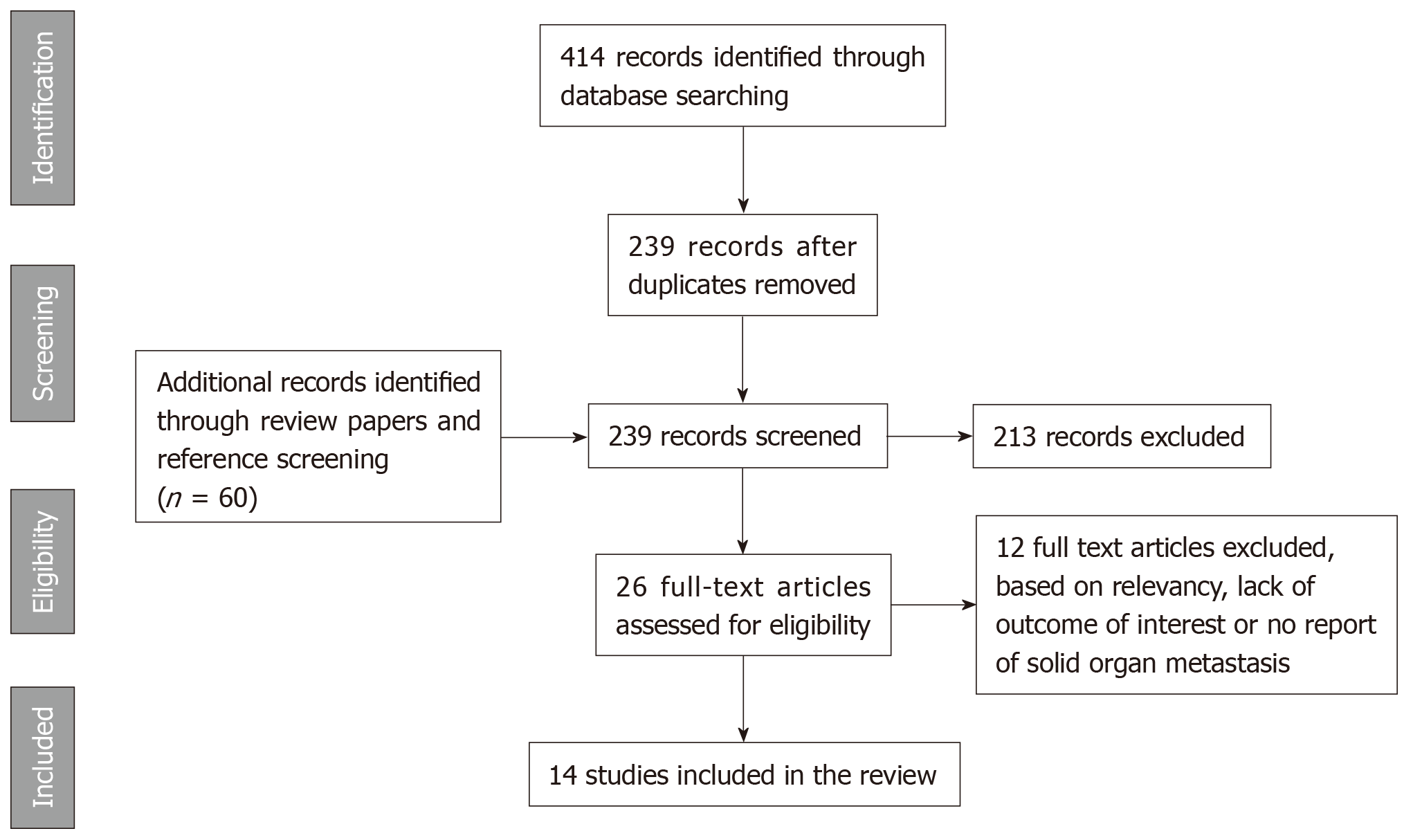
The systematic search yielded 399 initial results. After removal of duplicates, 235 references were screened on title and abstract. Subsequent assessment of full text resulted in inclusion of 14 articles. A graphical representation of the review process is demonstrated in a PRISMA flow chart (Figure 1)[23].
The results of the quality assessment of the studies included are summarised in the Supplementary Tables 1, 2 and 3 respectively. The majority of included studies were cohort studies. For all studies, both the case and control population consisted of patients treated for oesophageal cancer and data was retrospectively obtained from existing hospital databases. Furthermore, patients in the control group were not obtained as a random sample in the population and were selected from the same source as the cases. However, no information was given in the methodology of the studies regarding matching of both groups. The remaining articles had a retrospective cohort design or were case series. Again, the study population consisted of a selected group of patients. The comparability of the cohorts was well established, but the outcome of interest was already present at the start across all studies due to retrospective design.
Twelve studies included[24-35], eight of those assessed the survival outcomes of patients treated with resection of pulmonary metastases, three included multiple oligometastasis sites and one study only on liver oligometastasis (Supplementary Tables 4 and 5). The histological subtype of primary oesophageal cancer was squamous cell carcinoma (75.2%), adenocarcinoma (23.0%) and sarcoma/basaloid tumour (1.8%). The mean age was 63.3 years across all studies. The majority of patients has undergone resection for the primary oesophageal cancer with either chemotherapy or radiotherapy or a combination. In only one study patients were managed with definitive chemotherapy[27]. The Disease-Free Interval (DFI) was reported in 8 of the studies and the mean DFI was 19.6 months. The DFI duration was established as a prognostic factor is four studies. A disease-free survival (DFI) of less than 12 mo resulted in a 5-year survival rate of 15.7%, which was much lower than for patients with a DFI exceeding 12 months (39.2%, P = 0.048)[25].
Patients were investigated with various imaging modalities including Chest X-ray (CXR), computed tomography (CT) and positron emission tomography (PET) scan. However, the follow-up regimes were not specified in the studies included to assess variation in clinical practice and impact on detection. The management of oligometastasis involved surgery and mostly either chemotherapy or chemo-radiotherapy. The main reported outcomes were overall survival (OS) and the mean was 30.8 mo across all studies. Oligometastasis recurrence occurred in 32.1% of cases. Time to recurrence was identified as a predictor of survival and patients presenting with recurrence within 12 mo of definitive therapy for the primary tumour, had worse survival (P = 0.034)[35].
Chen et al[24] showed that patients with multiple pulmonary metastasis developed recurrence in comparison to those with solitary lesions, however, this was a small study of 5 patients. Regarding pulmonary metastasis, a larger diameter of the lesion (> 20 mm) was marginally associated with worse outcomes (P = 0.087)[27]. Presence of extra pulmonary metastases was established as unfavourable prognostic factor in five of the studies and Ichikawa et al[28] showed that none of the patients with extrapulmonary metastasis survived beyond 3 years, compared to 54.7% for patients presenting with a solitary pulmonary lesion (P = 0.0411).
A 2013 retrospective cohort study assessed survival following resection of liver and lung metastases. Patients with pulmonary recurrences had better outcomes (median survival of 13 mo) than metastases in the liver (medial survival of 5 mo) or other sites (median survival of 3 mo) and a surgical approach of these pulmonary lesions also seemed to beneficial with a median survival of 48 mo compared to 10 mo if not treated with resection (P = 0.009). Hepatic metastasectomy failed to establish a significant survival benefit (P = 0.06) [32]. The latter results were similar to the findings of Huddy et al[33] who assessed the outcomes of 4 patients treated with liver resection and to Hiyoshi et al[34]. In addition, the latter author could not demonstrate an improvement in survival following resection of lesions in the brain and bone. However, patients treated with pulmonary metastasectomy (solitary, bilateral or multiple lesions) showed a trend towards better outcomes. A recent study conducted by Ghaly et al[35] evaluated prognostic factors for survival of 56 patients following multimodal therapy of oligometastasis in the liver, bone, brain or adrenal glands. The median survival was not significantly different between both groups (P = 0.661). Time to recurrence was identified as a predictor of survival and patients presenting with recurrence within 12 mo of definitive therapy for the primary tumour, had worse survival (P = 0.034)[35].
Two studies assessed survival in oesophageal cancer patients presenting with synchronous oligometastasis[36,37] (Supplementary Tables 4 and 5). Onal et al[36] assessed the impact of an aggressive treatment approach of both primary tumour and solitary brain metastasis. Patients underwent definitive CRT of the primary tumour locally ablative treatment of the brain metastasis, consisting of radiotherapy, surgery or radiosurgery. The median time to progression was 8 mo and median survival was 18.9 mo, suggesting that this approach might improve survival in selected patients. A more recent study investigated the impact of suspicious lesions on pre-treatment imaging on the survival of patients undergoing oesophagectomy. The presence of suspected liver metastases had a 5-year survival rate of only 9.9% compared to 26.1% in patients with suspicious lesions at other sites or with no evidence of metastases on pre-treatment imaging (P = 0.014)[37].
Despite advances in diagnostic tools and treatment modalities, loco-regional and distant recurrences still occur frequently in oesophageal cancer. Survival rate is worse in the presence of haematogenous metastases (16 mo) compared to loco-regional recurrence (25.5 mo)[38]. Standard treatment modality for recurrences in oesophageal cancer often consists of systemic therapy. Patients presenting with oligometastatic disease may however benefit from aggressive local therapy with improvement in survival rates. To date, there is no guideline on the management of distant oligometastasis in oesophageal cancer and currently resection of distant metastases is mostly a personalised treatment.
The lungs are amongst the most common affected sites for metastasis in oesophageal cancer. Patients often present with multiple lesions and might have metastases at other sites as well. Furthermore, primary lung tumours commonly coexist with oesophageal cancer as smoking is a known risk factor in both malignancies[39,40]. Distinguishing metastases from second primary lung cancer requires genetic analysis, which was not performed in the included studies. Kanamori et al[31] excluded lesions suggestive of second primary lung cancer based on histological findings and Kozu et al[27] applied several clinical criteria. The latter author found poor long-term survival in these patients, confirming the aggressive nature of metastatic disease in oesophageal cancer. Consequently, survival rates could be affected in the other included studies as the number of patients with pulmonary metastases might be fewer than reported[41]. Pulmonary metastasectomy has proven its efficacy in other types of cancer, including colorectal, renal and head and neck malignancies. Both initial as repeated resections were encouraged in colorectal cancer as it could significantly improve survival rates [42]. This is consistent with the findings in the studies included in our review as the vast majority of the papers believed that pulmonary metastasectomy was a promising treatment option for improvement in survival following resection of a solitary pulmonary lesion. Ichikawa et al[28] has shown that it was a safe and feasible approach as the incidence of pulmonary complications remained low and no in-hospital mortality occurred. Hiyoshi et al[34] suggested that not only patients with a solitary lesion are good candidates for metastasectomy, but resection of bilateral and metachronous pulmonary multiple lesions might improve the prognosis as well. However, the study population only consisted of 9 patients with a solid organ metastasis. Furthermore, resection of pulmonary metastasis may have an improved prognostic value in metastatic gastric cancer[43].
Resection of liver metastases is common practice in colorectal cancer with a 5-year survival rates of more than 50% compared to patients receiving palliative treatment[44,45]. None of our included studies demonstrate a survival benefit of resection of liver metastasis, however, it is important to note that patient numbers were small. In addition, there is contrary evidence to survival benefit in patients undergoing resection for brain metastasis. Hiyoshi et al[34] did not show any benefit in the resection of lesions in the brain or bone. In contrast with this, Onal et al[36] suggested an improvement in survival outcome in patients with oligometastatic brain metastasis when treated with an aggressive approach of both the primary tumour and the metastatic lesion.
Recently, Kanamori et al[31] showed that the risk of re-recurrence of metastasis was 70% in those undergoing pulmonary metastectomy. However, smaller studies had lower recurrence rates of 22% Kobayashi et al[30], 50% Huddy et al[33] and 60% in Chen et al[24]. The rate of recurrence following treatment of oligometastasis was not reported in other studies. In all the included reports patients had surgical resection of their oligometastasis and either chemotherapy or chemoradiotherapy. It is thus unclear whether these patients significantly benefit from resection of metastases.
Hsu et al[9] showed that patients with more risk factors such as liver recurrence, early recurrence, and no treatment for recurrence would suffer from poorer post-recurrence survival. Therefore, patients with isolated, oligometastasis of EC after multimodality therapy may represent a subset of patients who will benefit from aggressive treatment of their metastatic disease and survival might be extended in this patient population. The majority of patients included had oesophageal squamous cell carcinoma (OSCC). The pattern of metastasis is different as OSCC has a higher incidence rate of lung metastasis in comparison to oesophageal adenocarcinoma which had a higher incidence rate of liver metastasis. The median disease free interval in patients was 18.6 months. The overall survival was 31.1 months. Furthermore, to our knowledge, there are currently no commonly accepted prognostic factors of metastatic oesophageal cancer indicating an improved prognosis. Several reports have reported favourable prognostic factors depending on their patient cohort, this included solitary metastasis, absence of extrapulmonary metastases and lack of nodal involvement and greater disease free interval > 12 months. In addition, the concept of the “test of time” is a convincing indicator of a more favourable biological cancer behaviour. However, there is an increasing shift toward individualized, multidisciplinary management of oligometastasis because it is difficult to conduct randomized controlled trials due to the variety of presentations[7]. The multicenter FLOT3 study with metastatic tumours of the oesophagogastric junction and gastric cancer suggests that well-selected patients may benefit from surgery following chemotherapy at the stage of limited metastases[46]. The results of the FLOT5 study is still awaiting to be published, assessing the effect of chemotherapy alone versus chemotherapy followed by surgical resection on survival and quality of life in patients with limited metastatic adenocarcinoma of the stomach or oesophagogastric junction. Only two papers assessed the presence of synchronous oligometastasis in patients with oesophageal cancer[36,37]. Hence, no comparison in the difference of between the presence of synchronous or metachronous oligometastatic disease could be made regarding survival outcomes.
The main limitation to this review is that all the studies included were retrospective observational studies. The majority of patients were OSCC and therefore not representing the other major oesophageal cancer subtype of adenocarcinoma. Therefore, there was clinical heterogeneity was present due to majority of patients being of Asian-predominant studies. Most of the studies included the proportion of patients receiving metastasectomy in their assessment. Hence, the sample size in the majority of the studies was small, which may introduce selection bias. Another limitation is that only papers published in English were included.
Aggressive treatment of oligometastatic disease in oesophageal cancer is performed on an individual basis. The lung and liver are amongst the most common sites of metastasis in oesophageal cancer. Several factors have been identified which might influence survival and should be taken into consideration in the management of oligometastasis. Most studies advocate a personalised approach to patient management until there are more studies to guide future decision making.
Oesophageal cancer is the eighth most common cancer worldwide with an associated poor prognosis. The 5-year survival rate rarely exceeds 5% in case of metastatic disease. Combined treatment modalities are used for the management of locally advanced disease, consisting of neoadjuvant chemotherapy with or without radiotherapy followed by surgery. Meanwhile, patients with recurrent or metastatic disease most commonly undergo systemic palliative therapy. However, to date there is a lack in specific guidelines regarding optimal management of patients presenting with oligometastatic oesophageal cancer. The European Society for Medical Oncology, suggests that patients with metastasis can be considered for different options of treatment depending on the clinical case. It is unclear for current studies whether resection improves the overall survival and what is the optimal management.
This systematic review focuses on the current practice regarding treatment of oligometastatic oesophageal cancer and factors affecting survival following treatment of oligometastasis.
This review aims to assess the current practice regarding the management of patients with oligometastatic oesophageal cancer and identify prognostic factors affecting survival following treatment for oligometastasis.
An extensive systematic search of the literature was performed in Cochrance Library, MEDLINE and EMBASE databases on January 4th, 2019. Relevant electronic databases were searched for studies assessing the clinical outcome of oligometastasis.
The main finding of this systematic review is that Oligometastatic oesophageal cancer in selected patients is amenable to loco-regional treatment, and the overall survival of this patient cohort may be improved with patient and tumour-specific treatments. However, there is an increasing shift toward individualized, multidisciplinary management of oligometastasis because it is difficult to conduct randomized controlled trials due to the variety of presentations.
The lung and liver are amongst the most common sites of metastasis in oesophageal cancer. Most studies advocate a personalised approach to patient management until there are more studies to guide future decision making. Aggressive treatment of oligometastatic disease in oesophageal cancer is performed on an individual basis. Several factors have been identified which might influence survival and should be taken into consideration in the management of oligometastasis. Most studies advocate a personalised approach in the management of oligometastatic oesophageal cancer.
The current management advocated by most studies is based on a personalised approach to patient management until there are more studies to guide future decision making. Larger scale future studies or randomised controlled trials to assess optimal management plan for oligometastatic disease is required to guide management of this patient cohort.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aurello P, Salati M S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 2. | Ai D, Zhu H, Ren W, Chen Y, Liu Q, Deng J, Ye J, Fan J, Zhao K. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis. 2017;9:3023-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 4. | American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016; . |
| 5. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1084] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 6. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 7. | Abate E, DeMeester SR, Zehetner J, Oezcelik A, Ayazi S, Costales J, Banki F, Lipham JC, Hagen JA, DeMeester TR. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Lee DH, Kim HR, Kim DK, Park SI, Kim YH. Outcomes of cervical lymph node recurrence in patients with esophageal squamous cell carcinoma after esophagectomy with 2-field lymph node dissection. J Thorac Cardiovasc Surg. 2013;146:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hsu PK, Wang BY, Huang CS, Wu YC, Hsu WH. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg. 2011;15:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Takefumi O, Saeki H, Oki E, Kakeji Y, Sakaguchi Y, Maehara Y. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today. 2012;42:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 306] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1823] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 13. | Grünberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705-2714. [PubMed] |
| 14. | Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F, Collette L, Sahmoud T. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216-1220. [PubMed] |
| 15. | Ilson DH, Wadleigh RG, Leichman LP, Kelsen DP. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol. 2007;18:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Bus P, Lemmens VE, van Oijen MG, Creemers GJ, Nieuwenhuijzen GA, van Baal JW, Siersema PD. Prognostic factors for medium- and long-term survival of esophageal cancer patients in the Netherlands. J Surg Oncol. 2014;109:465-471. [PubMed] |
| 17. | Parry K, Visser E, van Rossum PS, Mohammad NH, Ruurda JP, van Hillegersberg R. Prognosis and Treatment After Diagnosis of Recurrent Esophageal Carcinoma Following Esophagectomy with Curative Intent. Ann Surg Oncol. 2015;22 Suppl 3:S1292-S1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Wu SG, Zhang WW, He ZY, Sun JY, Chen YX, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 715] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 20. | Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med. 2012;2012:261096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 22. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1535] [Article Influence: 219.3] [Reference Citation Analysis (0)] |
| 23. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 844] [Reference Citation Analysis (0)] |
| 24. | Chen F, Sato K, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T, Date H. Pulmonary resection for metastasis from esophageal carcinoma. Interact Cardiovasc Thorac Surg. 2008;7:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Shiono S, Kawamura M, Sato T, Nakagawa K, Nakajima J, Yoshino I, Ikeda N, Horio H, Akiyama H, Kobayashi K; Metastatic Lung Tumor Study Group of Japan. Disease-free interval length correlates to prognosis of patients who underwent metastasectomy for esophageal lung metastases. J Thorac Oncol. 2008;3:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Kozu Y, Sato H, Tsubosa Y, Ogawa H, Yasui H, Kondo H. Surgical treatment for pulmonary metastases from esophageal carcinoma after definitive chemoradiotherapy: experience from a single institution. J Cardiothorac Surg. 2011;6:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Kozu Y, Oh S, Takamochi K, Suzuki K. Surgical outcomes of pulmonary metastases from esophageal carcinoma diagnosed by both pathological and clinical criteria. Surg Today. 2015;45:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ichikawa H, Kosugi S, Nakagawa S, Kanda T, Tsuchida M, Koike T, Tanaka O, Hatakeyama K. Operative treatment for metachronous pulmonary metastasis from esophageal carcinoma. Surgery. 2011;149:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Takemura M, Sakurai K, Takii M, Yoshida K. Metachronous pulmonary metastasis after radical esophagectomy for esophageal cancer: prognosis and outcome. J Cardiothorac Surg. 2012;7:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Kobayashi N, Kohno T, Haruta S, Fujimori S, Shinohara H, Ueno M, Udagawa H. Pulmonary metastasectomy secondary to esophageal carcinoma: long-term survival and prognostic factors. Ann Surg Oncol. 2014;21 Suppl 3:S365-S369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Kanamori J, Aokage K, Hishida T, Yoshida J, Tsuboi M, Fujita T, Nagino M, Daiko H. The role of pulmonary resection in tumors metastatic from esophageal carcinoma. Jpn J Clin Oncol. 2017;47:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ichida H, Imamura H, Yoshimoto J, Sugo H, Kajiyama Y, Tsurumaru M, Suzuki K, Ishizaki Y, Kawasaki S. Pattern of postoperative recurrence and hepatic and/or pulmonary resection for liver and/or lung metastases from esophageal carcinoma. World J Surg. 2013;37:398-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Huddy JR, Thomas RL, Worthington TR, Karanjia ND. Liver metastases from esophageal carcinoma: is there a role for surgical resection? Dis Esophagus. 2015;28:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Hiyoshi Y, Morita M, Kawano H, Otsu H, Ando K, Ito S, Miyamoto Y, Sakamoto Y, Saeki H, Oki E, Ikeda T, Baba H, Maehara Y. Clinical significance of surgical resection for the recurrence of esophageal cancer after radical esophagectomy. Ann Surg Oncol. 2015;22:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Ghaly G, Harrison S, Kamel MK, Rahouma M, Nasar A, Port JL, Stiles BM, Altorki NK. Predictors of Survival After Treatment of Oligometastases After Esophagectomy. Ann Thorac Surg. 2018;105:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Onal C, Akkus Yildirim B, Guler OC. Outcomes of aggressive treatment in esophageal cancer patients with synchronous solitary brain metastasis. Mol Clin Oncol. 2017;7:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Erhunmwunsee L, Englum BR, Onaitis MW, D'Amico TA, Berry MF. Impact of pretreatment imaging on survival of esophagectomy after induction therapy for esophageal cancer: who should be given the benefit of the doubt?: esophagectomy outcomes of patients with suspicious metastatic lesions. Ann Surg Oncol. 2015;22:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Kuang JJ, Jiang ZM, Chen YX, Ye WP, Yang Q, Wang HZ, Xie DR. Smoking Exposure and Survival of Patients with Esophagus Cancer: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2016;2016:7682387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Fékété F, Sauvanet A, Kaisserian G, Jauffret B, Zouari K, Berthoux L, Flejou JF. Associated primary esophageal and lung carcinoma: a study of 39 patients. Ann Thorac Surg. 1994;58:837-842. [PubMed] |
| 41. | Leong PP, Rezai B, Koch WM, Reed A, Eisele D, Lee DJ, Sidransky D, Jen J, Westra WH. Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst. 1998;90:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Iijima Y, Akiyama H, Atari M, Fukuhara M, Nakajima Y, Kinosita H, Uramoto H. Pulmonary Resection for Metastatic Gastric Cancer. Ann Thorac Cardiovasc Surg. 2016;22:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D'Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 45. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-25; discussion 825-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1364] [Cited by in RCA: 1287] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 46. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |