Published online Sep 15, 2019. doi: 10.4251/wjgo.v11.i9.686
Peer-review started: March 18, 2019
First decision: June 5, 2019
Revised: July 5, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: September 15, 2019
Processing time: 181 Days and 18.6 Hours
Despite improvements in the early diagnosis, prognosis and therapeutic strategies for gastric cancer (GC), human GC remains one of the most frequently diagnosed malignant tumors in the world, and the survival rate of GC patients remains very poor. Thus, a suitable therapeutic strategy for GC is important for prolonging survival. Both tumor cells themselves and the tumor microenvironment play an important role in tumorigenesis, including angiogenesis, inflammation, immunosuppression and metastasis. Importantly, these cells contribute to gastric carcinogenesis by altering the angiogenic phenotype switch. The development, relapse and spreading of tumors depend on new vessels that provide the nutrition, growth factors and oxygen required for continuous tumor growth. Therefore, a state of tumor dormancy could be induced by blocking tumor-associated angiogenesis. Recently, several antiangiogenic agents have been identified, and their potential for the clinical management of GC has been tested. Here, we provide an up-to-date summary of angiogenesis and the angiogenic factors associated with tumor progression in GC. We also review antiangiogenic agents with a focus on the anti-vascular endothelial growth factor receptor (VEGFR)-mediated pathway for endothelial cell growth and their angiogenesis ability in GC. However, most antiangiogenic agents have reported no benefit to overall survival (OS) compared to chemotherapy alone in local or advanced GC. In phase III clinical trials, only ramucirumab (anti-VEGFR blocker) and apatinib (VEGFR-TKI blocker) have reported an improved median overall response rate and prolonged OS and progression-free survival outcomes as a 2nd-line agent combined with chemotherapy treatment in advanced GC. By providing insights into the molecular mechanisms of angiogenesis associated with tumor progression in GC, this review will hopefully aid the optimization of antiangiogenesis strategies for GC therapy in combination with chemotherapy and adjuvant treatment.
Core tip: Tumor angiogenesis in gastric cancer (GC) and antiangiogenic therapies for GC, including information from their preclinical and/or application to clinical trials, are discussed. The antiangiogenic strategies for advanced GC include decreasing the expression of proangiogenic ligands and their receptors, increasing the level of angiogenic inhibitors, and directly targeting the inner walls of endothelial cells. Here, the antiangiogenic strategies mainly focus on decreasing the expression of vascular endothelial growth factor-mediated pathway constituents for advanced GC in phase III clinical trials. Thus, this review provides a brief description of various tumor angiogenic factors for the purposes of diagnosis, prognosis and therapeutics and describes the antiangiogenic agents that are currently being investigated in preclinical and phase III clinical trials. Hopefully, according to the molecular mechanism of tumor angiogenesis, we highlight the accuracy of the diagnosis and prognosis and the selection of the most appropriate therapy for GC patients.
- Citation: Hsieh HL, Tsai MM. Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol 2019; 11(9): 686-704
- URL: https://www.wjgnet.com/1948-5204/full/v11/i9/686.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i9.686
Gastric cancer (GC) has a high incidence throughout the world and a high mortality rate associated with malignant tumors[1-3]. GC might not cause any clinical symptoms at the early stage, resulting in the fact that GC is rarely detected at the early stage[2,3]. However, the five-year survival outcome for late-stage GC patients is only approximately 20%-30% after initial diagnosis[4], and gastrectomy is the major common treatment for GC. Thus, to improve the low survival outcome, it is necessary to develop novel therapeutic strategies for GC[5].
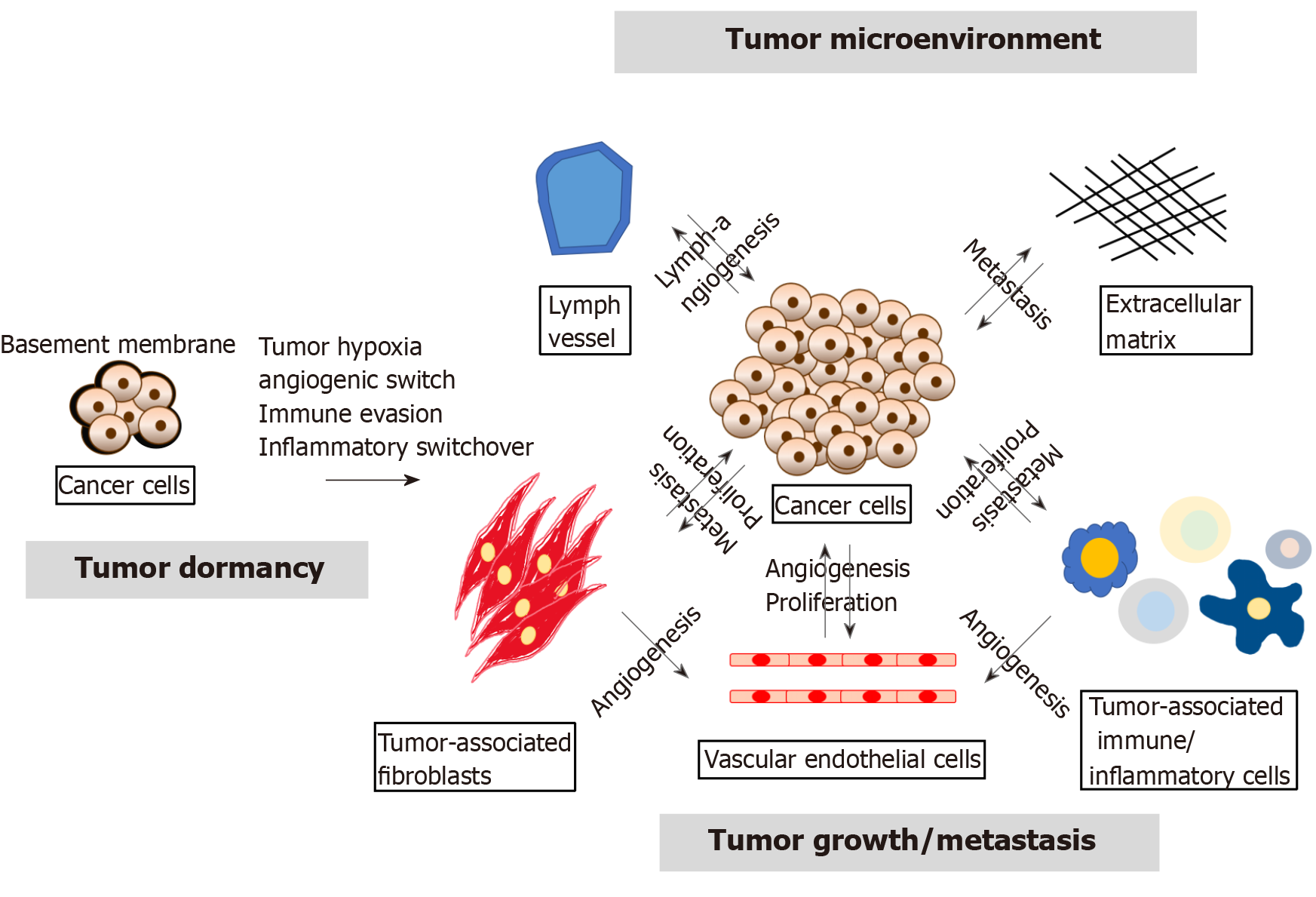
In recent decades, studies on the molecular mechanism of tumor development have focused on the genetic or epigenetic changes in tumor cells, such as the emergence of cancer stem cells (CSCs), epithelial-mesenchymal transition (EMT) and the expression of microRNAs (miRNAs)[6]. However, several studies conducted in recent years found that the tumor microenvironment (TME) strongly influences tumor growth and progression and revealed that the tumor-host interactions determine tumor progression[7,8]. The TME contains extracellular matrix and stromal cells, including ECs, tumor-associated fibroblasts and tumor-associated immune/inflammatory cells, which can regulate tumor progression through autocrine/paracrine cytokines or factors. Furthermore, cancer cells can support the angiogenesis of ECs, and ECs can also help cancer cell proliferation by releasing growth factors. Tumor-associated immune/inflammatory cells can control cancer cell proliferation and metastasis under different conditions, and cancer cells might induce immune cell dysfunction as well as proinflammatory cytokine release. Exosomal miRNAs can alter normal fibroblasts into TAFs for tumor survival, and TAFs can promote tumor proliferation and metastasis. Thus, the TME is also involved in multiple processes, including tumor angiogenesis, inflammation, immunosuppression and metastasis, as shown in Figure 1[9,10].
In 1971, Dr. Folkman and Klagsbrun[11] provided a novel theory stating that all phases of rapid tumor growth are dependent on tumor angiogenesis. At present, it is known that tumor angiogenesis plays a key role in tumor progression, and the angiogenic switch is necessary for tumor growth, relapse and metastasis. Herein, we provide a review of tumor-associated angiogenesis, explore the molecular regulation of angiogenesis, and discuss various antiangiogenic drugs and their potential applications based on preclinical and phase III clinical trials for GC.
An increasing number of studies has revealed that tumor growth is strongly associated with tumor angiogenesis[12]. Tumor growth, relapse and metastasis should turn on the “angiogenic switch” to induce tumor growth to a size greater than 1-2 mm. Numerous signals (e.g., epigenetic changes, the TME, CSCs, EMT, and miRNAs) can disturb tumor dormancy, resulting in local tumor proliferation/recurrence or metastasis at a secondary site[13]. The “angiogenic switch” is regulated by angiogenic activators and inhibitors[14,15], and the timing of the “angiogenic switch” can occur before, during or after tumor progression. As will be discussed in the following sections (Table 1), recent studies have shown that the available knowledge on the induction and molecular regulation of tumor angiogenesis has grown rapidly, and several growth factors, growth factor receptors, cytokines and signaling pathways have been identified in GC.
| Biological category | Gene name | Regulator of pro-/anti angiogenic types | Antiangiogenic drug | Drug direct target | Preclinical trials; cell line (in vitro)/animal (in vivo) | Clinical application | |
| Expression levels in GC patients | Prognostic factors (proangiogenic biomarker) | ||||||
| Transcription factor | Hypoxia | Activator | NSAID[82] | COX-1, COX-2 inhibitor | ● | ND | ND |
| HIF[16-19,22-25] | |||||||
| Growth factor | VEGF family[26-38] | Activator | Aflibercept[22] | Anti-VEGF-A | ● | VEGF-A, C | Lymph node metastasis |
| overexpres-sion[21,41-47] | (VEGF-A, C) | ||||||
| Distant metastasis | |||||||
| Anti-PIGF | (VEGF-A) | ||||||
| Poor survival | |||||||
| (VEGF-A) | |||||||
| Bevacizumab[83-89] | Anti-VEGF-A | ● | ND | ND | |||
| IFN[90] | Anti-IFNR | ● | ND | ND | |||
| Rapamycin[91] | Anti-rapamycin kinase | ● | ND | ND | |||
| Neovastat[92] | Anti-VEGF | ● | ND | ND | |||
| PIGF[29,30,35,48] | Activator | Aflibercept[22] | Anti-VEGF-A | ● | PIGF | ND | |
| Anti-PIGF | overexpres-sion[49-51] | ||||||
| FGF, EGF, HGF, IGF[31,52-55] | Activator | IFN[93] | Anti-IFNR | ● | ND | ND | |
| PDGF[56,57] | Activator | SU6668 | Multiple receptor | ● | ND | ND | |
| Orantinib[94] | TKI | ||||||
| Growth factor receptor | VEGFR[32,33] | Activator | Ramuci-rumab[95-97] | Anti-VEGFR2 | ● | ND | ND |
| Regorafenib[98,99] | VEGFR TKI | ● | ND | ND | |||
| Apatinib[98,99] | VEGFR TKI | ● | ND | ND | |||
| Foretinib[98,99] | VEGFR TKI | ● | ND | ND | |||
| SU5416 | Multiple receptor (KDR/FGFR/PDGFR) | ● | ND | ND | |||
| SU6668 | |||||||
| Orantinib[94] | |||||||
| Pazopanib[100] | Multiple receptor TKI | ● | ND | ND | |||
| Sorafenib (Nexavar)[101,102] | Multikinase inhibitor (the serine/threonine kinase Raf and receptor tyrosine kinases) | ● | ND | ND | |||
| Sunitinib | Multitargeting TKI | ● | ND | ND | |||
| (Sutent)[103,104] | |||||||
| Telatinib | Multitargeting TKI | ● | ND | ND | |||
| Erbitux (Cetuximab)[105] | |||||||
| GP130 | Activator | ND | ND | ● | ND | ND | |
| IL-6R[58] | |||||||
| Her2/ | Activator | Trastuzumab[59,62] | Anti-Her2/Neu | ● | ND | ND | |
| Neu[59-62] | |||||||
| Cytokine | Ang-1,3,4[63,64,66-73] | Activator | ND | ND | ● | Ang-1,2 | Lymph node metastasis |
| overexpres-sion[74-77] | Liver metastasis | ||||||
| Ang-2[65,66] | Activator | Poor survival | |||||
| Inhibitor | |||||||
| IL-6 [58] | Activator | ND | ND | ● | ND | ND | |
| IL-8[37,106] | Activator | ND | ND | ● | ND | ND | |
| IL-17[78] | Activator | ND | ND | ● | ND | ND | |
| Tryptase[79,80] | Activator | ND | ND | ● | Tryptase overexpres-sion[81] | ND | |
| ECM | MMP[92],[107,108] | Activator | Marimastat[107,108] | MMP inhibitor | ● | ND | ND |
| Bay 12-9566 | |||||||
| AG3340 | |||||||
| Neovastat[92] | |||||||
Preclinical trial: First, the basement membrane in growing tumor cells is injured locally, and tumor cells immediately experience destruction and hypoxia. Tumor hypoxia is a major force that triggers tumor angiogenesis and activates the expression of hypoxia-inducible factor-1 (HIF-1), which then induces the expression of various proangiogenic factors, including vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR), in cancer cells[16-19]. Moreover, HIF-2 isoforms have similar functions as HIF-1, but HIF-2 mainly activates the expression of erythropoietin (EPO) in kidney and liver cells[20]. Overall, HIF-1 is known as a potential target of anticancer therapy in many cancers[21]. In addition, treatment with HIF-1-specific inhibitors has been studied in animal models, and it has been shown that this treatment results in slowed growth of tumors, decreased angiogenesis and minor vessel maturation[22]. Stoeltzing et al[23] obtained similar results using the dominant negative form of HIF-1 in GC. Chronic infection with Helicobacter pylori induces DNA damage by generating reactive oxygen species (ROS) in GC cells[24]. Overaccumulation of ROS might stimulate HIF-1 accumulation and aid tumor angiogenesis in GC[25].
Preclinical trial: Growing cancer cells encourage the growth of new blood vessels by secreting VEGF and VEGFR into the surrounding TME, and secreted VEGF binds to VEGFR on the outer surface of ECs. ECs are activated by the VEGF signaling pathway, and this activation induces the growth, survival, vascular permeability and migration of ECs to encourage tumor angiogenesis[26]. To date, various cytokines and a major proangiogenic factor of ECs have been found to be members of the VEGF-A family. The VEGF (homodimers) family of growth factors contains VEGF-A, B, C, D and E and placental growth factor (PIGF), and during angiogenesis[27,28], these growth factors bind to and activate the tyrosine kinase receptors (TKRs) VEGFR-1, VEGFR-2, and VEGFR-3, which are specifically expressed on the surface of ECs and have different affinities for the ligands. Consequently, the downstream TKR signaling proteins activate proliferation-mediating signaling pathways, such as the phosphatidylinositol 3 kinase (PI3K)/AKT, protein kinase C (PKC), and mitogen-activated protein kinase (MAPK; p38 and p42/44) pathways[29-31]. In general, VEGF-A binds to VEGFR-1 and VEGFR-2, PlGF and VEGF-B bind to VEGFR-1, and VEGF-C and VEGF-D bind to VEGFR-2 and VEGFR-3[32-34]. Carmeliet et al[35] reported that among the VEGFs, the vegfa gene can lead to embryonic lethality due to serious vascular defects after the loss of only a single allele in mice[34-36]. An in vitro tube formation assay using GC cells cocultured with human umbilical vein endothelial cells (HUVECs) demonstrated proangiogenesis function due to the upregulation of VEGF in GC cells[37]. In a rat model, the blockage of VEGF by a specific siRNA led to reduced proliferation and cell cycle arrest[38]. Moreover, the coreceptor of neuropilins in signaling pathways is activated by other growth factors or VEGFs, and neuropilins bind several growth factors and enhance their function; however, the molecular mechanisms affected by neuropilins remain unclear[39,40]. The above data indicate that GC cells possess proangiogenic abilities by secreting angiogenic cytokines to both stimulate ECs and to support their own growth in an autocrine manner. Furthermore, the growth and invasion of GC cells are mainly controlled by the VEGF-mediated pathway.
Clinical application: These discoveries from in vitro and animal models were confirmed in GC patients, and their diagnostic or prognostic abilities were tested in GC patients. Through ELISA, significantly higher preoperative plasma or serum VEGF levels were detected in GC patients compared with healthy control subjects. Importantly, a clinicopathological analysis revealed that higher VEGF expression in the plasma or serum of GC patients was significantly associated with advanced stage, distant metastasis and worse survival outcomes[21,41-47].
Preclinical trial: PIGF is another member of the VEGF family and plays a proangiogenic role in the progression of some tumors[29,30,35,48]. Akrami et al[49,50] reported that the knockdown of PlGF in AGS and MKN-45 cells inhibited the proliferation, self-renewal capacity, MMP activity, transcription activity and migration of these cells.
Clinical application: Higher PIGF and VEGF levels were detected by ELISA in GC tissues compared with paired noncancerous mucosa tissues. A clinicopathological analysis showed that higher expression of only PIGF in GC patients was significantly associated with tumor stage, distant metastasis and worse survival outcomes [51].
Preclinical trial: The fibroblast growth factor (FGF) family is a large cytokine family, and some of these cytokines, e.g., FGF-1/-2, bind to different fibroblast growth factor receptors, e.g., FGFR 1-4, to activate the PI3K/AKT/mTOR (mammalian target of rapamycin) pathway. Furthermore, these cytokines can regulate tumor angiogenesis, proliferation, migration and antiapoptosis/survival activities both in vitro and in vivo[31,52-54]. epidermal growth factor (EGF), hepatocyte growth factor (HGF) and insulin-like growth factor (IGF) reportedly stimulate proangiogenic, proliferation and survival activities similarly to those induced by VEGF[55].
Preclinical trial: Pericytes and smooth muscle cells secrete platelet-derived growth factor (PDGF)-BB, which then binds to PDGFR-β and thereby modulates tumor angiogenesis in ECs[56,57].
Preclinical trial: In a mouse model, the blockage of GP130 inhibits tumor development in the epithelium of the glandular stomach via the STAT 1/3-mediated angiogenesis pathway. These results suggest that the TME and cancer cells secrete interleukin-6 (IL-6) via autocrine or paracrine binding to GP130 or IL-6R[58].
Preclinical trial: In tumor cells, EGF binds to EGFR and HER-2/neu to activate the PI3K/AKT and RAS-MAPK-mediated pathways, which are involved in the overexpression of VEGF-A. The secretion of VEGF-A from cancer cells can be mediated through the activation of various signaling pathways. Furthermore, these factors act as central regulators of tumor growth and tumor angiogenesis in GC[59-62].
Preclinical trial: Ang-1, -2, -3, and -4 biologically serve as growth factors for ECs and can strongly regulate competitive interaction with TIE-2 (TKR), which is expressed on the surface of ECs[63,64]. The binding of Ang-1 to TIR-2 activates TIE-2 phosphorylation via the Ang-1/Tie2-cascade pathway and is involved in the proliferation, migration, inflammation and survival of ECs. Ang-2 is then released from activated ECs and serves as a significant antagonist[65,66]. Additionally, TIE-1 (an orphan receptor) can form a complex with TIE-2 to form heterodimers and compete with Ang-1/TIE-2 interactions and thereby promote inflammation in ECs[66-69]. Inhibition of Ang-1 or Ang-2 shows similar inhibition of cell proliferation in GC cell lines[70-73].
Clinical application: Blank et al[74] found that high expression levels of Ang in serum and tissue from GC patients are associated with poor survival. In addition, the Ang/VEGF ratio in GC and esophageal cancer patients serves as an independent proangiogenic biomarker for the clinical response to chemotherapy[75]. Another group of researchers found that Ang-2 can serve as an independent predictor of OS and liver metastasis in GC patients[76]. Moreover, Aktaş et al[77] found that VEGF, PIGF, and Ang-1 are strongly correlated with OS; thus, these angiogenesis prognostic indices (APIs) could predict survival outcomes in GC patients.
Preclinical trial: Tumor-infiltrating macrophages secrete IL-8 and upregulate VEGF to activate EC angiogenesis in GC, as demonstrated in an in vitro assay[37].
Preclinical trial: IL-17 stimulates the STAT3-mediated angiogenesis pathway to upregulate VEGF in GC[78].
Preclinical trial: Tumor-infiltrating mast cells (TIMs) secrete tryptase by binding to proteinase-activated receptor-2 (PAR-2) and then produce VEGF to stimulate tumor angiogenesis and EC proliferation, as demonstrated through in vitro and in vivo assays[79,80].
Clinical application: TIMs can release tryptase via PAR-2 activation and are involved in tumor angiogenesis. Ammendola et al[81] suggested that an increased mast cell density positive for tryptase (MCDPT) and a higher general vascularized area are related to poor survival outcome and can thus serve as potential targets in both primary tumor and lymph node metastases in GC patients.
According to the results of studies on the molecular mechanism of tumor angiogenesis, we can develop a novel antiangiogenic strategy that could reduce tumor angiogenesis and limit tumor growth instead of eradicate the tumors and thereby delay the progression of precancer/primary lesion to metastases/aggressive cancers. The purpose of antiangiogenesis therapy is not to directly target cytotoxic tumor cells but rather block the supply of oxygen, growth factors and nutrition from blood vessels[109]. Thus, this section will focus on several tumor angiogenic factors that could serve as potential targets for antiangiogenic drugs that are currently being investigated in preclinical (the section only highlights the most common antiangiogenic drugs; Table 1) and clinical studies on GC patients. Due to the metabolic changes and stemness of malignant cells lacking oxygen supply in various tumors, tumors appear to escape antiangiogenic therapy within a short time owing to the manipulation of alternative pathways[110], vasculogenic imitation[111] and recruitment of bone marrow-derived cells[112,113]. Various clinical trials have not shown a statistically significant extension of survival outcomes. Thus, most of the antiangiogenesis strategy can be ineffective. In phase III clinical trials, only ramucirumab (anti-VEGFR) and apatinib (VEGFR-TKI) have reported to improve ORR and prolong OS and PFS outcomes when used as a 2nd-line regimen combined with chemotherapy treatment in advanced GC (Table 2).
| Tar-get C Cate-gory | Blockers | Country | Cancer type | Setting | Treat-ment | N | ORR (%) | DCR (%) | PFS (mo) | OS (mo) | Top 5 adverse events | Ref. |
| HR (95% CI)P value | P value | HR (95% CI)P value | HR (95% CI)P value | |||||||||
| Anti-VEGF | Bevaci-zumab (Mono-clonal Ab) | Multieth-nic | ●Metasta-tic GC | 1st-line | Bevaci-zumab | 387 | 46% | 76.90% | 6.7 | 12.1 | Neutro-penia | AVA-GAST[114] |
| Asia-Pacific | Unresec-table locally advanced GC | +Cis/Cap | Febrile neutrope-nia | |||||||||
| Europe | Recurrent GC | Anemia | ||||||||||
| Pan-America | Gastro-esophageal junction GC | Placebo | 387 | 37.40% | 67.70% | 5.3 | 10.1 | Decreased appetite | ||||
| +Cis/Cap | P = 0.0315 | ND | P = 0.0037 | P = 0.1002 | Diarrhea | |||||||
| China | Metastatic GC | 1st-line | Bevacizumab | 100 | 40.70% | 75.30% | 6.3 | 10.5 | Vomiting | AVA-TAR[115] | ||
| Unresectable locally advanced GC | +Cis/Cap | Neutrope-nia | ||||||||||
| Recurrent GC | Nausea | |||||||||||
| Gastro-esophageal junction GC | Anemia | |||||||||||
| Placebo | 102 | 33.70% | 72.10% | 6 | 11.4 | Intestinal obstruc-tion | ||||||
| +Cis/Cap | ||||||||||||
| P = 0.348 | ND | P = 0.4709 | P = 0.5567 | |||||||||
| China | Unresectable locally advanced GC | 1st-line | Bevacizumab | 40 | 65% | 30% | 15.2 | 17.6 | Nausea | [116] | ||
| +Doc/Oxa/5-FU | Vomiting | |||||||||||
| Sensory neuropa-thy | ||||||||||||
| Placebo | 40 | 42.50% | 42.50% | 12.3 | 16.4 | Leukope-nia | ||||||
| +Doc/Oxa/5-FU | P = 0.0436 | ND | P = 0.013 | P = 0.776 | Decreased hemoglo-bin | |||||||
| United Kingdom | Resectable GC | Peri-operative | Bevacizumab | 530 | ND | ND | ND | 48.10% | Lethargy | (United Kingdoms Medical Research Council ST03) [117] | ||
| Esophagogastric junction GC | +Cis/Cap/Epi | Nausea | ||||||||||
| Lower esophageal GC | Neutropenia | |||||||||||
| Diarrhea | ||||||||||||
| Placebo | 533 | ND | ND | ND | 50.30% | Alopecia | ||||||
| +Cis/Cap/Epi | ND | ND | P = 0.56 | P = 0.36 | ||||||||
| Anti-VEGFR | Ramucirumab | Multieth-nic | Advanced gastric GC | 2nd-line | Ramucirumab | 238 | 3% | 49% | 2.1 | 5.2 | Fatigue | REG-ARD[118] |
| (Monoclonal Ab) | North America, Europe, Australia, | Gastro-esophageal junction GC | + Pla/5-Fu | Abdomi-nal pain | ||||||||
| New Zealand | Decreased appetite | |||||||||||
| Asia | Vomiting | |||||||||||
| South and Central America, India, South Africa, Middle East | Placebo | 117 | 3% | 23% | 1.3 | 3.8 | Constipa-tion | |||||
| + Pla/5-Fu | ||||||||||||
| ND | P = 0.76 | P < 0.0001 | P = 0.047 | |||||||||
| Multieth-nic | Advanced gastric GC | 2nd-line | Ramucirumab | 330 | 28% | 80% | 4.4 | 9.63 | Fatigue | RAIN-BOW[119] | ||
| North and South America | Gastro-esophageal junction GC | + Pac | Neuropa-thy | |||||||||
| Europe | Decreased appetite | |||||||||||
| Australia, | Placebo | 335 | 16% | 64% | 2.86 | 7.4 | Abdomi-nal pain | |||||
| Asia | + Pac | Nausea | ||||||||||
| P < 0.0001 | P = 0.0001 | P < 0.0001 | P = 0.0169 | |||||||||
| Multieth-nic | Metastatic GC | 1st-line | Ramucirumab | 326 | 41.10% | 81.90% | 10.2 | 11.2 | Neutropenia | RAIN-FALL[120] | ||
| North America | Gastro-esophageal junction GC | + Cis/5-Fu | Anaemia | |||||||||
| Europe | Hypertension | |||||||||||
| Japan | Placebo | 319 | 36.40% | 76.50% | 9.2 | 10.7 | Palmar-plantar erythrodysesthesia syndrome | |||||
| + Cis/5-Fu | Fatigue | |||||||||||
| P = 0.17 | P = 0.095 | P = 0.4 | P = 0.68 | |||||||||
| VEGF | apatinib | China | Metastatic GC | 3rd-line | Apatinib | 176 | 2.84 | 42.05 | 2.6 | 6.5 | Hand-foot syndrome | [121] |
| TKI | Advanced GC | Proteinu-ria | ||||||||||
| Gastro-esophageal junction GC | Hyperten-sion | |||||||||||
| Placebo | 91 | 0 | 8.79 | 1.8 | 4.7 | Myelosuppression | ||||||
| P < 0.001 | P = 0.1695 | P < 0.001 | P = 0.0149 | Nausea and vomiting |
Preclinical trial: As demonstrated in a preclinical model, this drug, which is a recombinant monoclonal antibody against VEGF-A, serves as a powerful and effective antiangiogenesis agent in several cancers[83-85]. An in vitro study revealed that treatment with bevacizumab reduced cell growth and pro-apoptosis in GC cell lines[86]. Yamashita-Kashima et al[87] performed an in vivo study and found that bevacizumab could be effective against GC and select biomarkers in the MKN-45 human gastric xenograft model. A study with mouse models revealed that treatment with bevacizumab significantly reduced the tumor size[88,89]. In the future, we will explore the effects of the antibody-mediated blockage of VEGF-mediated tumor angiogenesis in GC to obtain a more in-depth understanding.
Clinical trial: Ohtsu et al[114] explored the effect of bevacizumab, which is a VEGF blocker. The AVAGAST clinical trial indicated that the 1st line treatment of advanced GC patients (multiethnic population) with bevacizumab in combination with chemotherapy (Cisplatin; Cis/Capecitabine; Cap) resulted in significantly improved ORR (P = 0.0315) and extended PFS (P = 0.0037) outcomes compared with those achieved with chemotherapy alone (Table 2). However, the AVATAR clinical trial showed that the 1st line treatment of advanced GC patients (China) with bevacizumab in combination with chemotherapy (Cis/Cap) did not significantly prolong the survival outcomes compared with those achieved with chemotherapy alone[115]. In contrast, Ma et al[116] assessed the effects of bevacizumab in combination with chemotherapy (Docetaxel; Doc/Oxaliplatin; Oxa/5-FU) compared with those of the 1st line treatment of chemotherapy alone in advanced GC patients (China) and observed significantly improved ORR (P = 0.0436) and extended PFS (P = 0.013) outcomes compared with those achieved with chemotherapy alone. The other group, the ST03 clinical trial, showed that the perioperative treatment of advanced GC patients (United Kingdom) with bevacizumab in combination with chemotherapy (Cis/Cap/Epirubicin; Epi) had no positive results compared with those achieved with chemotherapy alone[117]. However, the differences in the outcomes achieved after bevacizumab treatment among the different populations remain unknown.
Preclinical trial: The interferon family contains multifunctional cytokines that exhibit antiviral and antitumor properties, induce regulatory cell apoptosis and immune responses and inhibit proangiogenic factors. Abdel-Rahman et al[90] evaluated bevacizumab in combination with other anticancer agents, such as mTOR inhibitors and interferon (IFN), as a more effective treatment for gastrointestinal tract and pancreatic tissues. Preclinical and clinical trials showed that other mTOR inhibitors, such as rapamycin, also display antiangiogenic activity in GC[91]. Moreover, Neovastat is a multifunctional drug that blocks VEGF, MMPs and proapoptotic activity in ECs. One MMP inhibitor (Marimastat) has been shown to induce positive outcomes in phase III clinical trials with advanced GC patients. The other MMP inhibitors are continuing to be investigated in clinical trials[92].
Clinical trial: A clinical phase II trial showed that the treatment of advanced GC patients with interferon-alpha 2B (IFN) and folinic acid (FA) in combination with 5-fluorouracil (5-FU) chemotherapy also resulted in significantly prolonged PFS outcomes compared with those achieved with chemotherapy alone[122]. Al-Batran et al[123] demonstrated that mTOR-mediated inhibitors (e.g., rapamycin) blocked the growth of GC cells and delayed tumor progression in cell lines and mouse models. Additionally, the mTOR inhibitor rapamycin has also yielded better survival outcomes in phase I/II studies of metastatic GC patients than do treatment without rapamycin.
Preclinical trial: Ramucirumab is a VEGFR-2-targeted monoclonal antibody that inhibit VEGFR-2 signaling. An in vitro study showed that treatment with ramucirumab also inhibited cell growth and promoted apoptosis in GC cell lines and animal models[95,96]. Thus, both bevacizumab and ramucirumab inhibit VEGF-mediated pathways in GC. Additionally, an in vivo study showed that the effects of combination therapy involving anti-VEGFR and anti-EGFR agents resulted in a significantly decreased tumor size in a GC mouse model[97].
Clinical trial: Fuchs et al[118] attempted to explore the effect of ramucirumab, which blocks VEGFR signaling. The REGARD clinical trial indicated that the treatment of advanced GC patients (multiethnic) with ramucirumab in combination with chemotherapy (Pla/5-Fu) resulted in significantly extended PFS (P < 0.0001) and OS (P = 0.047) outcomes compared with those achieved with placebo. Moreover, the RAINBOW clinical trial showed that the treatment of advanced GC patients (multiethnic) with ramucirumab in combination with chemotherapy (Paclitaxel; Pac) also resulted in significantly improved ORR (P < 0.0001) and DCR (P < 0.0001), extended PFS (P < 0.0001) and OS (P = 0.0169) outcomes compared with those achieved with chemotherapy alone[119]. In contrast, the RAUNFALL clinical trial showed that the treatment of advanced GC patients (multiethnic) with bevacizumab in combination with chemotherapy (Cis/5-Fu) had no positive results compared with those achieved with chemotherapy alone[120]. Ramucirumab was approved by the United States Food and Drug Administration (FDA) in 2014 as a 2nd-line treatment of advanced GC due to the REGARD and RAINBOW clinical trials and has beneficial effects on PFS and OS for advanced GC.
Preclinical trial: Regorafenib, apatinib and foretinib belong to the family of multitargeting TKIs. Blockage of the effects of VEGF by silencing RNA in GC cell lines led to reduced tumor volume after implantation of these GC cells into nude mice[98]. The same effect was observed in mice treated with apatinib after tumor grafting[99].
Clinical trial: First, Li et al[121] explored the effect of apatinib, which VEGFR TKI blockade. A 116 clinical trial (3rd line) indicated that the treatment of advanced GC patients (China) with apatinib resulted in significantly improved ORR (P < 0.001), extended PFS (P < 0.001) and OS (P = 0.0149) outcomes compared with those achieved with placebo. In a phase II study, the tumor-angiogenesis inhibitor regorafenib, which targets VEGFR, TIE and multiple kinases, was evaluated in advanced GC patients, and the results showed that treatment with this inhibitor resulted in significantly prolonged PFS outcomes compared with those achieved with placebo[124]. Thus, regorafenib will be investigated in a phase III study. However, another antiangiogenic drug, foretinib, which inhibits VEGFR2 and TIE-2, did not yield any benefits in the survival outcomes of GC patients[125]. In addition, Shan et al[126] reviewed information from clinical trials evaluating antiangiogenic agents (with a focus on multitargeting TKIs) in advanced GC and found that only apatinib yielded a positive effect on PFS.
Preclinical trial: Orantinib (SU5416 SU6668)[94], pazopanib[100], sorafenib (Nexavar)[101,102], sunitinib (Sutent)[103,104] and telatinib (Erbitux, Cetuximab)[105] block tyrosine kinases and belong to the family of multitargeting TKIs. Suppressing the effects of VEGF by silencing RNA in GC cell lines led to decreased tumor angiogenesis and growth after these cells were implanted into nude mice.
Clinical trial: Chen et al[71] summarized the results from clinical trial phase II studies of antiangiogenic drugs, including VEGF ligands, VEGFRs and multitarget TKIs, in advanced GC. The treatment of advanced GC patients with orantinib[127], pazopanib[128,129], sorafenib[130-133], sunitinib[134-136], telatinib[137-141] and vandetanib resulted in significantly extended OS and PSF.
Preclinical trial: Aflibercept traps VEGF and PlGF in vivo and is currently being investigated in a clinical trial (NCT01747551) as a supplement to standard chemotherapy for GC patients[22]. In addition to VEGF-specific inhibition, the effect of HIF-1 blockage has been investigated in animal models in several studies. The treatment of subcutaneous xenografts with an inhibitory HIF-1 compound results in smaller and less vascularized tumors after implantation into nude mice.
Seidman et al[62] reported that the antibody trastuzumab blocks the Her2/neu receptor through the RAS-MAPK proliferation signaling pathway. A log-rank test showed improved survival outcomes in breast cancer patients. The comparison of two different Her2 and VEGF inhibitors revealed that the effect of tumor growth inhibition on Her2-overexpressing GC xenografts through the combination of Her2 and VEGF inhibitors was better than that achieved with either inhibitor alone[59].
In an animal model, nonsteroid anti-inflammatory drug (NSAID)-mediated cyclooxygenase (COX) inhibition resulted in reduced tumor angiogenesis, and decreased HIF-1 expression was detected in GC cells after treatment with NSAIDs[25].
In clinical phase trials, cancer patients are typically administered combination therapy consisting of antiangiogenic agents with chemotherapeutic agents. However, antiangiogenic therapy sometimes elicits several adverse effects, such as hypertension[142,143] or proteinuria[144], but the factors responsible for these adverse effects remain unknown. In general, the results from several studies on some antiangiogenic therapies, such as the inhibition of VEGF, Ang-1 and PlGF, indicate that antiangiogenic therapy not only inhibits EC migration and proliferation but also enhances chemotherapy ability. Hwang et al[145] indicated that the inhibition of VEGFR enhances paclitaxel sensitivity in GC cells. Another group of researchers showed that the upregulation of HIF-1 promotes chemotherapy and the antiapoptosis ability in GC cells by inducing miR-27a- or p53- and NF-kB-mediated pathways[146-148]. Additionally, compared with normal blood vessels, tumor vessels exhibit heterogeneity, versatility, high permeability and vascular properties that benefit chemotherapy[149]. Thus, antiangiogenic therapy could exert an adjuvant effect in chemotherapy.
Tumor angiogenesis involves a complex multistep process. In general, the available knowledge indicates that proangiogenic and pro-oncogenic (such as proliferation, anti-apoptosis, migration and invasion) pathways are linked to each other. Thus, tumor angiogenesis occurs at different stages of tumor progression, including tumor growth, metastasis and recurrence. This connection can be clearly observed by the administration of combination therapy against angiogenic and proliferative pathways, such as the VEGF-, EGFR- and STAT3-mediated pathways[16-19,31,52-54,58]. These transcription factors regulate cell growth, migration and angiogenesis in multiple ways.
First, we investigated the expression of angiogenic factors in GC through preclinical trials [cell line (in vitro)/animal model (in vivo)] and thus determined whether these factors could serve as predictive factors/biomarkers for proliferation, invasion or metastasis and/or have diagnostic or prognostic value[7,8]. An increasing number of studies has revealed that antiangiogenic agents attack tumor ECs as their target instead of tumor cells themselves, which is the final goal of tumor dormancy therapy. Moreover, the therapeutic target of antiangiogenic agents is tumor ECs, which are more genetically stable, show increased homogeneity and have a lower alteration level; antiangiogenic drugs can interact with ECs directly, resulting in higher potency, decreased drug resistance and fewer side effects[150]. We explored the combination of antiangiogenic drugs and cytotoxic anticancer (chemical) drugs to develop a highly effective strategy for the management of advanced GC[13-15]. Thus, antiangiogenic drugs might be valuable for the long-term management of tumor dormancy because they do not induce the development of antiangiogenic drug resistance, and these drugs present fewer side effects. A few recent clinical trials have revealed that antiangiogenic therapy could potentially extend the survival outcomes of advanced GC patients[109].
In assessing the effectiveness of antiangiogenesis therapy, a clinical phase III trial showed that only ramucirumab (an anti-VEGFR antibody) and apatinib (VEGFR TKI blocker) achieved positive results (Table 2). Although both ramucirumab and bevacizumab are anti-VEGF drugs, bevacizumab (AVAGAST, AVATAR, ST03, Ma et al[116]) had no positive results on OS, while ramucirumab (REGARD, RAINBOW) was more effective targeted drug and exerted more positive results for OS in advanced GC. We suggested that this is because bevacizumab only binds to VEGF-A, whereas ramucirumab binds to VEGFR-2, which blocks more VEGFs. Therefore, ramucirumab could exert more effective antiangiogenic function due to the inhibition of more VEGF molecules. One possible reason is the differences in the targets of the antiangiogenic action. However, the differences in the ability of these two anti-VEGF drugs remain partially unknown. Furthermore, the different populations of GC patients might be another factor that affects the benefits of these drugs. In the AVAGAST and RAINBOW studies, the non-Asian subgroup (66.5%; RAINBOW) achieved a greater benefit in OS from antiangiogenic therapy than did the Asian subgroup (51%; AVAGAST). However, the effect of ramucirumab still lacks 1st-line chemotherapy evidence. The extent of the usefulness of ramucirumab still requires exploration in further trials in different ethnicities and upon delivery as a 1st-, 2nd- or 3th-line chemotherapy. Additionally, in evaluating the safety of antiangiogenic therapy, most adverse events related to antiangiogenesis are tolerable and controllable, including hypertension, neutropenia and wound healing (Table 2). Conversely, the Cougar-02 trial, a Doc+best supportive care (BSC) study, has a similar result for OS as the REGARD trial and was more cost effective[151]. Finally, of the VEGFR TKIs, only apatinib in the phase III clinical trial showed extended PFS and OS in advanced GC patients. We recommend that chemotherapy in combination with ramucirumab (anti-VEGFR) and apatinib (VEGFR TKI) significantly improves the outcome in ORR, expended PFS, and OS in the management of advanced GC.
Here, this review only included phase III clinical trials published in English. Previous studies have found that the combination of antiangiogenic agents with chemotherapy may be beneficial for advanced GC in OS, but potential publication bias should be considered when construing these results. To reduce possible publication bias, we tried to search in multiple databases. Nevertheless, some restrictions were present in this systemic review and statistical analysis (e.g., meta-analysis)[152,153] such as the small size of included studies, multiple drugs implemented and the high heterogeneity between different studies. Therefore, a larger cohort size, more standardized research and high statistical quality should be implemented in future studies to identify patients who would most likely benefit from antiangiogenic treatment. Thus, this review will provide basic (tumor angiogenesis) and clinical (antiangiogenic drugs) research for the survey of the management of GC treatments.
Although several phase III clinical trials have reported positive results, new vessels in tumors have pleomorphic features, including heterogeneity, flexibility, penetrability, various vascular biomarkers, and turbulent blood flow with no lymphatic vessels, and these unusual features make the delivery of therapeutic drugs difficult. Hence, there remain several obstacles regarding the translation of antiangiogenic strategies from animal models to clinical trials[92,108,154].
The current problems regarding preclinical to clinical trials and the future directions for antiangiogenic therapy are discussed below.
In preclinical trials, we usually perform experiments in animals with xenografts of various tumor cells, but these models cannot represent spontaneous and orthotopic human cancers, particularly highly metastatic tumors[155]. Therefore, antiangiogenic drugs are not effective for every organ in the body. Antiangiogenic drugs often yield different results or side effects in preclinical and clinical trials.
In advanced GC, the tumor develops several ways of escaping treatment and rapidly activating angiogenic pathways. Ebos et al[156] reported that enhanced metastasis was treated with sunitinib in a mouse model. Another group found a similar result[157]. This may partly fail to translate to a survival benefit of antiangiogenic drugs in localized or nonmetastatic GC. Therefore, it is crucial to develop novel biomarkers that are able to predict the prognosis of antiangiogenic treatments for advanced GC. In clinical trials, to assess antiangiogenic therapies, newer imaging systems and/or substitute biomarkers should be established for monitoring tumor vessel functions. Antiangiogenic drugs induce tumor dormancy, which is different from the results of chemotherapy[155].
The aims of managing GC are to reduce drug toxicity and adverse events and prolong survival. Therefore, the optimal biological dose and therapeutic schedule of antiangiogenic drugs should be established. Moreover, antiangiogenic drugs can be combined with chemotherapy and/or radiotherapy[149].
According to previous studies, the clinical effect is quite different in individuals due to heterogeneity of the tumor. It is unclear which patients benefit most from angiogenesis inhibitors. The race/ethnicity of patients seems to influence the efficacy of antiangiogenic treatments on OS. The patients should be selected, and angiogenic factors should be detected before the administration of antiangiogenic drugs. Individual angiogenic profiling according to an individual’s genetic background remain a problem that need to be addressed.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang L, Tanabe S S-Editor: Ma YJ L-Editor: A E-Editor: Qi LL
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1254] [Article Influence: 66.0] [Reference Citation Analysis (8)] |
| 2. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P'eng FK. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg. 2000;24:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Vd Wurff AA, Bosscha K, Coebergh JW. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer. 2010;46:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration). Group Oba K, Paoletti X, Bang YJ, Bleiberg H, Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Shitara K, Tsuburaya A, Van Cutsem E, Buyse M. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013;49:1565-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2672] [Cited by in RCA: 2600] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 7. | Mbeunkui F, Johann DJ. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1724] [Cited by in RCA: 1699] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 9. | Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 11. | Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3324] [Cited by in RCA: 3185] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 12. | Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993;10:302-313. [PubMed] |
| 13. | Alsabti EA. Tumor dormancy: a review. J Cancer Res Clin Oncol. 1979;95:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2466] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 15. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5900] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 16. | Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3808] [Cited by in RCA: 3968] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 18. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 19. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. [PubMed] |
| 20. | Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4764] [Cited by in RCA: 5022] [Article Influence: 228.3] [Reference Citation Analysis (0)] |
| 22. | Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 369] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res. 2003;9:433-440. [PubMed] |
| 26. | Bose S, Deininger M, Gora-Tybor J, Goldman JM, Melo JV. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362-3367. [PubMed] |
| 27. | Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1482] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 28. | Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3027] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 29. | Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1801] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 30. | Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015-3025. [PubMed] |
| 31. | Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 1068] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 32. | Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1969] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 33. | Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 34. | Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2927] [Cited by in RCA: 2785] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 35. | Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2997] [Cited by in RCA: 2872] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 36. | Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2565] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 37. | Shi J, Wei PK. Interleukin-8: A potent promoter of angiogenesis in gastric cancer. Oncol Lett. 2016;11:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Xu WH, Ge YL, Li Q, Zhang X, Duan JH. Inhibitory effect of vascular endothelial growth factors-targeted small interfering RNA on proliferation of gastric cancer cells. World J Gastroenterol. 2007;13:2044-2047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2798] [Cited by in RCA: 2923] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 40. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6952] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 41. | Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, Karatzas G. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Kakeji Y, Koga T, Sumiyoshi Y, Shibahara K, Oda S, Maehara Y, Sugimachi K. Clinical significance of vascular endothelial growth factor expression in gastric cancer. J Exp Clin Cancer Res. 2002;21:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 43. | Wang TB, Wang J, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C combined with multi-detector CT in the preoperative diagnosis of lymph node metastasis of gastric cancer. Asia Pac J Clin Oncol. 2012;8:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Kikuchi S, Obata Y, Yagyu K, Lin Y, Nakajima T, Kobayashi O, Kikuichi M, Ushijima R, Kurosawa M, Ueda J. Reduced serum vascular endothelial growth factor receptor-2 (sVEGFR-2) and sVEGFR-1 levels in gastric cancer patients. Cancer Sci. 2011;102:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Sheng SL, Bao SH, Huang G, Wang LM. Development of time-resolved immunofluorometric assays for vascular endothelial growth factor and application on plasma of patients with gastric tumours. Clin Exp Immunol. 2008;151:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Vidal O, Metges JP, Elizalde I, Valentíni M, Volant A, Molina R, Castells A, Pera M. High preoperative serum vascular endothelial growth factor levels predict poor clinical outcome after curative resection of gastric cancer. Br J Surg. 2009;96:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Villarejo-Campos P, Padilla-Valverde D, Martin RM, Menéndez-Sánchez P, Cubo-Cintas T, Bondia-Navarro JA, Fernández JM. Serum VEGF and VEGF-C values before surgery and after postoperative treatment in gastric cancer. Clin Transl Oncol. 2013;15:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2373] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 49. | Akrami H, Mahmoodi F, Havasi S, Sharifi A. PlGF knockdown inhibited tumor survival and migration in gastric cancer cell via PI3K/Akt and p38MAPK pathways. Cell Biochem Funct. 2016;34:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Mahmoodi F, Akrami H. PlGF Knockdown Decreases Tumorigenicity and Stemness Properties of Spheroid Body Cells Derived from Gastric Cancer Cells. J Cell Biochem. 2017;118:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, Lee PH. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Liang G, Liu Z, Wu J, Cai Y, Li X. Anticancer molecules targeting fibroblast growth factor receptors. Trends Pharmacol Sci. 2012;33:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Forough R, Weylie B, Patel C, Ambrus S, Singh US, Zhu J. Role of AKT/PKB signaling in fibroblast growth factor-1 (FGF-1)-induced angiogenesis in the chicken chorioallantoic membrane (CAM). J Cell Biochem. 2005;94:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Sahin F, Celik HA, Aydin HH, Oktem G, Omay SB, Saydam G. The interaction between taxoids and serine/threonine protein phosphatase activities during taxan-induced apoptosis of HL 60 leukemic cells. Hematology. 2008;13:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 318] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Xue Y, Lim S, Yang Y, Wang Z, Jensen LD, Hedlund EM, Andersson P, Sasahara M, Larsson O, Galter D, Cao R, Hosaka K, Cao Y. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 58. | Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 59. | Singh R, Kim WJ, Kim PH, Hong HJ. Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med. 2013;45:e52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 968] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 61. | Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Le XF. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986-6996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D'Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis CA. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 63. | Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1070] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 64. | Saharinen P, Bry M, Alitalo K. How do angiopoietins Tie in with vascular endothelial growth factors? Curr Opin Hematol. 2010;17:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1404] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 66. | Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2548] [Cited by in RCA: 2531] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 67. | Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 68. | Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 69. | Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 70. | Wang J, Wu KC, Zhang DX, Fan DM. Antisense angiopoietin-1 inhibits tumorigenesis and angiogenesis of gastric cancer. World J Gastroenterol. 2006;12:2450-2454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Chen Z, Zhu S, Hong J, Soutto M, Peng D, Belkhiri A, Xu Z, El-Rifai W. Gastric tumour-derived ANGPT2 regulation by DARPP-32 promotes angiogenesis. Gut. 2016;65:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Ou XL, Chen HJ, Sun WH, Hang C, Yang L, Guan YY, Yan F, Chen BA. Effects of angiopoietin-1 on attachment and metastasis of human gastric cancer cell line BGC-823. World J Gastroenterol. 2009;15:5432-5441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Tang S, Wang D, Zhang Q, Li L. miR-218 suppresses gastric cancer cell proliferation and invasion via regulation of angiopoietin-2. Exp Ther Med. 2016;12:3837-3842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Blank S, Deck C, Dreikhausen L, Weichert W, Giese N, Falk C, Schmidt T, Ott K. Angiogenic and growth factors in gastric cancer. J Surg Res. 2015;194:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Dreikhausen L, Blank S, Sisic L, Heger U, Weichert W, Jäger D, Bruckner T, Giese N, Grenacher L, Falk C, Ott K, Schmidt T. Association of angiogenic factors with prognosis in esophageal cancer. BMC Cancer. 2015;15:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Hacker UT, Escalona-Espinosa L, Consalvo N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E, Coutelle O, Büning H. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial. Br J Cancer. 2016;114:855-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Aktaş SH, Akbulut Yazici HO, Zengin N, Akgün HN, Üstüner Z, Içli F. A new angiogenesis prognostic index with VEGFA, PlGF, and angiopoietin1 predicts survival in patients with advanced gastric cancer. Turk J Med Sci. 2017;47:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Wu X, Yang T, Liu X, Guo JN, Xie T, Ding Y, Lin M, Yang H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol. 2016;37:5493-5501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Ammendola M, Marech I, Sammarco G, Zuccalà V, Luposella M, Zizzo N, Patruno R, Crovace A, Ruggieri E, Zito AF, Gadaleta CD, Sacco R, Ranieri G. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int J Mol Sci. 2015;16:3237-3250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Ammendola M, Sacco R, Zuccalà V, Luposella M, Patruno R, Gadaleta P, Zizzo N, Gadaleta CD, De Sarro G, Sammarco G, Oltean M, Ranieri G. Mast Cells Density Positive to Tryptase Correlate with Microvascular Density in both Primary Gastric Cancer Tissue and Loco-Regional Lymph Node Metastases from Patients That Have Undergone Radical Surgery. Int J Mol Sci. 2016;17:E1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Dormond O, Foletti A, Paroz C, Rüegg C. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat Med. 2001;7:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Deissler HL, Lang GE. [In vitro studies on the mechanism of action of VEGF and its inhibitors]. Klin Monbl Augenheilkd. 2008;225:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Ziemssen F, Sobolewska B, Deissler H, Deissler H. Safety of monoclonal antibodies and related therapeutic proteins for the treatment of neovascular macular degeneration: addressing outstanding issues. Expert Opin Drug Saf. 2016;15:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Peters GJ. Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol. 2015;7:340-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Lv Y, Song L, Chang L, Liu Y, Zhang X, Li Q, Zhou X, Liu W. Inhibitory effects of bevacizumab monoclonal antibodies in combination with chemotherapy in different time sequences on a human gastric carcinoma cell line. Ir J Med Sci. 2017;186:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Yamashita-Kashima Y, Fujimoto-Ouchi K, Yorozu K, Kurasawa M, Yanagisawa M, Yasuno H, Mori K. Biomarkers for antitumor activity of bevacizumab in gastric cancer models. BMC Cancer. 2012;12:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Ninomiya S, Inomata M, Tajima M, Ali AT, Ueda Y, Shiraishi N, Kitano S. Effect of bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, on peritoneal metastasis of MNK-45P human gastric cancer in mice. J Surg Res. 2009;154:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Imaizumi T, Aoyagi K, Miyagi M, Shirouzu K. Suppressive effect of bevacizumab on peritoneal dissemination from gastric cancer in a peritoneal metastasis model. Surg Today. 2010;40:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Abdel-Rahman O, Fouad M. Bevacizumab-based combination therapy for advanced gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): a systematic review of the literature. J Cancer Res Clin Oncol. 2015;141:295-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Pinto MP, Owen GI, Retamal I, Garrido M. Angiogenesis inhibitors in early development for gastric cancer. Expert Opin Investig Drugs. 2017;26:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs. 2000;9:2167-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 93. | Xu RH, Kalechman Y, Albeck M, Sredni B. The cytoprotective effect of the immunomodulator AS101 against hydrochloride induced gastric lesions. Res Commun Mol Pathol Pharmacol. 1995;87:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 94. | Muehlbauer PM. Anti-angiogenesis in cancer therapy. Semin Oncol Nurs. 2003;19:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Falcon BL, Chintharlapalli S, Uhlik MT, Pytowski B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther. 2016;164:204-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 96. | Arakawa Y, Tamura M, Aiba K, Morikawa K, Aizawa D, Ikegami M, Yuda M, Nishikawa K. Significant response to ramucirumab monotherapy in chemotherapy-resistant recurrent alpha-fetoprotein-producing gastric cancer: A case report. Oncol Lett. 2017;14:3039-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 98. | Sun P, Yu H, Zhang WQ, Hu M, Lv R. Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer growth in vivo. Oncol Rep. 2012;28:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Lin Y, Zhai E, Liao B, Xu L, Zhang X, Peng S, He Y, Cai S, Zeng Z, Chen M. Autocrine VEGF signaling promotes cell proliferation through a PLC-dependent pathway and modulates Apatinib treatment efficacy in gastric cancer. Oncotarget. 2017;8:11990-12002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett. 2018;16:687-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 101. | Xu X, Tang X, Wu X, Feng X. Biosynthesis of sorafenib coated graphene nanosheets for the treatment of gastric cancer in patients in nursing care. J Photochem Photobiol B. 2019;191:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Yang F, Li A, Liu H, Zhang H. Gastric cancer combination therapy: synthesis of a hyaluronic acid and cisplatin containing lipid prodrug coloaded with sorafenib in a nanoparticulate system to exhibit enhanced anticancer efficacy and reduced toxicity. Drug Des Devel Ther. 2018;12:3321-3333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Lyros O, Mueller A, Heidel F, Schimanski CC, Gockel I, Galle PR, Lang H, Moehler M. Analysis of anti-proliferative and chemosensitizing effects of sunitinib on human esophagogastric cancer cells: Synergistic interaction with vandetanib via inhibition of multi-receptor tyrosine kinase pathways. Int J Cancer. 2010;127:1197-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Fuereder T, Jaeger-Lansky A, Hoeflmayer D, Preusser M, Strommer S, Cejka D, Koehrer S, Crevenna R, Wacheck V. mTOR inhibition by everolimus counteracts VEGF induction by sunitinib and improves anti-tumor activity against gastric cancer in vivo. Cancer Lett. 2010;296:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Chen LT, Oh DY, Ryu MH, Yeh KH, Yeo W, Carlesi R, Cheng R, Kim J, Orlando M, Kang YK. Anti-angiogenic Therapy in Patients with Advanced Gastric and Gastroesophageal Junction Cancer: A Systematic Review. Cancer Res Treat. 2017;49:851-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Kishida O, Miyazaki Y, Murayama Y, Ogasa M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyohara T, Shimomura I, Shinomura Y. Gefitinib (Iressa, ZD1839) inhibits SN38-triggered EGF signals and IL-8 production in gastric cancer cells. Cancer Chemother Pharmacol. 2005;55:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Folkman J. Fighting cancer by attacking its blood supply. Sci Am. 1996;275:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Vecchione L, Orditura M, Ciardiello F, De Vita F. Novel investigational drugs for gastric cancer. Expert Opin Investig Drugs. 2009;18:945-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol Rep. 2017;38:625-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 960] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 111. | Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 490] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 112. | van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67:441-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 113. | Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 114. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 115. | Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y, Xu R, Chen L, Liu Y, Yu S, Bu L, Piao Y. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 116. | Ma J, Yao S, Li XS, Kang HR, Yao FF, Du N. Neoadjuvant Therapy of DOF Regimen Plus Bevacizumab Can Increase Surgical Resection Ratein Locally Advanced Gastric Cancer: A Randomized, Controlled Study. Medicine (Baltimore). 2015;94:e1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 118. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1574] [Article Influence: 143.1] [Reference Citation Analysis (0)] |
| 119. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1768] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 120. | Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, Ilson DH, Alsina M, Chau I, Lacy J, Ducreux M, Mendez GA, Alavez AM, Takahari D, Mansoor W, Enzinger PC, Gorbounova V, Wainberg ZA, Hegewisch-Becker S, Ferry D, Lin J, Carlesi R, Das M, Shah MA; RAINFALL Study Group. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 121. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 122. | Jäger E, Bernhard H, Klein O, Wächter B, Theiss F, Dippold W, Meyer zum Büschenfelde KH, Knuth A. Combination 5-fluorouracil (FU), folinic acid (FA), and alpha-interferon 2B in advanced gastric cancer: results of a phase II trial. Ann Oncol. 1995;6:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Al-Batran SE, Ducreux M, Ohtsu A. mTOR as a therapeutic target in patients with gastric cancer. Int J Cancer. 2012;130:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 124. | Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, Bang YJ, Alcindor T, O'Callaghan CJ, Burnell MJ, Tebbutt NC, Rha SY, Lee J, Cho JY, Lipton LR, Wong M, Strickland A, Kim JW, Zalcberg JR, Simes J, Goldstein D. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol. 2016;34:2728-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 125. | Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe DC, Keer H, Martin AM, Liu Y, Gagnon R, Bonate P, Liu L, Gilmer T, Bottaro DP. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013;8:e54014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 126. | Shan F, Miao R, Xue K, Li Z, Li Z, Bu Z, Wu A, Zhang L, Wu X, Zong X, Wang X, Li S, Ji X, Jia Z, Li Z, Ji J. Controlling angiogenesis in gastric cancer: A systematic review of anti-angiogenic trials. Cancer Lett. 2016;380:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 127. | Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1121] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 128. | Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 129. | Liu SX, Xia ZS, Zhong YQ. Gene therapy in pancreatic cancer. World J Gastroenterol. 2014;20:13343-13368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 130. | Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med. 2013;15:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 131. | Ortiz R, Melguizo C, Prados J, Álvarez PJ, Caba O, Rodríguez-Serrano F, Hita F, Aránega A. New gene therapy strategies for cancer treatment: a review of recent patents. Recent Pat Anticancer Drug Discov. 2012;7:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Cao S, Cripps A, Wei MQ. New strategies for cancer gene therapy: progress and opportunities. Clin Exp Pharmacol Physiol. 2010;37:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Tseng SJ, Liao ZX, Kao SH, Zeng YF, Huang KY, Li HJ, Yang CL, Deng YF, Huang CF, Yang SC, Yang PC, Kempson IM. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat Commun. 2015;6:6456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 134. | Tazawa H, Kagawa S, Fujiwara T. Advances in adenovirus-mediated p53 cancer gene therapy. Expert Opin Biol Ther. 2013;13:1569-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Prabha S, Sharma B, Labhasetwar V. Inhibition of tumor angiogenesis and growth by nanoparticle-mediated p53 gene therapy in mice. Cancer Gene Ther. 2012;19:530-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 136. | Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med (Berl). 2007;85:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 137. | Zhang C, Wang QT, Liu H, Zhang ZZ, Huang WL. Advancement and prospects of tumor gene therapy. Chin J Cancer. 2011;30:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 138. | Allen JW, Moon J, Redman M, Gadgeel SM, Kelly K, Mack PC, Saba HM, Mohamed MK, Jahanzeb M, Gandara DR. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol. 2014;32:2463-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 139. | Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui J, Nott LM, Charpentier D, Liauw W, Sawyer MB, Jefford M, Magoski NM, Haydon A, Walters I, Ringash J, Tu D, O'Callaghan CJ. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol. 2013;31:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 140. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M; Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 681] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 141. | Hitre E, Budai B, Takácsi-Nagy Z, Rubovszky G, Tóth E, Remenár É, Polgár C, Láng I. Cetuximab and platinum-based chemoradio- or chemotherapy of patients with epidermal growth factor receptor expressing adenoid cystic carcinoma: a phase II trial. Br J Cancer. 2013;109:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 142. | Österlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer. 2011;104:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 143. | Tahover E, Uziely B, Salah A, Temper M, Peretz T, Hubert A. Hypertension as a predictive biomarker in bevacizumab treatment for colorectal cancer patients. Med Oncol. 2013;30:327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 144. | Khoja L, Kumaran G, Zee YK, Murukesh N, Swindell R, Saunders MP, Clamp AR, Valle JW, Wilson G, Jayson GC, Hasan J. Evaluation of hypertension and proteinuria as markers of efficacy in antiangiogenic therapy for metastatic colorectal cancer. J Clin Gastroenterol. 2014;48:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Hwang JE, Lee JH, Park MR, Kim DE, Bae WK, Shim HJ, Cho SH, Chung IJ. Blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in gastric cancer cells. Yonsei Med J. 2013;54:374-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 146. | Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 147. | Zhao Q, Li Y, Tan BB, Fan LQ, Yang PG, Tian Y. HIF-1α Induces Multidrug Resistance in Gastric Cancer Cells by Inducing MiR-27a. PLoS One. 2015;10:e0132746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 148. | Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, Cramer T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5:e12038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 149. | Groenewald P, Bradshaw D, Neethling I, Martin LJ, Dempers J, Morden E, Zinyakatira N, Coetzee D. Linking mortuary data improves vital statistics on cause of death of children under five years in the Western Cape Province of South Africa. Trop Med Int Health. 2016;21:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 150. | Kakeji Y, Maehara Y, Sumiyoshi Y, Oda S, Emi Y. Angiogenesis as a target for gastric cancer. Surgery. 2002;131:S48-S54. [PubMed] |
| 151. | Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, Swinson D, Falk S, Chau I, Cunningham D, Kareclas P, Cook N, Blazeby JM, Dunn JA; COUGAR-02 Investigators. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 152. | Lei X, Wang F, Ke Y, Wei D, Gu H, Zhang Z, Jiang L, Lv L, Lin J, Wang L. The role of antiangiogenic agents in the treatment of gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e6301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 153. | Bai ZG, Zhang ZT. A systematic review and meta-analysis on the effect of angiogenesis blockade for the treatment of gastric cancer. Onco Targets Ther. 2018;11:7077-7087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 154. | Nelson NJ. Inhibitors of angiogenesis enter phase III testing. J Natl Cancer Inst. 1998;90:960-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 156. | Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1414] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 157. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1901] [Cited by in RCA: 1903] [Article Influence: 118.9] [Reference Citation Analysis (0)] |