Published online Aug 15, 2019. doi: 10.4251/wjgo.v11.i8.622
Peer-review started: May 21, 2019
First decision: July 22, 2019
Revised: July 31, 2019
Accepted: August 3, 2019
Article in press: August 3,2019
Published online: August 15, 2019
Processing time: 91 Days and 22.4 Hours
Histone Lysine Specific Demethylase 1 (LSD1) is the first histone demethylase to be discovered, which regulates various biological functions by making lysine of histone H3K4, H3K9 and non-histone substrates demethylated. Abnormal regulation of LSD1 is closely related to the occurrence and development of gastric cancer. The change of LSD1 expression level plays an important role in the proliferation and metastasis of gastric cancer cells. The study of its function and mechanism may provide a theoretical basis for early diagnosis and targeted therapy of gastric cancer.
To investigate the effect of downregulation of lysine-specific demethylase 1 (LSD1) expression on proliferation and invasion of gastric cancer cells and the possible regulatory mechanisms of the VEGF-C/PI3K/AKT signaling pathway.
The LSD1-specific short hairpin RNA (shRNA) interference plasmid was transiently transfected, and expression of LSD1 was downregulated. The cell proliferation ability of LSD1 was observed by CCK-8 assay after downregulating expression of LSD1. Transwell invasion assay was used to observe the change of cell invasion ability after downregulating expression of LSD1. Expression of phosphorylated phosphoinositide 3-kinase (p-PI3K), PI3K, p-AKT, AKT, vascular endothelial growth factor receptor (VEGFR)-3, matrix metalloproteinase (MMP)-2 and MMP-9 in each group was detected by Western blotting.
The cell proliferation ability of transiently transfected LSD1-shRNA interference plasmid group was significantly lower than that of the control group (P < 0.05). Transwell invasion assay showed that the number of cells across the membrane of the LSD1-shRNA transfection group (238.451 ± 5.216) was significantly lower than that of the control group (49.268 ± 6.984) (P < 0.01). Western blotting showed that expression level of VEGF-C, p-PI3K, PI3K, p-AKT, AKT, VEGFR-3, MMP-2 and MMP-9 in the LSD1-shRNA group was significantly lower than that in the control group (P < 0.05).
Downregulation of LSD1 expression inhibits metastatic potential of gastric cancer cells, and VEGF-C-mediated activation of PI3K/AKT signaling pathway, which may be an important mechanism for inhibiting lymph node metastasis in gastric cancer cells.
Core tip: The abnormal regulation of Lysine Specific Demethylase 1 (LSD1) is closely related to the occurrence and development of various cancers, such as gastric cancer. LSD1 is highly expressed in gastric cancer tissues, and the change in LSD1 expression level plays an important role in the proliferation and metastasis of gastric cancer cells. The VEGF-C/PI3K/AKT signaling pathway plays an important role in lymphangiogenesis and metastasis of gastric cancer. Therefore, this study investigated downregulation of LSD1 expression in order to observe the changes in gastric cancer cell proliferation and invasion, and further explored the role of the VEGF-C/PI3K/AKT signaling pathway. This study explores the role and mechanism of LSD1 in inhibiting gastric cancer cell metastasis from the perspective of epigenetics, providing a basis for early clinical diagnosis of gastric cancer metastasis.
- Citation: Pan HM, Lang WY, Yao LJ, Wang Y, Li XL. shRNA-interfering LSD1 inhibits proliferation and invasion of gastric cancer cells via VEGF-C/PI3K/AKT signaling pathway. World J Gastrointest Oncol 2019; 11(8): 622-633
- URL: https://www.wjgnet.com/1948-5204/full/v11/i8/622.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i8.622
Lysine-specific demethylase 1 (LSD1) is the first histone lysine demethylase discovered and belongs to the family of amine oxidases, also known as KDM1, NPAO, BHC110 and AOF1[1]. LSD1 demethylates lysine from histone (H3K4 and H3K9) and nonhistone substrates, further regulating various biological functions. In recent years, research on the impact of LSD1 on tumorigenesis and development has become a hotspot. Studies have shown that the abnormal regulation of LSD1 is closely related to the occurrence and development of various cancers, such as gastric cancer[2,3]. It has been reported that LSD1 is highly expressed in gastric cancer tissues, and the change in LSD1 expression level plays an important role in the proliferation and metastasis of gastric cancer cells[4-7]. The lymphatic system is an important pathway for tumor metastasis, such as in gastric cancer, lymphatic vessels are most commonly invaded in the process of metastasis. Gastric tumors generally have a poor prognosis and early detection is difficult, so molecular markers to improve early detection and predict outcomes are greatly needed[8].
The VEGF-C/PI3K/AKT signaling pathway plays an important role in lymphangiogenesis and metastasis of gastric cancer. Vascular endothelial growth factor (VEGF)-C binds to VEGF receptor (VEGFR)-3 and activates it, and further activates phosphoinositide 3-kinase (PI3K). The activated PI3K produces a second messenger phosphatidylinositol 3,4,5 trisphosphate in the cell membrane, which further binds to the PH-protein-containing signaling protein AKT in the cell, resulting in AKT activation. Activated AKT translocates to the cytoplasm or nucleus, upregulates expression of matrix metalloproteinase (MMP)-2 and MMP-9, and promotes lymphangiogenesis[9,10]. The effect of LSD1 on the VEGF-C/PI3K/AKT signaling pathway in gastric cancer cell proliferation and invasion has not been reported. Therefore, this study investigated downregulation of LSD1 expression in order to observe the changes in gastric cancer cell proliferation and invasion, and further explored the role of the VEGF-C/PI3K/AKT signaling pathway. The covalent modification of histones belongs to the category of epigenetics. This study explores the role and mechanism of LSD1 in inhibiting gastric cancer cell metastasis from the perspective of epigenetics, providing a basis for early clinical diagnosis of gastric cancer metastasis and providing new potential molecular targets for drug development.
Human gastric cancer MKN-45 cells (Chinese Academy of Sciences Cell Bank, Shanghai); Ham's F-12K medium (Sigma, St. Louis, MO, USA); β-actin monoclonal antibody (ab8226; Abcam, Cambridge, UK,); LSD1 goat anti-human polyclonal antibody (ab17721; Abcam); p-PI3K rabbit anti-goat antibody (ab32089; Abcam); PI3K rabbit anti-goat antibody (ab32089; Abcam); p-AKT rabbit anti-goat antibody (ab38449; Abcam); AKT rabbit anti-goat antibody (ab179463; Abcam); VEGFR-3 rabbit anti-goat antibody (ab213926; Abcam); MMP-2 rabbit anti-goat antibody (ab213910; Abcam); MMP-9 rabbit anti-goat antibody (ab73734; Abcam); Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA); and LSD1-shRNA plasmid (GenePharma, Shanghai, China).
A total of 50 specimens of gastric cancer patients who underwent surgery in our hospital between January 2016 and January 2019 were collected. The patients included 29 men and 21 women, aged 50–75 years, with a median age of 63 years. Thirty adjacent tissues (> 3 cm from the edge of the tumor) were taken as controls. Specimens were stored in an ultralow temperature freezer at − 80 °C.
The logarithmic growth phase cells were seeded in a six-well plate to adjust the cell density to 5 × 104. When the cell confluence was about 60%, the LSD1-shRNA plasmid was transiently transfected with Lipofectamine 2000. After 4 h, the complete medium without the double antibody was replaced and the culture was continued. After 24 and 48 h, expression of fluorescence was observed under a fluorescent inverted microscope. The cells were divided into two groups, transfected LSD1-shRNA group and control group. The transfected LSD1-shRNA group was transiently transfected with LSD1-shRNA interference plasmid, and the control group was normal cultured cells.
After 24 h transfection, the two groups of cells were harvested and RNA was extracted by TRIzol reagent. RNA was reverse transcribed into cDNA using the Prime Script RT Reagent Kit, and then frozen at −20 °C. The LSD1 primer was designed using Primer 5.0; the upstream primer was 5'-GACTTCTTGGCAGAGTTGTC-3', and the downstream primer was 5'-GTGAAAGAGTTGCAGATC-3'. Quantitative reverse transcription polymerase chain reaction (RT-PCR) was carried out using the TaKaRa Taq kit. The total reaction system was 25.0 μL; the reaction conditions were 95 °C for 30 s, 58 °C for 35 s and 72 °C for 30 s, for a total of 40 cycles. The reaction product was subjected to 1.5% agarose gel electrophoresis, photographed by a gel imager, and analyzed by gray value using the Image Lab software provided by the gel imager.
The cells were digested at 24, 48 and 72 h after transfection, and the cell suspension was adjusted to a density of 2×104 cells/mL. The cells were added to a 96-well plate for 2 h, 100 μL per well. Ten microliters of CCK8 solution was added to each well and the cells were incubated for 4 h. Absorbance at 450 nm was measured using a microplate reader.
Cell viability was calculated as follows: cell viability* (%) = [A (transfection group) − A (blank)] / [A (control group) − A (blank)] × 100; where A (transfection group) has absorbance of wells with transfected cells and CCK8 solution; A (blank) has absorbance of wells with medium and CCK8 solution, without cells; and A (control group) has absorbance of wells with control group cells and CCK8 solution.
After 48 h transfection, the cells in each group were collected, washed several times with pre-cooled phosphate-buffered saline, and the cells were scraped off, and the pellet was centrifuged at 1000 rpm/min. The cells were lysed, centrifuged, and the supernatant was collected. The extracted protein concentration was determined using the BCA method. Fifty micrograms of protein and loading buffer (5×) were mixed at a ratio of 4: 1, heated in a boiling water bath, fully denatured, cooled to room temperature, and loaded. SDS-PAGE was performed and the film was transferred to a PVDF membrane, which was removed, labeled, shaken at 37 °C, and 5% blocking solution (30 mL TBST and 1.5 g skimmed milk powder) was added and the membrane was blocked for 2 h. Primary antibodies to LSD1 1: 1000, VEGF-C 1: 300, p-PI3K 1: 500, PI3K 1: 400, p-AKT 1: 400, AKT 1: 1000, VEGFR-3 1: 600, MMP-2 1: 600, MMP-9 1: 800, and β-actin 1: 2000 were added. The PVDF membrane was placed in a solution of horseradish-peroxidase-conjugated secondary antibody (1: 5000) diluted with 5% skimmed milk powder and TBST, and incubated at 37 °C for 1 h. The film was washed, developed, and fixed, and the image was observed and saved using a gel imaging system. Gray value analysis was performed using Quantity One software.
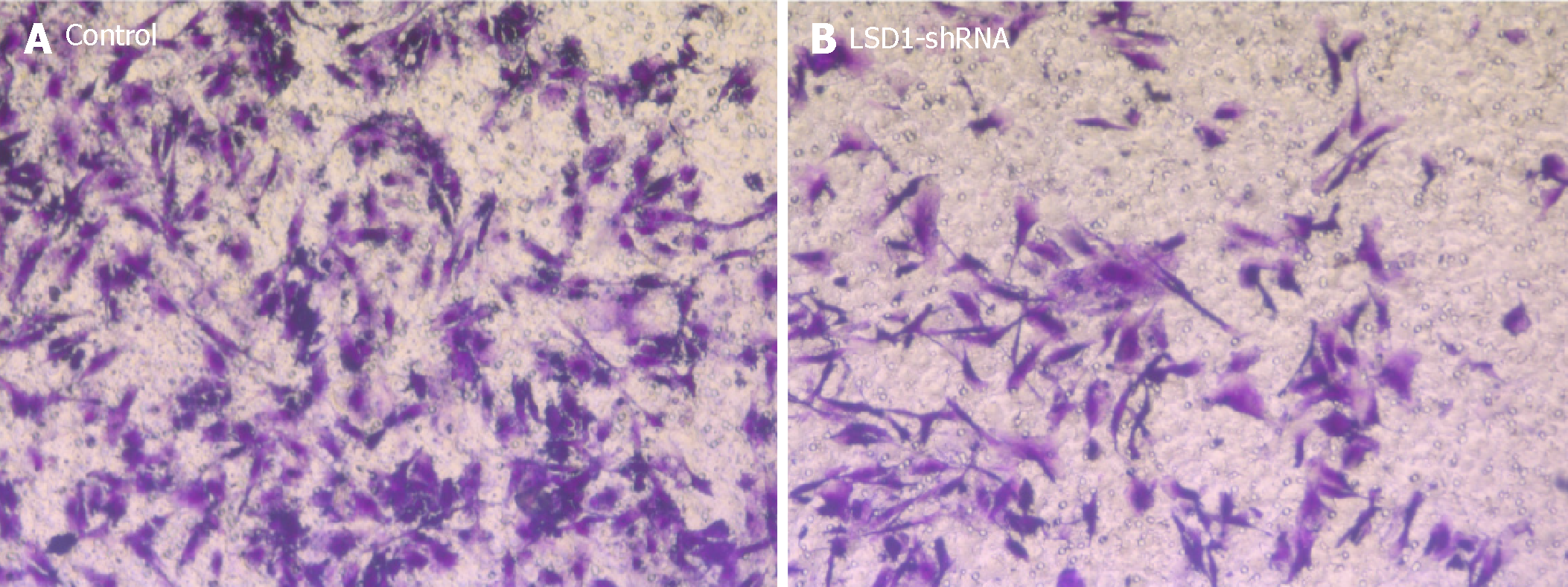
Two groups of cells in logarithmic growth phase were taken, resuspended in serum-free medium, and 200 μL of cell suspension was added to a Transwell chamber coated with Matrigel matrix. Six hundred microliters of complete medium was added to the lower chamber. After 24 h, the cells in the upper chamber were removed with a cotton swab, and the chamber was air dried, placed on a glass slide, fixed in 4% paraformaldehyde, stained with 0.1% crystal violet, and observed under an inverted microscope, and 10 fields of view were selected to count and photographs were taken.
Statistical analysis was performed using SPSS version 20.0. All values were expressed as median (M) and interquartile range (Q) for the differences in protein expression, cell proliferation and cell invasion between the two groups. Mann–Whitney U test was performed, α = 0.05. The relationship between LSD1 expression and clinical and pathological parameters was tested by χ2 test. P < 0.05 was considered statistically significant.
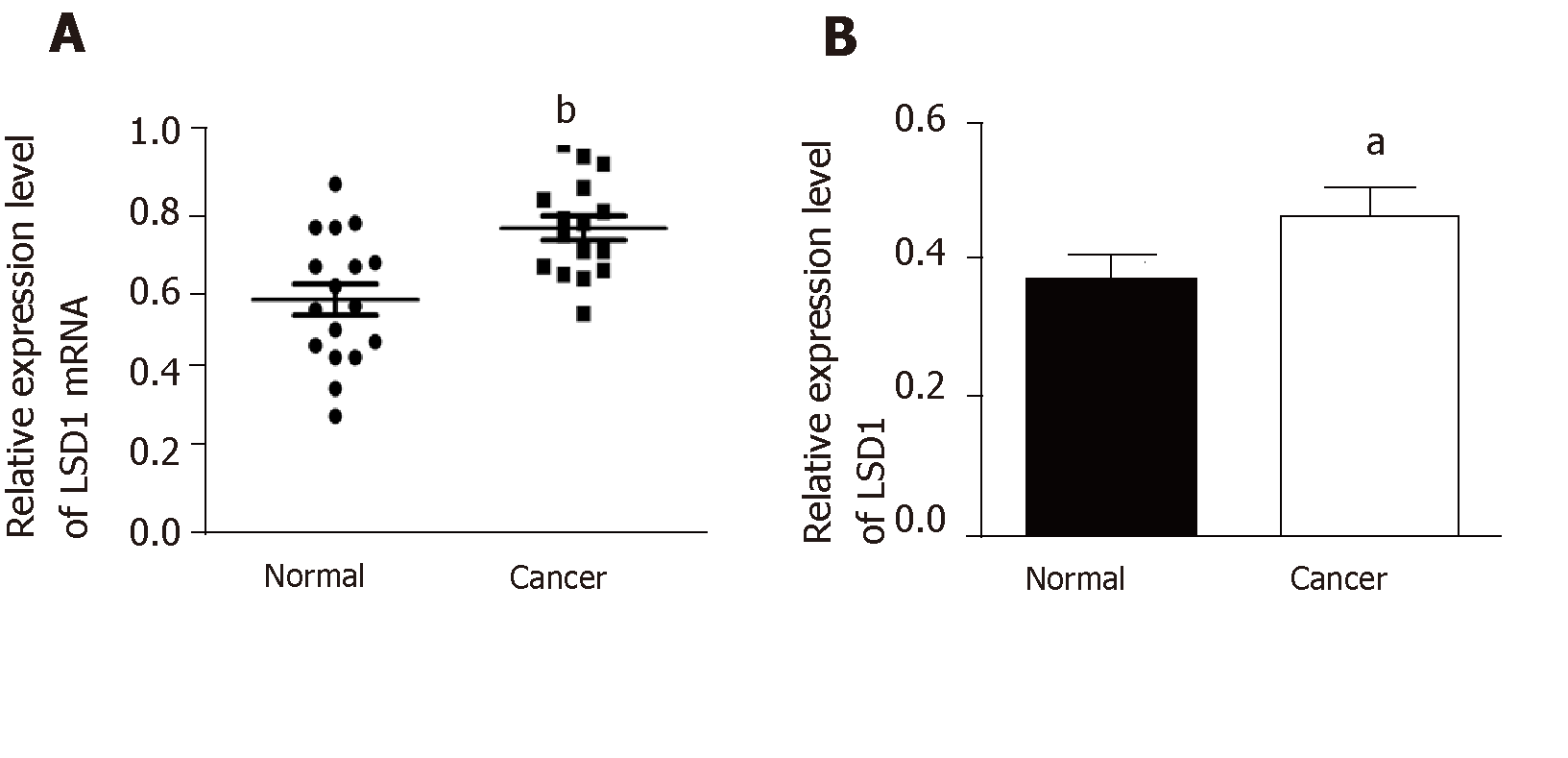
Quantitative RT-PCR and Western blotting analysis showed that expression of LSD1 mRNA and protein in gastric cancer tissues was significantly higher than that in adjacent tissues (Figure 1A and 1B, P < 0.05).
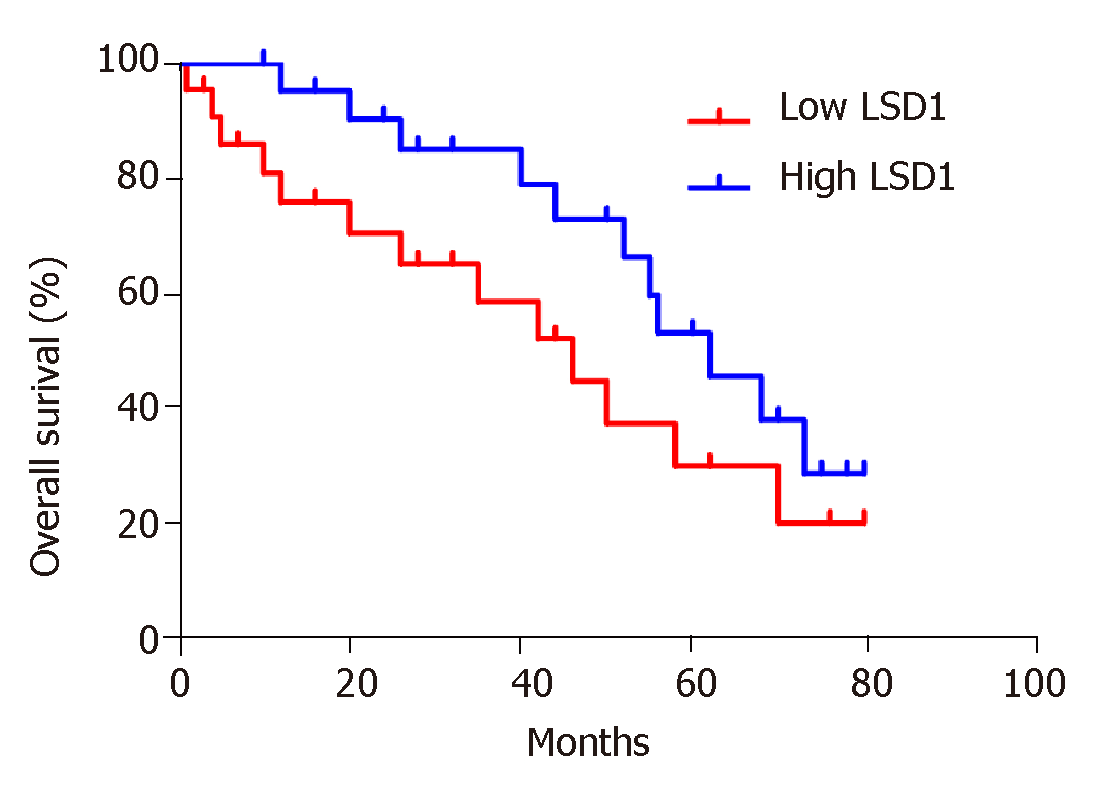
Based on the median value of LSD1 expression in 50 patients with gastric cancer, patients were divided into a high LSD1 expression group (greater than median value) and low LSD1 expression group (less than median value). LSD1 expression was not associated with gender, age, and degree of invasion in patients with gastric cancer. Tumor size, differentiation, Duke stage, and lymph node metastasis rate were higher in the high LSD1 expression group than in the low LSD1 expression group (Table 1). Survival analysis showed that the overall survival rate of patients with high LSD1 expression was lower than that of patients with low LSD1 expression (Figure 2).
| Pathological parameters | n | Low LSD1 expression | High LSD1 expression | P value |
| Gender | 0.779 | |||
| Male | 29 | 12 | 17 | |
| Female | 21 | 12 | 9 | |
| Age (yr) | 0.528 | |||
| ≥ 60 | 23 | 13 | 10 | |
| ≤ 60 | 27 | 8 | 19 | |
| Tumor diameter (cm) | 0.143 | |||
| > 5 | 26 | 19 | 7 | |
| ≤ 5 | 24 | 11 | 13 | |
| Local lymph node metastasis | 0.006 | |||
| Yes | 18 | 9 | 9 | |
| No | 32 | 10 | 22 | |
| Infiltration depth | 0.285 | |||
| T1 | 9 | 3 | 6 | |
| T2 | 8 | 5 | 3 | |
| T3 | 23 | 5 | 18 | |
| T4 | 10 | 3 | 7 | |
| Differentiation | 0.026 | |||
| high | 26 | 6 | 20 | |
| medium | 14 | 2 | 12 | |
| low | 10 | 3 | 7 | |
| Duke stage | 0.017 | |||
| A | 10 | 1 | 9 | |
| B | 13 | 3 | 10 | |
| C | 9 | 0 | 9 | |
| D | 17 | 4 | 13 |
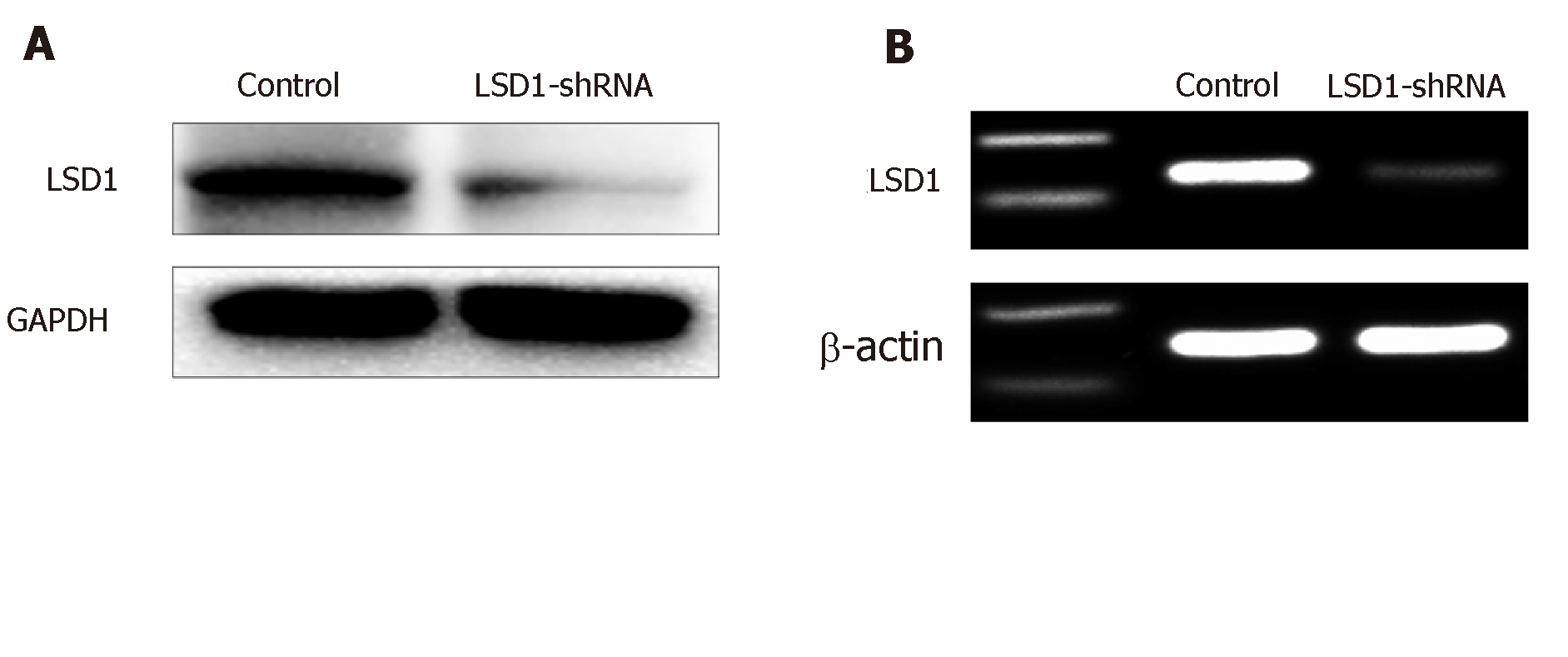
RT-PCR showed that expression of LSD1 mRNA in the LSD1-shRNA transfection group (0.33 ± 0.05, n = 10) was significantly lower than that in the control group (0.63 ± 0.02, n = 10) (P < 0.05). Western blotting showed that protein expression in the LSD1-shRNA transfection group (0.25 ± 0.015, n = 10) was significantly lower than that in the control group (0.963 ± 0.037, n = 10) (P < 0.05). It is suggested that transient transfection of LSD1-shRNA interference plasmid can downregulate expression of LSD1 (Figure 3, Table 2).
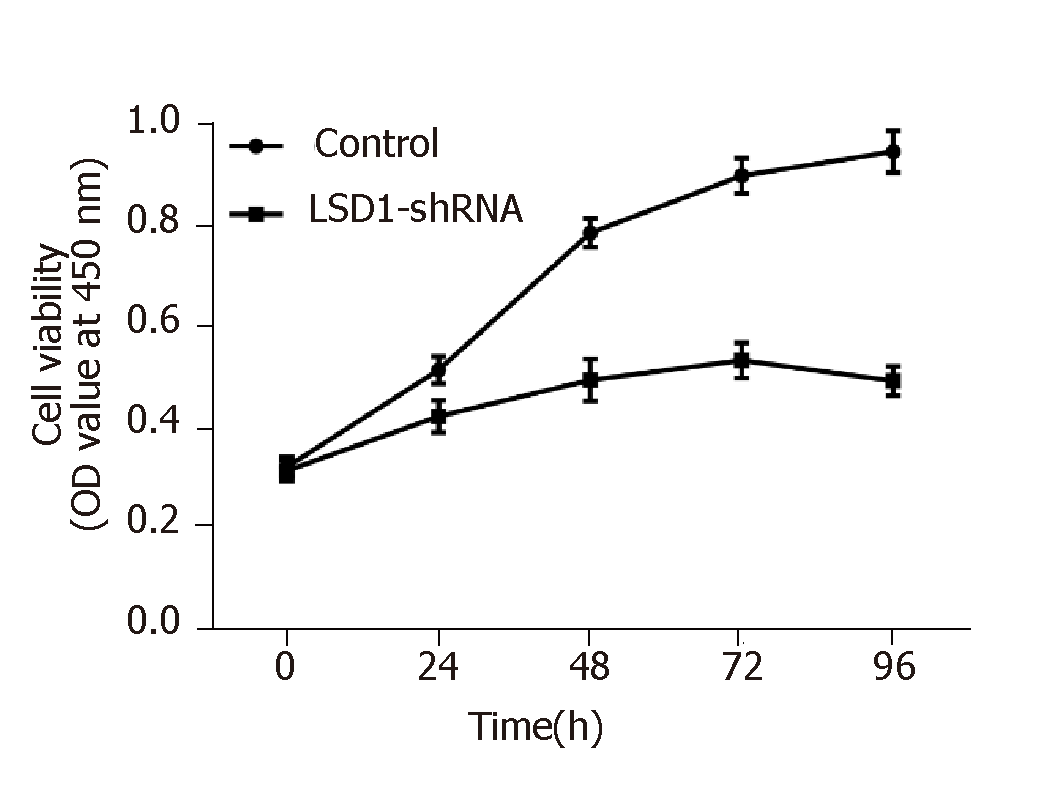
CCK-8 assay showed that cell viability of the LSD1-shRNA transfection group was significantly lower than that of the Control group at the same time point (P < 0.05). It is suggested that downregulation of LSD1 expression can inhibit proliferation of gastric cancer cells (Figure 4, Table 3).
After transfecting the cells with LSD1-shRNA plasmid for 48 h, the cells were digested and resuspended in serum-free medium for Transwell invasion assay. The number of cells across the membrane in the LSD1-shRNA transfection group (238.451 ± 5.216) was significantly lower than that in the Control group (49.268 ± 6.984) (P < 0.01). It is suggested that downregulation of LSD1 expression can inhibit the invasiveness of gastric cancer cells (Figure 5).
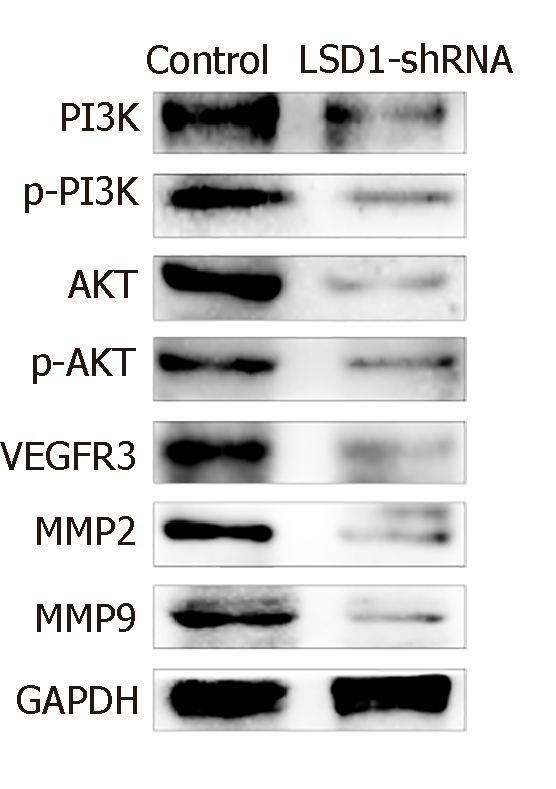
After transfection for 48 h, expression of phosphorylated (p)-PI3K, PI3K, p-AKT, AKT, VEGFR-3, MMP-2 and MMP-9 protein in LSD1-shRNA group was significantly lower than that in the Control group (P < 0.05). It is suggested that downregulation of LSD1 expression can inhibit expression of VEGF-C in gastric cancer cells and inhibit activation of the VEGF-C-mediated PI3K/AKT signaling pathway (Figure 6).
LSD1 was the first histone lysine demethylase to be discovered. This finding confirms the dynamic balance of histone methylation. This finding also provides new research ideas for epigenetic development of histone modifications [1]. Studies have shown that LSD1 can demethylate H3K4mel/2 and H3K9mel/2, thereby regulating downstream gene transcription. LSDl can remove methylation of the tumor suppressor protein p53 K370, inhibit p53 activity, and inhibit p53 target gene expression[11-14].
Studies have shown that LSD1 plays an important role in the development of many tumors[15-18]. Wang et al[2] found that downregulation of LSD1 expression can inhibit breast cancer invasion and metastasis by affecting the transforming growth factor-β1 signaling pathway. It was found that LSD1 is highly expressed in prostate cancer, and downregulation of LSD1 expression inhibits androgen-induced transcriptional activation, thereby inhibiting cell proliferation. LSD1 inhibitor pargyline blocks LSD1 from demethylating H3K9, thereby inhibiting androgen-receptor-dependent transcription[19,20]. Studies in colon cancer cells have found that inhibition of LSD1 expression can inhibit the metastatic potential of colon cancer cells, and most of the genes silenced in the development of colon cancer will be re-expressed[21]. LSD1 inhibitors can inhibit epithelial–mesenchymal transition by inhibiting the activity of LSD1, thereby inhibiting the metastasis and invasion of MGC-80 gastric cancer cells[22]. Our research found that LSD1 is highly expressed in gastric cancer tissues, consistent with existing reports[22-25]. Clinical data analysis showed that the expression of LSD1 in gastric cancer cases with different degrees of differentiation, different stages, and with or without lymph node metastasis is significant, suggesting that LSD1 plays an important role in the differentiation and metastasis of gastric cancer. In this study, human gastric cancer MKN-45 cell line was selected to study the proliferation and invasiveness of human gastric cancer cells by transiently transfecting LSD1-shRNA interference plasmid to downregulate expression of LSD1. The effect of LSD1 on the proliferation and metastatic potential of gastric cancer cells has been reported previously; therefore, this study aimed to investigate further the mechanism of action. Therefore, only the LSD1-shRNA group and control group were established in this study, and no negative control group[23-26]. Proliferation of transfected LSD1-shRNA cells was significantly lower than that in the control group. The number of cells crossing the membrane in the LSD1-shRNA transfected group was significantly lower than that in the control group. These results suggest that downregulation of LSD1 expression can inhibit proliferation and invasion of human gastric cancer MKN-45 cells, suggesting that LSD1 expression is closely related to the metastatic potential of gastric cancer. The results of this study are consistent with previous studies[27].
In the study of LSD1 expression and the metastatic potential mechanism of gastric cancer, there is no report on the VEGF-C/PI3K/AKT signaling pathway. Lymph node metastasis is the most common form of metastasis in gastric cancer, and it is also one of the important factors leading to poor prognosis and recurrence. Early detection of lymph node metastasis is also difficult[28-31]. It is closely related to lymphangiogenesis in gastric or other cancers[32,33]. VEGF-C is an important lymphangiogenic factor and plays an important role in lymphangiogenesis[34,35]. Our study found that expression of VEGF-C protein in gastric cancer cells decreased 48 h after transfection of LSD-shRNA interference plasmid, suggesting that downregulation of LSD1 expression inhibits tumor metastasis by inhibiting VEGF-C expression.
Studies have shown that VEGF-C-mediated activation of the PI3K/AKT signaling pathway plays an important regulatory role in lymph node metastasis of tumors[36,37]. VEGFR-3 can bind to VEGF-C, and is induced to be phosphorylated and activated, thereby activating the PI3K/AKT signaling pathway, upregulating MMP-2 and MMP-9 expression, and playing an important regulatory role. In this study, we found that expression of p-PI3K, PI3K, p-AKT, AKT, VEGFR-3, MMP-2 and MMP-9 in gastric cancer cells decreased 48 h after transfection of LSD1-shRNA interference plasmid, suggesting that downregulation of LSD1 expression can be inhibited. VEGF-C-mediated activation of the PI3K/AKT signaling pathway, downregulation of LSD1 expression, and inhibition of the VEGF-C/PI3K/AKT signaling pathway are some of the important mechanisms for inhibition of gastric cancer cell metastasis. In this study, we found that expression of p-PI3K, PI3K, p-AKT, AKT, VEGFR-3, MMP-2 and MMP-9 in gastric cancer cells decreased 48 h after transfection of LSD1-shRNA interference plasmid, suggesting that downregulation of LSD1 expression can inhibit VEGF-C-mediated activation of the PI3K/AKT signaling pathway; and downregulation of LSD1 expression inhibits the VEGF-C/PI3K/AKT signaling pathway, which is one of the important mechanisms that inhibit metastasis of gastric cancer cells.
In a follow-up study, we aim to investigate the effect of downregulating expression of LSD1 on lymphangiogenesis of gastric cancer cells during metastasis to supplement the results of this study, by studying the effects of downregulating LSD1 expression on the growth, migration and lumen formation of gastric cancer cells.
In summary, downregulation of LSD1 expression can inhibit proliferation and invasion of human gastric cancer cell line MKN-45. Downregulation of LSD1 expression inhibits the VEGF-C/PI3K/AKT signaling pathway, which is one of the important mechanisms that may inhibit gastric cancer cell metastasis. The results of this study will provide a new experimental basis for the early diagnosis and treatment of gastric cancer and the development of new targets for antitumor drugs.
Epigenetics means that the DNA sequence is unchanged, and the cell phenotype or gene expression is genetically altered, mainly including DNA methylation, histone covalent modification, and chromatin remodeling. Among them, histone covalent modification activates or inhibits gene expression by regulating chromatin structure. Histone Lysine Specific Demethylase 1 (LSD1) is the first histone demethylase to be discovered, which regulates various biological functions by making lysine of histone H3K4, H3K9 and non-histone substrates demethylated. Abnormal regulation of LSD1 is closely related to the occurrence and development of gastric cancer. At present, there are few reports on the role of LSD1 expression level in the proliferation and metastasis of gastric cancer cells, and there is no literature reports on the role of VEGF-C/PI3K/AKT signaling pathway in LSD1 expression and the metastatic potential of gastric cancer. This research is innovative and can provide a theoretical basis for early diagnosis and targeted therapy of gastric cancer.
The main content of this study was to down-regulate the expression of LSD1 to observe the changes in the proliferation and invasion of human gastric cancer MKN-45 cells, and the role of VEGF-C/PI3K/AKT signaling pathway in inhibiting the metastasis of gastric cancer cells by down-regulating the expression of LSD1. Key questions to be addressed in this study: (1) The changes on the proliferation and invasion of human gastric cancer MKN-45 cells after down-regulating the expression of LSD1; and (2) The role of the VEGF-C/PI3K/AKT signaling pathway in it. Research significance: The results of this study will provide a new experimental basis for the early diagnosis and treatment of gastric cancer and the development of new targets for anti-tumor drugs, and add a new experimental basis for the study of epigenetics in cancer therapy.
The main goal was to investigate the down-regulation of the expression of LSD1 on the proliferation and invasion of human gastric cancer MKN-45 cell line and its mechanism. It has been found that down-regulation of LSD1 expression inhibits VEGF-C/PI3K/AKT signaling pathway and thereby inhibits gastric cancer cell metastasis. The results of this study will provide a new experimental basis for the early diagnosis and treatment of clinical gastric cancer and the development of new targets for anti-tumor drugs.
In this study, human gastric cancer MKN-45 cell line was selected and transiently transfected with LSD1 shRNA interference plasmid to down-regulate the expression of LSD1. The proliferation and invasion ability of human gastric cancer MKN-45 cell line were observed by CCK-8 cell proliferation assay and Transwell invasion assay. Western Blot was used to detect changes in molecular protein levels associated with gastric cancer metastasis in the VEGF-C/PI3K/AKT signaling pathway.
This study found that down-regulation of LSD1 expression inhibits VEGF-C/PI3K/AKT signaling pathway and further inhibits gastric cancer cell metastasis. No relevant literature reports have been reported. In order to further study the effect of down-regulating LSD1 expression on lymphangiogenesis during gastric cancer cell metastasis, we need to study the effect of down-regulating LSD1 expression on the growth, migration and lumen formation of gastric cancer cell lymphatic endothelial cells in the future, and thus supplement the results of this study.
Down-regulation of LSD1 expression inhibits the proliferation and invasion of human gastric cancer MKN-45 cell line. Down-regulation of LSD1 expression can inhibit VEGF-C/PI3K/AKT signaling pathway, which may be one of the important mechanisms for its inhibition of gastric cancer cell metastasis. Down-regulation of LSD1 expression can inhibit the proliferation and invasion of human gastric cancer MKN-45 cell line; down-regulation of LSD1 expression inhibits VEGF-C/PI3K/AKT signaling pathway, thereby inhibiting gastric cancer cell metastasis. Is down-regulation of LSD1 expression promoting or inhibiting the proliferation and invasion of human gastric cancer MKN-45 cell line. Down-regulation of LSD1 expression inhibits VEGF-C/PI3K/AKT signaling pathway and inhibits gastric cancer cell metastasis. Down-regulation of LSD1 expression inhibits VEGF-C/PI3K/AKT signaling pathway and thereby inhibits gastric cancer cell metastasis. Down-regulation of LSD1 expression can inhibit the proliferation and invasion of human gastric cancer MKN-45 cell line, and also can inhibit the metastasis of gastric cancer cells by restraining VEGF-C/PI3K/AKT signal pathway. Epigenetic research is important for the early diagnosis and treatment of tumors.
This study has produced innovative results through common basic cell experiments. In this study, we intend to study the effect of down-regulating the expression of LSD1 on the growth, migration and lumen formation of lymphatic endothelial cells in gastric cancer cells, and to study the effect of down-regulating the expression of LSD1 on lymphangiogenesis during gastric cancer cell metastasis in order to supplement the results of this study. Animal experiments will also be performed to verify and supplement the results.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Georgescu EF, Parakkal D, Raisch KP S-Editor: Wang JL L-Editor: A E-Editor: Zhou BX
| 1. | Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3213] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 544] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Jie D, Zhongmin Z, Guoqing L, Sheng L, Yi Z, Jing W, Liang Z. Positive expression of LSD1 and negative expression of E-cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Bajenova O, Chaika N, Tolkunova E, Davydov-Sinitsyn A, Gapon S, Thomas P, O'Brien S. Carcinoembryonic antigen promotes colorectal cancer progression by targeting adherens junction complexes. Exp Cell Res. 2014;324:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Liu YW, Xia R, Lu K, Xie M, Yang F, Sun M, De W, Wang C, Ji G. LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Mol Cancer. 2017;16:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Cao C, Vasilatos SN, Bhargava R, Fine JL, Oesterreich S, Davidson NE, Huang Y. Functional interaction of histone deacetylase 5 (HDAC5) and lysine-specific demethylase 1 (LSD1) promotes breast cancer progression. Oncogene. 2017;36:133-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Ding J, Xie M, Lian Y, Zhu Y, Peng P, Wang J, Wang L, Wang K. Long noncoding RNA HOXA-AS2 represses P21 and KLF2 expression transcription by binding with EZH2, LSD1 in colorectal cancer. Oncogenesis. 2017;6:e288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Zhou Y, Hu Z. The Functions of Circulating Tumor Cells in Early Diagnosis and Surveillance During Cancer Advancement. J Transl Int Med. 2017;5:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Sun S, Gong F, Liu P, Miao Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene. 2018;664:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Ma X, Yao H, Yang Y, Jin L, Wang Y, Wu L, Yang S, Cheng K. miR-195 suppresses abdominal aortic aneurysm through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. Int J Mol Med. 2018;41:2350-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Wang P, Fan F, Li X, Sun X, Ma L, Wu J, Shen C, Zhu H, Dong Z, Wang C, Zhang S, Zhao X, Ma X, Zou Y, Hu K, Sun A, Ge J. Riboflavin attenuates myocardial injury via LSD1-mediated crosstalk between phospholipid metabolism and histone methylation in mice with experimental myocardial infarction. J Mol Cell Cardiol. 2018;115:115-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Local A, Huang H, Albuquerque CP, Singh N, Lee AY, Wang W, Wang C, Hsia JE, Shiau AK, Ge K, Corbett KD, Wang D, Zhou H, Ren B. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat Genet. 2018;50:73-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 13. | Zhang L, Carnesecchi J, Cerutti C, Tribollet V, Périan S, Forcet C, Wong J, Vanacker JM. LSD1-ERRα complex requires NRF1 to positively regulate transcription and cell invasion. Sci Rep. 2018;8:10041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li A, You D, Li W, Cui Y, He Y, Li W, Chen Y, Feng X, Sun S, Chai R, Li H. Novel compounds protect auditory hair cells against gentamycin-induced apoptosis by maintaining the expression level of H3K4me2. Drug Deliv. 2018;25:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Fu X, Zhang P, Yu B. Advances toward LSD1 inhibitors for cancer therapy. Future Med Chem. 2017;9:1227-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Zheng YC, Yu B, Jiang GZ, Feng XJ, He PX, Chu XY, Zhao W, Liu HM. Irreversible LSD1 Inhibitors: Application of Tranylcypromine and Its Derivatives in Cancer Treatment. Curr Top Med Chem. 2016;16:2179-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Duan YC, Ma YC, Qin WP, Ding LN, Zheng YC, Zhu YL, Zhai XY, Yang J, Ma CY, Guan YY. Design and synthesis of tranylcypromine derivatives as novel LSD1/HDACs dual inhibitors for cancer treatment. Eur J Med Chem. 2017;140:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Liu C, Liu L, Chen X, Cheng J, Zhang H, Zhang C, Shan J, Shen J, Qian C. LSD1 Stimulates Cancer-Associated Fibroblasts to Drive Notch3-Dependent Self-Renewal of Liver Cancer Stem-like Cells. Cancer Res. 2018;78:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Liang Y, Ahmed M, Guo H, Soares F, Hua JT, Gao S, Lu C, Poon C, Han W, Langstein J, Ekram MB, Li B, Davicioni E, Takhar M, Erho N, Karnes RJ, Chadwick D, van der Kwast T, Boutros PC, Arrowsmith CH, Feng FY, Joshua AM, Zoubeidi A, Cai C, He HH. LSD1-Mediated Epigenetic Reprogramming Drives CENPE Expression and Prostate Cancer Progression. Cancer Res. 2017;77:5479-5490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Sehrawat A, Gao L, Wang Y, Bankhead A, McWeeney SK, King CJ, Schwartzman J, Urrutia J, Bisson WH, Coleman DJ, Joshi SK, Kim DH, Sampson DA, Weinmann S, Kallakury BVS, Berry DL, Haque R, Van Den Eeden SK, Sharma S, Bearss J, Beer TM, Thomas GV, Heiser LM, Alumkal JJ. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc Natl Acad Sci U S A. 2018;115:E4179-E4188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Xi J, Xu S, Zhang L, Bi X, Ren Y, Liu YC, Gu Y, Xu Y, Lan F, Zha X. Design, synthesis and biological activity of 4-(4-benzyloxy)phenoxypiperidines as selective and reversible LSD1 inhibitors. Bioorg Chem. 2018;78:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sivanandhan D, Rajagopalan S, Nair S. Novel dual inhibitors of LSD1-HDAC for treatment of cancer. Cancer Research. 2015;75:3509-3509. [DOI] [Full Text] |
| 23. | Zheng YC, Duan YC, Ma JL, Xu RM, Zi X, Lv WL, Wang MM, Ye XW, Zhu S, Mobley D, Zhu YY, Wang JW, Li JF, Wang ZR, Zhao W, Liu HM. Triazole-dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J Med Chem. 2013;56:8543-8560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Ma JL, Zhang T, Suo FZ, Chang J, Wan XB, Feng XJ, Zheng YC, Liu HM. Lysine-specific demethylase 1 activation by vitamin B2 attenuates efficacy of apatinib for proliferation and migration of gastric cancer cell MGC-803. J Cell Biochem. 2018;119:4957-4966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Hong X, Huang H, Qiu X, Ding Z, Feng X, Zhu Y, Zhuo H, Hou J, Zhao J, Cai W, Sha R, Hong X, Li Y, Song H, Zhang Z. Targeting posttranslational modifications of RIOK1 inhibits the progression of colorectal and gastric cancers. Elife. 2018;7:e29511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Huang M, Hou J, Wang Y, Xie M, Wei C, Nie F, Wang Z, Sun M. Long Noncoding RNA LINC00673 Is Activated by SP1 and Exerts Oncogenic Properties by Interacting with LSD1 and EZH2 in Gastric Cancer. Mol Ther. 2017;25:1014-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 27. | Li Y, Tian X, Sui CG, Jiang YH, Liu YP, Meng FD. Interference of lysine-specific demethylase 1 inhibits cellular invasion and proliferation in vivo in gastric cancer MKN-28 cells. Biomed Pharmacother. 2016;82:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Choudhary NS, Bodh V, Kumar N, Puri R, Sarin H, Guleria M, Piplani T, Krishan S, Rai R, Sud R. Yield of endoscopic ultrasound-guided fine needle aspiration for subcentimetric lymph nodes: A comparison to larger nodes. Endosc Ultrasound. 2017;6:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ge N, Zhang S, Jin Z, Sun S, Yang A, Wang B, Wang G, Xu G, Hao J, Zhong L, Zhong N, Li P, Zhu Q, Nian W, Li W, Zhang X, Zhou X, Yang X, Cui Y, Ding Z. Clinical use of endoscopic ultrasound-guided fine-needle aspiration: Guidelines and recommendations from Chinese Society of Digestive Endoscopy. Endosc Ultrasound. 2017;6:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Hocke M, Ignee A, Dietrich C. Role of contrast-enhanced endoscopic ultrasound in lymph nodes. Endosc Ultrasound. 2017;6:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Cartana ET, Gheonea DI, Cherciu IF, Streaţa I, Uscatu CD, Nicoli ER, Ioana M, Pirici D, Georgescu CV, Alexandru DO, Şurlin V, Gruionu G, Săftoiu A. Assessing tumor angiogenesis in colorectal cancer by quantitative contrast-enhanced endoscopic ultrasound and molecular and immunohistochemical analysis. Endosc Ultrasound. 2018;7:175-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Yamane S, Katada C, Tanabe S, Azuma M, Ishido K, Yano T, Wada T, Watanabe A, Kawanishi N, Furue Y, Kondo Y, Komori S, Ishiyama H, Hayakawa K, Koizumi W. Clinical Outcomes in Patients with Cancer of Unknown Primary Site Treated By Gastrointestinal Oncologists. J Transl Int Med. 2017;5:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Mohri J, Katada C, Ueda M, Sugawara M, Yamashita K, Moriya H, Komori S, Hayakawa K, Koizumi W, Atsuda K. Predisposing Factors for Chemotherapy-induced Nephrotoxicity in Patients with Advanced Esophageal Cancer Who Received Combination Chemotherapy with Docetaxel, Cisplatin, and 5-fluorouracil. J Transl Int Med. 2018;6:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Montagnani F, Di Leonardo G, Pino M, Perboni S, Ribecco A, Fioretto L. Protracted Inhibition of Vascular Endothelial Growth Factor Signaling Improves Survival in Metastatic Colorectal Cancer: A Systematic Review. J Transl Int Med. 2017;5:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Gousopoulos E, Proulx ST, Bachmann SB, Dieterich LC, Scholl J, Karaman S, Bianchi R, Detmar M. An Important Role of VEGF-C in Promoting Lymphedema Development. J Invest Dermatol. 2017;137:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Liao XZ, Tao LT, Liu JH, Gu YY, Xie J, Chen Y, Lin MG, Liu TL, Wang DM, Guo HY, Mo SL. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Fernández JG, Rodríguez DA, Valenzuela M, Calderon C, Urzúa U, Munroe D, Rosas C, Lemus D, Díaz N, Wright MC, Leyton L, Tapia JC, Quest AF. Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription. Mol Cancer. 2014;13:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |